Abstract
Laboratory-reared beagles were vaccinated with a placebo or a bacterin comprised of Borrelia burgdorferi S-1-10 and ospA-negative/ospB-negative B. burgdorferi 50772 and challenged after 1 year with B. burgdorferi-infected Ixodes scapularis ticks. For the placebo recipients, spirochetes were recovered from 9 (60%) skin biopsy specimens collected after 1 month, and the organisms persisted in the skin thereafter. Ten (67%) dogs also developed joint infection (3 dogs), lameness or synovitis (7 dogs), or B. burgdorferi-specific antibodies (8 dogs). For the vaccine recipients, spirochetes were recovered from 6 (40%) skin biopsy specimens collected after 1 month. However, subsequent biopsy specimens were negative, and the dogs failed to develop joint infection (P = 0.224), lameness/synovitis (P = 0.006), or Lyme disease-specific antibody responses (P = 0.002). The bacterin provided a high level of protection for 1 year after immunization, and the addition of the OspC-producing B. burgdorferi 50772 provided enhanced protection.
Lyme disease, an Ixodes sp. tick-associated zoonosis caused by Borrelia burgdorferi, causes significant morbidity in humans and dogs. In contrast to the case for human illness, however, a classical Lyme disease scenario in dogs has been difficult to document, especially since many of the animals exposed to B. burgdorferi fail to develop clinical abnormalities (17). However, infected dogs may also develop fever, anorexia, fatigue, renal failure, and, most commonly, limb and joint disorders (5, 28-30).
Canine vaccines provide protection by inducing anti-OspA borreliacidal antibodies that kill B. burgdorferi in the tick midgut as the infected parasite ingests blood (6, 9). The vaccines have been used commercially for years, and the approach has been effective (3, 4, 23, 27). However, a recombinant OspA (rOspA) vaccine (15) or whole-cell bacterins (4, 16) that provide protection by this mechanism failed to prevent B. burgdorferi infection in 20% to 40% of recipients. The failures could be expected because B. burgdorferi downregulates the expression of OspA immediately after the infected tick begins feeding (26), borreliacidal anti-OspA antibodies are genospecies specific (20, 32), and ticks may be infected with OspA-negative Lyme disease spirochetes (8).
Another effective target for antibody-mediated protection is OspC (10, 25). This protein is expressed in the tick midgut and salivary gland (24) and also during the early stages of infection in the mammalian host (31). Moreover, this protein is susceptible to anti-OspC borreliacidal antibodies, especially those specific for the C terminus (12, 21). In addition, researchers showed recently that a bivalent bacterin that induced high levels of both anti-OspA and anti-OspC borreliacidal antibodies provided complete protection from Lyme disease spirochetes shortly after immunization (14). In this study, we evaluated the ability of the bivalent bacterin (14) to provide protection against B. burgdorferi-infected ticks for at least 1 year after vaccination.
Vaccination and tick challenge.
Two groups (n = 15) of 8-week-old, laboratory-reared beagle puppies were randomized without regard to sex, vaccinated subcutaneously in the neck with a 1-ml dose of bacterin or placebo, and boosted after 21 days with an additional 1-ml dose. The bacterin was prepared as described previously (14). Briefly, B. burgdorferi S-1-10, a sensu stricto isolate that induces high levels of anti-OspA borreliacidal antibodies, and B. burgdorferi 50772, an ospA-negative/ospB-negative sensu stricto isolate that induces high levels of anti-OspC borreliacidal antibodies (14), were grown separately in modified Barbour-Stoenner-Kelly (BSK) medium (4) at 35°C until the spirochetes were replicating logarithmically. The replicating organisms were inactivated by a 24- to 48-h incubation with 10 mM binary ethylenimine that was then neutralized with sodium thiosulfate for 6 to 12 h. The killed spirochetes were then concentrated by continuous-flow centrifugation (model RC-5B/26Plus centrifuge; Sorvall) at 25,000 × g with a flow rate of 40 to 60 ml/min and combined in a balanced salt solution that contained 30 μg of gentamicin/ml, 30 units of nystatin/ml, 5% Emulsigen (MVP Laboratories, Inc., Omaha, NE), and 1% HEPES so that a 1-ml dose contained at least 2.5 × 107 spirochetes of each isolate. At harvest, the spirochetes were counted with a Petroff-Hausser counting chamber, and the number of organisms/ml was adjusted by the concentration factor to achieve a final titer. The placebo was balanced salt solution with gentamicin, nystatin, Emulsigen, and HEPES. In addition, the dogs were vaccinated and boosted at 7 and 11 weeks of age with Galaxy DA2PPv+Cv (Schering-Plough Animal Health). The dogs were provided food and water ad libitum, and the experiments were approved by the Intervet/Schering-Plough Institutional Animal Care and Use Committee.
Adult Ixodes scapularis ticks were collected from wooded areas by flagging the underbrush in the focus of high disease endemicity (11) near Ettrick, WI. To confirm infection, the midguts from 25 ticks were examined by fluorescence microscopy after staining with fluorescein isothiocyanate-labeled OspA monoclonal antibody H5332 (22), and B. burgdorferi was detected in 6 (24%) ticks. Ten male ticks and 10 female ticks were then selected randomly and placed into a rubber cup that was secured to the left dorsal-anterior region of each animal for 1 week. The dogs were isolated during the tick challenge but then housed communally in groups of 4 to 6 thereafter.
Anti-OspA and anti-OspC borreliacidal antibody responses after vaccination.
Blood samples were obtained 7 days (day 28) after the booster and immediately prior (day 394) to the tick challenge and tested for anti-OspA or anti-OspC borreliacidal antibodies as described previously (2). Briefly, 5 × 105 low-passage B. burgdorferi S-1-10 (OspA) or 50772 (OspC) organisms were combined with serum and guinea pig complement (Rockland Immunochemical, Gilbertsville, PA), and the suspension was incubated at 35°C. Following incubation, 100 μl of each assay suspension was combined with phosphate-buffered saline (PBS) and acridine orange (Sigma-Aldrich), and the spirochetes were analyzed for killing by using a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA). The borreliacidal antibodies were detected indirectly by monitoring the increased fluorescence intensity that occurs when the acridine orange intercalates into blebbed, nonviable spirochetes. A ≥13% shift in the mean fluorescence intensity compared to a normal serum control was considered positive (2). The presence of blebbed nonmotile B. burgdorferi was confirmed by dark-field microscopy. A positive control was also included, and serum samples from individual animals were assayed concurrently.
Similar to previous findings (14), the sera from the placebo recipients did not contain borreliacidal antibodies (titers < 1:80). In contrast, the immune serum samples collected 1 week after the booster vaccination (day 28) each contained high levels of borreliacidal activity that could be detected by using B. burgdorferi S-1-10 (titer range, 1:1,280 to >1:10,240) or 50772 (titer range, 1:640 to 1:5,120) (Table 1). We then confirmed that the borreliacidal activity was due to anti-OspA or anti-OspC borreliacidal antibodies. Briefly, a 1-ml volume of immune serum was passed four times over separate columns that contained rOspA bound to Sepharose 4B (Sigma-Aldrich) or rOspC bound to Tetralink tetrameric avidin resin (Promega, Madison, WI). As reported previously (14), removing the anti-OspA or anti-OspC antibodies caused the borreliacidal activity detected by using the S-1-10 or 50772 isolates, respectively, to decrease significantly (data not shown). Immediately prior to the tick challenge (day 394), however, the anti-OspC borreliacidal antibody titers had waned to undetectable levels. In addition, the levels of anti-OspA borreliacidal antibodies had also decreased significantly, but the response was still detected (titer range, 1:80 to 1:640) in the immune sera from 13 (87%) vaccine recipients.
TABLE 1.
Borreliacidal antibody titers in immune sera collected at times after vaccination and booster with the bivalent bacterin
| Dog | Borreliacidal antibody titera detected by using: |
|||
|---|---|---|---|---|
|
B. burgdorferi S-1-10 (OspA) |
B. burgdorferi 50772 (OspC) |
|||
| 7 days | 1 yr | 7 days | 1 yr | |
| 1 | 2,560 | 80 | 640 | NDb |
| 2 | 5,120 | 160 | 640 | ND |
| 3 | ≥10,240 | 160 | 640 | ND |
| 4 | ≥10,240 | 640 | 2,560 | ND |
| 5 | ≥10,240 | 160 | 640 | ND |
| 6 | ≥10,240 | ND | 2,560 | ND |
| 7 | ≥10,240 | 80 | 2,560 | ND |
| 8 | ≥10,240 | 320 | 5,120 | ND |
| 9 | ≥10,240 | 640 | 2,560 | ND |
| 10 | ≥10,240 | 320 | 1,280 | ND |
| 11 | ≥10,240 | 640 | 1,280 | ND |
| 12 | ≥10,240 | 320 | 2,560 | ND |
| 13 | ≥10,240 | 160 | 2,560 | ND |
| 14 | 2,560 | ND | 1,280 | ND |
| 15 | 1,280 | 80 | 1,280 | ND |
Reciprocal dilution.
ND, none detected.
Ability of the vaccination to prevent infection.
After the tick challenge, we examined the tick midguts for B. burgdorferi. Midguts were fixed on glass slides and overlaid sequentially with B. burgdorferi-specific rabbit polyclonal antibodies diluted 1:500 in PBS (pH 7.2) and incubated for 30 min at 37°C. After the slides were washed, goat anti-rabbit fluorescein isothiocyanate-labeled immunoglobulin G (IgG) antibodies (Sigma-Aldrich, St. Louis, MO) diluted 1:200 in PBS were laid over them, and the slides were masked and examined with fluorescence microscopy. The midguts from 15 (16%) of 95 engorged ticks recovered from the placebo recipients contained B. burgdorferi, and at least one positive tick was recovered from 11 (73%) dogs. In contrast, spirochetes were detected in only 2 (3%) of 75 engorged ticks from the bacterin recipients (P = 0.003). In addition, each positive tick was recovered from a dog that was producing anti-OspA borreliacidal antibodies (titers, 1:160 and 1:320).
We then collected skin biopsy specimens from areas adjacent to the tick bite sites after 1, 2, and 4 months. The skin was anesthetized with 0.5 ml of lidocaine (2%), and a biopsy specimen was removed with a disposable 4-mm dermal punch (Miltex, Inc., York, PA). The biopsy specimens were then removed from the punch with sterile forceps and crushed immediately prior to placement of the sample in separate tubes that contained BSK medium supplemented with gelatin (20%), rifampin (40 μg/ml), and kanamycin (8 μg/ml). The cultures were then incubated at 35°C and examined microscopically for 4 weeks. The ability of the BSK medium to support growth from an inoculum of one organism (1) was confirmed prior to culture.
B. burgdorferi was recovered from the skin biopsy specimens collected from 9 (60%), 8 (53%), and 7 (47%) placebo recipients after 1, 2, and 4 months, respectively. In contrast, spirochetes were recovered from the initial (1-month) skin biopsy specimens from 6 (40%) vaccine recipients but were not recovered after 2 (P = 0.002) or 4 (P = 0.006) months. In addition, 5 dogs infected with B. burgdorferi after 1 month had anti-OspA borreliacidal antibody titers (titers, 1:80 to 1:640) at the time of tick challenge.
We also cultured the joints by removing approximately 1-cm3 sections of the joint capsules from the left stifle, tarsus, elbow, and carpus at necropsy. Half the tissue sample was combined with 9 ml of BSK medium in a sterile bag and emulsified by passage through a laboratory blender (Stomacher 80; Seward Medical, London, United Kingdom). One milliliter of the suspension was then transferred to 9 ml of fresh BSK, and the culture was incubated at 35°C and examined microscopically for 4 weeks. The other half of the sample was placed into 10% formalin, blinded with regard to vaccination status, and forwarded for histopathology studies. Spirochetes were recovered from the joints of 3 (20%) placebo recipients but were not recovered from the vaccine recipients (P = 0.224). The collective results therefore demonstrated that placebo-vaccinated dogs became infected with B. burgdorferi, and the infection could occur despite the presence of anti-OspA borreliacidal antibodies. In addition, some vaccine recipients became infected, but the spirochetes were cleared rapidly, most likely because of the immune response generated by the unique OspC-expressing B. burgdorferi 50772.
Ability of vaccination to prevent limb/joint disorder.
Dogs were observed daily for 8 months after the tick challenge for Lyme disease-related joint stiffness or lameness (16, 17). To exacerbate the abnormality, dexamethasone (AmTech, St. Joseph, MO) was injected intramuscularly at a dose of 0.4 mg/lb of body weight for five consecutive days beginning at week 19 after challenge (14). In response, 2 (13%) dogs that received the placebo developed lameness shortly after the immunosuppression. The formalin-fixed joint tissues were processed by routine methods, stained with hematoxylin-eosin, and examined for cellular infiltrates and tissue damage. Five (33%) placebo recipients had infiltrations of lymphocytes and/or plasma cells in the synovial or subsynovial tissues of one or more joint capsules, characteristic of canine Lyme disease (14, 30). In contrast, the bacterin recipients did not develop lameness, and their joint capsules remained normal (P = 0.006).
Ability of the vaccination to prevent seroconversion.
Immune serum was collected 90 days after the tick challenge and tested by Western blotting and use of the SNAP 4Dx test (IDEXX Laboratories, Westbrook, ME) for B. burgdorferi-specific antibodies that bound a 20-kDa protein (22) and the C6 peptide (18), respectively. The SNAP 4Dx test was used according to the manufacturer's directions. Western blotting was performed using standard techniques and B. burgdorferi 297, which accurately detects the anti-20-kDa-protein antibodies (14, 22). Briefly, B. burgdorferi was boiled in treatment buffer, and 225 μg of protein was loaded onto a 10%-to-20% gradient polyacrylamide gel (Bio-Rad, Hercules, CA) and electrophoresed. The proteins were then transferred to a polyvinylidene difluoride membrane, cut into strips, blocked with 1% bovine serum albumin (BSA) in PBS-0.1% Tween 20, and then incubated sequentially at room temperature with serum diluted 1:100 and horseradish peroxidase-labeled anti-dog IgG (Kirkegaard & Perry Laboratories, Gaithersburg, MD). Reactions were detected by development with the TMB (3,3′,5,5′tetramethylbenzidine) membrane peroxidase substrate system (Kirkegaard & Perry). Serum from a healthy dog was used as a control.
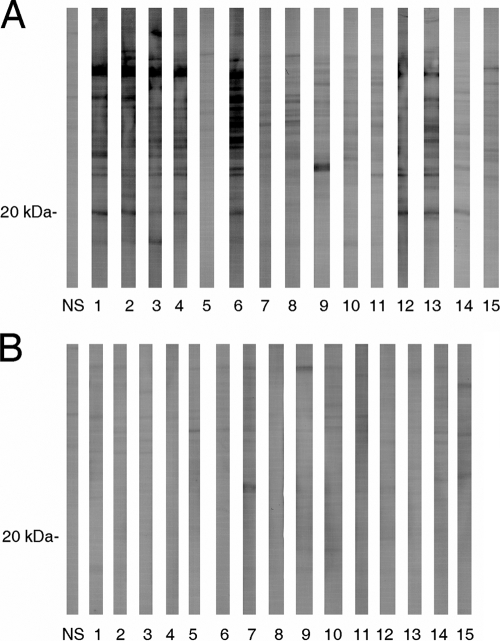
Sera from 14 (93%) placebo recipients contained antibodies against numerous B. burgdorferi proteins, and 7 (47%) contained sufficient levels of antibodies to confirm that the banding patterns were identical to those obtained in a previous study (22) that used immune sera from culture-confirmed B. burgdorferi-infected beagles (Fig. 1A). Moreover, 8 (53%) and 7 (47%) sera contained antibodies that bound the B. burgdorferi-specific 20-kDa protein (22) and C6 peptide (18), respectively. In contrast, the immune sera from the vaccinated dogs contained only low levels of antibodies against a few nonspecific proteins (Fig. 1B).
FIG. 1.
Antibody responses detected by Western blotting in normal serum (NS) or sera collected postchallenge from dogs vaccinated with a placebo (panel A) or the bivalent bacterin (panel B).
Conclusions.
Researchers showed previously that a bivalent bacterin comprised of B. burgdorferi S-1-10 and B. burgdorferi 50772 induced high levels of anti-OspA and anti-OspC borreliacidal antibodies that provided complete protection against canine Lyme disease shortly after immunization. In this study, we extended these findings by confirming that the bacterin provides protection from B. burgdorferi-infected ticks for 1 year after vaccination.
Similar to what was previously found (14), the bacterin induced high levels of anti-OspA and anti-OspC borreliacidal antibodies shortly after vaccination. After 1 year, the anti-OspC borreliacidal antibodies were no longer detectable. In contrast, anti-OspA borreliacidal antibodies were detected in the sera from 13 (87%) vaccine recipients, but the levels had waned significantly. When the dogs were challenged with infected ticks, however, the vaccination still provided a high level of protection. Lyme disease spirochetes colonized the skin of 6 (40%) bacterin recipients, despite the presence of anti-OspA borreliacidal antibodies in the immune sera from 5 dogs. However, the infection was cleared thereafter, and the animals failed to seroconvert or develop Lyme disease-related lameness or synovitis. In contrast, B. burgdorferi was recovered from the initial skin biopsy specimens from 9 placebo recipients, and the infection persisted. More significantly, 8 (53%, P = 0.002) dogs developed two or more clinical signs or symptoms of Lyme disease, including persistent skin infection, infected joint tissues, lameness, synovitis, and B. burgdorferi-specific anti-20-kDa-protein or anti-C6-peptide antibody responses (Table 2).
TABLE 2.
Recovery of B. burgdorferi, development of clinical abnormalities, and development of Lyme disease-specific antibody responses in placebo recipients after tick challenge
| Dog |
B. burgdorferi in skin after: |
B. burgdorferi in joints | Lameness | Synovitis | Antibodies specific for: |
Totala | |||
|---|---|---|---|---|---|---|---|---|---|
| 1 mo | 2 mo | 4 mo | 20-kDa protein | C6 | |||||
| 1 | + | + | + | + | − | + | + | + | 5 |
| 2 | + | + | − | − | − | − | + | + | 3 |
| 3 | + | + | + | − | − | − | + | + | 3 |
| 4 | + | + | + | + | − | + | + | + | 5 |
| 5 | − | − | + | − | − | − | − | − | 1 |
| 6 | + | + | + | + | − | + | + | + | 5 |
| 7 | − | − | − | − | − | − | − | − | 0 |
| 8 | − | − | − | − | + | − | − | − | 1 |
| 9 | + | + | − | − | + | − | − | − | 2 |
| 10 | + | − | − | − | − | − | − | − | 1 |
| 11 | − | − | − | − | − | − | − | − | 0 |
| 12 | + | + | + | − | − | + | + | + | 4 |
| 13 | + | + | + | − | − | + | + | + | 4 |
| 14 | − | − | − | − | − | − | + | − | 1 |
| 15 | − | − | − | − | − | − | − | − | 0 |
Total number of Lyme disease parameters positive.
The ability to recover B. burgdorferi from the initial biopsy specimens from 6 vaccine recipients is significant. For example, the finding confirmed the ability of the spirochetes to escape anti-OspA borreliacidal antibodies, possibly due to antigenic variation. In support of this, Fikrig et al. (8) recovered B. burgdorferi from OspA-vaccinated mice and showed ospA alterations that abrogated the binding of anti-OspA borreliacidal antibodies. However, the spirochetes also persisted without alterations in OspA (8), so other mechanisms are also likely to play a role.
In addition, the results provide compelling evidence of the enhanced protection afforded by including the OspC-expressing B. burgdorferi 50772 isolate in the bacterin. The mechanism(s) responsible for eliminating the spirochetes from the infected vaccine recipients remains unknown. For example, the organisms may have been eliminated by antibody-mediated phagocytosis or cell-mediated responses enhanced by the vaccination (31). Another explanation may be that the absence of anti-OspC borreliacidal antibodies allowed a small number of OspC-expressing spirochetes to invade the skin cells (7, 13, 19), the anti-OspC borreliacidal antibody-producing cells in the immediate vicinity were reactivated as the infection progressed, and the response eliminated the spirochetes before they could disseminate. Regardless of the mechanism, however, the results confirmed that infection with B. burgdorferi occurs despite the presence of OspA borreliacidal antibodies, and the bacterin overcomes this shortcoming by providing a high level of protection for at least 1 year.
Footnotes
Published ahead of print on 17 March 2010.
REFERENCES
- 1.Callister, S. M., K. L. Case, W. A. Agger, R. F. Schell, R. C. Johnson, and J. L. E. Ellingson. 1990. Effects of bovine serum albumin on the ability of Barbour-Stoenner-Kelly medium to detect Borrelia burgdorferi. J. Clin. Microbiol. 28:363-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callister, S. M., D. A. Jobe, W. A. Agger, R. F. Schell, T. J. Kowalski, S. D. Lovrich, and J. A. Marks. 2002. Ability of the borreliacidal antibody test to confirm Lyme disease in clinical practice. Clin. Diagn. Lab. Immunol. 9:908-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang, Y. F., M. J. Appel, R. H. Jacobson, S. J. Shin, P. Harpending, R. Straubinger, L. A. Patrican, H. Mohammed, and B. A. Summers. 1995. Recombinant OspA protects dogs against infection and disease caused by Borrelia burgdorferi. Infect. Immun. 63:3543-3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu, H. J., L. G. Chavez, B. M. Blumer, R. W. Sebring, T. L. Wasmoen, and W. M. Acree. 1992. Immunogenicity and efficacy study of a commercial Borrelia burgdorferi bacterin. J. Am. Vet. Med. Assoc. 201:403-411. [PubMed] [Google Scholar]
- 5.Dambach, D. M., C. A. Smith, R. M. Lewis, and T. J. Van Wickle. 1997. Morphologic, immunohistochemical, and ultrastructural characterization of a distinctive renal lesion in dogs putatively associated with Borrelia burgdorferi infection: 49 cases (1987-1992). Vet. Pathol. 34:85-96. [DOI] [PubMed] [Google Scholar]
- 6.de Silva, A. M., S. R. Telford III, L. R. Brunet, S. W. Barthold, and E. Fikrig. 1996. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J. Exp. Med. 83:271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duray, P. H., S. Yin, Y. Ito, L. Bezrukov, C. Cox, M. Cho, W. Fitzgerald, D. Dorward, J. Zimmerberg, and L. Margolis. 2005. Invasion of human tissue ex vivo by Borrelia burgdorferi. J. Infect. Dis. 191:1747-1754. [DOI] [PubMed] [Google Scholar]
- 8.Fikrig, E., H. Tao, S. W. Barthold, and R. A. Flavell. 1995. Selection of variant Borrelia burgdorferi isolates from mice immunized with outer surface protein A or B. Infect. Immun. 63:1658-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fikrig, E., S. R. Telford III, S. W. Barthold, F. S. Kantor, A. Spielman, and R. A. Flavell. 1992. Elimination of Borrelia burgdorferi from vector ticks feeding on OspA-immunized mice. Proc. Natl. Acad. Sci. U. S. A. 89:5418-5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilmore, R. D., Jr., K. J. Kappel, M. C. Dolan, T. R. Burkot, and B. J. B. Johnson. 1996. Outer surface protein C (OspC), but not P39, is a protective immunogen against a tick-transmitted Borrelia burgdorferi challenge: evidence for a conformational protective epitope in OspC. Infect. Immun. 64:2234-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson, C. A., S. D. Lovrich, W. A. Agger, and S. M. Callister. 2002. Reassessment of a Midwestern Lyme disease focus for Borrelia burgdorferi and the human granulocytic ehrlichiosis agent. J. Clin. Microbiol. 40:2070-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jobe, D. A., S. D. Lovrich, R. F. Schell, and S. M. Callister. 2003. C-terminal region of outer surface protein C binds borreliacidal antibodies in sera from patients with Lyme disease. Clin. Diagn. Lab. Immunol. 10:573-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klempner, M. S., R. Noring, and R. A. Rogers. 1993. Invasion of human skin fibroblasts by the Lyme disease spirochete, Borrelia burgdorferi. J. Infect. Dis. 167:2532-2536. [DOI] [PubMed] [Google Scholar]
- 14.LaFleur, R. L., J. C. Dant, T. L. Wasmoen, S. M. Callister, D. A. Jobe, S. D. Lovrich, T. F. Warner, O. Abdelmagid, and R. F. Schell. 2009. Bacterin that induces anti-OspA and anti-OspC borreliacidal antibodies provides a high level of protection against canine Lyme disease. Clin. Vaccine Immunol. 16:253-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy, S. A., K. K. Clark, and L. T. Glickman. 2005. Infection rates in dogs vaccinated and not vaccinated with an OspA Borrelia burgdorferi vaccine in a Lyme disease-endemic area of Connecticut. Int. J. Appl. Res. Vet. Med. 3:1-5. [Google Scholar]
- 16.Levy, S. A., B. A. Lissman, and C. M. Ficke. 1993. Performance of a Borrelia burgdorferi bacterin in borreliosis-endemic areas. J. Am. Vet. Med. Assoc. 202:1834-1838. [PubMed] [Google Scholar]
- 17.Levy, S. A., and L. A. Magnarelli. 1992. Relationship between development of antibodies to Borrelia burgdorferi in dogs and the subsequent development of limb/joint borreliosis. J. Am. Vet. Med. Assoc. 200:344-347. [PubMed] [Google Scholar]
- 18.Levy, S. A., T. P. O'Connor, J. L. Hanscom, and P. Shields. 2002. Utility of an in-house C6 ELISA test kit for determination of infection status of dogs naturally exposed to Borrelia burgdorferi. Vet. Ther. 3:308-315. [PubMed] [Google Scholar]
- 19.Livengood, J. A., and R. D. Gilmore, Jr. 2006. Invasion of human neuronal and glial cells by an infectious strain of Borrelia burgdorferi. Microbes Infect. 8:2832-2840. [DOI] [PubMed] [Google Scholar]
- 20.Lovrich, S. D., S. M. Callister, B. K. DuChateau, L. C. L. Lim, J. Winfrey, S. P. Day, and R. F. Schell. 1995. Abilities of OspA proteins from different seroprotective groups of Borrelia burgdorferi to protect hamsters from infection. Infect. Immun. 63:2113-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lovrich, S. D., D. A. Jobe, R. F. Schell, and S. M. Callister. 2005. Borreliacidal OspC antibodies specific for a highly conserved epitope are immunodominant in human Lyme disease and do not occur in mice or hamsters. Clin. Diagn. Lab. Immunol. 12:646-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lovrich, S. D., R. L. LaFleur, D. A. Jobe, J. C. Johnson, K. E. Asp, R. F. Schell, and S. M. Callister. 2007. Borreliacidal OspC antibody response of canines with Lyme disease differs significantly from that of humans with Lyme disease. Clin. Vaccine Immunol. 14:635-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma, J., P. M. Hine, E. R. Clough, D. Fish, R. T. Coughlin, G. A. Beltz, and M. G. Shew. 1996. Safety, efficacy, and immunogenicity of a recombinant Osp subunit canine Lyme disease vaccine. Vaccine 14:1366-1374. [DOI] [PubMed] [Google Scholar]
- 24.Piesman, J., N. S. Zeidner, and B. S. Schneider. 2003. Dynamic changes in Borrelia burgdorferi populations in Ixodes scapularis (Acari: Ixodidae) during transmission: studies at the mRNA level. Vector Borne Zoonotic Dis. 3:125-132. [DOI] [PubMed] [Google Scholar]
- 25.Rousselle, J. C., S. M. Callister, R. F. Schell, S. D. Lovrich, D. A. Jobe, J. A. Marks, and C. A. Weinecke. 1998. Borreliacidal antibody production against outer surface protein C of Borrelia burgdorferi. J. Infect. Dis. 178:733-741. [DOI] [PubMed] [Google Scholar]
- 26.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. U. S. A. 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Straubinger, R. K., T. Dharma Rao, E. Davison, B. A. Summers, R. H. Jacobson, and A. B. Frey. 2001. Protection against tick-transmitted Lyme disease in dogs vaccinated with a multiantigenic vaccine. Vaccine 20:181-193. [DOI] [PubMed] [Google Scholar]
- 28.Straubinger, R. K., A. F. Straubinger, L. Harter, R. H. Jacobson, Y. F. Chang, B. A. Summers, H. N. Erb, and M. J. Appel. 1997. Borrelia burgdorferi migrates into joint capsules and causes an upregulation of interleukin-8 in synovial membranes of dogs experimentally infected with ticks. Infect. Immun. 65:1273-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Straubinger, R. K., A. F. Straubinger, B. A. Summers, H. N. Erb, L. Harter, and M. J. Appel. 1998. Borrelia burgdorferi induces the production and release of proinflammatory cytokines in canine synovial explant cultures. Infect. Immun. 66:247-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Summers, B. A., A. F. Straubinger, R. H. Jacobson, Y. F. Chang, M. J. Appel, and R. K. Straubinger. 2005. Histopathological studies of experimental Lyme disease in the dog. J. Comp. Pathol. 133:1-13. [DOI] [PubMed] [Google Scholar]
- 31.Tilly, K., J. K. Krum, A. Bestor, M. W. Jewett, D. Grimm, D. Bueschel, R. Byram, D. Dorward, M. J. VanRaden, P. Stewart, and P. Rosa. 2006. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect. Immun. 74:3554-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilske, B., U. Busch, V. Fingerle, F. Jauris-Heipke, V. Preac-Mursic, D. Rossler, and G. Will. 1996. Immunological and molecular variability of OspA and OspC. Implication for Borrelia vaccine development. Infection 24:208-212. [DOI] [PubMed] [Google Scholar]