Abstract
Specific humoral immune responses in a clinical trial on cattle for vaccines against contagious bovine pleuropneumonia (CBPP) were investigated. The trial included a subunit vaccine consisting of five recombinant putative variable surface proteins of the infectious agent Mycoplasma mycoides subsp. mycoides small colony type (M. mycoides SC) compared to the currently approved attenuated vaccine strain T1/44 and untreated controls. Humoral immune responses to 65 individual recombinant surface proteins of M. mycoides SC were monitored by a recently developed bead-based array assay. Responses to the subunit vaccine components were found to be weak. Animals vaccinated with this vaccine were not protected and had CBPP lesions similar to those of the untreated controls. In correlating protein-specific humoral responses to T1/44-induced immunity, five proteins associated with a protective immune response were identified by statistical evaluation, namely, MSC_1046 (LppQ), MSC_0271, MSC_0136, MSC_0079, and MSC_0431. These five proteins may be important candidates in the development of a novel subunit vaccine against CBPP.
Contagious bovine pleuropneumonia (CBPP) is a severe respiratory disease in cattle caused by Mycoplasma mycoides subsp. mycoides small colony type (M. mycoides SC). CBPP has a major impact on livestock production, and its potential for serious and rapid spread across national borders makes the disease notifiable to the World Organization for Animal Health (OIE). Affected countries are excluded from the international cattle trade. The disease is causing vast problems, with severe socioeconomic consequences in many African countries (12, 39). CBPP was eradicated from Europe in the beginning of the 20th century (11) but has reemerged every decade since (26). Slaughtering of all infected herds enabled the European eradication (40). Although the strategy was successfully used in Botswana during 1995 (40), it was directly correlated with decreased public health (4) and is not considered to be a reasonable strategy for CBPP control in Africa (9, 20, 21, 39). The two remaining options are chemotherapy and vaccination campaigns. The use of chemotherapy in CBPP treatment is controversial and officially discouraged due to the risk of creating silent carriers of the disease (32), although recent antibiotic trials have shown positive effects (16, 25).
Extensive vaccination is the preferred method for CBPP prevention in Africa (22, 39), and the currently approved vaccine, T1/44 (and its streptomycin-resistant derivate, T1-SR), is an attenuated live M. mycoides SC strain obtained from 44 passages in eggs that has been in use for nearly 60 years. It has several drawbacks, such as short-term immunity (11), poor protection in recent trials (26, 34), and even pathogenicity (23). Changes in the formulation have been suggested to easily improve the live vaccines (22), but most recent work has been done to find completely new vaccine formulas. Trials with a saponin-inactivated whole-cell antigen (27) and immunostimulating complex (ISCOM) formulations from the whole mycoplasma cell membrane (2, 17) have so far been unsuccessful. Two subunit vaccine candidates have been evaluated: the first based on the capsular polysaccharide of M. mycoides SC (36) and the second based on the immunogenic lipoprotein LppQ (27). The polysaccharide antigen has only been evaluated in mice, and the LppQ immunizations seemed to exacerbate the CBPP symptoms in cattle. Subunit vaccines are still desired for CBPP prevention (10), and possible components can be proteins of known pathogenicity mechanisms, as recently reviewed (30). Among these are the well-characterized lipoproteins LppA (6), LppB (38), LppC (31), and LppQ (1) and the variable surface protein Vmm (29). LppQ is the predominant antigen during infection in cattle (3) and has been subcloned to develop an enzyme-linked immunosorbent assay (ELISA) for CBPP diagnostics (5). It has also recently been suggested as part of a multiprotein ELISA with LppA and other surface proteins (24).
In this study, we performed a clinical trial in cattle with a recombinant antigen vaccine candidate and analyzed the protein-specific humoral immune responses evoked by this potential vaccine, the T1/44 vaccine, and control cattle with CBPP. The antigen for immunizations consisted of five previously studied putative variable surface proteins expressed as recombinant proteins in Escherichia coli (15). Since, historically, only those vaccines of live M. mycoides SC strains have conferred satisfactory protective immune responses, the aim was to make a vaccine of surface components that may differ between M. mycoides SC organisms in a host environment and those cultivated in growth media. Variable surface proteins are generally believed to enhance colonization of the host tissue and help evade host immune responses by antigenic variation, as summarized in reference 29. In a subunit vaccine, they may therefore trigger immune responses that inhibit or prevent the above-mentioned mechanisms.
Monitoring of protein-specific humoral responses was accomplished with the recently developed M. mycoides SC surface protein bead-based array (14). The assay is based on a platform from Luminex Corp. using spectrally distinguishable microspheres (13) to form an array in suspension. Binding of serum antibodies to 65 recombinant mycoplasma surface proteins, each coupled to a unique bead, was analyzed using a flow cytometer-like instrument. This enabled monitoring of the humoral immune responses at regular time points throughout the vaccine trial and statistical identification of proteins that were associated with a protective immune response in the T1/44-vaccinated cattle, after the clinical evaluation and postmortem reports were completed.
MATERIALS AND METHODS
Vaccine trial. (i) Cattle.
The experimental protocol, including justification, was approved by the Director of Veterinary Services in Windhoek, Namibia. Cattle from a government research farm in an area free of CBPP for 70 years were selected and tested to be serologically negative to M. mycoides SC by complement fixation test (CFT) and competitive ELISA (cELISA). They were then moved to the Okavango region in the northern region of Namibia, and a total of 35 cattle were housed together outdoors in an enclosed pasture. Ten of the cattle were infected by intubation with M. mycoides SC, five were given the recombinant antigens, and five were given the T1/44 vaccine. Five cattle were untreated in-contact infection controls, and the 10 remaining cattle were given vaccines from a trial held in parallel.
(ii) Immunizations.
The antigen mixture for the recombinant vaccine candidate consisted of recombinant versions of the following putative variable surface proteins of M. mycoides SC: MSC_0117, MSC_0364, MSC_0816, MSC_0847, and MSC_1033 (37). The proteins were expressed as fusion proteins in E. coli with a hexahistidine and albumin binding protein tag (His6-ABP) as previously described (15). Equal amounts of each protein were mixed to a total protein concentration of 1 mg/ml (0.2 mg/ml of each protein) and incubated for 1 h with an adsorbent aluminum hydroxide gel (5% [vol/vol] Rehydragel LV; General Chemical Corp.). An oil-in-water emulsion was added (15% [vol/vol] Emulsigen; MVP Technologies), and the subunit vaccine was stored at +4°C until use, approximately 3 weeks. At immunization, each animal was given a single 1-ml dose subcutaneously in the left side of the neck. The T1/44 PERIBOV (Panvac modified strain) freeze-dried vaccine, with a minimum of 107 CFU, was obtained from the Botswana Vaccine Institute. It was reconstituted immediately before use, and 1 ml was given subcutaneously in the left side of the neck, as recommended, to five cattle.
(iii) CBPP challenge.
Ten untreated cattle were infected with the “Matapi” strain of M. mycoides SC as a source of infection 1 month after vaccinations in the other groups. The “Matapi” strain was grown to a titer of 109/ml in modified Hayflick's medium (MHM). The infection was carried out once by intubation to the bifurcation of the bronchi with 20 ml culture followed by 30 ml MHM agar washed down with 50 ml MHM broth. The experiment continued for 123 days after intubation, which was denoted as day zero. The trial thus spanned days −36 (immunization) to 125 (final sacrifice). All animals were kept together outdoors in an enclosed pasture throughout the trial.
(iv) Disease monitoring during the experimental period.
All cattle had regular veterinarian inspections, including daily temperature recordings and weekly blood sampling. The CFT was carried out as described in the OIE manual (28). The cattle were sacrificed by skilled personnel and examined postmortem by veterinary pathologists at the end of the experimental period.
Monitoring of protein-specific humoral immune responses.
Serum samples were transported on dry ice and kept at −80°C until analysis with the bead-based M. mycoides SC recombinant surface protein array (14). During these experiments, the array consisted of 66 recombinant proteins with predicted surface localization and mostly full-length size, including the control protein and the fusion tag His6-ABP, which is present in all recombinant proteins (for details, see Table S1 in the supplemental material). Experiments were conducted as previously described (14). In brief, the Luminex suspension array technology was used as follows. Sera were diluted 1:3,000 and preadsorbed with His6-ABP and an E coli lysate to reduce undesired signals. Each serum sample was incubated for 1 h with 5 μl of bead mixture (a solution containing the 66 proteins covalently bound to magnetic beads of separate identifications [IDs]). Beads were sedimented magnetically, washed, and resuspended in a biotinylated anti-bovine IgG solution (30 μl, 0.5 μg/ml; Jackson Immunoresearch). After 45 min of incubation, beads were again washed and resuspended in a phycoerythrin-labeled streptavidin solution (30 μl, 0.5 μg/ml; Pierce) and incubated for 20 min. Finally, the beads were washed and mixed with a stop solution (0.2% [vol/vol] paraformaldehyde in 100 μl of 0.1 M phosphate-buffered saline [PBS; pH 7.2]) for 5 min followed by a last sedimentation and resuspension in PBS-0.1% Tween 20 (PBST). Samples were thereafter analyzed in a Luminex LX200 system using Luminex IS 2.3 software counting 50 events per bead ID and sample. The median fluorescence intensity (MFI) was chosen to display serum antibody-protein interactions; data analysis and graphical presentation were performed in Microsoft Excel 2008 or R, an environment for statistical computing and graphics (18).
RESULTS
Observations during the vaccine trial. (i) Directly infected cattle.
Nine out of the 10 cattle that were intubated with M. mycoides SC as a source of infection were serologically positive for CBPP by CFT and cELISA during the trial. At postmortem examination, the intubated cattle showed lesions typical of CBPP, including eight cattle with encapsulated sequestra and one with a marbled lung. Postmortem examination of the tenth cow showed a hypertrophic lung only. In all, these results show a successful establishment of CBPP within the vaccine trial.
(ii) In-contact infection control cattle.
All five cattle were serologically positive for CBPP by day 90 of the trial, about 13 weeks after exposure to the intubated cattle (see Table S2 in the supplemental material). At postmortem examination, all five cattle manifested acute and/or subacute CBPP lesions (Table 1). Chronic lesions were also present in all cattle, small sequestra (<5 cm) in two cows, and large sequestra (>20 cm) in the remaining three. These results demonstrate that CBPP was spread among the cattle in the trial, thus challenging both vaccine groups.
TABLE 1.
Summary of vaccine trial resultsa
| Group and animal | No. of days with temp of >39°C (cough) | CFT at withdrawalb | Pathologyc |
|---|---|---|---|
| T1/44 | |||
| 146 | 0 (yes) | 0 | PF, PCF |
| 178 | 0 (no) | 0 | PF |
| 179 | 0 (no) | 0 | PF, HPZ/M-DL |
| 181 | 0 (yes) | 0 | PF, PCF |
| 192 | 1 (no) | 160 | PF, SS-CL, AD |
| Recombinant antigens | |||
| 150 | 1 (no) | 2,560 | PF, SS-DLx2, HLN, AD |
| 155 | 12 (yes) | 1,280 | PF, LS-CL, AD, PCF |
| 161 | 13 (yes) | 2,560 | PF, LS-AL/CL/DL, AD |
| 162 | 9 (no) | 320 | HLN, LS-DL, AD |
| 189 | 10 (no) | 320 | PF, SS-AL, AD |
| In contact | |||
| 145 | 2 (no) | 640 | PF, HPZ/M-AL/DL/CL, LS-DL, HLN, AD |
| 165 | 1 (yes) | 160 | PF, SS-DL |
| 175 | 1 (yes) | 160 | PF, SS-AL, SS-CL, AD |
| 177 | 8 (yes) | 5,120 | PF, LS-AL/CL, AD, PCF |
| 188 | 11 (no) | 1,280 | LS-DL, HLN, AD |
Disease records were obtained during the CBPP vaccine trial or at postmortem exam. CFT is expressed as the reciprocal of the serum dilution.
One week prior to withdrawal for the in-contact group.
PF, pleural fluid; LS, large sequester; SS, small sequester; AL, apical lobe; DL, diaphragmatic lobe; HLN, hyperplastic lymph nodes; HPZ/M, hepatization/marbling; AD, pleural adhesion; CL, cardiac lobe; PCF, pericardic fluid.
(iii) T1/44-vaccinated cattle.
All five cattle had immune responses to the vaccine as judged by CFT prior to CBPP exposure to intubated cattle at day zero and onwards (see Table S2 in the supplemental material). One cow had low CFT titers (1/40 or less) throughout, for 104 days, (15 weeks) and three cows had stronger CFT titers that declined after day 55 (week 8). The last cow, no. 192, was CFT positive throughout the trial, peaking at a titer of 1/10,240 on day 69 (week 10). Postmortem examinations revealed adhesion between the visceral and parietal pleura and a small sequestra (5 cm) in this animal. All five cattle had pleural fluid, and two had pericardic fluid (Table 1).
(iv) Cattle receiving the recombinant vaccine candidate.
One of the five cattle immunized with the recombinant antigens was CFT positive by day 0. The remaining cattle became CFT positive following challenge by the intubated cattle (from intubation on day zero and onwards); all were CFT positive by day 90 (week 13; see Table S2 in the supplemental material). Postmortem examinations revealed CBPP lesions including pleural adhesion and sequestra in all cattle; four also had pleural fluid (Table 1). Three cattle had large sequestra, almost encompassing the whole lung in two cases. The last two cattle had one and two small sequestra, respectively.
Multiplex analysis using surface protein array. (i) Identification of protein-specific responses.
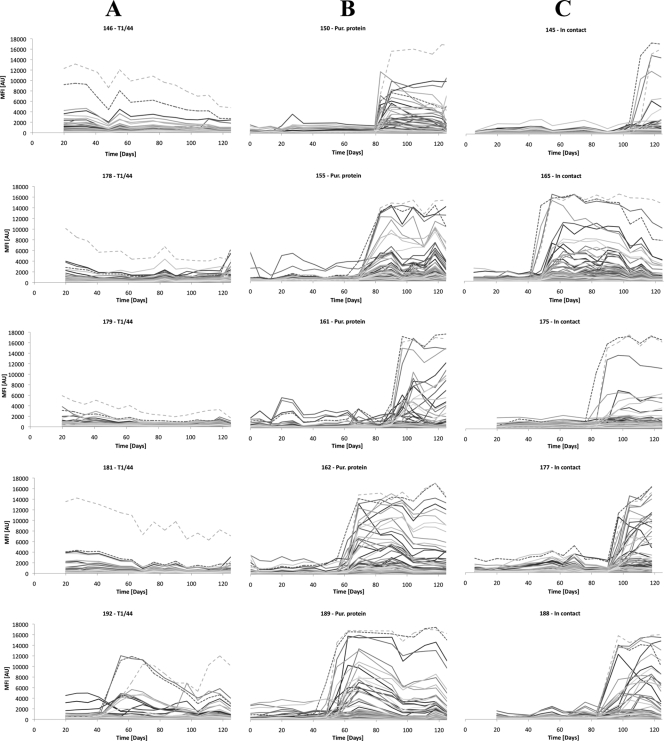
Serum samples were collected on a weekly basis from the three groups of five cattle in the vaccine trial, and all 237 samples were analyzed using the suspension bead array assay. Analysis was divided into three runs and results were reported as median fluorescence intensity (MFI) for each recombinant protein ID. The reproducibility of the experiment determined by a serum pool used in all three runs generated an interassay coefficient of variation (CV) of 23%. For each animal, the MFI signals during the whole trial were visualized in charts as a time-sequential humoral (IgG) response curve for each protein. Both vaccinated groups and the untreated in-contact group are shown in Fig. 1. The T1/44-vaccinated cattle (Fig. 1A) had similar signal patterns with generally low IgG titers throughout the study, with the exception of an initial and persistent raised titer to MSC_1046. However, cow 192 differs from the others as it lacked the initial high titer to MSC_1046 and had a pattern of raised IgG titers to several proteins later in the trial. A sequester was revealed at the postmortem examination of this cow, and the peak CFT titer of 1/10,240 was noted within a week of the detected protein-specific titers to several proteins.
FIG. 1.
Time line analysis. Protein-specific IgG titers to 65 recombinant surface proteins monitored during 125 days from when vaccinated cattle were put in contact with CBPP carriers. Results are displayed as line charts for the following groups: five cattle vaccinated with the T1/44 vaccine (A); five cattle immunized with a recombinant protein candidate vaccine, both of which were given 36 days before contact (B); and five untreated in-contact control cattle (C). Dashed lines mark titers to R1046, corresponding to MSC_1046 (LppQ) in light gray and R816 (MSC_0816) in dark gray. These proteins are highly immunogenic, and initial and persistent IgG titers to LppQ are induced by T1/44 vaccination. The raised titers to several proteins seen during the later stages of the trial in panel B and Fig. 2 coincide with CFT titers of 1/320 or more, indicating onset of clinical CBPP. Pur., purified.
Interestingly, cattle that had received the recombinant vaccine candidate had no persisting antibody titers at day 0 from any of the five antigens, showing that the immunization administered in this way did not raise a long-lasting humoral immune response. The groups of cattle immunized with the recombinant vaccine candidate (Fig. 1B) and untreated controls (Fig. 1C) in fact showed similar responses, characterized by a dramatic increase in antibody titers to many M. mycoides SC recombinant surface proteins in the latter part of the trial. As all these cattle had CBPP lesions including sequestra at postmortem examination (Table 1), this elevated M. mycoides SC-specific response signifies onset of clinical CBPP in the animal. This raised response is seen within a week (on average) of CFT titers of 1/320 or more (see Table S2 in the supplemental material), which further verifies onset of clinical CBPP.
By using the internal control, fusion tag His6-ABP, the binding of serum antibodies to the fusion tag was monitored in each serum sample. Its signal was generally low, with a mean at 230 arbitrary units (AU), although in 18 samples from five cattle in the later stages of the trial, there were peaks at ±500 to 2,000 AU when general antibody titers were high (day 69 and onwards). This can be compared to the maximum signals from these serum samples that approached 20,000 AU.
To obtain an overview of the complete data set, the results were visualized in a heat map (Fig. 2) in which proteins and sera were hierarchically clustered based on Euclidian distances of log2-transformed signals. Three major clusters of sera were formed: (i) a cluster of sera with high antibody titers to M. mycoides SC taken during the progression of CBPP in the recombinant vaccine candidate and control groups; (ii) a cluster of all but one serum from the T1/44-vaccinated group and sera from the other groups with slightly raised titers, taken just before the onset of CBPP; and (iii) a cluster of sera with low IgG titers taken early in the trial from same two groups. The remaining sample from the T1/44 group is the last sample from cow 179, characterized by overall lower signals which were comparable to the low-signal serum cluster (Fig. 1A). Serum-free controls clustered separately. Replicates of the two CBPP-positive controls clustered together in the CBPP-affected cluster, and replicates of the CBPP-negative control clustered into two groups within the low-signal serum cluster.
FIG. 2.
Cluster analysis of the complete data set. A heat map displaying hierarchical cluster analysis of Euclidean distances of log2-transformed signals was used to visualize the whole data set. The serum sample dendrogram on top forms major clusters of (i) samples taken during clinical CBPP (denoted by black bars), (ii) all samples but one from T1/44-vaccinated cattle (green bars) and samples from recombinant protein immunized cattle (dark blue bars) and controls (light blue bars) taken prior to onset of CBPP, and finally (iii) samples taken early in the trial. The protein dendrogram on the left forms two clusters of immunogenic proteins and proteins demonstrating low signals (native counterpart of recombinant protein displayed to the right). Among the immunogenic proteins, recombinants corresponding to MSC_1046 (LppQ) and MSC_0816 form a subcluster with the high titers in clinical CBPP samples and T1/44-vaccinated cattle. The five components of the recombinant protein vaccine candidate are marked in bold. Replicates of two positive control sera (red and dark red) and serum-free blanks (gray) cluster as expected. Replicates of the negative control serum (yellow) are found in the cluster of low-signal sera. Color intensity denotes signal intensity: white is low, orange is medium, and red is high.
The proteins formed two major clusters. The first contained immunodominant surface proteins that had evoked strong humoral immune responses shown by medium to high signals. The two most potent stimulators of the humoral immune response formed their own subcluster, namely, MSC_1046 and MSC_0816. These proteins have also been identified in several other screening analyses of CBPP-diseased cattle (1, 14, 15, 24). The high antibody titers to MSC_1046 and MSC_0816 were found among the diseased as well as the T1/44-vaccinated cattle. The top subcluster of immunodominant proteins obtained predominantly strong signals in sera from diseased cattle and some sera from T1/44-vaccinated cattle, while signals from the remaining subclusters were more scattered and varied from one serum to another. In the second major protein cluster, proteins that may be poor immunogens or at least have triggered low titers of IgG were found. Four of the five components of the recombinant vaccine candidate were found in the cluster of presumably immunodominant proteins, while the last (MSC_1033) was found in the cluster of presumably poor immunogens. The internal control protein His6-ABP (fusion partner of all recombinant proteins) was found in this cluster and indicated the threshold level for mycoplasma-specific signals in each serum. This analysis indicates that the CBPP-diseased cattle evoked strong humoral immune responses to about half (36 of 65) of the native counterparts of the proteins included in the array. To further identify protein-specific responses associated with the T/44 vaccine and recombinant vaccine candidate, more thorough analyses were done.
(ii) Characterization of the T1/44-induced humoral response.
To investigate the humoral responses directed toward the T1/44 surface proteins, protein-specific differences in the IgG levels of this group were compared to the others. For this analysis, the data set was reduced by removing time points corresponding to clinical CBPP in the purified protein vaccine group, controls, and T1/44-vaccinated cow 192. The Welch's t test was used to identify significant mean signal differences (per animal) for each protein, and the results were visualized in a volcano plot (Fig. 3). Here, four proteins were identified with significantly (P < 0.05) higher antibody titers of at least 3-fold magnitude in T1/44 receivers. These recombinant proteins were designed with the following native counterparts, in descending order: MSC_1046, MSC_0271, MSC_0136, and MSC_0079. A fifth protein, MSC_0431, had significantly higher antibody titers (P < 0.05) and was close to the 3-fold criterion. MSC_1046 corresponds to the LppQ protein and seems to be most important in the T1/44-induced humoral response. It had the highest significance (P < 0.01) and largest signal differences (an average of 5,800 AU to 400 AU). From the time line graphs in Fig. 1A, antibody titers for MSC_1046 are high from the beginning and throughout the trial, with the exception of cow 192, in which a small CBPP lesion was found. In this cow and all cattle of both other groups (Fig. 1B and C) high antibody titers to R1046 concurred with the onset of clinical CBPP, also evident in Fig. 2. In this context, an initial strong antibody response to MSC_1046 (LppQ) seems to be an indicator in creating a protective immune response. The remaining four proteins were not as evident as MSC_1046 (LppQ) in the time line graphs (Fig. 1) since their absolute signals were lower (an average of <1,700 AU) in the T1/44-vaccinated group. Like MSC_1046 (LppQ), these four proteins also generated strong immune responses in cattle with clinical CBPP (Fig. 2). None of the four latter immunodominant proteins have been extensively studied before, MSC_0079 is a putative phosphonate ABC transporter, and MSC_0136 is a putative protein. The MSC_0136 gene has previously been used in a phylogenetic study of the Mycoplasma mycoides cluster (33). MSC_0431 and MSC_0271 are both predicted to be lipoproteins. Three of these proteins (except MSC_0271) and MSC_1046 have recently been suggested as parts of novel CBPP diagnostics based on eight or nine recombinant proteins (24).
FIG. 3.
Significant differences in responses of T1/44-vaccinated cattle compared to other groups. Welch's t test was used to determine significant differences in protein-specific mean IgG titers, and the results were displayed as the volcano plot seen here. Dashed lines form two upper-right sections of proteins with P values of <0.05 or 0.01 and over 3-fold differences in antibody titer of T1/44-vaccinated cattle. Four proteins meet these criteria, one with a P value of <0.01. These are marked in gray: 1, R1046; 2, R271; 3, R0136; and 4, R0079. A fifth protein is significant (P < 0.05) but barely misses the signal fold criteria (protein 5, R0431). The recombinant fusion partner of all proteins, His6-ABP, is marked in solid black.
(iii) Effects of the recombinant vaccine candidate.
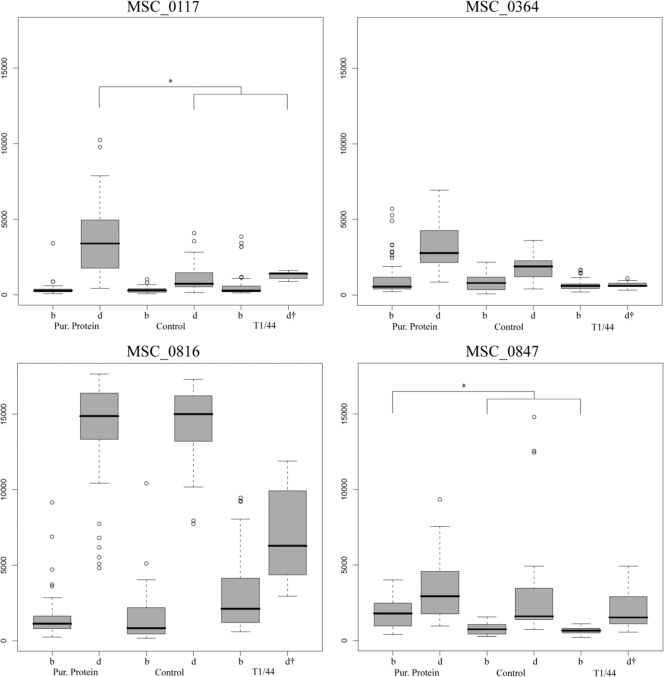
According to the clinical data, the immunizations with the recombinant vaccine candidate had no protective effect and CBPP progressed as in the control group. To investigate whether this was due to a poor immunization scheme or a nonprotective immune response, antibody titers to the subcomponents of the vaccine candidate were examined. Results are summarized as box plots in Fig. 4, showing results from all time points grouped as before and during CBPP infection. MSC_1033 had uniformly low titers throughout the vaccine trial (signals of ±1,000 AU) and was excluded from the analysis. For one protein, MSC_0847, significantly (P < 0.05) higher mean IgG titers were observed in the recombinant vaccine candidate group compared to the controls before onset of CBPP. During the manifested disease, mean titers to MSC_0117 were significantly (P < 0.05) higher in the recombinant vaccine candidate group and MSC_0364 had seemingly higher mean titers. Judging from these results, the vaccination induced a stronger immune response to three of the five proteins, but it was not as high as the IgG titer to MSC_1046 (LppQ) that was seen throughout the study when induced by T1/44 vaccination (Fig. 1A). Interestingly, IgG titers to MSC_0816 during CBPP were lower in the T1/44 group than those in the other groups.
FIG. 4.
Responses to subcomponents of the recombinant protein vaccine candidate. Four of the five recombinant surface proteins in the vaccine candidate had generated IgG titers of interest in the protein-specific analysis, here displayed as box plots for three groups of cattle before (b) and during (d) CBPP infection. Only two proteins had significantly (P < 0.05) different titers between the purified (Pur.) protein vaccine group and the two other groups, as measured by mean IgG titers and marked by asterisks. The bold line represents the median signal, the boxes comprise 50% of the data set, and the whiskers extend to the furthest data point within 1.5 times of the box length. Circles mark outliers. †, only one animal in the T1/44-vaccinated group developed CBPP.
DISCUSSION
This study had two objectives: to evaluate the candidate recombinant vaccine and more importantly to find novel protein targets to include in a second-generation recombinant vaccine. Judging from the clinical data, it is obvious that the recombinant vaccine candidate did not confer protection against CBPP. Clinical observations during the trial and at postmortem (Table 1) were indistinguishable from those of the untreated control group, while the T1/44 vaccine conferred protection but not full prevention of lung lesions. However, failure of the recombinant antigens could be due to an insufficient immune response under the present adjuvant and administration conditions. Comparisons of the CFT titers to both vaccines (see Table S2 in the supplemental material) show an earlier and stronger response to T1/44, although it could partly be explained by the nature of the CFT, as this and the T1/44 vaccine are both based on whole-cell M. mycoides SC.
The protein-specific responses (Fig. 1 and 2) also separate the T1/44-vaccinated cattle from the cattle receiving the recombinant vaccine candidate and the control group. However, scrutiny of the five recombinant antigen components (Fig. 4) shows significant differences for one protein prior to the onset of the disease and for one protein during clinical CBPP, indicating that the recombinant vaccine candidate did trigger a weak immune response. Disregarding whether responses to these proteins confer immunity or not, a successful immunization should elicit a stronger immune response upon contact with the pathogen. In this sense, the antigen concentration needs to be titrated in order to generate stronger immune responses that might be protective. Booster immunizations, inoculum size, and alternative adjuvants should be considered when assessing and optimizing novel vaccines.
As a way of identifying novel potential vaccine components and expanding the knowledge of immunity to CBPP, responses specific to the protected cattle in the T1/44 group were identified (Fig. 3). Here, four or five proteins were identified, depending on the cutoff for signal differences between the groups. The criterion of a 3-fold-higher signal in T1/44-vaccinated cattle was imposed in recognition of the small sample set of five cattle per group to reduce the impact of individual animals and to isolate proteins with the comparably largest increase in IgG titers. There are probably additional proteins among the 65 included in this assay that could be potential vaccine candidates. These could be identified by using larger groups of cattle or by measuring other immune response components such as IgM, IgA, or cellular responses.
LppQ is known to be a marker of CBPP (1), but its usefulness as a vaccine is questionable based on recent studies (19, 27). The results of this study indicate that MSC_1046 (LppQ) is the most significant component of the T1/44-induced response (Fig. 1 and 3). In this context, it is important to remember that the presented results are protein-specific systemic IgG responses associated with T1/44-induced immunity by statistical significance. Whether the humoral responses to MSC_1046 (LppQ) or any other of the proteins identified here are protective is yet to be determined. The cell-mediated immunity also appears to be important (7, 8, 35) and emphasizes that further studies are needed. The recombinant proteins produced and used in this assay could of course be used for analysis of protein-specific cellular responses in a suitable assay format. Such a study was recently done on four recombinant lipoproteins, in which no cellular response to LppQ was induced but responses to LppA were observed (9).
Serum samples taken in the period prior to exposure to CBPP by intubation of 10 cattle were unfortunately not kept for analysis of protein-specific IgG titers. This prevented comparison of the initial immune responses to both vaccines, which would have given further insights into whether a sufficient immune response was triggered to the recombinant vaccine candidate. Furthermore, study of the primary immune response induced by T1/44 and comparison to the subsequent secondary immune response induced by the CBPP challenge (reported here) would possibly yield even more insights into the T1/44-induced protection against CBPP.
To our knowledge, no direct evidence has yet been presented for the role of a specific protein in CBPP immunity. This study makes a contribution by identifying several proteins (MSC_1046 (LppQ), MSC_0271, MSC_0136, MSC_0079, and MSC_0431) that are associated with immune responses to CBPP of interest for further evaluation in a second-generation recombinant vaccine. The next phase would be to evaluate cellular immune responses to the suggested proteins and evaluate other adjuvants or administration protocols of vaccines to produce protective immune responses.
Supplementary Material
Acknowledgments
We thank The Swedish International Development Cooperation Agency (SIDA) and the Department for Environment Food and Rural Affairs (Defra, United Kingdom) for funding this study, along with the Ministry of Agriculture, Water and Forestry of Namibia for facilitating the vaccine trial.
Footnotes
Published ahead of print on 31 March 2010.
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1.Abdo, E. M., J. Nicolet, and J. Frey. 2000. Antigenic and genetic characterization of lipoprotein LppQ from Mycoplasma mycoides subsp. mycoides SC. Clin. Diagn. Lab. Immunol. 7:588-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abusugra, I., G. Wolf, G. Bölske, F. Thiaucourt, and B. Morein. 1997. ISCOM vaccine against contagious bovine pleuropneumonia (CBPP). 1. Biochemical and immunological characterization. Vet. Immunol. Immunopathol. 59:31-48. [DOI] [PubMed] [Google Scholar]
- 3.Bonvin-Klotz, L., E. M. Vilei, K. Kühni-Boghenbor, N. Kapp, J. Frey, and M. H. Stoffel. 2008. Domain analysis of lipoprotein LppQ in Mycoplasma mycoides subsp. mycoides SC. Antonie Van Leeuwenhoek 93:175-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boonstra, E., M. Lindbaek, B. Fidzani, and D. Bruusgaard. 2001. Cattle eradication and malnutrition in under five's: a natural experiment in Botswana. Public Health Nutr. 4:877-882. [DOI] [PubMed] [Google Scholar]
- 5.Bruderer, U., J. Regalla, E. M. Abdo, O. J. Huebschle, and J. Frey. 2002. Serodiagnosis and monitoring of contagious bovine pleuropneumonia (CBPP) with an indirect ELISA based on the specific lipoprotein LppQ of Mycoplasma mycoides subsp. mycoides SC. Vet. Microbiol. 84:195-205. [DOI] [PubMed] [Google Scholar]
- 6.Cheng, X., J. Nicolet, R. Miserez, P. Kuhnert, M. Krampe, T. Pilloud, E. M. Abdo, C. Griot, and J. Frey. 1996. Characterization of the gene for an immunodominant 72 kDa lipoprotein of Mycoplasma mycoides subsp. mycoides small colony type. Microbiology 142:3515-3524. [DOI] [PubMed] [Google Scholar]
- 7.Dedieu, L., V. Balcer-Rodrigues, O. Cisse, M. Diallo, and M. Niang. 2006. Characterisation of the lymph node immune response following Mycoplasma mycoides subsp. mycoides SC infection in cattle. Vet. Res. 37:579-591. [DOI] [PubMed] [Google Scholar]
- 8.Dedieu, L., V. Balcer-Rodrigues, A. Yaya, B. Hamadou, O. Cisse, M. Diallo, and M. Niang. 2005. Gamma interferon-producing CD4 T-cells correlate with resistance to Mycoplasma mycoides subsp. mycoides S.C. infection in cattle. Vet. Immunol. Immunopathol. 107:217-233. [DOI] [PubMed] [Google Scholar]
- 9.Dedieu, L., P. Totte, V. Rodrigues, E. M. Vilei, and J. Frey. 31 January 2009. Comparative analysis of four lipoproteins from Mycoplasma mycoides subsp. mycoides small colony identifies LppA as a major T-cell antigen. Comp. Immunol. Microbiol. Infect. Dis. [Epub ahead of print.] doi: 10.1016/j.cimid.2008.011. [DOI] [PubMed]
- 10.Dedieu-Engelmann, L. 2008. Contagious bovine pleuropneumonia: a rationale for the development of a mucosal sub-unit vaccine. Comp. Immunol. Microbiol. Infect. Dis. 31:227-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egwu, G. O., R. A. J. Nicholas, J. A. Ameh, and J. B. Bashiruddin. 1996. Contagious bovine pleuropneumonia: an update. Vet. Bull. 66:875-888. [Google Scholar]
- 12.Food and Agriculture Organization of the United Nations. 2003. Contagious bovine pleuropneumonia. EMPRESS Transboundary Anim. Dis. Bull. 24:2-7. [Google Scholar]
- 13.Fulton, R. J., R. L. McDade, P. L. Smith, L. J. Kienker, and J. R. Kettman, Jr. 1997. Advanced multiplexed analysis with the FlowMetrix system. Clin. Chem. 43:1749-1756. [PubMed] [Google Scholar]
- 14.Hamsten, C., M. Neiman, J. M. Schwenk, M. Hamsten, J. B. March, and A. Persson. 2009. Recombinant surface proteomics as a tool to analyze humoral immune responses in bovines infected by Mycoplasma mycoides subsp. mycoides SC. Mol. Cell. Proteomics 8:2544-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamsten, C., J. Westberg, G. Bolske, R. Ayling, M. Uhlen, and A. Persson. 2008. Expression and immunogenicity of six putative variable surface proteins in Mycoplasma mycoides subsp. mycoides SC. Microbiology 154:539-549. [DOI] [PubMed] [Google Scholar]
- 16.Huebschle, O. J., R. D. Ayling, K. Godinho, O. Lukhele, G. Tjipura-Zaire, T. G. Rowan, and R. A. Nicholas. 2006. Danofloxacin (Advocin) reduces the spread of contagious bovine pleuropneumonia to healthy in-contact cattle. Res. Vet. Sci. 81:304-309. [DOI] [PubMed] [Google Scholar]
- 17.Huebschle, O. J., G. Tjipura-Zaire, I. Abusugra, G. di Francesca, F. Mettler, A. Pini, and B. Morein. 2003. Experimental field trial with an immunostimulating complex (ISCOM) vaccine against contagious bovine pleuropneumonia. J. Vet. Med. 50:298-303. [DOI] [PubMed] [Google Scholar]
- 18.Ihaka, R., and R. Gentleman. 1996. R: a language for data analysis and graphics. J. Comput. Graphical Stat. 5:299-314. [Google Scholar]
- 19.Janis, C., D. Bischof, G. Gourgues, J. Frey, A. Blanchard, and P. Sirand-Pugnet. 2008. Unmarked insertional mutagenesis in the bovine pathogen Mycoplasma mycoides subsp. mycoides SC: characterization of a lppQ mutant. Microbiology 154:2427-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jores, J., I. Nkando, A. Sterner-Kock, W. Haider, J. Poole, H. Unger, C. Muriuki, H. Wesonga, and E. L. Taracha. 2008. Assessment of in vitro interferon-gamma responses from peripheral blood mononuclear cells of cattle infected with Mycoplasma mycoides ssp. mycoides small colony type. Vet. Immunol. Immunopathol. 124:192-197. [DOI] [PubMed] [Google Scholar]
- 21.Kusiluka, L. J., and F. F. Sudi. 2003. Review of successes and failures of contagious bovine pleuropneumonia control strategies in Tanzania. Prev. Vet. Med. 59:113-123. [DOI] [PubMed] [Google Scholar]
- 22.March, J. B. 2004. Improved formulations for existing CBPP vaccines—recommendations for change. Vaccine 22:4358-4364. [DOI] [PubMed] [Google Scholar]
- 23.Mbulu, R. S., G. Tjipura-Zaire, R. Lelli, J. Frey, P. Pilo, E. M. Vilei, F. Mettler, R. A. Nicholas, and O. J. Huebschle. 2004. Contagious bovine pleuropneumonia (CBPP) caused by vaccine strain T1/44 of Mycoplasma mycoides subsp. mycoides SC. Vet. Microbiol. 98:229-234. [DOI] [PubMed] [Google Scholar]
- 24.Neiman, M., C. Hamsten, J. M. Schwenk, G. Bölske, and A. Persson. 2009. Multiplex screening of surface proteins from Mycoplasma mycoides subsp. mycoides SC for an antigen cocktail ELISA. Clin. Vaccine Immunol. 16:1665-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niang, M., A. Sery, O. Cissé, M. Diallo, M. Doucouré, M. Koné, C. F. Simbé, W. Amanfu, and F. Thiaucourt. 2006. Effect of antibiotic therapy on the pathogenesis of CBPP. Experimental transmission of the disease by contact from infected animals treated with oxytetracycline, p. 25-32. In FAO-OIE-AU/IBAR-IAEA. Antibiotics to the rescue? Consultative Group Meeting on CBPP in Africa. FAO, Rome, Italy.
- 26.Nicholas, R., J. Bashiruddin, R. Ayling, and R. Miles. 2000. Contagious bovine pleuropneumonia: a review of recent developments. Vet. Bull. 70:827-838. [Google Scholar]
- 27.Nicholas, R. A. J., G. Tjipura-Zaire, M. Scacchia, J. Frey, and O. J. B. Hübschle. 2004. An inactivated whole cell vaccine and LppQ subunit vaccine appear to exacerbate the effects of CBPP in adult cattle, p. 91-97. In Towards sustainable CBPP control programmes for Africa. FAO Animal Production and Health Proceedings. FAO, Rome, Italy.
- 28.OIE. 2008. Manual of diagnostic tests and vaccines for terrestrial animals. Office International des Epizooties, Paris, France.
- 29.Persson, A., K. Jacobsson, L. Frykberg, K. E. Johansson, and F. Poumarat. 2002. Variable surface protein Vmm of Mycoplasma mycoides subsp. mycoides small colony type. J. Bacteriol. 184:3712-3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pilo, P., J. Frey, and E. M. Vilei. 2007. Molecular mechanisms of pathogenicity of Mycoplasma mycoides subsp. mycoides SC. Vet. J. 174:513-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pilo, P., S. Martig, J. Frey, and E. M. Vilei. 2003. Antigenic and genetic characterisation of lipoprotein lppC from Mycoplasma mycoides subsp. mycoides SC. Vet. Res. 34:761-775. [DOI] [PubMed] [Google Scholar]
- 32.Provost, A. 1996. Strategies for prevention and eradication of contagious bovine pleuropneumonia with or without vaccination. Rev. Sci. Tech. 15:1355-1371. (In French.) [PubMed] [Google Scholar]
- 33.Thiaucourt, F., S. Lorenzon, A. David, and A. Breard. 2000. Phylogeny of the Mycoplasma mycoides cluster as shown by sequencing of a putative membrane protein gene. Vet. Microbiol. 72:251-268. [DOI] [PubMed] [Google Scholar]
- 34.Thiaucourt, F., A. Yaya, H. Wesonga, O. J. Hübschle, J. J. Tulasne, and A. Provost. 2000. Contagious bovine pleuropneumonia. A reassessment of the efficacy of vaccines used in Africa. Ann. N. Y. Acad. Sci. 916:71-80. [DOI] [PubMed] [Google Scholar]
- 35.Totté, P., V. Rodrigues, A. Yaya, B. Hamadou, O. Cisse, M. Diallo, M. Niang, F. Thiaucourt, and L. Dedieu. 2008. Analysis of cellular responses to Mycoplasma mycoides subsp. mycoides small colony biotype associated with control of contagious bovine pleuropneumonia. Vet. Res. 39:8. [DOI] [PubMed] [Google Scholar]
- 36.Waite, E. R., and J. B. March. 2002. Capsular polysaccharide conjugate vaccines against contagious bovine pleuropneumonia: immune responses and protection in mice. J. Comp. Pathol. 126:171-182. [DOI] [PubMed] [Google Scholar]
- 37.Westberg, J., A. Persson, A. Holmberg, A. Goesmann, J. Lundeberg, K. E. Johansson, B. Pettersson, and M. Uhlén. 2004. The genome sequence of Mycoplasma mycoides subsp. mycoides SC type strain PG1T, the causative agent of contagious bovine pleuropneumonia (CBPP). Genome Res. 14:221-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vilei, E. M., E. M. Abdo, J. Nicolet, A. Botelho, R. Goncalves, and J. Frey. 2000. Genomic and antigenic differences between the European and African/Australian clusters of Mycoplasma mycoides subsp. mycoides SC. Microbiology 146:477-486. [DOI] [PubMed] [Google Scholar]
- 39.Windsor, R. S. 2000. The eradication of contagious bovine pleuropneumonia from south western Africa. A plan for action. Ann. N. Y. Acad. Sci. 916:326-332. [DOI] [PubMed] [Google Scholar]
- 40.Windsor, R. S., and A. Wood. 1998. Contagious bovine pleuropneumonia. The costs of control in central/southern Africa. Ann. N. Y. Acad. Sci. 849:299-306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.