Abstract
In Saccharomyces cerevisiae, the TEA transcription factor Tec1 is known to regulate target genes together with a second transcription factor, Ste12. Tec1-Ste12 complexes can activate transcription through Tec1 binding sites (TCSs), which can be further combined with Ste12 binding sites (PREs) for cooperative DNA binding. However, previous studies have hinted that Tec1 might regulate transcription also without Ste12. Here, we show that in vivo, physiological amounts of Tec1 are sufficient to stimulate TCS-mediated gene expression and transcription of the FLO11 gene in the absence of Ste12. In vitro, Tec1 is able to bind TCS elements with high affinity and specificity without Ste12. Furthermore, Tec1 contains a C-terminal transcriptional activation domain that confers Ste12-independent activation of TCS-regulated gene expression. On a genome-wide scale, we identified 302 Tec1 target genes that constitute two distinct classes. A first class of 254 genes is regulated by Tec1 in a Ste12-dependent manner and is enriched for genes that are bound by Tec1 and Ste12 in vivo. In contrast, a second class of 48 genes can be regulated by Tec1 independently of Ste12 and is enriched for genes that are bound by the stress transcription factors Yap6, Nrg1, Cin5, Skn7, Hsf1, and Msn4. Finally, we find that combinatorial control by Tec1-Ste12 complexes stabilizes Tec1 against degradation. Our study suggests that Tec1 is able to regulate TCS-mediated gene expression by Ste12-dependent and Ste12-independent mechanisms that enable promoter-specific transcriptional control.
In Saccharomyces cerevisiae and related yeast species, the transcription factors Tec1 and Ste12 are a paradigm for studying the mechanisms of combinatorial and promoter-specific target gene control (5, 30, 37, 53). These DNA binding proteins can interact with each other and control various developmental programs, including vegetative adhesion, filament formation, and pheromone-induced sexual mating (13, 15–17, 35, 37, 41, 42, 46). Tec1 belongs to the family of the TEA transcription factors, which control several transcriptional programs governing cellular differentiation in many eukaryotes. Common to this family is the TEA DNA binding domain (DBD) composed of a three-helix bundle, which binds to conserved TEA consensus sequence (TCS) elements (2, 37). S. cerevisiae Ste12 is the founding member of the Ste12-like transcription factors that are found preferentially in fungi, where they control cellular development and pathogenesis (15, 33, 34, 50). Ste12 binds to pheromone response elements (PREs), and two or more of these elements are necessary to confer pheromone-responsive transcriptional control by the Fus3/Kss1 mitogen-activated protein kinase (MAPK) pathway (13, 14, 23, 52).
For combinatorial target gene control, Tec1 and Ste12 form a complex through the C-terminal part of Tec1 (10). For target gene binding, initial in vitro studies have shown that Tec1-Ste12 complex formation enables cooperative binding to combined filamentation and invasion response elements (FREs), which consist of a TCS and a PRE (37). More recent studies show that the promoter regions of many Tec1 target genes do not contain FREs but contain one or several TCSs (10). In addition, Tec1 and Ste12 are often present at the same promoters in vivo (5, 6, 53). It has thus been suggested that Tec1-Ste12 complexes regulate transcription predominantly via TCS elements, with Tec1 providing the DNA binding domain and Ste12 the transcriptional activation domain (AD) (10). Tec1-Ste12 complexes can be further regulated by the protein Dig1, whose inhibitory function on Ste12 is relieved during mating by phosphorylation through the pheromone-stimulated MAPKs Fus3 and Kss1 (4, 10, 12, 45, 49). In addition, Tec1 is directly phosphorylated by the MAPK Fus3 in response to pheromone, which triggers ubiquitin-mediated degradation of the transcription factor (3, 8, 9). Finally, Ste12 directly controls TEC1 gene expression via PREs, and TEC1 transcript levels are pheromone inducible (43). As a consequence, TEC1 transcript and Tec1 protein levels are drastically reduced in the absence of Ste12 (30).
In the study presented here, we have explored the possibility that Tec1 confers Ste12-independent transcriptional regulation, because we have previously shown that overexpression of TEC1 in ste12Δ mutant strains leads to activation of TCS-driven reporter genes (30). We now demonstrate that physiological levels of Tec1 can bind to TCS elements in vitro and regulate TCS-driven genes in vivo in the absence of Ste12. On a genome-wide scale we show that Tec1 is able to control a subset of its target genes without Ste12 through a C-terminal transcriptional activation domain. We also show that complex formation between Tec1 and Ste12 provides Tec1 stability control. Our study suggests that Tec1 is able to regulate TCS-mediated transcription by Ste12-dependent as well as Ste12-independent mechanisms to confer promoter-specific control.
MATERIALS AND METHODS
Yeast strains and growth conditions.
All yeast strains used in this study are listed in Table 1. Yeast strains carrying the dig1Δ::kanMX4 deletion allele were obtained by transformation with a corresponding deletion cassette and verified by Southern blot analysis. Different TEC1 versions and TEC1-STE12 hybrid constructs were introduced into the yeast genome by targeting of the appropriate plasmid to the leu2::hisG locus. TCS and PRE reporter gene plasmids were targeted to the ura3-52 locus. Yeast culture medium was prepared as described previously (21). Adhesive growth tests were as described earlier (46). For cycloheximide-induced translational shutoff experiments, yeast cultures grown to an optical density at 600 nm (OD600) of 1 were treated with 200 μg/ml cycloheximide and samples were taken at the indicated time points.
Table 1.
Yeast strains used in this study
| Strain | Relevant genotype | Reference |
|---|---|---|
| RH2500 | MATatec1Δ::HIS3 ura3-52 leu2::hisG trp1::hisG | 30 |
| RH2501 | MATatec1Δ::HIS3 ste12Δ::TRP1 ura3-52 leu2::hisG | 30 |
| RH2765 | RH2500 with TCS-CYC1-lacZ::URA3 | 30 |
| RH2767 | RH2500 with CYC1(ΔUAS)-lacZ::URA3 | 30 |
| RH2768 | RH2500 with TCS-PRE-CYC1-lacZ::URA3 | 30 |
| RH2774 | RH2501 with TCS-CYC1-lacZ::URA3 | 30 |
| RH2777 | RH2501 with TCS-PRE-CYC1-lacZ::URA3 | 30 |
| RH2662 | MATaflo11Δ::kanR ura3-52 | 7 |
| EGY48-p1840 | MATα ura3 his3 trp1 leu2 lexAop-lacZ-URA3 | 22 |
| YHUM1631 | RH2765 with PURA3-TEC1::LEU2 | This study |
| YHUM1634 | RH2765 with PURA3-TEC11–280::LEU2 | This study |
| YHUM1635 | RH2765 with PURA3-TEC11–280-TEC1377–486::LEU2 | This study |
| YHUM1640 | RH2774 with PURA3-TEC11–280::LEU2 | This study |
| YHUM1641 | RH2774 with PURA3-TEC11–280-TEC1377–486::LEU2 | This study |
| YHUM1642 | RH2774 with PURA3-TEC11–280-STE121–688::LEU2 | This study |
| YHUM1643 | RH2774 with PURA3-TEC11–280-STE121–250::LEU2 | This study |
| YHUM1644 | RH2774 with PURA3-TEC11–280-STE121–400::LEU2 | This study |
| YHUM1645 | RH2774 with PURA3-TEC11–280-STE12380–688::LEU2 | This study |
| YHUM1646 | RH2774 with PURA3-TEC11–280-STE121–688-TEC1377–486::LEU2 | This study |
| YHUM1647 | RH2774 with PURA3-TEC11–280-STE121–250-TEC1377–486::LEU2 | This study |
| YHUM1648 | RH2774 with PURA3-TEC11–280-STE121–400-TEC1377–486::LEU2 | This study |
| YHUM1649 | RH2774 with PURA3-TEC11–280-STE12380–688-TEC1377–486::LEU2 | This study |
| YHUM1676 | RH2501 with PURA3-TEC1::LEU2 | This study |
| YHUM1694 | RH2500 with PTEC1-TEC1::LEU2 | This study |
| YHUM1696 | RH2500 with tcs(ACATTCcT)-CYC1-lacZ::URA3 | This study |
| YHUM1697 | RH2500 with tcs(ACATTCaT)-CYC1-lacZ::URA3 | This study |
| YHUM1698 | RH2500 with tcs(AtATTCTT)-CYC1-lacZ::URA3 | This study |
| YHUM1699 | RH2500 with tcs(ACATgCTT)-CYC1-lacZ::URA3 | This study |
| YHUM1700 | MATatec1Δ::HIS3 ura3-52 trp1::hisG | This study |
| YHUM1701 | MATatec1Δ::HIS3 ste12Δ::TRP1 ura3-52 | This study |
| YHUM1712 | RH2774 with PTEC1-TEC1::LEU2 | This study |
| YHUM1713 | RH2774 with PURA3-TEC1T273 M::LEU2 | This study |
| YHUM1718 | YHUM1676 with TCS-CYC1-lacZ::URA3 | This study |
| YHUM1720 | YHUM1649 with dig1Δ::kanMX4 | This study |
| YHUM1721 | MATatec1Δ::HIS3 PTEC1-TEC1::LEU2 TCS-CYC1-lacZ::URA3 | This study |
| YHUM1723 | MATatec1Δ::HIS3 PURA3-TEC1T273 M::LEU2 TCS-CYC1-lacZ::URA3 | This study |
| YHUM1724 | MATatec1Δ::HIS3 TCS-CYC1-lacZ::URA3 | This study |
| YHUM1727 | MATatec1Δ::HIS3 PURA3-TEC1::LEU2 TCS-CYC1-lacZ::URA3 | This study |
| YHUM1731 | RH2500 with tcs(ACtTTCTT)-CYC1-lacZ::URA3 | This study |
| YHUM1732 | RH2500 with tcs(ACAgTCTT)-CYC1-lacZ::URA3 | This study |
| YHUM1733 | RH2768 with PURA3-TEC11–280-TEC1377–486::LEU2 | This study |
| YHUM1734 | RH2777 with PURA3-TEC11–280-TEC1377–486::LEU2 | This study |
| YHUM1735 | RH2777 with PURA3-TEC11–280::LEU2 | This study |
| YHUM1736 | RH2768 with PURA3-TEC11–280::LEU2 | This study |
| YHUM1737 | RH2777 with PURA3-TEC11–280-STE121–688-TEC1377–486::LEU2 | This study |
| YHUM1738 | RH2777 with PURA3-TEC11–280-STE12380–688-TEC1377–486::LEU2 | This study |
| YHUM1739 | RH2777 with PURA3-TEC1::LEU2 | This study |
| YHUM1740 | RH2768 with PURA3-TEC1::LEU2 | This study |
| YHUM1743 | YHUM1727 with dig1Δ::kanMX4 | This study |
| YHUM1744 | YHUM1718 with dig1Δ::kanMX4 | This study |
| YHUM1745 | YHUM1644 with dig1Δ::kanMX4 | This study |
| YHUM1746 | YHUM1645 with dig1Δ::kanMX4 | This study |
| YHUM1747 | YHUM1648 with dig1Δ::kanMX4 | This study |
| YHUM1749 | RH2768 with PTEC1-TEC1::LEU2 | This study |
| YHUM1750 | RH2777 with PTEC1-TEC1::LEU2 | This study |
| YHUM1751 | RH2777 with PURA3-TEC11–280-STE121–250-TEC1377–486::LEU2 | This study |
| YHUM1752 | RH2777 with PURA3-TEC11–280-STE121–400-TEC1377–486::LEU2 | This study |
| YHUM1846 | RH2777 with PURA3-TEC11–280-STE121–688::LEU2 | This study |
| YHUM1847 | RH2777 with PURA3-TEC11–280-STE121–250::LEU2 | This study |
| YHUM1848 | RH2777 with PURA3-TEC11–280-STE121–400::LEU2 | This study |
| YHUM1849 | RH2777 with PURA3-TEC11–280-STE12380–688::LEU2 | This study |
Plasmids.
All plasmids used in this study are listed in Table 2. Plasmids BHUM1622 to BHUM1627, carrying point-mutated TCS elements upstream of the CYC1-lacZ reporter gene, were obtained by substituting a 430-bp XhoI fragment containing the CYC1 UAS of plasmid pLI4 for synthetic linkers with the indicated mutated TCS element and XhoI-cohesive ends. Linkers were prepared by annealing of the corresponding primers (see Table S3 in the supplemental material), and a single insertion was confirmed by sequencing. Plasmids BHUM1384, BHUM1388, BHUM1389, BHUM1394, and BHUM1401 were obtained by site-directed mutagenesis using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) and BHUM388 as the template. Similarly, plasmids BHUM1480 to BHUM1484 were obtained by site-directed mutagenesis using pME2289 as the template. Plasmid BHUM1616 was constructed by introduction of a 3.1-kb PstI/BamHI fragment from pME2068, carrying PTEC1-TEC1, into YIplac128. For BHUM1258, BHUM1259, BHUM1347, BHUM1350, BHUM1357 to BHUM1365, BHUM1464, and BHUM1465, the URA3 promoter region was amplified from the yeast genome by PCR with primers BHURA3-1 and BHURA3-2, yielding a 430-bp product with a newly created upstream SphI site and a new SalI site behind the ATG start codon. The fragment was inserted into YIplac128 to obtain plasmid YIplac128-PURA3. A 2.1-kb SalI/BamHI fragment carrying TEC1 or TEC1T273M was released from pME2068 or pME2102 and placed downstream of the URA3 promoter to create BHUM1258 and BHUM1259. Plasmids with truncated TEC1 versions and TEC1-STE12 fusions were constructed by the following strategy. (i) First, a BglII restriction site was inserted into the TEC1 open reading frame (ORF) behind codon 280 by whole-vector PCR with primers JFTEC1-280-1 and -2 using the 2.1-kb SalI/BamHI TEC1 ORF from pME2068 inserted into pBluescript II KS+ as a template. The PCR product served as a whole-vector PCR template to introduce an additional XhoI site behind codon 377 (primers JFTEC1-377-1 and -2) or behind codon 486 (primers JFTEC1-486-1 and -2). Mutagenized TEC1 ORFs were released by SalI/BamHI digestion and inserted into YIplac128-PURA3. To replace a natural XhoI site in the TEC1 terminator region by BamHI, PURA3-TEC1Bgl280Xho486 and PURA3-TEC1Bgl280Xho377 were amplified from these plasmids with primers BHURA3-1 and JFTEC1-1918-BamHI, resulting in 2.4-kb SphI/BamHI cassettes that were subcloned into YIplac128 to obtain BHUM1347 and BHUM1350. (ii) BHUM1464 and BHUM1465 were obtained by replacing the BglII/XhoI TEC1 fragments in BHUM1347 and BHUM1350 by a synthetic linker which was prepared by annealing of primers BglII/XhoI linker 1 and BglII/XhoI linker 2. (iii) STE12 ORF fragments were amplified from the yeast genome by PCR with primers generating a new BglII site at the 5′ end and a XhoI site at the 3′ end of each product. To obtain the TEC1-STE12 fusion constructs BHUM1357 to BHUM1365, STE12 fragments were inserted into BglII/XhoI sites of plasmids BHUM1347 and BHUM1350.
Table 2.
Plasmids used in this study
| Plasmid | Genotypea | Reference |
|---|---|---|
| BHUM29 | PGAL1-TEC1 in pRS316 | 30 |
| BHUM30 | TEC1 in pRS202 | 30 |
| BHUM212 | pLG669-Z with TCS-PRE-CYC1-lacZ | 37 |
| BHUM213 | pLG669-Z with TCS-pre-CYC1-lacZ | 37 |
| BHUM214 | pLG669-Z with tcs-PRE-CYC1-lacZ | 37 |
| BHUM388 | MBP-TEC1-FLAG in pMAL-c2 | 37 |
| BHUM389 | MBP-STE12-FLAG in pMAL-c2 | 37 |
| BHUM752 | TEC1281–486 in pEG202 | This work |
| BHUM1258 | PURA3-TEC1 in YIplac128 | This work |
| BHUM1259 | PURA3-TEC1T273M in YIplac128 | This work |
| BHUM1347 | PURA3-TEC1Bgl280Xho486 in YIplac128 | This work |
| BHUM1350 | PURA3-TEC1Bgl280Xho377 in YIplac128 | This work |
| BHUM1357 | PURA3-TEC11–280-STE121–688::LEU2 in YIplac128 | This work |
| BHUM1358 | PURA3-TEC11–280-STE121–250::LEU2 in YIplac128 | This work |
| BHUM1359 | PURA3-TEC11–280-STE121–400::LEU2 in YIplac128 | This work |
| BHUM1360 | PURA3-TEC11–280-STE12380–688::LEU2 in YIplac128 | This work |
| BHUM1362 | PURA3-TEC11–280-STE121–688-TEC1377–486::LEU2 in YIplac128 | This work |
| BHUM1363 | PURA3-TEC11–280-STE121–250-TEC1377–486::LEU2 in YIplac128 | This work |
| BHUM1364 | PURA3-TEC11–280-STE121–400-TEC1377–486::LEU2 in YIplac128 | This work |
| BHUM1365 | PURA3-TEC11–280-STE12380–688-TEC1377–486::LEU2 in YIplac128 | This work |
| BHUM1384 | MBP-TEC1R164A-FLAG in pMAL-c2 | This work |
| BHUM1388 | MBP-TEC1L144A-FLAG in pMAL-c2 | This work |
| BHUM1389 | MBP-TEC1L146A-FLAG in pMAL-c2 | This work |
| BHUM1394 | MBP-TEC1G163A-FLAG in pMAL-c2 | This work |
| BHUM1401 | MBP-TEC1S187A-FLAG in pMAL-c2 | This work |
| BHUM1464 | PURA3-TEC11–280::LEU2 in YIplac128 | This work |
| BHUM1465 | PURA3-TEC11–280-TEC1377–486::LEU2 in YIplac128 | This work |
| BHUM1480 | PTEC1-TEC1L146A in YCplac111 | This work |
| BHUM1482 | PTEC1-TEC1G163A in YCplac111 | This work |
| BHUM1483 | PTEC1-TEC1S187A in YCplac111 | This work |
| BHUM1484 | PTEC1-TEC1R164A in YCplac111 | This work |
| BHUM1616 | PTEC1-TEC1 in YIplac128 | This work |
| BHUM1622 | pLI4 with tcs(ACATTCcT)-CYC1-lacZ | This work |
| BHUM1623 | pLI4 with tcs(ACATTCaT)-CYC1-lacZ | This work |
| BHUM1624 | pLI4 with tcs(ACATgCTT)-CYC1-lacZ | This work |
| BHUM1625 | pLI4 with tcs(AtATTCTT)-CYC1-lacZ | This work |
| BHUM1626 | pLI4 with tcs(ACtTTCTT)-CYC1-lacZ | This work |
| BHUM1627 | pLI4 with tcs(ACAgTCTT)-CYC1-lacZ | This work |
| BHUM1687 | TEC1350–486 in pEG202 | This work |
| pEG202 | HIS3-based two-hybrid vector with lexA-DBD | 22 |
| pJG4-5 | TRP1-based two-hybrid vector with B42-AD | 22 |
| pLI4 | CYC1-lacZ in URA3-based integrative vector | 30 |
| pME2051 | pLI4 with TCS-CYC1-lacZ | 30 |
| pME2055 | pLI4 with TCS-TCS-CYC1-lacZ | 30 |
| pME2068 | PTEC1-TEC1 in YCplac33 | 30 |
| pME2102 | PTEC1-TEC1T273M in YCplac33 | 30 |
| pME2289 | PTEC1-TEC1 in YIplac111 | 30 |
| YIplac128 | LEU2-based integrative vector | 19 |
| YCplac111 | LEU2-based CEN vector | 19 |
| YEplac195 | URA3-based 2μm vector | 19 |
TCS-PRE corresponds to the sequence of FRE(Ty1) (37).
Protein analysis. (i) Preparation of yeast cell extracts.
Preparation of total cell yeast extracts was performed as previously described (32, 51). Briefly, yeast cultures grown to an OD600 of 1 were concentrated to 1 ml, mixed with 150 μl lysis buffer (1.85 M NaOH, 7.5% [vol/vol] β-mercaptoethanol), and incubated on ice for 10 min. Samples were mixed with 150 μl trichloroacetic acid (55%) and centrifuged for 10 min at 13,000 rpm. One hundred microliters of urea buffer (5% SDS, 8 M urea, 92.6 mM Na2HPO4, 107.4 mM NaH2PO4, 1.5% dithiothreitol, 0.1 mM EDTA [pH 6.8], bromophenol blue) and 2 μl of 2 M Tris-Cl were added to the pellet and shaken for 20 min at 37°C. Samples were centrifuged for 5 min at 13,000 rpm, and supernatants were further analyzed.
(ii) Generation of polyclonal anti-Tec1 antibodies.
GST-Tec11–280 was purified by cultivation of Escherichia coli BL21(DE3) cells (Novagen, NJ) containing plasmid pME2676 (GST-TEC11–280 in pETM30) (8) in LB medium supplemented with kanamycin. Target protein expression was induced with 0.1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 4 h at 20°C, and cells were resuspended in collection buffer (50 mM Tris-HCl [pH 7.5], 500 mM NaCl, 5 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride) and disrupted in a fluidizer. GST-Tec1M1-E280 was purified by column chromatography on a glutathione-Sepharose column, and the glutathione S-transferase (GST) tag was proteolytically removed by TEV protease digestion and Ni-nitrilotriacetic acid (NTA) purification. Purified Tec1M1-E280 was used for standard immunization procedure by the Pineda Antibody Service (Berlin, Germany) to obtain polyclonal rabbit anti-Tec11–280 antibodies.
(iii) Immunoblot analysis.
Equal amounts of proteins were separated by 12% SDS-PAGE and transferred to nitrocellulose membranes. Tec1 and Tec1-Ste12 fusion proteins or Tub1 was detected using enhanced chemiluminescence (ECL) technology after incubation of membranes with polyclonal rabbit anti-Tec11–280 or monoclonal mouse anti-Tub1 antibodies (Calbiochem, Darmstadt, Germany). As secondary antibodies, horseradish peroxidase-coupled goat anti-rabbit (Santa Cruz, CA) or goat anti-mouse (Dianova, Hamburg, Germany) antibodies were used, respectively. Signals were quantified using a scanner and the ImageQuant TL software (GE Healthcare, Freiburg, Germany).
Electrophoretic mobility shift assays.
Maltose binding protein (MBP), MBP-Tec1 and MBP-Ste12 proteins were prepared from E. coli using plasmids pMAL-c2, BHUM388, and BHUM389 essentially as described earlier (37). E. coli protein extracts containing different Tec1 variants were obtained using appropriate plasmids. 32P-labeled DNA was prepared by PCR amplification of 97-bp (TCS), 113-bp (TCS-TCS), or 111-bp (TCS-PRE, TCS-pre, and tcs-PRE) DNA fragments using the primer pair BHCYC1-2/BHCYC1-3 and plasmid BHUM212, BHUM213, BHUM214, pME2051, or pME2055. Five picomoles of the resulting DNA was incubated with 100 μCi of [γ-32P]ATP and T4 polynucleotide kinase, followed by purification using a QIAquick nucleotide removal kit (Qiagen, Hilden, Germany). Cy5-labeled 46-bp (TCS), 50-bp (PRE-tcs* and PRE-PRE-PRE) and 51-bp (TCS-PRE, TCS-pre, and tcs-PRE) DNA fragments were obtained by annealing synthetic 5′ Cy5-labeled (Metabion, Martinsried, Germany) and unlabeled complementary oligonucleotides. To obtain unlabeled competitor DNA, 31-bp synthetic oligonucleotides were annealed.
For protein-DNA complex formation, the indicated amounts of labeled DNA fragments and purified proteins or E. coli protein extract were incubated for 30 min at room temperature with 0.2 μg poly(dI-dC)·poly(dI-dC), Complete protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany), 20 μg bovine serum albumin (BSA), 2 mM dithiothreitol, 50 mM NaCl, 10 mM Tris-Cl (pH 7.5), and 0.1 mM EDTA. When used together, MBP-Tec1 and MBP-Ste12 proteins were premixed and incubated on ice for 30 min before addition to DNA. Nonlabeled competitor DNA was added in a 100, 500, or 1,000 molar excess before addition of the proteins. After incubation, samples were mixed with loading buffer and separated on 6% native polyacrylamide gels for 1.5 h at 110 to 150 V. Gels with Cy5-labeled DNA were directly scanned on a Typhoon Trio variable-mode manager (GE Healthcare, Freiburg, Germany). Gels with 32P-labeled DNA were fixed in 10% acetic acid solution for 15 min, dried, and subjected to autoradiography. Signals were quantified using the ImageQuant TL software and analyzed using the Prism5 software (GraphPad, La Jolla, CA).
β-Galactosidase assays.
Yeast strains carrying TCS-CYC1-lacZ or FRE-CYC1-lacZ reporters were grown to exponential phase in appropriate media, and extracts were prepared and assayed for β-galactosidase activity as previously described (8). β-Galactosidase activity was normalized to the total protein in each extract with the following formula: (optical density at 420 nm × 0.304)/(0.0045 × protein concentration × extract volume × time). Assays were performed in triplicate on at least three transformants, and the mean values were calculated. Standard deviations did not exceed 20%.
Yeast one-hybrid assay.
Yeast strain EGY48-p1840 (20) carrying plasmid pEG202, pEG-TEC1281–486 (BHUM752), or pEG-TEC1350–486 (BHUM1687) and plasmid pJG4-5 (22) was cultivated to exponential growth in liquid SC-Trp-His medium supplemented with 2% galactose before β-galactosidase was measured as described above.
Microarray analysis.
Transcriptional profiling of yeast strains YHUM1642, YHUM1676, YHUM1694, YHUM1700, and YHUM1701 was performed using Yeast Genome 2.0 expression arrays (Affymetrix, High Wycombe, United Kingdom) following standard protocols. Yeast strains were grown in synthetic complete medium lacking leucine and histidine with 2% glucose. For each strain two independent colonies were used. Overnight cultures were diluted into 30 ml of fresh medium to an OD600 of 0.25 and grown to an OD600 of 1 at 30°C before cells were harvested by centrifugation and rapidly frozen in liquid nitrogen. Total RNA was prepared using an RNeasy mini kit (Qiagen, Hilden, Germany) following Yeast Protocol 1c for mechanical disruption. RNA yield and purity were determined using an Agilent 2100 Bioanalyzer (Agilent Technologies, Böblingen, Germany). All subsequent steps were conducted according to the Affymetrix Gene Chip Expression Technical Manual (Affymetrix, High Wycombe, United Kingdom). Briefly, one cycle of cDNA synthesis was performed with 8 μg of total RNA. In vitro transcription labeling was carried out for 16 h. The fragmented samples were hybridized for 16 h on Affymetrix Yeast Genome 2.0 expression arrays, washed, and stained using the GeneChip Hybridization, Wash, and Stain Kit (P/N 900720) and an Affymetrix Fluidics 450 station. Arrays were scanned on an Affymetrix GeneArrayScanner 3000 7G, and nonscaled RNA signal intensity (CEL) files were generated using the Affymetrix GeneChip Command Console software. The resulting CEL files were loaded into the Affymetrix Expression Console software, and CHP files were created using Quantile normalization and Probe Logarithmic Intensity Error Estimation (PLIER). Differentially expressed genes were defined as having an average signal intensity of more than 50 and a fold change of at least 1.5 (calculated as the fold change of the average expression in the duplicate measurements).
Quantitative real-time PCR.
Quantitative real-time PCR was performed by reverse transcription of 8 μg of total RNA into cDNA using the GeneChip Expression 3′-Amplification One Cycle cDNA synthesis kit (Affymetrix, High Wycombe, United Kingdom). Quantitative real-time PCR experiments were performed in duplicate in 96-well plates using the MyiQ single-color real-time PCR detection system (Bio-Rad, Munich, Germany). Independent PCRs were performed using the same cDNA for both the gene of interest and CDC28 as a reference. Gene-specific primers are shown in Table S3 in the supplemental material. The reaction mix (25-μl final volume) consisted of 12.5 μl of 2× iQ SYBR green supermix (Bio-Rad, Munich, Germany), 1 μl of each primer (0.4 μM final concentration), 0.5 μl fluorescein isothiocyanate (FITC) (0.02 μM final concentration), 9 μl H2O, and 1 μl of a 1/10 dilution of the cDNA preparation. A control without template was incorporated in each assay. The thermocycling program consisted of one hold at 95°C for 3 min, followed by 45 cycles of 15 s at 95°C, 30 s at 60°C, and 30 s at 72°C. After completion of these cycles, melting curve data were collected to determine PCR specificity, contamination, and the absence of primer dimers. The detection of the threshold cycle by was done automatically by the cycler software. Quantification of gene expression was carried out with the Gene Expression Analysis for iQ real-time PCR detection system software (Bio-Rad, Munich, Germany).
Microarray data accession number.
Array data are available from the ArrayExpress database (http://www.ebi.ac.uk) under accession number E-MEXP-2207.
RESULTS
In vivo, physiological amounts of Tec1 are sufficient to activate TCS-mediated gene expression and FLO11-mediated adhesion in the absence of Ste12.
We have previously shown that Tec1 is able to activate expression of TCS-driven reporters and natural target genes in ste12Δ mutant strains (30), suggesting that Tec1 can regulate transcription independently of Ste12. These former experiments were done by expression of TEC1 from high-copy plasmids, because efficient TEC1 transcription depends on Ste12. To more accurately measure the influence of Ste12 on the transcriptional activity of Tec1 in vivo, we created yeast strains that contain physiological amounts of Tec1 even when Ste12 is absent. These strains carry a single genomic copy of a TEC1 gene under the control of the Ste12-independent URA3 promoter to uncouple TEC1 transcription from Ste12 control. Using highly specific Tec1 antibodies directed against the TEA domain, we found that PURA3-TEC1 expressing STE12 strains contain Tec1 protein levels that very closely match the Tec1 amounts detected in normal TEC1 STE12 strains (Fig. 1A). In strains lacking STE12, Tec1 protein levels dropped more than 14-fold when TEC1 was driven by the TEC1 promoter but only 2-fold in the case of PURA3-TEC1.
Fig. 1.
Activation of TCS- and FRE-driven reporter genes and FLO11-dependent adhesion by Tec1 in the presence and absence of Ste12. (A) Reporter gene expression was determined by measuring β-galactosidase activities in STE12 or ste12Δ strains expressing no TEC1 (tec1Δ) or single genomic copies of TEC1 driven from the endogenous promoter (TEC1) or the Ste12-independent URA3 promoter (PURA3-TEC1) and carrying integrated TCS- or FRE-driven CYC1-lacZ genes. Tec1 protein levels were determined by quantitative immunoblot analysis using specific antibodies against Tec1 and Tub1 as an internal control and are indicated as percentages of Tec1 protein present in a control TEC1 STE12 strain. Relative reporter gene activation corrected to Tec1 protein levels is shown in italic. (B) Adhesive growth of yeast strains of the indicated genotypes was determined after growth on solid SC-Ura medium. Plates were photographed before (total growth) and after (adhesive growth) removal of nonadhesive cells by a wash assay. For high-copy TEC1 expression (hc TEC1) plasmid BHUM30 was used; all other strains carry control plasmid YEplac195. (C) FLO11 expression in strains of the indicated genotypes was determined by quantitative real-time PCR. Expression is presented as the percentage of the level measured in a TEC1 STE12 control strain, which was set to 100. Error bars indicate standard deviations.
We next used the PURA3-TEC1-expressing yeast strains to quantify Tec1-dependent activation of TCS- and FRE-driven transcriptional reporters, and we related these activities to the Tec1 protein levels present in the different strains. These quantitative measurements revealed that the presence or absence of Ste12 had no obvious effect on the Tec1-dependent expression of the TCS reporter (Fig. 1A). For the FRE reporter, we found that Tec1 was able to stimulate expression even in the absence of Ste12 (Fig. 1A, compare tec1Δ and PURA3-TEC1) and that Tec1-mediated expression was similar to that found for the TCS reporter. However, Tec1-mediated expression of the FRE reporter, but not that of the TCS reporter, was further stimulated by the presence of Ste12, which reflects the cooperative binding of Tec1 and Ste12 to the combined FRE element. These data suggest that there may be no fundamental difference between the functions of Tec1 at the TCS versus the FRE reporter.
We also measured adhesive growth and FLO11 expression in the different TEC1 and PURA3-TEC1 expressing yeast strains in order to relate these processes to the different Tec1 protein levels. As expected, TEC1 strains grow adhesively in the presence, but not in the absence, of STE12 (Fig. 1B). In contrast, PURA3-TEC1 confers adhesive growth even in a ste12Δ strain, although with lower efficiency than in a STE12 strain or compared to a ste12Δ strain carrying TEC1 on a high-copy plasmid (Fig. 1B). In agreement with these data, we found that Tec1 was able to significantly activate FLO11 transcription in the absence of Ste12 (Fig. 1C) and that in general FLO11 transcript levels correlated with Tec1 protein levels.
Together, these data demonstrate that Tec1 is able to activate TCS-mediated gene expression in the absence of Ste12 and they suggest that Tec1 can bind to TCS elements independently of Ste12.
In vitro, Tec1 binds to TCS elements with high affinity and specificity independently of Ste12.
We next investigated the in vitro binding of Tec1 to TCS elements in the absence and presence of Ste12. For this purpose, we performed quantitative gel retardation analysis using Tec1 and Ste12 proteins purified from E. coli and different TCS-containing DNA fragments. Importantly, we used Tec1 protein at nanomolar concentrations, which corresponds to the in vivo amounts of approximately 530 Tec1 molecules per cell (18). Under these conditions, Tec1 efficiently bound to single TCSs without Ste12, and binding was not influenced by a neighboring PRE (Fig. 2A). However, Tec1 DNA binding was much more efficient when a second TCS element was present on the target DNA, and it was drastically reduced when the TCS sequence was altered from the proposed consensus sequence CATTCTT to CAaaCTT (Fig. 2A).
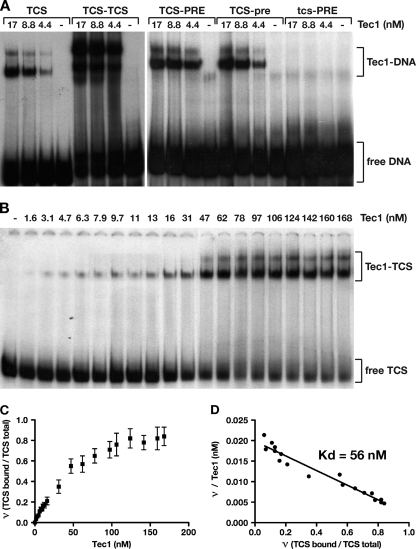
Fig. 2.
Tec1 DNA binding affinity in the absence of Ste12. (A) MBP-Tec1 protein at the indicated concentration was mixed with approximately 0.5 nM 32P-labeled DNA fragments carrying TCS and/or PRE sequences or mutated versions (tcs and pre). Tec1-DNA complexes and free DNA were visualized by autoradiography after separation by native gel electrophoresis. MBP alone was added as a negative control at 100 nM in lanes without MBP-Tec1 (−). (B) Titration analysis of Tec1 binding to single-TCS-containing DNA was performed by adding increasing amounts of MPB-Tec1 at the indicated concentrations to 0.5 nM 32P-labeled TCS DNA fragments as described for panel A. (C) Tec1-TCS complex formation obtained for panel B was quantified by measuring bound and free TCS DNA and visualized by plotting the ratio ν (TCS bound/TCS total) versus the Tec1 protein concentration. Error bars indicate standard deviations obtained from two independent measurements. (D) Scatchard plot analysis of the data obtained for panel C was used to calculate the apparent dissociation constant Kd of the Tec1-TCS complex.
We further quantified the binding affinity of Tec1 to a single TCS by performing titration gel shift analysis (Fig. 2B) and calculating the apparent dissociation constant (Kd) of the Tec1-TCS complex. These experiments revealed that Tec1 binds to the consensus TCS with an approximate Kd of 56 nM (Fig. 2C and D). This value is comparable to that found for mammalian TEAD family transcription factors, which bind to TCS elements with Kd values of 16 to 45 nM (27).
In higher eukaryotes, systematic analysis of target sequences for TEAD family proteins revealed strong binding to the consensus sequence ANATDCHN (2). Here, we determined the effects of all possible single-nucleotide exchanges in an ACATTCTT target sequence on the Tec1 binding affinity by performing a quantitative competition titration gel retardation analysis (Fig. 3 A and B). This systematic analysis revealed RMATTCYY as the consensus sequence for strong and Ste12-independent Tec1 DNA binding (Fig. 3B). We also found a good correlation between in vitro DNA binding affinity and in vivo target gene recognition when measuring Tec1-dependent activation of CYC1-lacZ reporter genes driven by the consensus TCS ACATTCTT or mutated versions (Fig. 3C).
Fig. 3.
Quantitative analysis of sequence requirement for Tec1-TCS binding in vitro and TCS-mediated reporter gene activation by Tec1 in vivo. (A) Effects of single-nucleotide exchanges on Tec1 binding. Systematic competition gel shift analysis was performed with 50 nM Tec1 protein and approximately 1 nM Cy5-labeled TCS DNA premixed with increasing amounts (100-, 500-, or 1,000-fold excess) of nonlabeled competitor DNA carrying the indicated TCS sequences. Nucleotides differing from the consensus TCS sequence ACATTCTT are shown in lowercase letters. The ratio of bound TCS to total TCS was determined by quantitative fluorescence imaging. Values multiplied by 100 are shown to indicate the percentage of bound DNA. (B) Relative Tec1 binding affinities to different mutant TCS sequences. Values are shown as percentage of the control TCS affinity and were obtained by building the ratios between the ν values (1,000-fold excess of competitor) of control and mutant sequences multiplied by 100. The derived consensus sequence conferring strong Tec1 binding is shown at the bottom and was compared to the consensus sequence for the human TEF-1 TEAD (2). (C) Reporter gene activation by different TCS sequences was determined by measuring β-galactosidase activities in yeast strains carrying integrated CYC1-lacZ genes driven by the indicated TCS sequences or no TCS element and expressing TEC1 from a low-copy plasmid. tec1Δ indicates expression of the control TCS reporter gene in the absence of Tec1. Error bars indicate standard deviations.
We next asked whether (i) Ste12 affects binding of Tec1 to single TCSs and (ii) Tec1 influences the binding of Ste12 to repeated PREs. To address these issues, we performed gel retardation experiments using Tec1 together with Ste12. We found that Ste12 did not affect Tec1 binding to individual TCSs in vitro (Fig. 4A), suggesting that Tec1-Ste12 complex formation does not increase the Tec1 DNA binding affinity. As previously described (37), we found that FREs are cooperatively bound by Tec1 and Ste12 but not by Ste12 alone (Fig. 4A and B). Mutation of the PRE sequence in the FRE from TGAAACG to acttACG (pre) resulted in loss of cooperativity and led to a binding behavior of Tec1 as found for the individual TCS (Fig. 4B). However, mutation of the TCS in the FRE from CATTCT to CAaaCT (tcs) did not completely disrupt cooperative binding of Tec1 and Ste12 (Fig. 4B). This indicates that Ste12 can stimulate Tec1 binding at low-affinity TCSs that are flanked by PREs. To further test this hypothesis, we looked for such configurations of Tec1 and Ste12 binding sites in natural yeast promoters. We found that the TEC1 promoter itself contains a TGAAACAGGAGATTCTT sequence element at positions −93 to −77 relative to the translational start site, which consists of a PRE and a low-affinity TCS (Fig. 4C, PRE-tcs*). Gel retardation analysis revealed significant cooperative binding of Tec1 and Ste12 to this element (Fig. 4C), indicating that low-affinity TCSs might be relevant in vivo if they are flanked by Ste12 binding sites. Finally, we measured the effect of Tec1 on Ste12 binding to repeated PREs, which are found in pheromone-stimulated genes. Here, we analyzed a FUS1 promoter element that consists of three tandemly repeated PREs. We found that Ste12 binding was very efficient in both the absence and the presence of Tec1 (Fig. 4D), indicating that Tec1-Ste12 complex formation does not affect Ste12 DNA binding to repeated PREs.
Fig. 4.
Influence of Ste12 on Tec1 DNA binding and vice versa. (A to D) MBP-Tec1 and MBP-Ste12 proteins at the concentrations shown were mixed with approximately 1 nM Cy5-labeled DNA fragments containing the indicated combinations of TCS and/or PRE elements or mutated tcs, tcs*, or pre versions (for sequences, see the text). The percentage of bound DNA is shown at the bottom and was determined by quantitative fluorescence imaging as described for Fig. 2.
TCS-mediated transcriptional regulation by Tec1 depends on conserved residues in the TEA domain.
In mammalian TEF proteins, structural and mutational analyses of the TEA domain have revealed a number of residues that are crucial for DNA binding (2). Because no comparable analysis has been performed for Tec1, we analyzed a number of amino acid residues in the three helices that form the TEA domain for their involvement in TCS binding in vitro and TCS recognition in vivo. We focused on residues either that are conserved between Tec1 and mammalian TEA domains or that in mammalian TEA domains were associated with DNA binding (Fig. 5A). This analysis revealed that two single mutations introduced in helix H1, L144A and L146A, did not affect DNA binding affinity in vitro, indicating that these residues are not crucial for TCS recognition (Fig. 5B). In vivo, the L146A mutation led to a 2-fold reduction in TCS reporter expression (Fig. 5C), which might be caused by the slightly decreased protein levels of this Tec1 variant when expressed in yeast (Fig. 5D). We also introduced two mutations in the highly conserved GRNEL sequence of helix H2 found in most TEA family transcription factors. Both mutations, G163A and R164A, abolished TCS binding in vitro (Fig. 5B) and reporter gene activation in vivo (Fig. 5C), demonstrating the crucial role of these conserved residues in DNA binding. Finally, we found that the conserved residue S187 in helix H3, which has been suggested to confer DNA recognition, is essential for DNA binding and TCS-mediated gene expression.
Fig. 5.
Characterization of the Tec1 TEA domain. (A) Sequence alignment of Tec1 and human TEF-1 TEA domains. The diagram indicates the positions of helices and intermediate loops. Numbering refers to Tec1 residues, identical amino acids are marked by lines, triangles indicate Tec1 residues analyzed by point mutagenesis, and asterisks refer to TEF-1 residues involved in DNA binding (2). (B) The effects of single amino acid exchanges on Tec1 DNA binding were measured by quantitative gel retardation analysis using identical amounts of bacterially expressed Tec1 proteins and Cy5-labeled TCS DNA fragments. Percentages of bound DNA and relative DNA binding activities compared to unaltered Tec1 are shown at the bottom. (C) TCS reporter gene activation by different TEC1 variants was determined by measuring β-galactosidase activities in yeast strain YHUM1363 carrying an integrated TCS-CYC1-lacZ gene and expressing no TEC1 (tec1Δ) or TEC1 variants with the indicated mutations from low-copy plasmids. Error bars indicate standard deviations. (D) Protein levels of different Tec1 variants in the strains described for panel C were determined by quantitative immunoblot analysis using specific antibodies against Tec1 and Cdc28 (as an internal control) and are indicated as percentages of Tec1 protein present in the control TEC1 strain. (E) Activation of adhesive growth by different TEC1 variants with the indicated mutations in the yeast strains described for panel C was determined using a wash assay after growth on solid SC-Leu medium for 5 days at 30°C.
We also determined the effects of selected mutations in the Tec1 TEA domain on yeast adhesive growth in order to analyze the correlation between in vitro DNA binding, in vivo TCS reporter gene activation, and regulation of a Tec1-dependent developmental process. We found that G163A, R164A, and S187A, which had severe defects in DNA binding, were also defective in adhesive growth. In contrast, the L146A mutant, which did not exhibit obvious defects in binding to TCS, was competent in adhesive growth (Fig. 5E).
The C-terminal domain of Tec1 confers Ste12-independent transcriptional activation and can be replaced by a Ste12 activation domain.
We have previously shown that deletions in the C-terminal part of Tec1 cause a loss of TCS-mediated gene activation (30). Therefore, we further analyzed the function of this part of Tec1 with respect to reporter gene activation. For this purpose, we expressed TEC11–280 from the URA3 promoter, a construct that encodes the TEA DNA binding domain (DBD) and lacks the C-terminal Ste12 interaction domain (Fig. 6A and B). We found that the relative transcriptional activity of Tec11–280 was drastically reduced in the presence and absence of Ste12 (Fig. 6C). We then fused the C-terminal TEC1377–486 domain back to TEC11–280 and found a significant, yet not a full, reconstitution of the transcriptional activity in both the presence and the absence of Ste12 (Fig. 6C). Together, these data indicate that the C-terminal part of Tec1 carries a Ste12-independent transcriptional activation domain. To independently corroborate this hypothesis, we performed a yeast one-hybrid analysis using the lexA-based yeast two-hybrid system (22). Here, we found that fusion of the lexA DNA binding domain to the C-terminal Tec1281–486 or Tec1350–486 portions led to a significant stimulation of a lexAop-driven lacZ reporter gene compared to lexA alone (Fig. 6D). Also, Tec1281–486 led to a better activation than Tec1350–486, a finding that correlates with the results obtained with the TCS reporter.
Fig. 6.
Activities of different Tec1 and Ste12 proteins. (A) Diagrams showing Tec1 with TEA and Ste12-binding domains and Ste12 with the DNA binding domain (DBD) and activation domains (ADI and ADII) (44). (B) Diagrams showing different TEC1-STE12 hybrid constructs driven by the URA3 promoter. Encoded Tec1 and Ste12 amino acid residues and functional domains are indicated. (C) Relative activation of TCS and FRE reporter genes by Tec1 (lanes 1), Tec11–280 (lanes 2), and Tec11–280-Tec1377–486 (lanes 3) was determined in STE12 or ste12Δ strains expressing single genomic copies of corresponding TEC1 variants and TCS- or FRE-driven CYC1-lacZ genes as described for Fig. 5. (D) Activation of a lexAop-lacZ reporter gene by lexA (pEG202), lexA-TEC1281–486 (BHUM752), or lexA-TEC1350–486 (BHUM1687). Yeast strain EGY48-p1840 (22) was transformed with these plasmids and pJG4-5, followed by quantitative measurement of β-galactosidase activities using SC-Trp-His medium supplemented with 2% galactose. Error bars indicate standard deviations. (E) Relative activation of TCS and FRE reporter genes by different Tec1-Ste12 hybrid proteins was determined in ste12Δ tec1Δ strains expressing single genomic copies of corresponding TEC1-STE12 variants and TCS- or FRE-driven CYC1-lacZ reporter genes. (F) Adhesive growth of the yeast strains described for panels C and E was determined after 5 days of incubation on solid yeast extract-peptone-dextrose (YEPD) medium.
We next asked whether fusion of Ste12 or different Ste12 domains that were previously identified as a DNA binding domain and two independent transcriptional activation domains, ADI and ADII (29), would confer transcriptional activity to the Tec1 TEA domain. For this purpose, we constructed a series of TEC1TEA-STE12 hybrid genes (Fig. 6A and B) and measured the activity of the encoded synthetic proteins with respect to TCS- and FRE-mediated reporter gene activation and adhesion. All genes were expressed as single genomic copies driven by the URA3 promoter in tec1Δ ste12Δ mutant strains.
With respect to TCS-mediated reporter gene activation, we found that addition of the DBD carrying Ste121–250 portion to Tec11–280 did not render an efficient transcriptional activity (Fig. 6E, construct 4). In contrast, fusion of the Ste12 ADI or ADII to Tec11–280 resulted in hybrid proteins that activated the TCS-driven reporter gene as efficiently as the full-length Tec1 protein in the absence of Ste12 (Fig. 6E, constructs 6 and 8). An even better effect was obtained by fusion of the full-length Ste12 protein to Tec11–280, which stimulated TCS-driven reporter expression more than 6-fold (Fig. 6E, construct 10). We also fused the Tec1377–486 domain to the different Tec11–280-Ste12 hybrid proteins, and in all cases we found an increase in the relative TCS-mediated transcriptional activities (Fig. 6E, constructs 5, 7, 9, and 11). This finding further supports the view that the C-terminal part of Tec1 carries an AD. We also determined the relative transcriptional activities of different Tec1-Ste12 hybrid proteins at the FRE reporter. As found for the TCS reporter, we measured a strong stimulation of activities by inserting either Ste12 ADI, Ste12 ADII, or full-length Ste12 into Tec11–280-Tec1377–486. Interestingly, insertion of the Ste12 DBD alone into Tec11–280-Tec1377–486 conferred a 9.1-fold stimulation of the relative transcriptional activity at the FRE (compare Fig. 6C, construct 3, with Fig. 6E, construct 5), indicating cooperative DNA binding of this construct at FRE sites.
We then determined activation of FLO11-mediated adhesive growth by the different Tec1-Ste12 hybrid proteins. We did not observe a full correlation between stimulation of adhesion and activation of the TCS and FRE reporter genes. Specifically, fusions between the Tec1 TEA domain and Ste12 ADII were unable to activate adhesive growth (Fig. 6F, constructs 6 and 7), although their transcriptional activities at isolated TCS and FRE sites were significantly higher than that of the natural Tec1-Ste12 complex. This unexpected finding indicates that the activity of this hybrid protein not only requires a TCS but might be additionally influenced by neighboring sequence elements for, e.g., further DNA binding proteins that appear to differ between the TCS reporter and the natural FLO11 gene. In the case of fusions between the Tec1 TEA domain and Ste12 ADI or full-length Ste12, reporter gene activation was mirrored by a strong stimulation of adhesion (Fig. 6F, constructs 8, 9, 10, and 11). These data point to a specific function of Ste12 ADI in FLO11-mediated adhesion.
In vivo, Tec1 has been found to form a complex with Dig1 via association with Ste12. Therefore, we measured how Dig1 affects the Ste12-dependent and Ste12-independent activity of Tec1 and that of different Tec1-Ste12 hybrid proteins. In agreement with previous studies, we found that TCS-mediated activity of the natural Tec1-Ste12 complex and adhesive growth are under negative control of Dig1 (see Fig. S1A and B in the supplemental material). However, the Ste12-independent stimulation of the TCS reporter and of adhesion by Tec1 was not influenced by Dig1. We also found a negative effect of Dig1 on the transcriptional activities of hybrids between the Tec1 TEA domain and Ste12 ADII (see Fig. S1A, constructs 6 and 7, in the supplemental material) but not for the Tec1-Ste12 ADI hybrids (Fig. S1A, constructs 8 and 9), indicating that Dig1 inhibits Ste12 activity via ADII.
In summary, the data in this section show that Tec1 is able to activate TCS-mediated transcription in the absence of Ste12 depending on its C-terminal domain, which can be replaced by an activation domain of Ste12.
On a genome-wide scale, Ste12-dependent and Ste12-independent Tec1-regulated genes constitute two distinct classes.
Our strains expressing TEC1 from the Ste12-independent URA3 promoter enabled us to analyze and compare genome-wide Tec1-regulated gene expression in the presence and absence of Ste12, an issue which was not addressed in previous studies (6, 38). For this purpose, we compared transcriptional profiles of TEC1 STE12 and tec1Δ STE12 strains as well as of PURA3-TEC1 ste12Δ and tec1Δ ste12Δ strains grown under nutrient-rich conditions using high-density oligonucleotide arrays (Affymetrix GeneChips). We found 289 genes that are regulated at least 1.5-fold by Tec1 in the presence of Ste12 and 48 genes that are regulated by Tec1 in the absence of Ste12, with 35 genes overlapping between the two groups (Fig. 7A and B; see Table S1 in the supplemental material). Thus, the total of 302 Tec1 target genes can be divided into two distinct classes, with 254 genes (84%) being Tec1 regulated in a strictly Ste12-dependent manner (Fig. 7C, class I) and 48 genes (16%) that are Ste12-independently regulated by Tec1 (Fig. 7C, class II).
Fig. 7.
Classification of Tec1-regulated genes by transcriptional profiling. (A) Venn diagram indicating the number of genes differentially expressed in yeast strains YHUM1694 (TEC1 STE12) and YHUM1700 (tec1Δ STE12) (blue) or YHUM1676 (PURA3-TEC1 ste12Δ) and YHUM1701 (tec1Δ ste12Δ) (red). Numbers are given for genes whose expression differs at least 1.5-fold between the yeast strains compared and for genes shared between the different groups. (B) Display of a subset of Tec1 target genes differentially expressed in strains of the indicated genotypes (combinations A to C). Numbers indicate the fold changes in expression levels when comparing the different strain pairs. (C) Classification of all 302 Tec1-regulated genes identified in this study into two major classes. Numbers in parentheses indicate the number of genes present in the different classes. (D) Concurrent enrichment analysis of 203 transcription factors bound by 302 Tec1-regulated genes was performed using the ChIPCodis web server (1) based on available yeast chip-on-chip (25) and TFBS (36) data. Statistically overrepresented transcription factors (P < 0.02) bound by the indicated class I and class II genes are shown. Transcription factor binding is indicated in green. The presence of high-affinity RMATTCYY TCS sequences in the promoter regions of the different genes is indicated by red boxes. Normal regulation by the Tec1TEA-Ste12 fusion protein is shown by blue boxes.
To uncover possible differences in the promoters of class I and class II genes, we performed a concurrent enrichment analysis using the ChIPCodis web server (1), which is based on available chip-on-chip (25) and TFBS data (36) for 203 yeast transcription factors. We found that class I was specifically enriched for a total of 38 genes that are bound by Tec1, Ste12, and Dig1 under nutrient-rich conditions (Fig. 7D) (25). We then analyzed these class I genes for the presence of Tec1 binding sites and found high-affinity TCS elements matching the RMATTCYY consensus sequence in 50% of the promoters (Fig. 7D; see Table S2 in the supplemental material). This indicates that these class I genes are regulated by Tec1-Ste12 complexes via TCS binding. In contrast, class II was specifically enriched for 32 genes that were previously identified to be bound by the transcription factors Yap6, Nrg1, Cin5, Skn7, Hsf1, and Msn4 under hyperoxic stress conditions (Fig. 7D) (25). Notably, most of these class II genes were previously not found to bind Tec1 under nutrient-rich conditions (25). Nevertheless, we detected high-affinity RMATTCYY TCS elements in the promoter regions of 59% of these genes (Fig. 7D). Thus, efficient in vivo binding of Tec1 to these class II genes might depend on nonstandard conditions or additional transcription factors.
Because we found that the Tec11–280-Ste121–688 hybrid protein, which represents the Tec1 TEA domain fused to the full-length Ste12 (Tec1TEA-Ste12), is a very potent activator of TCS-driven reporters, we wondered how this protein would regulate the different classes of Tec1 target genes. Transcriptional profiling revealed that 199 (78%) of class I genes are normally regulated by Tec1TEA-Ste12, whereas only 55 genes (22%) are deregulated (Fig. 7C and D). This indicates that a large proportion of class I genes are regulated by Tec1-Ste12 complexes without the need of the Tec1 C-terminal domain. A significantly higher proportion (72%) of class II genes enriched for Yap6, Nrg1, Cin5, Skn7, Hsf1, or Msn4 binding were deregulated by Tec11–280-Ste121–688 (Fig. 7D), indicating that normal regulation of these genes specifically depends on the Tec1 C-terminal activation domain.
In summary, our genome-wide analysis reveals that Tec1 target genes constitute two distinct classes that are defined by their Ste12-dependent and Ste12-independent regulatory patterns and their promoter characteristics.
Ste12 controls Tec1 protein stability independently of Dig1 and the Cdc4 phosphodegron (CPD) at Thr273 but involves the Tec1 C-terminal part.
We noticed that Tec1 protein levels are lower in the absence of Ste12, even when TEC1 is expressed from the Ste12-independent URA3 promoter (Fig. 1A). Because Ste12 does not affect TEC1 transcript levels in PURA3-TEC1-carrying strains, we tested whether Ste12 might influence Tec1 protein stability. Therefore, we determined in vivo decay rates of Tec1 in the presence and absence of Ste12 by performing cycloheximide-induced translational shutoff experiments. We followed the decrease of endogenous Tec1 protein levels in the PURA3-TEC1-expressing STE12 and ste12Δ strains after addition of cycloheximide by quantitative immunoblot analysis (Fig. 8A). These experiments revealed that the rate of decay of Tec1 increases more than 5-fold when STE12 is deleted (Fig. 8B), indicating that Ste12 positively controls Tec1 protein stability. To corroborate this finding, we performed a promoter shutoff experiment using the TEC1 gene under the control of the glucose-repressible GAL1 promoter. Again, we found that the Tec1 half-life decreases roughly 5-fold in strains lacking STE12 (see Fig. S2 in the supplemental material), supporting the view that Tec1-Ste12 complex formation significantly enhances Tec1 protein stability. We also explored the possibility that the Ste12-dependent Tec1 stability control is conferred by Dig1, which associates with Tec1-Ste12 complexes (10). However, Dig1 had no effect on the Tec1 half-life in either the presence or absence of Ste12 (Fig. 8C).
Fig. 8.
Dependence of Tec1 protein stability on Ste12 and Dig1. (A) The rate of decay of Tec1 in yeast strains YHUM1727 (STE12 PURA3-TEC1) and YHUM1718 (ste12Δ PURA3-TEC1) was determined after addition of cycloheximide to exponentially growing cultures by measuring Tec1 protein levels at the indicated time points using quantitative immunoblot analysis. (B) The Ste12-dependent half-life of Tec1 was calculated by plotting the relative Tec1 protein levels obtained in panel A versus time, with starting amounts set to 100%. (C) Dig1-dependent decay of Tec1 in yeast strains of the indicated genotype and expressing PURA3-driven TEC1 was measured as described for panel A. (D) Ste12-dependent decay of Tec1T273M was determined in strains YHUM1723 (STE12 PURA3-TEC1T273M) and YHUM1713 (ste12Δ PURA3-TEC1T273M). (E) The Ste12-dependent half-lives of Tec11–280 and Tec11–280-Tec1377–486 proteins were determined using the strains described for Fig. 6.
Previous studies have shown that Tec1 stability is decreased in response to pheromone by Fus3-mediated phosphorylation of a Cdc4 phosphodegron (CPD) motif centered at position Thr273 of Tec1 and that mutation of Thr273 or absence of Fus3 increases Tec1 stability (3, 8, 9). Therefore, we tested whether Ste12-dependent Tec1 stability control might also be mediated by Thr273. For this purpose, we constructed STE12 and ste12Δ strains carrying a single genomic copy of the TEC1T273M allele under the control of the URA3 promoter as the single source for functional Tec1 protein. We found that Ste12 affected the decay rate of Tec1T273M comparably to that of regular Tec1 (Fig. 8D), indicating that the CPD motif at T273 is not involved in regulation of Tec1 stability by Ste12.
Finally, we tested whether the C-terminal part of Tec1 might affect protein stability, because we noticed that steady-state levels of the Tec11–280 variant are significantly increased (Fig. 6C). Indeed, we found that the half-life of Tec11–280 lacking the C-terminal part is 3-fold higher than that of full-length Tec1 and, more importantly, completely uncoupled from Ste12 control (Fig. 8E). Moreover, fusing the Tec1377–486 part back to Tec11–280 not only lowered the half-life 3-fold to match the value observed for regular Tec1 but also reconstituted stability control by Ste12 (Fig. 8E).
In summary these data indicate that Ste12-mediated Tec1 stability control involves the C-terminal part of Tec1 but not Dig1 or the CPD at Thr273.
DISCUSSION
The mechanisms by which the S. cerevisiae transcription factors Tec1 and Ste12 regulate their target genes have become a valuable paradigm for combinatorial and program-specific transcriptional control. Previous studies have shown that these mechanisms include cooperative binding of Tec1 and Ste12 to combined TCS-PRE target sequences (37), Tec1-Ste12 complex formation and interaction with the inhibitor protein Dig1 (4, 10, 12, 45, 49), control of Tec1 protein stability by the MAPK Fus3 (3, 8, 9), and regulation of TEC1 transcription by Ste12 (30, 43). In addition, both regulators have been found to colocalize at many target gene promoters in vivo, suggesting that Tec1-Ste12 complex formation confers program-specific target gene control (6, 53). Our study provides (i) new insights into the mechanisms by which Tec1-Ste12 complex formation contributes to TCS-mediated target gene control and (ii) evidence that Tec1 is capable of controlling target gene expression by previously unknown Ste12-independent mechanisms.
With respect to regulation by Tec1-Ste12 complexes, we have identified a large class of Tec1 target genes that are regulated by Ste12-dependent mechanisms. Our bioinformatic analysis reveals that many of these genes contain high-affinity TCS elements, often without a flanking PRE, and that many class I genes are bound by Tec1, Ste12, and Dig1 in vivo (25, 53). We further find that Tec1 efficiently binds to TCSs in vitro and that this activity is not affected by Ste12. Also, our Tec1 deletion and Tec1-Ste12 hybrid analyses have revealed that TCS reporters and many TCS-carrying class I genes are normally regulated by chimeras between the Tec1 TEA domain and Ste12. Together, these findings suggest that TCS-containing class I genes can be regulated by Tec1-Ste12 complexes by at least two different mechanisms (Fig. 9). We conclude that at TCS promoters without PREs, Tec1-Ste12 complexes can bind via the Tec1 TEA domain and activate transcription through the different ADs of Ste12. This conclusion is in agreement with the study performed by Chou et al. (10). Our data do not rule out, however, that additional factors present at TCS promoters might affect recruitment or transcriptional activity of Tec1-Ste12 complexes. At promoters carrying TCSs flanked by a PRE, we find that Tec1-Ste12 complexes bind cooperatively, which is in line with initial studies (37). However, our study has revealed that there might be no fundamental difference in Tec1 functions at TCS- versus TCS-PRE-containing promoters. Finally, we show that class I genes can be regulated by Dig1. Our data indicate that Dig1 regulation is mediated by Ste12 ADII (Fig. 9), a finding that is supported by the fact that Ste12 associates with Dig1 through the ADII domain (44).
Fig. 9.
Mechanisms for promoter-specific gene regulation by Tec1 and Ste12. Deg, degradation; Txn, transcription; AD, activation domain; DBD, DNA binding domain; CPD, Cdc4 phosphodegron; TEA, TEA DNA binding domain. For further details, see the text.
A completely novel outcome of our study is that Tec1-Ste12 complex formation confers Tec1 stability. So far, only a few examples of stability control by interacting transcription factors have been found in S. cerevisiae, e.g., in the case of the mating type transcription factors MATa1 and MATα2 (26). Here, we find that Ste12-mediated Tec1 stability control is independent of T273 phosphorylation and Fus3 (3, 8, 9) but instead involves the C-terminal part of Tec1. Given the fact that Tec1-Ste12 complex formation depends on the C-terminal portion of Tec1 (10), our results indicate that Ste12 might mask a destabilizing signal in this part of Tec1 (Fig. 9). What is the role of this mechanism? An interesting possibility is that this might be a regulatory device that ensures appropriate discrimination between class I and class II Tec1 target genes. For instance, such a mechanism might prevent erroneous activation of class I genes in the absence of Ste12 and/or ensure efficient expression in its presence. It might also stabilize class I gene expression over a broader range of Tec1 concentrations, e.g., when Tec1 protein levels fluctuate due to varying Fus3 activity within a population (11). Thus, future studies of Ste12-mediated Tec1 stability control could reveal novel insights into the significance of such mechanisms in population development and dynamics.
A central and novel finding of our investigation is the discovery of a second class of Tec1 target genes that can be regulated by Ste12-independent mechanisms. How does Tec1 regulate these genes? Our biochemical and genetic analyses have shown that Tec1 efficiently binds TCSs without Ste12 and that class II gene activation depends on the C-terminal domain of Tec1. Our bioinformatic analysis has revealed that a large number of class II gene promoters contain high-affinity TCSs. Yet, we find that only a few class II promoters have previously been identified to be bound by Tec1 under nutrient-rich conditions (25). Therefore, efficient Tec1 recruitment to class II promoters might depend on nonstandard conditions and on the interaction with further regulators. So far, no genome-wide Tec1 binding analysis has been performed under a regimen of different stress conditions. However, we found that class II is enriched for genes that are bound by the transcriptional regulators Yap6, Nrg1, Cin5, Skn7, Hsf1, and Msn4 under hyperoxic stress (25). This opens the possibility that Tec1 might be recruited to class II promoters under certain stress conditions and interact with these stress-related DNA binding proteins. Based on previously identified functions of these regulators, such conditions might include oxidative and salt stress (e.g., Yap6, Cin5, and Skn7) (40, 48), heat shock (e.g., Hsf1 and Msn4) (24, 39) or glucose starvation (e.g., Nrg1) (31). Whether and how Tec1 might interact with any of these regulators remains to be investigated. Alternatively, Tec1 might regulate class II genes also indirectly via transcription factors that are under direct Tec1 control. With respect to this possibility, we noticed that class II includes RPI1, a gene which encodes a putative transcriptional regulator involved in the heat shock response and entry into stationary phase (28, 47). Interestingly, the promoter of RPI1 contains high-affinity TCS elements and is bound by Tec1, but not Ste12, under nutrient-rich conditions (25). This opens the possibility that class II genes might be regulated by Tec1 via Rpi1.
In summary, our study reveals that the mechanisms by which Tec1 is able to control target gene expression in S. cerevisiae are much more complex than previously anticipated. Because we find that TCS-mediated transcriptional control by Tec1 depends on conserved residues in the TEA domain, our findings also contribute to a better understanding of the functions of TEA transcription factors in eukaryotes in general.
Supplementary Material
ACKNOWLEDGMENTS
We thank Hiten Madhani and Gerald R. Fink for generous gifts of plasmids and strains. We are grateful to Diana Kruhl for technical assistance and to Gunther Döhlemann for help in performing microarray analysis and quantitative real-time PCR.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG).
Footnotes
Supplemental material for this article may be found at http://ec.asm.org/.
Published ahead of print on 29 January 2010.
REFERENCES
- 1.Abascal F., Carmona-Saez P., Carazo J. M., Pascual-Montano A. 2008. ChIPCodis: mining complex regulatory systems in yeast by concurrent enrichment analysis of chip-on-chip data. Bioinformatics 24:1208–1209 [DOI] [PubMed] [Google Scholar]
- 2.Anbanandam A., Albarado D. C., Nguyen C. T., Halder G., Gao X., Veeraraghavan S. 2006. Insights into transcription enhancer factor 1 (TEF-1) activity from the solution structure of the TEA domain. Proc. Natl. Acad. Sci. U. S. A. 103:17225–17230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao M. Z., Schwartz M. A., Cantin G. T., Yates J. R., III, Madhani H. D. 2004. Pheromone-dependent destruction of the Tec1 transcription factor is required for MAP kinase signaling specificity in yeast. Cell 119:991–1000 [DOI] [PubMed] [Google Scholar]
- 4.Bardwell L., Cook J. G., Voora D., Baggott D. M., Martinez A. R., Thorner J. 1998. Repression of yeast Ste12 transcription factor by direct binding of unphosphorylated Kss1 MAPK and its regulation by the Ste7 MEK. Genes Dev. 12:2887–2898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borneman A. R., Gianoulis T. A., Zhang Z. D., Yu H., Rozowsky J., Seringhaus M. R., Wang L. Y., Gerstein M., Snyder M. 2007. Divergence of transcription factor binding sites across related yeast species. Science 317:815–819 [DOI] [PubMed] [Google Scholar]
- 6.Borneman A. R., Leigh-Bell J. A., Yu H., Bertone P., Gerstein M., Snyder M. 2006. Target hub proteins serve as master regulators of development in yeast. Genes Dev. 20:435–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braus G. H., Grundmann O., Brückner S., Mösch H. U. 2003. Amino acid starvation and Gcn4p regulate adhesive growth and FLO11 gene expression in Saccharomyces cerevisiae. Mol. Biol. Cell 14:4272–4284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brückner S., Köhler T., Braus G. H., Heise B., Bolte M., Mösch H. U. 2004. Differential regulation of Tec1 by Fus3 and Kss1 confers signaling specificity in yeast development. Curr. Genet. 46:331–342 [DOI] [PubMed] [Google Scholar]
- 9.Chou S., Huang L., Liu H. 2004. Fus3-regulated Tec1 degradation through SCFCdc4 determines MAPK signaling specificity during mating in yeast. Cell 119:981–990 [DOI] [PubMed] [Google Scholar]
- 10.Chou S., Lane S., Liu H. 2006. Regulation of mating and filamentation genes by two distinct Ste12 complexes in Saccharomyces cerevisiae. Mol. Cell. Biol. 26:4794–4805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colman-Lerner A., Gordon A., Serra E., Chin T., Resnekov O., Endy D., Pesce C. G., Brent R. 2005. Regulated cell-to-cell variation in a cell-fate decision system. Nature 437:699–706 [DOI] [PubMed] [Google Scholar]
- 12.Cook J. G., Bardwell L., Thorner J. 1997. Inhibitory and activating functions for MAPK Kss1 in the S. cerevisiae filamentous-growth signalling pathway. Nature 390:85–88 [DOI] [PubMed] [Google Scholar]
- 13.Dolan J. W., Kirkman C., Fields S. 1989. The yeast Ste12 protein binds to the DNA sequence mediating pheromone induction. Proc. Natl. Acad. Sci. U. S. A. 86:5703–5707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Errede B., Ammerer G. 1989. Ste12, a protein involved in cell-type-specific transcription and signal transduction in yeast, is part of protein-DNA complexes. Genes Dev. 3:1349–1361 [DOI] [PubMed] [Google Scholar]
- 15.Fields S., Herskowitz I. 1985. The yeast STE12 product is required for expression of two sets of cell-type specific genes. Cell 42:923–930 [DOI] [PubMed] [Google Scholar]
- 16.Fields S., Herskowitz I. 1987. Regulation by the yeast mating-type locus of STE12, a gene required for cell-type-specific expression. Mol. Cell. Biol. 7:3818–3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gavrias V., Andrianopoulos A., Gimeno C. J., Timberlake W. E. 1996. Saccharomyces cerevisiae TEC1 is required for pseudohyphal growth. Mol. Microbiol. 19:1255–1263 [DOI] [PubMed] [Google Scholar]
- 18.Ghaemmaghami S., Huh W. K., Bower K., Howson R. W., Belle A., Dephoure N., O'Shea E. K., Weissman J. S. 2003. Global analysis of protein expression in yeast. Nature 425:737–741 [DOI] [PubMed] [Google Scholar]
- 19.Gietz R. D., Sugino A. 1988. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74:527–534 [DOI] [PubMed] [Google Scholar]
- 20.Golemis E. A., Brent R. 1992. Fused protein domains inhibit DNA binding by LexA. Mol. Cell. Biol. 12:3006–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guthrie C., Fink G. R. (ed.). 1991. Guide to yeast genetics and molecular biology. Methods Enzymol. 194:1–863 [PubMed] [Google Scholar]
- 22.Gyuris J., Golemis E., Chertkov H., Brent R. 1993. Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell 75:791–803 [DOI] [PubMed] [Google Scholar]
- 23.Hagen D. C., McCaffrey G., Sprague G., Jr 1991. Pheromone response elements are necessary and sufficient for basal and pheromone-induced transcription of the FUS1 gene of Saccharomyces cerevisiae. Mol. Cell. Biol. 11:2952–2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahn J. S., Hu Z., Thiele D. J., Iyer V. R. 2004. Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Mol. Cell. Biol. 24:5249–5256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harbison C. T., Gordon D. B., Lee T. I., Rinaldi N. J., Macisaac K. D., Danford T. W., Hannett N. M., Tagne J. B., Reynolds D. B., Yoo J., Jennings E. G., Zeitlinger J., Pokholok D. K., Kellis M., Rolfe P. A., Takusagawa K. T., Lander E. S., Gifford D. K., Fraenkel E., Young R. A. 2004. Transcriptional regulatory code of a eukaryotic genome. Nature 431:99–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson P. R., Swanson R., Rakhilina L., Hochstrasser M. 1998. Degradation signal masking by heterodimerization of MATalpha2 and MATa1 blocks their mutual destruction by the ubiquitin-proteasome pathway. Cell 94:217–227 [DOI] [PubMed] [Google Scholar]
- 27.Kaneko K. J., DePamphilis M. L. 1998. Regulation of gene expression at the beginning of mammalian development and the TEAD family of transcription factors. Dev. Genet. 22:43–55 [DOI] [PubMed] [Google Scholar]
- 28.Kim J. H., Powers S. 1991. Overexpression of RPI1, a novel inhibitor of the yeast Ras-cyclic AMP pathway, down-regulates normal but not mutationally activated ras function. Mol. Cell. Biol. 11:3894–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirkman-Correia C., Stroke I. L., Fields S. 1993. Functional domains of the yeast STE12 protein, a pheromone-responsive transcriptional activator. Mol. Cell. Biol. 13:3765–3772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Köhler T., Wesche S., Taheri N., Braus G. H., Mösch H. U. 2002. Dual role of the Saccharomyces cerevisiae TEA/ATTS family transcription factor Tec1p in regulation of gene expression and cellular development. Eukaryot. Cell 1:673–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuchin S., Vyas V. K., Carlson M. 2002. Snf1 protein kinase and the repressors Nrg1 and Nrg2 regulate FLO11, haploid invasive growth, and diploid pseudohyphal differentiation. Mol. Cell. Biol. 22:3994–4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kushnirov V. V. 2000. Rapid and reliable protein extraction from yeast. Yeast 16:857–860 [DOI] [PubMed] [Google Scholar]
- 33.Li D., Bobrowicz P., Wilkinson H. H., Ebbole D. J. 2005. A mitogen-activated protein kinase pathway essential for mating and contributing to vegetative growth in Neurospora crassa. Genetics 170:1091–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu H., Köhler J., Fink G. R. 1994. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science 266:1723–1726 [DOI] [PubMed] [Google Scholar]
- 35.Liu H., Styles C. A., Fink G. R. 1993. Elements of the yeast pheromone response pathway required for filamentous growth of diploids. Science 262:1741–1744 [DOI] [PubMed] [Google Scholar]
- 36.MacIsaac K. D., Wang T., Gordon D. B., Gifford D. K., Stormo G. D., Fraenkel E. 2006. An improved map of conserved regulatory sites for Saccharomyces cerevisiae. BMC Bioinformatics 7:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madhani H. D., Fink G. R. 1997. Combinatorial control required for the specificity of yeast MAPK signaling. Science 275:1314–1317 [DOI] [PubMed] [Google Scholar]
- 38.Madhani H. D., Galitski T., Lander E. S., Fink G. R. 1999. Effectors of a developmental mitogen-activated protein kinase cascade revealed by expression signatures of signaling mutants. Proc. Natl. Acad. Sci. U. S. A. 96:12530–12535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez-Pastor M. T., Marchler G., Schuller C., Marchler-Bauer A., Ruis H., Estruch F. 1996. The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress response element (STRE). EMBO J. 15:2227–2235 [PMC free article] [PubMed] [Google Scholar]
- 40.Mendizabal I., Rios G., Mulet J. M., Serrano R., de Larrinoa I. F. 1998. Yeast putative transcription factors involved in salt tolerance. FEBS Lett. 425:323–328 [DOI] [PubMed] [Google Scholar]
- 41.Mösch H.-U., Fink G. R. 1997. Dissection of filamentous growth by transposon mutagenesis in Saccharomyces cerevisiae. Genetics 145:671–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mösch H.-U., Kübler E., Krappmann S., Fink G. R., Braus G. H. 1999. Crosstalk between the Ras2p-controlled mitogen-activated protein kinase and cAMP pathways during invasive growth of Saccharomyces cerevisiae. Mol. Biol. Cell 10:1325–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oehlen L., Cross F. R. 1998. The mating factor response pathway regulates transcription of TEC1, a gene involved in pseudohyphal differentiation of Saccharomyces cerevisiae. FEBS Lett. 429:83–88 [DOI] [PubMed] [Google Scholar]
- 44.Olson K. A., Nelson C., Tai G., Hung W., Yong C., Astell C., Sadowski I. 2000. Two regulators of Ste12p inhibit pheromone-responsive transcription by separate mechanisms. Mol. Cell. Biol. 20:4199–4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pi H., Chien C. T., Fields S. 1997. Transcriptional activation upon pheromone stimulation mediated by a small domain of Saccharomyces cerevisiae Ste12p. Mol. Cell. Biol. 17:6410–6418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts R. L., Fink G. R. 1994. Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Genes Dev. 8:2974–2985 [DOI] [PubMed] [Google Scholar]
- 47.Sobering A. K., Jung U. S., Lee K. S., Levin D. E. 2002. Yeast Rpi1 is a putative transcriptional regulator that contributes to preparation for stationary phase. Eukaryot. Cell 1:56–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sollner S., Schober M., Wagner A., Prem A., Lorkova L., Palfey B. A., Groll M., Macheroux P. 2009. Quinone reductase acts as a redox switch of the 20S yeast proteasome. EMBO Rep. 10:65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tedford K., Kim S., Sa D., Stevens K., Tyers M. 1997. Regulation of the mating pheromone and invasive growth responses in yeast by two MAP kinase substrates. Curr. Biol. 7:228–238 [DOI] [PubMed] [Google Scholar]
- 50.Vallim M. A., Miller K. Y., Miller B. L. 2000. Aspergillus SteA (sterile12-like) is a homeodomain-C2/H2-Zn+2 finger transcription factor required for sexual reproduction. Mol. Microbiol. 36:290–301 [DOI] [PubMed] [Google Scholar]
- 51.Yaffe M. P., Schatz G. 1984. Two nuclear mutations that block mitochondrial protein import in yeast. Proc. Natl. Acad. Sci. U. S. A. 81:4819–4823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuan Y. L., Fields S. 1991. Properties of the DNA-binding domain of the Saccharomyces cerevisiae Ste12 protein. Mol. Cell. Biol. 11:5910–5918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeitlinger J., Simon I., Harbison C. T., Hannett N. M., Volkert T. L., Fink G. R., Young R. A. 2003. Program-specific distribution of a transcription factor dependent on partner transcription factor and MAPK signaling. Cell 113:395–404 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.