Abstract
Toxoplasma gondii CDPK1 (TgCDPK1) was found to be the target of the toxoplasmocidal compound 1NM-PP1. When TgCDPK1 was mutated at position 128 from glycine to methionine, resistance was gained. Inhibition of gliding motility without inhibition of micronemal secretion by 1NM-PP1 suggests a function for TgCDPK1 in gliding motility.
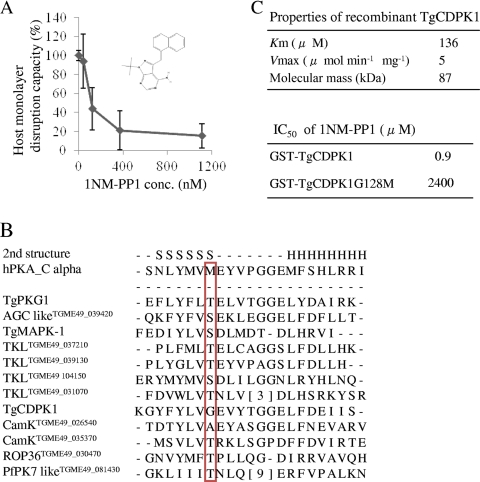
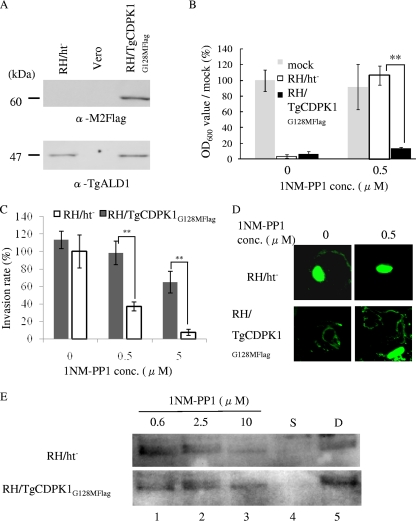
Toxoplasma gondii is an obligate intracellular parasite of the phylum Apicomplexa, which includes the causative pathogens of toxoplasmosis, malaria, and cryptosporidiosis. Although infection with this parasite is typically asymptomatic, infection rates are high. In addition, acute toxoplasmosis can be fatal in immunocompromised individuals and can cause severe birth defects or abortion during the first trimester of pregnancy (3). Several reports have shown that Toxoplasma gondii kinases function during the invasion steps, including attachment (17), secretion of micronemes, and subsequent invasion (5, 16). To identify the candidate, a chemical genetics approach using cyclic GMP (cGMP)-dependent protein kinase (PGK) inhibitor compound 2 successfully showed the T. gondii PKG (TgPKG) to be a target in the invasion steps (7). To date, however, candidate kinases have not been fully described. We used a protein kinase inhibitor analog, 1NM-PP1 (Merck KGaA, Darmstadt, Germany) (2), to help identify potential contributing kinases. In order to confirm the 1NM-PP1 effect on the T. gondii life cycle overall, a host monolayer disruption assay was performed as described elsewhere (22). Briefly, 96-well plates with confluent Vero cells were inoculated with 1.0 × 104 parasites in 200 μl of infectious medium (Dulbecco's modified Eagle's medium with 2% fetal calf serum [FCS]) containing the tested reagent or dimethyl sulfoxide (DMSO) at each concentration. About 4 days after inoculation, host cell debris were washed with phosphate-buffered saline (PBS) three times, fixed with methanol for 5 min, and stained using crystal violet, and the optical density at 600 nm (OD600) was measured using densitometry. 1NM-PP1 successfully inhibited the T. gondii life cycle at >500 nM (Fig. 1A). 1NM-PP1 has been reported to inhibit analog-sensitive kinases (as-kinases), which have a small amino acid at the gatekeeper residue of the ATP binding site instead of a bulky amino acid (23). Therefore, we predicted the target of 1NM-PP1 by searching for such kinases in the T. gondii genome. From the T. gondii genome, we obtained a list of 114 functional kinases by using SMART (19) (http://smart.embl-heidelberg.de/) and KinG (18) (http://hodgkin.mbu.iisc.ernet.in/∼king/), which all have full kinase domains and catalytic aspartic acid. Using the alignment data from ClustalW (11) and secondary structure data predicted by Psipred (13) (http://bioinf.cs.ucl.ac.uk/psipred/), kinase subdomains (11) were assigned and the gatekeeper residues in subdomain V were predicted. We found 12 predicted as-kinases that had the following amino acids at the gatekeeper residue: T, S, A, or G (2) (Fig. 1B). Among these predicted as-kinases, TgCDPK1 (GenBank ID AF333958) was the only kinase with the smallest amino acid, glycine, at the gatekeeper residue. Therefore, TgCDPK1 was predicted to be one of the targets of 1NM-PP1. Next, we checked the susceptibility of TgCDPK1 to 1NM-PP1 using an in vitro kinase assay. The open reading frame (ORF) of TgCDPK1 was PCR amplified from total RNA of the T. gondii RH strain using the primers 5′-CCGCCTCGAGCGGGCAGCAGGAAAGCACTCT-3′ and 5′-CCCAAGCTTAGTTTCCGCAGAGCTTCA-3′ and was cut with XhoI/HindIII and inserted into the same site of pBluescript II KS(+) (Stratagene, La Jolla, CA). The resultant plasmid was cut with XhoI-NotI and inserted into the same site of pAcGHLT-C (BD Biosciences, San Jose, CA) and designated as pGST-wt-CDPK1. For the expression of 128G-M-mutated CDPK1, pGST-CDPK1G128M was constructed from pGST-wt-CDPK1 using the QuikChange site-directed mutagenesis kit (Stratagene) with the primers 5′-CTACTTCTACCTCGTCATGGAAGTGTACACGGGAGGC-3′ and 5′-CTACTTCTACCTCGTCHTSGAAGTGTACACGGGAGGC-3′ in accordance with the manufacturer's instructions. Wild-type glutathione S-transferase (GST)–TgCDPK1 and mutated GST- TgCDPK1G128M were expressed as GST fusion proteins, purified by using a baculovirus expression system, and used for an in vitro kinase assay, as described elsewhere (24). In vitro kinase reactions were performed as described elsewhere (17) with syntide-2 (Sigma) as substrate and 100 μM CaCl2. GST-TgCDPK1 showed appropriate kinetics properties (Km, 136 μM; Vmax, 5 μmol min−1 mg−1) (Fig. 1C) with other recombinant TgCDPK1 forms as reported previously (8, 17). GST-TgCDPK1 and GST-TgCDPK1G128M were inhibited by 1NM-PP1 with 50% inhibitory concentrations (IC50s, in μM) of 0.9 and 2,400, respectively. Next, we constructed a C-terminal Flag-tagged TgCDPK1G128M stably expressing parasite to clarify the target of 1NM-PP1. First, the green fluorescent protein (GFP)-coding sequence of pMini.GFP.ht (14) (kindly provided by G. Arrizabalaga) was replaced with a 3xFlag sequence from the p3xFlag-CMV vector (Sigma, St. Louis, MO) and designated as pMini.3xFlag.ht. The analog-insensitive CDPK1 (ai-CDPK1) ORF was amplified from pGST-CDPK1G128M with the primers 5′-GAAGATCTGGGGCAGCAGGAAAGC-3′ and 5′-GGGGTACCGAGTTTCCGCAGAGCTTC-3′, cut with BglII/KpnI, inserted into the same site of pMini.3xFlag.ht before the 3xFlag coding sequence, and designated as pMini.ai-C1.3xFlag. RH/ht− (kindly provided by X. Xuan) was transfected with pMini.ai-C1.3xFlag and selected with a hypoxanthine-xanthine-guanine phosphoribosyl transferase selection marker, as described elsewhere (14). A clone that stably expressed C-terminal 3xFlag-tagged TgCDPK1G128M, which was designated as RH/TgCDPK1G128MFlag, was selected by limited dilution, and expression was confirmed by Western blotting of whole lysate from the infected Vero cells (Fig. 2A). RH/TgCDPK1G128MFlag showed no apparent differences from the parental strain in either proliferation rate or invasion rate (data not shown). When the host monolayer disruption capacities of RH/ht− and RH/TgCDPK1G128MFlag were assayed under 0.5 μM 1NM-PP1 or without 1NM-PP1, the OD600 value, which stood for the crystal violet-stained viable host cells, of RH/TgCDPK1G128MFlag was low (Fig. 2B, right) as well as that of 1NM-PP1 nontreated infected wells (Fig. 2B, left), whereas that of the parental strain RH/ht− with 0.5 μM 1NM-PP1 didn't change from that of mock-infected wells (Fig. 2B). These results indicated that RH/TgCDPK1G128MFlag could grow normally under 0.5 μM 1NM-PP1 treatment and disrupt host cells. Therefore, TgCDPK1G128M was thought to give parasites resistance to 1NM-PP1, and TgCDPK1 is thought to be the main target of 1NM-PP1. TgCDPK1 may be involved in the invasion process and is thought to play a role in Ca2+ signal transduction (1). We took advantage of the instant effects of small-compound-based gene functional suppression to reveal the contribution of TgCDPK1 over a short time frame, as seen during invasion (4). Invasion assays were performed as described elsewhere (17). Briefly, host monolayers were infected with drug-treated parasites (1.0 × 106/ml) in drug-containing medium for 30 min and fixed. External (attached) parasites were stained with anti-SAG1 monoclonal antibody (TP3; Hytest, Finland) before Triton X-100 permeabilization and detection of internal (invaded) parasites with rabbit anti-tachyzoite polyclonal antibody (Funakoshi, Japan). Anti-rabbit IgG goat antibody conjugated with Alexa 488 and anti-mouse IgG goat antibody conjugated with Alexa 546 (Invitrogen, Carlsbad, CA) were used for the secondary antibodies. Treatment with 0.5 μM 1NM-PP1 reduced the invasion rate of RH/ht− to less than 40%, whereas no apparent changes were detected with the RH/TgCDPK1G128MFlag strain in the 0.5 μM 1NM-PP1 treatment group (Fig. 2C). The effect of 1NM-PP1 on the invasion might be partly due to the inhibition of as-kinases other than TgCDPK1 (among them, TgPKG has been reported to play a role in invasion [7]). However, the difference between 1NM-PP1 effects on invasion of RH/ht− and RH/TgCDPK1G128MFlag clones suggested the role of TgCDPK1 in the invasion. The contribution of TgCDPK1 to the invasion was consistent with a previous report described with KT5926 (17). Next, gliding motility assays were performed as described elsewhere (25). Briefly, parasites were allowed to glide for 15 min at 37°C on the slide glasses coated with 50% FCS in PBS. Gliding trails were stained with anti-SAG1 monoclonal antibody (TP3) and anti-mouse IgG goat antibody conjugated with Alexa 488 (Invitrogen). Treatment with 0.5 μM 1NM-PP1 also reduced the gliding motility of RH/ht−, but not of RH/TgCDPK1G128MFlag (Fig. 2D). Finally, ethanol-induced micronemal secretion was assayed as described elsewhere (5). In brief, parasites were incubated at 18°C for 10 min with test reagent, followed by incubation at 37°C for 30 min after the addition of 1% (vol/vol) ethanol. Cells were removed by centrifugation at 4,000 × g, and the supernatant was used for Western blotting. For the detection of secreted proteins, anti-TgM2AP rabbit antibody (21) (kindly provided by V. Carruthers) was used. Surprisingly, however, there was no concentration-dependent change in secreted TgM2AP with 1NM-PP1 treatment and DMSO treatment (Fig. 2E, lanes 1 to 3 and 5), whereas the treatment with 1 μM staurosporine resulted in undetectable secreted TgM2AP (Fig. 2E, lane 4). This result suggested that TgCDPK1 can contribute to gliding motility directly. Recently, it has been reported that some glideosome complex members are phosphorylated (9, 10). Therefore, TgCDPK1 might phosphorylate the glideosome complex directly and change its activity. Inhibitor-refractory TgCDPK1G128M-expressing parasites in the present report gained resistance against 1NM-PP1. However, the previously reported TgCDPK1G128Q-expressing parasite (7) has been suggested not to gain resistance to cGMP-dependent protein kinase inhibitor compound 2 and to not function in the invasion step. This difference might result from the differences between the inhibitors used. To examine the essential genes of this pathogen via genetic approaches, several tools have been developed, including conditional knockouts, RNA interference (20), and conditional proteolysis-based gene suppression (12). These methods are very useful for analyzing indispensable genes; however, one weakness is that gene suppression is not instant in the conditional knockout and is dependent on the genes to be analyzed. Any protein kinases of T. gondii mutated to be analog sensitive as well as Plasmodium falciparum protein kinases reported in a review (6) can also be functionally downregulated with high specificity and over a short time span of several minutes by using 1NM-PP1 and RH/TgCDPK1G128MFlag. This strategy provides us with an additional conditional gene regulation tool in T. gondii.
Fig. 1.
Toxoplasmocidal properties of 1NM-PP1 and its putative targets. (A) The reduction in OD600 values from mock-infected wells was calculated as the monolayer disruption capacity. Capacity in the absence of 1NM-PP1 was estimated to be 100%. The structure of 1NM-PP1 is also illustrated. The standard errors of the means from triplicate experiments are shown. (B) Alignment of subdomain V of 12 predicted as-kinases and the human protein kinase A (PKA) catalytic subunit alpha are shown. The gatekeeper residues are shown in the red box. Predicted secondary structures are indicated with an S (β-sheet) or H (α-helix) on the first line. (C) Kinetics properties of GST-TgCDPK1 with substrate peptide syntide-2 and effects of 1NM-PP1 on GST-TgCDPK1 and GST-TgCDPK1G128M. Reactions were performed with 1.0 ng of kinase in 30 μl of reaction buffer (20 mM HEPES [pH 7.5], 10 mM MgCl2, 1 mg/ml bovine serum albumin, 100 μM CaCl2, 2 μM dithiothreitol, 2 μM ATP, and 5.0 μCi [γ-32P]ATP). For the inhibitory assay, 100 μM syntide-2 was used.
Fig. 2.
1NM-PP1-resistant strain RH/ai-C1. (A) Confirmation of Flag-tagged TgCDPK1G128M expression in RH/TgCDPK1G128MFlag based on Western blotting. Vero cells infected with RH/ht−, RH/TgCDPK1G128MFlag, or mock infected were subjected to a Western blot assay with the anti-M2 Flag antibody (α-M2Flag; Sigma, St. Louis, MO) (upper panel) or anti-T. gondii aldolase rabbit antibody newly raised as described previously (15) against myelin basic protein-TgALD1 expressed in Escherichia coli (24), in order to load adequate amounts of parasites. (B) Effects of 1NM-PP1 on the overall life cycle with RH/ht− or RH/TgCDPK1G128MFlag in the host monolayer disruption assay. OD600 values in the absence of infection and without 1NM-PP1 were estimated as 100%. (C) Effects of 1NM-PP1 on RH/ht− and RH/TgCDPK1G128MFlag during parasite invasion. Invasion rates were calculated from the number of completely invaded parasites per number of whole parasites counted. Invasion rates of RH/ht− in the absence of 1NM-PP1 were estimated as 100%. (D) Effects of 1NM-PP1 on gliding motility with RH/ht− or RH/TgCDPK1G128MFlag. Gliding trails were visualized with anti-SAG1 antibody. (E) Effects of 1NM-PP1 on secretions. The 35-kDa bands of TgM2AP are shown. In panels C to E, parasites were pretreated with 1NM-PP1, 1 μM staurosporine (S), or DMSO (D) at the listed concentrations. In panels B and C, the standard errors of the means from triplicate experiments are shown. **, P < 0.01, two-tailed Student's t test.
Acknowledgments
We thank X. Xuan (Obihiro University of Agriculture and Veterinary Medicine), G. Arrizabalaga (University of Idaho), and V. Carruthers (University of Michigan Medical School) for useful technical advice and help with the materials.
This study was supported by a Grant-in-Aid for Young Scientists from the Ministry of Education, Culture, Science, Sports and Technology of Japan and the Bio-oriented Technology Research Advancement Institution.
Footnotes
Published ahead of print on 19 February 2010.
REFERENCES
- 1.Billker O., Lourido S., Sibley L. D. 2009. Calcium-dependent signaling and kinases in apicomplexan parasites. Cell Host Microbe 5:612–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bishop A. C., Ubersax J. A., Petsch D. T., Matheos D. P., Gray N. S., Blethrow J., Shimizu E., Tsien J. Z., Schultz P. G., Rose M. D., Wood J. L., Morgan D. O., Shokat K. M. 2000. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature 407:395–401 [DOI] [PubMed] [Google Scholar]
- 3.Black M. W., Boothroyd J. C. 2000. Lytic cycle of Toxoplasma gondii. Microbiol. Mol. Biol. Rev. 64:607–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carruthers V., Boothroyd J. C. 2007. Pulling together: an integrated model of Toxoplasma cell invasion. Curr. Opin. Microbiol. 10:83–89 [DOI] [PubMed] [Google Scholar]
- 5.Carruthers V. B., Giddings O. K., Sibley L. D. 1999. Secretion of micronemal proteins is associated with toxoplasma invasion of host cells. Cell. Microbiol. 1:225–235 [DOI] [PubMed] [Google Scholar]
- 6.Doerig C., Billker O., Pratt D., Endicott J. 2005. Protein kinases as targets for antimalarial intervention: kinomics, structure-based design, transmission-blockade, and targeting host cell enzymes. Biochim. Biophys. Acta 1754:132–150 [DOI] [PubMed] [Google Scholar]
- 7.Donald R. G., Zhong T., Wiersma H., Nare B., Yao D., Lee A., Allocco J., Liberator P. A. 2006. Anticoccidial kinase inhibitors: identification of protein kinase targets secondary to cGMP-dependent protein kinase. Mol. Biochem. Parasitol. 149:86–98 [DOI] [PubMed] [Google Scholar]
- 8.Donald R. G., Zhong T., Meijer L., Liberator P. A. 2005. Characterization of two T. gondii CK1 isoforms. Mol. Biochem. Parasitol. 141:15–27 [DOI] [PubMed] [Google Scholar]
- 9.Gilk S. D., Gaskins E., Ward G. E., Beckers C. J. 2009. GAP45 phosphorylation controls assembly of the Toxoplasma myosin XIV complex. Eukaryot. Cell 8:190–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green J. L., Rees-Channer R. R., Howell S. A., Martin S. R., Knuepfer E., Taylor H. M., Grainger M., Holder A. A. 2008. The motor complex of Plasmodium falciparum: phosphorylation by a calcium-dependent protein kinase. J. Biol. Chem. 283:30980–30989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanks S. K., Hunter T. 1995. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 9:576–596 [PubMed] [Google Scholar]
- 12.Herm-Gotz A., Agop-Nersesian C., Munter S., Grimley J. S., Wandless T. J., Frischknecht F., Meissner M. 2007. Rapid control of protein level in the apicomplexan Toxoplasma gondii. Nat. Methods 4:1003–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones D. T. 1999. Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 292:195–202 [DOI] [PubMed] [Google Scholar]
- 14.Karasov A. O., Boothroyd J. C., Arrizabalaga G. 2005. Identification and disruption of a rhoptry-localized homologue of sodium hydrogen exchangers in Toxoplasma gondii. Int. J. Parasitol. 35:285–291 [DOI] [PubMed] [Google Scholar]
- 15.Kato K., Sudo A., Kobayashi K., Tohya Y., Akashi H. 2008. Characterization of Plasmodium falciparum protein kinase 2. Mol. Biochem. Parasitol. 162:87–95 [DOI] [PubMed] [Google Scholar]
- 16.Kato N., Sakata T., Breton G., Le Roch K. G., Nagle A., Andersen C., Bursulaya B., Henson K., Johnson J., Kumar K. A., Marr F., Mason D., McNamara C., Plouffe D., Ramachandran V., Spooner M., Tuntland T., Zhou Y., Peters E. C., Chatterjee A., Schultz P. G., Ward G. E., Gray N., Harper J., Winzeler E. A. 2008. Gene expression signatures and small-molecule compounds link a protein kinase to Plasmodium falciparum motility. Nat. Chem. Biol. 4:347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kieschnick H., Wakefield T., Narducci C. A., Beckers C. 2001. Toxoplasma gondii attachment to host cells is regulated by a calmodulin-like domain protein kinase. J. Biol. Chem. 276:12369–12377 [DOI] [PubMed] [Google Scholar]
- 18.Krupa A., Abhinandan K. R., Srinivasan N. 2004. KinG: a database of protein kinases in genomes. Nucleic Acids Res. 32:D153–D155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Letunic I., Doerks T., Bork P. 2009. SMART 6: recent updates and new developments. Nucleic Acids Res. 37:D229–D232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meissner M., Agop-Nersesian C., Sullivan W. J., Jr 2007. Molecular tools for analysis of gene function in parasitic microorganisms. Appl. Microbiol. Biotechnol. 75:963–975 [DOI] [PubMed] [Google Scholar]
- 21.Rabenau K. E., Sohrabi A., Tripathy A., Reitter C., Ajioka J. W., Tomley F. M., Carruthers V. B. 2001. TgM2AP participates in Toxoplasma gondii invasion of host cells and is tightly associated with the adhesive protein TgMIC2. Mol. Microbiol. 41:537–547 [DOI] [PubMed] [Google Scholar]
- 22.Roos D. S., Donald R. G., Morrissette N. S., Moulton A. L. 1994. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 45:27–63 [DOI] [PubMed] [Google Scholar]
- 23.Shokat K., Velleca M. 2002. Novel chemical genetic approaches to the discovery of signal transduction inhibitors. Drug Discov. Today 7:872–879 [DOI] [PubMed] [Google Scholar]
- 24.Sugi T., Kato K., Kobayashi K., Pandey K., Takemae H., Kurokawa H., Tohya Y., Akashi H. 2009. Molecular analyses of Toxoplasma gondii calmodulin-like domain protein kinase isoform 3. Parasitol. Int. 58:416–423 [DOI] [PubMed] [Google Scholar]
- 25.Wetzel D. M., Hakansson S., Hu K., Roos D., Sibley L. D. 2003. Actin filament polymerization regulates gliding motility by apicomplexan parasites. Mol. Biol. Cell 14:396–406 [DOI] [PMC free article] [PubMed] [Google Scholar]