Abstract
In Europe, ixodid ticks are important arthropod vectors of human and animal pathogens, but comprehensive studies of the prevalence of all relevant pathogens in Central Europe are scarce. As a result of ecological changes, the incidences of tick-borne infections are expected to increase. In this study, 1,394 nymphal and adult Ixodes ricinus ticks sampled monthly during the active season from 33 ecologically distinct collection sites throughout Luxembourg were screened for all human tick-borne pathogens relevant in Central Europe. Species were identified by sequence analysis of detection PCR amplicons. Mean infection rates of ticks were 11.3% for Borrelia burgdorferi sensu lato, 5.1% for Rickettsia sp., 2.7% for Babesia sp., and 1.9% for Anaplasma phagocytophilum. No tick was found to be infected with Coxiella sp., Francisella tularensis subsp., or Tick-borne encephalitis virus (TBEV). A total of 3.2% of ticks were infected with more than one pathogen species, including mixed Borrelia infections (1.5%). Seasonal variations of tick infection rates were observed for Borrelia, Babesia, and Anaplasma, possibly reflecting a behavioral adaptation strategy of questing ticks. A positive correlation between the grade of urbanization and Borrelia infection rate of ticks was observed, suggesting an established urban zoonotic cycle. We also found Hepatozoon canis (0.1%) and Bartonella henselae (0.3%), which so far have not been found in questing Ixodes ricinus ticks in Central Europe.
In Western Europe, the hard tick Ixodes ricinus is the main arthropod vector of various human and animal pathogens, causing several tens of thousands of severe infections in humans every year (25, 37). The most common tick-borne infection is Lyme borreliosis. This multisystemic disorder is caused by spirochetes of the Borrelia burgdorferi sensu lato complex, which is comprised of at least 12 species worldwide (45). Among the 6 European species, only Borrelia garinii, Borrelia afzelii, and Borrelia burgdorferi sensu stricto are known as human pathogens, whereas the significance of Borrelia valaisiana, Borrelia spielmanii, and Borrelia lusitaniae for human health is not clear (24). In a metaanalysis of 154 European studies, a mean of 13.7% of ticks were found to be infected with Borrelia spp., predominantly with B. afzelii and B. garinii. However, the prevalence of Borrelia species varies from 2 to 49% between different regions (43).
Other tick-borne bacteria which cause disease in humans are Rickettsia sp., Anaplasma phagocytophilum, Bartonella henselae and Bartonella quintana, Coxiella burnetii, and Francisella tularensis subsp., all of which show only relatively low prevalence rates of 0.1 to 4.8% for European ticks (16, 23, 26, 42, 48, 52). In addition, three species of the parasitic protozoan Babesia are known to infect humans, namely, B. divergens, B. microti, and the newly described Babesia sp. EU1 (5). Also, in Western Europe, Tick-borne encephalitis virus (TBEV) has a relatively low prevalence; however, this pathogen deserves special attention because of the severe disease it causes in humans. Tick-borne encephalitis affects at least 10,000 humans in Europe annually (13, 37), and up to 5% of ixodid ticks are infected in areas where it is endemic (44, 54).
As a result of climatic changes and the human impact on the environment, the prevalence of ticks and tick-borne infections in Central Europe is expected to increase (20, 57). Nevertheless, recent studies of human pathogens are rare in Central Europe (14, 15, 17, 31, 39, 48, 49), and comprehensive surveys to assess risks to human health are warranted.
Here we present such a comprehensive study in Central Europe which investigates all relevant human tick-borne pathogens in questing nymphal and adult ticks from 33 representative collection sites throughout the 2007 season.
MATERIALS AND METHODS
Tick collection and identification.
A total of 33 representative tick habitats distributed over all of Luxembourg were selected on the basis of plant cover, microclimate, and other ecological parameters. From May to October 2007, these sites were sampled every month for questing ticks using the cloth-dragging method. This method samples mainly exophilic ticks, such as Ixodes ricinus, that actively quest for hosts. After morphological identification of tick species, developmental stages, and sex of adults under a stereomicroscope, ticks were stored individually at −80°C. Only adults and nymphs were further investigated for the presence of tick-borne pathogens.
Nucleic acid extraction, PCR, and sequencing.
Adult and nymphal ticks were disrupted and homogenized individually with a rotor-stator homogenizer with exchangeable generators (PRO Scientific Inc., Oxford, CT) or the TissueLyser II (Qiagen, Venlo, Netherlands) in 300 μl lysis buffer of the QIAamp DNA blood minikit (Qiagen, Venlo, Netherlands). Nucleic acid extraction was performed according to the manufacturer's protocol. As a quality control of the nucleic acid extraction, the reverse transcription, and potential PCR inhibition, 20-μl aliquots of 5 tick homogenates were pooled and spiked with measles virus culture supernatant. A total of 200 μl of eluted nucleic acids were divided into 3 aliquots and stored at −80°C. Reverse transcription of total RNA with random primers (Invitrogen, Merelbeke, Belgium) and the measles virus PCR (for quality control) were performed as described previously (27). Specific detection PCRs of all pathogens were carried out with previously published primers using 5 μl of raw DNA or cDNA (references and details in Table 1). PCR products were purified directly using the Jet Quick PCR purification spin kit (Genomed, Loehne, Germany), or in the case of multiple bands, fragments of the expected size were extracted from 1.5% agarose gels (QIAquick gel extraction kit, Qiagen, Venlo, Netherlands). Purified products were sequenced in both directions with a BigDye Terminator (version 3.1) cycle sequencing kit (Applied Biosystems, Nieuwerkerk, Netherlands) on a capillary sequencer (Model 3100 Avant; Applied Biosystems) with PCR primers as sequencing primers.
TABLE 1.
Primers and PCR conditions used for the detection of the eight different pathogen groupsa
| Pathogen | Primer name | Primer orientation | Target gene | 5′-3′ sequence | Reference | Primer concn (μM) | MgCl2 concn (mM) | Annealing step (temp [°C], time [s]) | Elongation step (temp [°C], time [s]) |
|---|---|---|---|---|---|---|---|---|---|
| Anaplasma phagocytophilum | EL(569)F | Forward | groEL | ATGGTATGCAGTTTGATCGC | 0.8 | 2 | 61, 30 | 72, 45 | |
| EL(1193)R | Reverse | groEL | TCTACTCTGTCTTTGCGTTC | ||||||
| EL(569)F | Forward | groEL | ATGGTATGCAGTTTGATCGC | 0.8 | 2 | 56, 30 | 72, 45 | ||
| EL(1142)R | Reverse | groEL | TTGAGTACAGCAACACCACCGGAA | 1 | |||||
| Babesia sp. | BJ1 | Forward | 18S rRNA | GTCTTGTAATTGGAATGATGG | 0.8 | 3 | 61, 30 | 70, 60 | |
| BN2 | Reverse | 18S rRNA | TAGTTTATGGTTAGGACTACG | 8 | |||||
| Bartonella sp. | 321s | Forward | 16S-23S | AGATGATGATCCCAAGCCTTCTGC | 0.8 | 1.5 | 60, 30 | 72, 45 | |
| 983as | Reverse | 16S-23S | TGTTCTYACAACAATGATGATG | 36 | |||||
| Borrelia burgdorferi sensu lato | Outer1 | Forward | flaB | AARGAATTGGCAGTTCAATC | 0.8 | 2 | 59, 30 | 72, 30 | |
| Outer2 | Reverse | flaB | GCATTTTCWATTTTAGCAAGTGATG | ||||||
| Inner1 | Forward | flaB | ACATATTCAGATGCAGACAGAGGTTCTA | 0.8 | 2 | 59, 30 | 72, 30 | ||
| Inner2 | Reverse | flaB | GAAGGTGCTGTAGCAGGTGCTGGCTGT | 9 | |||||
| V1a | Forward | OspA gene | GGGAATAGGTCTAATATTAGC | 0.8 | 2 | 50, 45 | 72, 60 | ||
| V1b | Forward | OspA gene | GGGGATAGGTCTAATATTAGC | ||||||
| R2 | Reverse | OspA gene | CATAAATTCTCCTTATTTTAAAGC | ||||||
| R37 | Reverse | OspA gene | CCTTATTTTAAAGCGGC | ||||||
| V3a | Forward | OspA gene | GCCTTAATAGCATGTAAGC | 0.8 | 2 | 52, 45 | 72, 60 | ||
| V3b | Forward | OspA gene | GCCTTAATAGCATGCAAGC | ||||||
| R2 | Reverse | OspA gene | CATAAATTCTCCTTATTTTAAAGC | ||||||
| R37 | Reverse | OspA gene | CCTTATTTTAAAGCGGC | 38 | |||||
| Coxiella sp. | Q5 | Forward | htpB | GCGGGTGATGGTACCACAACA | 0.4 | 1.5 | 58, 30 | 72, 30 | |
| Q3 | Reverse | htpB | GGCAATCACCAATAAGGGCCG | ||||||
| Q6 | Forward | htpB | TTGCTGGAATGAACCCCA | 0.8 | 2 | 56, 30 | 72, 30 | ||
| Q4 | Reverse | htpB | TCAAGCTCCGCACTCATG | 56 | |||||
| Francisella tularensis subsp. | Fr153F0.1 | Forward | 16S rRNA | GCCCATTTGAGGGGGATACC | 0.4 | 2 | 60, 30 | 72, 60 | |
| Fr1281R0.1 | Reverse | 16S rRNA | GGACTAAGAGTACCTTTTTGAGT | 3 | |||||
| Rickettsia sp. | Rr17k.1p | Forward | 17 kDa | TTTACAAAATTCTAAAAACCAT | 0.8 | 2 | 55, 30 | 72, 45 | |
| Rr17k.539n | Reverse | 17 kDa | TCAATTCACAACTTGCCATT | ||||||
| Rr17k.90p | Forward | 17 kDa | GCTCTTGCAACTTCTATGTT | 0.8 | 2 | 54, 30 | 72, 45 | ||
| Rr17k.539n | Reverse | 17 kDa | TCAATTCACAACTTGCCATT | 28 | |||||
| Tick-borne encephalitis virus | 283F1 | Forward | E protein | GAGAYCAGAGTGAYCGAGGCTGG | 0.4 | 2 | 57, 30 | 72, 45 | |
| 827R1 | Reverse | E protein | AGGTGGTACTTGGTTCCMTCAAGT | ||||||
| 349F2 | Forward | E protein | GTCAAGGCGKCTTGTGAGGCAA | 0.8 | 2 | 58, 30 | 72, 30* | ||
| 814R2 | Reverse | E protein | TTCCCTCAATGTGTGCCACAGG | 50 |
PCR protocol was as follows: 94°C for 3 min; 40 cycles of 94°C for 30 s, specific annealing conditions, and 72°C for specific elongation time; and subsequent incubation at 72°C for 10 min. *, 25 cycles instead of 40 were performed.
Borrelia species were further characterized by sequencing the outer surface protein A (OspA) gene (Table 1). In order to identify mixed Borrelia infections, sequences with nucleotide ambiguities were cloned into pCR4-TOPO plasmid vector (Invitrogen, Merelbeke, Belgium) and transformed into OneShot TOP10 electrocompetent Escherichia coli (Invitrogen, Merelbeke, Belgium) using the manufacturer's protocol. Sixteen clones per sample were picked, and the insert was sequenced with M13 primers (Invitrogen, Merelbeke, Belgium).
Data analysis.
A BLAST search was performed for all sequences, and species identity was confirmed by phylogenetic analysis and distance calculations using MEGA version 3.1 (33). Phylogenetic analysis was based on the neighbor-joining method using the Kimura 2-parameter model with 1,000 bootstrap replicates and pairwise deletion. To test for differences in the tick infection rates among geographic regions, the data of 4 to 8 collection sites in the North, Northeast, East, South, West, and Center of Luxembourg were pooled (Table 2). Additionally, the habitat of each collection site was characterized by the percentage of forest, agricultural plains, water bodies, and urbanized areas (buildings and sealed surfaces) in a 1-km2 area, with the collection site as the centroid using aerial photographs (Google Earth). Thus, four ecological categories were defined (category I, 0 to 4%; II, 5 to 9%; III, 10 to 24%; IV, 25 to 60% of urbanized area) with 6 to 11 collection sites per category (Table 2). One-way analysis of variance (ANOVA) tests were performed with SigmaStat3.1 (Systat Software, Erkrath, Germany) on tick infection rates for geographic groups and habitat categories. Pearson's goodness of fit chi-square (GFX) test was performed on prevalence data of nymphs and adults and of males and females. Below, in cases of statistical significance, P values are given in parentheses.
TABLE 2.
Infection rates of ticks for geographic groups and habitat categoriesa
| Geographic group or habitat category | CS | TDb | TIR (%) |
|||||
|---|---|---|---|---|---|---|---|---|
| Total | Borrelia | Rickettsia | Babesia | Anaplasma | Bartonella | |||
| Geographic groups | ||||||||
| North | 6 | 7.7 | 17.6 | 12.1 | 4.6 | 3.9 | 0 | 0.7 |
| Northeast | 4 | 5.9 | 27.1 | 21.9 | 6.5 | 4.5 | 0.6 | 0 |
| East | 6 | 9.3 | 20.1 | 9.1 | 7.8 | 1.6 | 4.5 | 0.3 |
| West | 4 | 3.7 | 10.3 | 2.8 | 4.7 | 1.9 | 2.8 | 0 |
| Center | 8 | 8.1 | 15.6 | 10.3 | 2.4 | 2.9 | 1.3 | 0 |
| South | 5 | 4.4 | 20.7 | 10.0 | 7.9 | 1.4 | 2.1 | 0.7 |
| Habitat categories | ||||||||
| I | 6 | 5.5 | 16.9 | 8.9 | 4.7 | 3.0 | 1.3 | 0.8 |
| II | 11 | 8.7 | 18.1 | 9.0 | 5.3 | 1.1 | 2.5 | 0.4 |
| III | 10 | 7.8 | 22.9 | 14.0 | 5.1 | 2.7 | 2.4 | 0 |
| IV | 6 | 6.1 | 20.6 | 14.6 | 5.1 | 2.8 | 0.8 | 0 |
CS, number of collection sites per group; TD, tick density; TIR, tick infection rate. Note that for the total tick infection rates, mixed infected ticks were counted only once.
Ticks per 100 m2.
Nucleotide sequence accession numbers.
Sequences were submitted to GenBank under accession numbers GU826702 to GU827130.
RESULTS
Tick numbers.
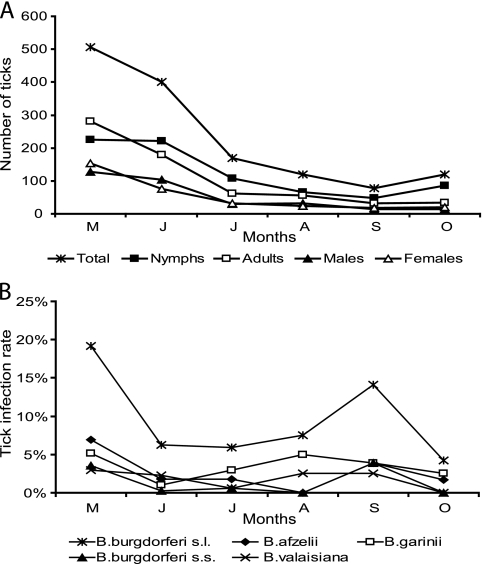
A total of 1,500 ticks, including 106 larvae, 752 nymphs, and 642 adults (320 males and 322 females) were collected. All ticks belonged to the species Ixodes ricinus. Tick density ranged from 3.7 ticks per 100 m2 in the West to 9.3 in the East. Higher densities were found in the habitats of categories II and III (8.7 and 7.8 ticks) than in the those of the others (Table 2). The nymphal and adult tick activity was highest in May and June (Fig. 1A). Larvae showed their main activity in August (9 sites, 1 to 56 larvae/site). Despite considerable variability in tick numbers per collection site (14 to 134 ticks/site and mean of 46), geographic region, and habitat category, the highest numbers of nymphs and adults were always observed in spring (data not shown).
FIG. 1.
Overall seasonal activity of the developmental stages of I. ricinus from 33 collection sites collected from May to October 2007 (A) and Borrelia infection rates of ticks (B). The letters M, J, J, A, S, and O represent the months May, June, July, August, September, and October, respectively. s.l., sensu lato; s.s., sensu stricto.
Tick infection rates.
Of the 1,394 adult and nymphal ticks, a total of 19.5% (n = 272) were infected with at least one pathogen. Nymphs had a significantly lower overall infection rate (16.4%) than adults (23.2%, P < 0.01), with females showing a significantly higher infection rate (26.7%) than males (19.7%, P < 0.05). A comparison of the infection rates by geographic regions and habitat categories revealed considerable variations (Table 2).
Borrelia.
B. burgdorferi sensu lato was the predominant pathogen group and was detected in 11.3% (n = 157) of all ticks. As expected, the tick infection rate was significantly higher in adults (15.0%; males, 14.7%; females, 15.2%) than in nymphs (8.1%) (P < 0.01). Borrelia infection rates were highest in the Northeast (21.9%) and lowest in the West (2.8%) (Table 2). The habitat classification showed a positive correlation between infection rates and the extent of urbanization, ranging from 8.9% in category I to 14.6% in category IV (Table 2).
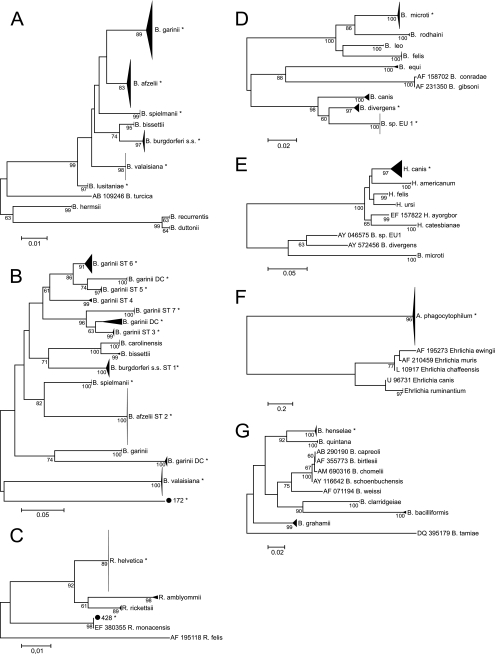
Six different Borrelia species were identified based on the flagellin B (FlaB) gene (Fig. 2A). B. afzelii (33.1%; n = 52) and B. garinii (29.9%; n = 47) were the most prevalent species, followed by B. valaisiana (19.1%; n = 30), B. burgdorferi sensu stricto (14.6%; n = 23), B. spielmanii (2.5%; n = 4), and B. lusitaniae (0.6%; n = 1). Sequences for the outer surface protein A (OspA) were obtained for 133 FlaB-positive samples, resulting in 9.3% B. burgdorferi sensu stricto, 37.7% B. afzelii, 19.1% B. garinii (serotypes 3 to 7, 2.6%, 0%, 2.6%, 10.6%, and 3.3%, respectively. Additionally, 11.9% of sequences formed three distinct clusters most closely related to B. garinii strains (Fig. 2B). OspA sequences of B. valaisiana and B. spielmanii formed distinct clusters. The OspA sequence derived from tick no. 172 (FlaB sequence clustered with B. lusitaniae) clustered distinctly from all reference and sample sequences, suggesting that the OspA sequence is also from B. lusitaniae. A total of 24 samples (1.7%) with nucleotide ambiguities were cloned, and 19 mixed infections were confirmed. In 2 additional samples, the FlaB and the OspA fragments corresponded to different Borrelia species. The most frequent combinations were B. valaisiana and B. garinii (10/21) (Table 3).
FIG. 2.
Phylogenetic trees for identification of pathogens to the species level based on 209 nucleotides of the FlaB gene of B. burgdorferi sensu lato (nucleotides 151 to 359 of GQ918147.1), including 157 samples and 71 reference sequences (A), on 462 to 465 nucleotides of the OspA gene of B. burgdorferi sensu lato (nucleotides 9441 to 9905 of CP001433.1), including 133 samples and 59 reference sequences (B), on 190 nucleotides of the 17-kDa antigen gene of Rickettsia species (nucleotides 140 to 329 of GU292313.1), including 72 samples and 14 reference sequences (C), on 343 to 370 nucleotides of the 18 rRNA of Babesia species (nucleotides 481 to 850 of EF413181.1), including 36 samples and 32 reference sequences (D), on 293 nucleotides of the 18 rRNA of Hepatozoon species (nucleotides 171 to 461 of FJ608736.1), including 1 sample and 34 reference sequences (E), on 466 nucleotides of the groEL gene of Anaplasma species (nucleotides 45 to 510 of GQ988761.1), including 26 samples and 55 reference sequences (F), and on 313 nucleotides of the 16S-23S region of Bartonella species (nucleotides 1782 to 2094 of AJ749669.1), including 4 samples and 26 reference sequences (G). Bootstrap values above 60 are shown. Asterisks represent (clusters including) our sequences. s.s., sensu stricto; DC, distinct cluster; ST, serotype.
TABLE 3.
Coinfections of Ixodes ricinus, potential reservoir hosts, and mode of acquisitiona
| Developmental stage/sex of ticks | Pathogen species |
Reservoir host preferences | Acquisition of coinfection | ||||
|---|---|---|---|---|---|---|---|
| Borrelia | Borrelia | Rickettsia | Babesia | Anaplasma | |||
| F | afzelii | microti | R + R | SIM | |||
| F | afzelii | microti | R + R | SIM | |||
| F | afzelii | helvetica | R + R/D | SIM or CON | |||
| F | valaisiana | helvetica | R + R/D | SIM or CON | |||
| F | valaisiana | helvetica | R + R/D | SIM or CON | |||
| F | valaisiana | helvetica | R + R/D | SIM or CON | |||
| F | valaisiana | phagocytophilum | R + B/D | CON | |||
| F | helvetica | phagocytophilum | R/D + B/D | SIM or CON | |||
| F | helvetica | sp. EU1 | R/D + D | SIM or CON | |||
| F | garinii ST3 | garinii ST7 | B + B | SIM | |||
| F | garinii ST6 | valaisiana | B + B | SIM | |||
| F | garinii ST6 | valaisiana | B + B | SIM | |||
| F | garinii DC | valaisiana | Unclear + B | SIM or CON | |||
| F | burgdorferi sensu stricto | Species unclear | R/B + Unclear | SIM or CON | |||
| M | afzelii | microti | R + R | SIM | |||
| M | afzelii | sp. EU1 | R + D | CON | |||
| M | afzelii | sp. EU1 | R + D | CON | |||
| M | garinii | helvetica | R/B + R/D | SIM or CON | |||
| M | garinii | helvetica | B + R/D | CON | |||
| M | garinii | sp. EU1 | phagocytophilum | B + D + B/D | CON | ||
| M | afzelii | burgdorferi sensu stricto | R + R/B | SIM or CON | |||
| M | afzelii | spielmanii | R + R | SIM | |||
| M | afzelii | spielmanii | R + R | SIM | |||
| M | garinii | burgdorferi sensu stricto | R/B + R/B | SIM or CON | |||
| M | garinii | valaisiana | R/B + B | SIM or CON | |||
| M | garinii | valaisiana | R/B + B | SIM or CON | |||
| M | garinii ST7 | valaisiana | B + B | SIM | |||
| M | garinii DC | valaisiana | Unclear + B | SIM or CON | |||
| M | valaisiana | burgdorferi sensu stricto | B + R/B | SIM or CON | |||
| N | afzelii | helvetica | R + R/D | SIM or CON | |||
| N | afzelii | helvetica | R + R/D | SIM or CON | |||
| N | afzelii | microti | R + R | SIM | |||
| N | afzelii | microti | R + R | SIM | |||
| N | afzelii | microti | R + R | SIM | |||
| N | garinii ST6 | valaisiana | sp. EU1 | B + B + D | CON | ||
| N | helvetica | phagocytophilum | R/D + B/D | SIM or CON | |||
| N | helvetica | phagocytophilum | R/D + B/D | SIM or CON | |||
| N | helvetica | sp. EU1 | R/D + B/D | SIM or CON | |||
| N | afzelii | garinii | R + R/B | SIM or CON | |||
| N | afzelii | burgdorferi sensu stricto | R + R/B | SIM or CON | |||
| N | garinii ST3 | valaisiana | B + B | SIM | |||
| N | garinii ST5 | garinii ST6 | B + B | SIM | |||
| N | garinii ST5 | garinii ST6 | B + B | SIM | |||
| N | garinii DC | valaisiana | Unclear + B | SIM or CON | |||
Seasonal evolution of Borrelia infections in ticks showed a bimodal seasonal activity beginning with high numbers in May and a second peak in September (Fig. 1B). On a species level, different patterns of seasonality were observed (Fig. 1B).
Regional differences in the prevalence of Borrelia species were also observed. B. afzelii was predominant in the North (59.5%) and South (35.7%), whereas B. garinii was most prevalent (53.6%) in the East. B. valaisiana was the predominant species in the Northeast (29.4%). In the West, B. garinii, B. afzelii, and B. burgdorferi sensu stricto were equally prevalent (33.3%).
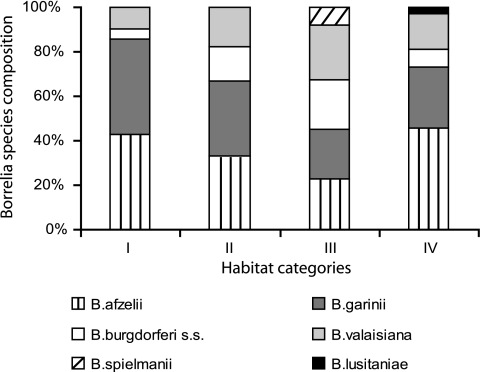
Species composition varied between habitat categories (Fig. 3): B. afzelii and B. garinii were equally prevalent in all categories, except in IV, in which B. afzelii was predominant. In category III, B. garinii, B. afzelii, B. burgdorferi sensu stricto, and B. valaisiana showed similar prevalence rates (23.4 to 25.5%). Tick infection rates of B. garinii were similar in all categories (3.0 to 4.0%), whereas B. afzelii seemed to prefer category IV (6.7%) to the others (2.7 to 3.8%). The 1.6-fold higher infection rates of categories III and IV (14.3%) in comparison to I and II (9.0%) are caused by B. valaisiana and B. burgdorferi (III) and by B. afzelii (IV), respectively.
FIG. 3.
Borrelia species composition by habitat category. s.s., sensu stricto.
Infection rates of adult ticks with B. garinii and B. valaisiana were significantly higher (P < 0.01) than those for nymphs. A higher adult infection rate was found with habitat categories II to IV, whereas in category I both infection rates were similar (data not shown).
Rickettsia.
In 5.1% (n = 71) of ticks, Rickettsia species were detected. These were identified as R. helvetica (n = 70) and R. monacensis (n = 1) (Fig. 2C). No clear trend in the seasonal variation of infected tick activity was observed (data not shown). The highest tick infection rates were found in the South, and the lowest rates in the Center (Table 2). All habitats had similar infection rates (Table 2). Nymphal and adult tick infection rates were similar (4.9% versus 5.3%), but the prevalence of Rickettsia-infected females (7.8%) was significantly higher than that of males (2.8%) (P < 0.01).
Babesia.
Babesia species were detected in 2.7% (n = 37) of ticks, with Babesia sp. EU1 being predominant (59.5%) and B. microti being the second most common species (35.1%). B. divergens and Hepatozoon canis were each detected in a single tick only (2.7%) (Fig. 2D and E). The highest prevalence was found in September (data not shown). Tick infection rates ranged from 1.4% in the South to 4.5% in the Northeast and from 1.1% to 3.0% in the different habitat categories (Table 2). B. microti infection rates were twice higher for adults (1.3%) than for nymphs (0.7%), whereas Babesia sp. EU1 was more prevalent in nymphs (1.9%) than in adults (1.3%).
Anaplasma phagocytophilum.
A total of 1.9% (n = 27) of ticks were infected with A. phagocytophilum (Fig. 2F). There was a clear unimodal seasonality for infected adult ticks with a peak in September, but no such pattern was found for nymphs (data not shown). The highest infection rate of A. phagocytophilum was found for ticks collected in the East (4.5%) (Table 2). Tick infection rates were lowest in habitat category IV (0.8%) and highest in II (2.5%) (Table 2). Female ticks seem to be more often infected (3.4%) than male (1.6%) or nymphal ticks (1.5%).
Bartonella.
Bartonella henselae was detected in 0.3% of all ticks (Fig. 2G), and the 4 infected ticks were found at different collection sites and in different months (data not shown). B. henselae was found only in the North, East, and South and in habitat categories I and II (Table 2).
Coxiella sp., Francisella tularensis subsp., and TBEV.
Coxiella sp., Francisella tularensis, and TBEV were not detected in any of the 1,394 ticks analyzed.
Mixed infections.
Infections with more than one pathogen occurred in 3.2% of all ticks (n = 44), most of which were coinfections with two pathogens (n = 42) (Table 3). Combinations of B. burgdorferi sensu lato and Babesia sp. (22.7%) and B. burgdorferi sensu lato and R. helvetica (18.2%) were most frequent. All coinfections involving Babesia microti were exclusively with B. afzelii (n = 6). Additionally, no Anaplasma-infected tick was coinfected with B. afzelii. Almost half of the observed coinfections (n = 21) involved different Borrelia species (also see above). Two coinfections with 3 pathogens (B. garinii, A. phagocytophilum, and Babesia sp. EU1 as well as B. garinii ST6, B. valaisiana, and Babesia sp. EU1) were found in a male and nymph, respectively. The adult coinfection rate (4.5%) was twice as high as the nymphal (2.0%) (P < 0.01), and the great majority of multiply infected ticks (75%) were collected in May and June (data not shown).
DISCUSSION
The present study is the most complete survey of all relevant tick-borne human pathogens in Central Europe. Additionally, it is among the very few studies with a monthly sampling of multiple collection sites.
The densities of I. ricinus at most collection sites (3.6 to 9.5 ticks/100 m2) correspond to the category “low tick abundance” (3 to 10 ticks/100 m2) according to Schwarz et al. (47). The observed infection rates of B. burgdorferi sensu lato (11.3%), R. helvetica (5.1%), and A. phagocytophilum (1.9%) in Luxembourg are comparable to those reported from neighboring countries Germany (2002 to 2005: Borrelia, 13.9 to 24%; Rickettsia, 8.9%; Anaplasma, 1.0% [23, 35, 40]), Belgium (1998: Borrelia, 23% [39]), and France (2006: Borrelia, 20.4%; Anaplasma, 0.5%; Rickettsia, 16% of tick pools [16, 22]).
In Europe, the most prevalent Borrelia species is either B. afzelii (7, 29, 35) or B. garinii (6, 39). We observed marked differences in the prevalence levels of Borrelia species for both the geographic regions and the habitat categories, which may be related to the specific host preferences of the different Borrelia species. Based on their sensitivity to reservoir host complement, Borrelia species have been divided into three ecological groups (34). Thus, B. afzelii and certain B. garinii strains (OspA serotype 4) are associated mainly with rodents and B. valaisiana and other B. garinii strains (OspA serotypes 3, 5, 6, and 7) with birds, whereas B. burgdorferi sensu stricto is found in both rodents and birds (34). In categories III and IV, higher Borrelia infection rates are caused by B. burgdorferi sensu stricto, B. valaisiana, and B. afzelii, suggesting an established urban zoonotic cycle with synanthropic rodents and songbirds as main hosts. Urban zoonoses have been described for other arthropod- and tick-borne pathogens, e.g., Bartonella, Coxiella, Ehrlichia, and Rickettsia, and their increasing incidence has been linked to various extrinsic and intrinsic factors (10).
The prevalence rates of Babesia species in our study are similar to those in reports from Germany (1%) (8, 23) but are much lower than those in France (20.0%) (21). However, in Germany, B. divergens is by far the most prevalent species (23), whereas in Luxembourg Babesia sp. EU1 and B. microti are predominant. We also detected H. canis, which has never been found in questing I. ricinus ticks from Central Europe before. The causative agent of canine hepatozoonosis is endemic in Southern Europe, Africa, and the Middle and Far East, where it is transmitted to dogs by oral uptake of infected Rhipicephalus sanguineus ticks during grooming (2). International tourism, including the importation of pet animals (2), may explain the introduction of pathogens to areas where they are nonendemic. The finding of H. canis in a questing female I. ricinus tick suggests the successful transmission from an infected dog to a feeding instar in Luxembourg that maintained the infection transstadially. Whether I. ricinus is a competent vector and whether ecological factors favor the establishment and spread of this pathogen in Central Europe require further attention.
Bartonella henselae (0.3%) has not been found in questing I. ricinus ticks in Central Europe before. This pathogen is commonly transmitted by infected cats and causes the cat scratch disease in humans. Only recently, the role of I. ricinus as a competent vector for B. henselae has been confirmed experimentally (11).
Although no TBEV-infected tick was found in this study, findings from France and recently also from Luxembourg's two neighboring German states Saarland and Rhineland-Palatinate (46, 55) suggest a further spread of this virus.
Since transovarial transmissions are rare, coinfections in I. ricinus ticks may shed some light on the route of infection, e.g., consecutive feedings, coinfected hosts, or cofeeding. Interestingly, analysis of reservoir host preferences of each pathogen (Table 3) revealed that pathogen combinations which normally do not occur in the same host were about eight times more frequent in adults (0.8%) than in nymphs (0.1%). In contrast, pathogen combinations that occur in the same host had slightly higher rates for adults (1.4%) than for nymphs (0.8%). This suggests that coinfections of nymphs are acquired during larval feeding on coinfected hosts, while in adults consecutive feedings are the main source of coinfections.
Only few studies have taken the seasonal variations of tick infection rates into account. Intriguingly, tick infection rates of Borrelia sp. and Babesia sp. were low in summer (July and August) and significantly increased in September (6.7 to 14.1% [P < 0.05] and 1.7 to 3.9% [P < 0.05], respectively). For A. phagocytophilum, a similar pattern was observed (1.4 to 5.1%), which may reflect a behavioral adaptation strategy of ticks. Aridity can force ticks to undergo quiescence in order to avoid critical loss of energy, which may be exacerbated by pathogen infections (19, 32, 41, 51) and thus contribute to preferential collection of uninfected ticks and to the observed seasonal variations in the tick infection rates.
In conclusion, the habitat influences not only tick densities and vertebrate host population but also the prevalence of Borrelia species. The observed seasonality of Borrelia, Anaplasma, and Babesia species has not been reported before, and, together with the possibility of urban zoonoses, it has major implications for human health. In addition, imported or neglected pathogens, like H. canis and B. henselae, as well as coinfections with various pathogen combinations, may represent new potential threats to human and animal health.
Acknowledgments
We thank Bettina Wilske, Cecilia Hizo-Teufel, Irina Golovljova, Nigel J. Silman, Mardjan Arvand, Bruno Gottstein, Horacio Gil, and Edward Siński for providing positive controls, Nick Aschman for his help with tick collection, and Emilie Charpentier for her experimental assistance.
This work was funded by the Centre de Recherche Public-Santé. A. L. Reye was supported by a fellowship from the Bourse Formation Recherche and the Aides à la Formation Recherche of the Fonds National de la Recherche Luxembourg.
Footnotes
Published ahead of print on 12 March 2010.
REFERENCES
- 1.Alberti, A., R. Zobba, B. Chessa, M. F. Addis, O. Sparagano, M. L. Pinna Parpaglia, T. Cubeddu, G. Pintori, and M. Pittau. 2005. Equine and canine Anaplasma phagocytophilum strains isolated on the island of Sardinia (Italy) are phylogenetically related to pathogenic strains from the United States. Appl. Environ. Microbiol. 71:6418-6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baneth, G., J. R. Barta, V. Shkap, D. S. Martin, D. K. Macintire, and N. Vincent-Johnson. 2000. Genetic and antigenic evidence supports the separation of Hepatozoon canis and Hepatozoon americanum at the species level. J. Clin. Microbiol. 38:1298-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barns, S. M., C. C. Grow, R. T. Okinaka, P. Keim, and C. R. Kuske. 2005. Detection of diverse new Francisella-like bacteria in environmental samples. Appl. Environ. Microbiol. 71:5494-5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker, C. A., A. Bouju-Albert, M. Jouglin, A. Chauvin, and L. Malandrin. 2009. Natural transmission of zoonotic Babesia spp. by Ixodes ricinus ticks. Emerg. Infect. Dis. 15:320-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaschitz, M., M. Narodoslavsky-Gfoller, M. Kanzler, G. Stanek, and J. Walochnik. 2008. Babesia species occurring in Austrian Ixodes ricinus ticks. Appl. Environ. Microbiol. 74:4841-4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaschitz, M., M. Narodoslavsky-Gfoller, M. Kanzler, J. Walochnik, and G. Stanek. 2008. Borrelia burgdorferi sensu lato genospecies in questing Ixodes ricinus ticks in Austria. Int. J. Med. Microbiol. 298:168-176. [Google Scholar]
- 7.Casati, S., M. V. Bernasconi, L. Gern, and J. C. Piffaretti. 2004. Diversity within Borrelia burgdorferi sensu lato genospecies in Switzerland by recA gene sequence. FEMS Microbiol. Lett. 238:115-123. [DOI] [PubMed] [Google Scholar]
- 8.Casati, S., H. Sager, L. Gern, and J. C. Piffaretti. 2006. Presence of potentially pathogenic Babesia sp. for human in Ixodes ricinus in Switzerland. Ann. Agric. Environ. Med. 13:65-70. [PubMed] [Google Scholar]
- 9.Clark, K., A. Hendricks, and D. Burge. 2005. Molecular identification and analysis of Borrelia burgdorferi sensu lato in lizards in the southeastern United States. Appl. Environ. Microbiol. 71:2616-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comer, J. A., C. D. Paddock, and J. E. Childs. 2001. Urban zoonoses caused by Bartonella, Coxiella, Ehrlichia, and Rickettsia species. Vector Borne Zoonotic Dis. 1:91-118. [DOI] [PubMed] [Google Scholar]
- 11.Cotte, V., S. Bonnet, D. Le Rhun, E. Le Naour, A. Chauvin, H. J. Boulouis, B. Lecuelle, T. Lilin, and M. Vayssier-Taussat. 2008. Transmission of Bartonella henselae by Ixodes ricinus. Emerg. Infect. Dis. 14:1074-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De La Fuente, J., V. Naranjo, F. Ruiz-Fons, U. Hofle, I. G. Fernandez De Mera, D. Villanua, C. Almazan, A. Torina, S. Caracappa, K. M. Kocan, and C. Gortazar. 2005. Potential vertebrate reservoir hosts and invertebrate vectors of Anaplasma marginale and A. phagocytophilum in central Spain. Vector Borne Zoonotic Dis. 5:390-401. [DOI] [PubMed] [Google Scholar]
- 13.Dobler, G. 2010. Zoonotic tick-borne flaviviruses. Vet. Microbiol. 140:221-228. [DOI] [PubMed] [Google Scholar]
- 14.Doudier, B., J. Olano, P. Parola, and P. Brouqui. 2010. Factors contributing to emergence of Ehrlichia and Anaplasma spp. as human pathogens. Vet. Parasitol. 167:149-159. [DOI] [PubMed] [Google Scholar]
- 15.Faulde, M. K., and R. G. Robbins. 2008. Tick infestation risk and Borrelia burgdorferi s.l. infection-induced increase in host-finding efficacy of female Ixodes ricinus under natural conditions. Exp. Appl. Acarol. 44:137-145. [DOI] [PubMed] [Google Scholar]
- 16.Ferquel, E., M. Garnier, J. Marie, C. Bernede-Bauduin, G. Baranton, C. Perez-Eid, and D. Postic. 2006. Prevalence of Borrelia burgdorferi sensu lato and Anaplasmataceae members in Ixodes ricinus ticks in Alsace, a focus of Lyme borreliosis endemicity in France. Appl. Environ. Microbiol. 72:3074-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fingerle, V., U. C. Schulte-Spechtel, E. Ruzic-Sabljic, S. Leonhard, H. Hofmann, K. Weber, K. Pfister, F. Strle, and B. Wilske. 5 July 2007. Epidemiological aspects and molecular characterization of Borrelia burgdorferi s.l. from southern Germany with special respect to the new species Borrelia spielmanii sp. nov. Int. J. Med. Microbiol. [Epub ahead of print.] doi: 10.1016/j.ijmm.2007.05.002. [DOI] [PubMed]
- 18.Foldvari, G., R. Farkas, and A. Lakos. 2005. Borrelia spielmanii erythema migrans, Hungary. Emerg. Infect. Dis. 11:1794-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Futse, J. E., M. W. Ueti, D. P. Knowles, Jr., and G. H. Palmer. 2003. Transmission of Anaplasma marginale by Boophilus microplus: retention of vector competence in the absence of vector-pathogen interaction. J. Clin. Microbiol. 41:3829-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray, J. S., H. Dautel, A. Estrada-Pena, O. Kahl, and E. Lindgren. 2009. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip. Perspect. Infect. Dis. 2009:593232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halos, L., T. Jamal, R. Maillard, F. Beugnet, A. Le Menach, H. J. Boulouis, and M. Vayssier-Taussat. 2005. Evidence of Bartonella sp. in questing adult and nymphal Ixodes ricinus ticks from France and co-infection with Borrelia burgdorferi sensu lato and Babesia sp. Vet. Res. 36:79-87. [DOI] [PubMed] [Google Scholar]
- 22.Halos, L., G. Vourc'h, V. Cotte, P. Gasqui, J. Barnouin, H. J. Boulous, and M. Vayssier-Taussat. 2006. Prevalence of Anaplasma phagocytophilum, Rickettsia sp. and Borrelia burgdorferi sensu lato DNA in questing Ixodes ricinus ticks from France. Ann. N. Y. Acad. Sci. 1078:316-319. [DOI] [PubMed] [Google Scholar]
- 23.Hartelt, K., R. Oehme, H. Frank, S. O. Brockmann, D. Hassler, and P. Kimmig. 2004. Pathogens and symbionts in ticks: prevalence of Anaplasma phagocytophilum (Ehrlichia sp.), Wolbachia sp., Rickettsia sp., and Babesia sp. in Southern Germany. Int. J. Med. Microbiol. 293(Suppl. 37):86-92. [DOI] [PubMed] [Google Scholar]
- 24.Herzberger, P., C. Siegel, C. Skerka, V. Fingerle, U. Schulte-Spechtel, A. van Dam, B. Wilske, V. Brade, P. F. Zipfel, R. Wallich, and P. Kraiczy. 2007. Human pathogenic Borrelia spielmanii sp. nov. resists complement-mediated killing by direct binding of immune regulators factor H and factor H-like protein 1. Infect. Immun. 75:4817-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hubalek, Z. 2009. Epidemiology of Lyme borreliosis. Curr. Probl. Dermatol. 37:31-50. [DOI] [PubMed] [Google Scholar]
- 26.Hubalek, Z., W. Sixl, J. Halouzka, and M. Mikulaskova. 1997. Prevalence of Francisella tularensis in Dermacentor reticulatus ticks collected in adjacent areas of the Czech and Austrian Republics. Cent. Eur. J. Public Health 5:199-201. [PubMed] [Google Scholar]
- 27.Hubschen, J. M., J. R. Kremer, S. De Landtsheer, and C. P. Muller. 2008. A multiplex TaqMan PCR assay for the detection of measles and rubella virus. J. Virol. Methods 149:246-250. [DOI] [PubMed] [Google Scholar]
- 28.Ishikura, M., S. Ando, Y. Shinagawa, K. Matsuura, S. Hasegawa, T. Nakayama, H. Fujita, and M. Watanabe. 2003. Phylogenetic analysis of spotted fever group rickettsiae based on gltA, 17-kDa, and rOmpA genes amplified by nested PCR from ticks in Japan. Microbiol. Immunol. 47:823-832. [DOI] [PubMed] [Google Scholar]
- 29.Jenkins, A., B. E. Kristiansen, A. G. Allum, R. K. Aakre, L. Strand, E. J. Kleveland, I. van de Pol, and L. Schouls. 2001. Borrelia burgdorferi sensu lato and Ehrlichia spp. in Ixodes ticks from southern Norway. J. Clin. Microbiol. 39:3666-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karbowiak, G. 2004. Zoonotic reservoir of Babesia microti in Poland. Pol. J. Microbiol. 53(Suppl.):61-65. [PubMed] [Google Scholar]
- 31.Klaus, C., B. Hoffmann, U. Hering, B. Mielke, K. Sachse, M. Beer, and J. Suss. 2009. Tick-borne encephalitis (TBE) virus prevalence and virus genome characterization in field-collected ticks (Ixodes ricinus) from risk, non-risk and former risk areas of TBE, and in ticks removed from humans in Germany. Clin. Microbiol. Infect. 16:238-244. [DOI] [PubMed] [Google Scholar]
- 32.Kocan, K. M., J. de la Fuente, E. F. Blouin, and J. C. Garcia-Garcia. 2004. Anaplasma marginale (Rickettsiales: Anaplasmataceae): recent advances in defining host-pathogen adaptations of a tick-borne rickettsia. Parasitology 129(Suppl.):S285-S300. [DOI] [PubMed] [Google Scholar]
- 33.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 34.Kurtenbach, K., S. De Michelis, S. Etti, S. M. Schafer, H. S. Sewell, V. Brade, and P. Kraiczy. 2002. Host association of Borrelia burgdorferi sensu lato—the key role of host complement. Trends Microbiol. 10:74-79. [DOI] [PubMed] [Google Scholar]
- 35.Maetzel, D., W. A. Maier, and H. Kampen. 2005. Borrelia burgdorferi infection prevalences in questing Ixodes ricinus ticks (Acari: Ixodidae) in urban and suburban Bonn, western Germany. Parasitol. Res. 95:5-12. [DOI] [PubMed] [Google Scholar]
- 36.Maggi, R. G., and E. B. Breitschwerdt. 2005. Potential limitations of the 16S-23S rRNA intergenic region for molecular detection of Bartonella species. J. Clin. Microbiol. 43:1171-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mansfield, K. L., N. Johnson, L. P. Phipps, J. R. Stephenson, A. R. Fooks, and T. Solomon. 2009. Tick-borne encephalitis virus—a review of an emerging zoonosis. J. Gen. Virol. 90:1781-1794. [DOI] [PubMed] [Google Scholar]
- 38.Michel, H., B. Wilske, G. Hettche, G. Gottner, C. Heimerl, U. Reischl, U. Schulte-Spechtel, and V. Fingerle. 2004. An ospA-polymerase chain reaction/restriction fragment length polymorphism-based method for sensitive detection and reliable differentiation of all European Borrelia burgdorferi sensu lato species and OspA types. Med. Microbiol. Immunol. 193:219-226. [DOI] [PubMed] [Google Scholar]
- 39.Misonne, M. C., G. Van Impe, and P. P. Hoet. 1998. Genetic heterogeneity of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected in Belgium. J. Clin. Microbiol. 36:3352-3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oehme, R., K. Hartelt, H. Backe, S. Brockmann, and P. Kimmig. 2002. Foci of tick-borne diseases in southwest Germany. Int. J. Med. Microbiol. 291(Suppl. 33):22-29. [DOI] [PubMed] [Google Scholar]
- 41.Pal, U., and E. Fikrig. 2003. Adaptation of Borrelia burgdorferi in the vector and vertebrate host. Microbes Infect. 5:659-666. [DOI] [PubMed] [Google Scholar]
- 42.Psaroulaki, A., D. Ragiadakou, G. Kouris, B. Papadopoulos, B. Chaniotis, and Y. Tselentis. 2006. Ticks, tick-borne rickettsiae, and Coxiella burnetii in the Greek Island of Cephalonia. Ann. N. Y. Acad. Sci. 1078:389-399. [DOI] [PubMed] [Google Scholar]
- 43.Rauter, C., and T. Hartung. 2005. Prevalence of Borrelia burgdorferi sensu lato genospecies in Ixodes ricinus ticks in Europe: a metaanalysis. Appl. Environ. Microbiol. 71:7203-7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rendi-Wagner, P. 2004. Risk and prevention of tick-borne encephalitis in travelers. J. Travel Med. 11:307-312. [DOI] [PubMed] [Google Scholar]
- 45.Richter, D., D. Postic, N. Sertour, I. Livey, F. R. Matuschka, and G. Baranton. 2006. Delineation of Borrelia burgdorferi sensu lato species by multilocus sequence analysis and confirmation of the delineation of Borrelia spielmanii sp. nov. Int. J. Syst. Evol. Microbiol. 56:873-881. [DOI] [PubMed] [Google Scholar]
- 46.RKI. 2008. FSME: Risikogebiete in Deutschland. Epidemiologisches Bull. 17:133-140. [Google Scholar]
- 47.Schwarz, A., W. A. Maier, T. Kistemann, and H. Kampen. 2009. Analysis of the distribution of the tick Ixodes ricinus L. (Acari: Ixodidae) in a nature reserve of western Germany using Geographic Information Systems. Int. J. Hyg. Environ. Health 212:87-96. [DOI] [PubMed] [Google Scholar]
- 48.Silaghi, C., J. Gilles, M. Hohle, V. Fingerle, F. T. Just, and K. Pfister. 2008. Anaplasma phagocytophilum infection in Ixodes ricinus, Bavaria, Germany. Emerg. Infect. Dis. 14:972-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silaghi, C., J. Gilles, M. Hohle, I. Pradel, F. T. Just, V. Fingerle, H. Kuchenhoff, and K. Pfister. 2008. Prevalence of spotted fever group rickettsiae in Ixodes ricinus (Acari: Ixodidae) in southern Germany. J. Med. Entomol. 45:948-955. [DOI] [PubMed] [Google Scholar]
- 50.Skarpaas, T., I. Golovljova, S. Vene, L. Unn, H. Sjursen, A. Plyusnin, and A. Lundkvist. 2006. Tickborne encephalitis virus, Norway and Denmark. Emerg. Infect. Dis. 12:1136-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skotarczak, B. 2009. Adaptation factors of Borrelia for host and vector. Ann. Agric. Environ. Med. 16:1-8. [PubMed] [Google Scholar]
- 52.Smetanova, K., K. Schwarzova, and E. Kocianova. 2006. Detection of Anaplasma phagocytophilum, Coxiella burnetii, Rickettsia spp., and Borrelia burgdorferi s. l. in ticks, and wild-living animals in western and middle Slovakia. Ann. N. Y. Acad. Sci. 1078:312-315. [DOI] [PubMed] [Google Scholar]
- 53.Sprong, H., P. R. Wielinga, M. Fonville, C. Reusken, A. H. Brandenburg, F. Borgsteede, C. Gaasenbeek, and J. W. van der Giessen. 2009. Ixodes ricinus ticks are reservoir hosts for Rickettsia helvetica and potentially carry flea-borne Rickettsia species. Parasit. Vectors 2:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stark, K., M. Niedrig, W. Biederbick, H. Merkert, and J. Hacker. 2009. Climate changes and emerging diseases. What new infectious diseases and health problem can be expected? Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 52:699-714. (In German.) [DOI] [PubMed] [Google Scholar]
- 55.Thorin, C., E. Rigaud, I. Capek, G. Andre-Fontaine, B. Oster, G. Gastinger, and G. Abadia. 2008. Seroprevalence of Lyme borreliosis and tick-borne encephalitis in workers at risk, in eastern France. Med. Mal. Infect. 38:533-542. (In French.) [DOI] [PubMed] [Google Scholar]
- 56.To, H., N. Kako, G. Q. Zhang, H. Otsuka, M. Ogawa, O. Ochiai, S. V. Nguyen, T. Yamaguchi, H. Fukushi, N. Nagaoka, M. Akiyama, K. Amano, and K. Hirai. 1996. Q. fever pneumonia in children in Japan. J. Clin. Microbiol. 34:647-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vorou, R. M., V. G. Papavassiliou, and S. Tsiodras. 2007. Emerging zoonoses and vector-borne infections affecting humans in Europe. Epidemiol. Infect. 135:1231-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]