Abstract
Until recently, Amblyomma maculatum (the Gulf Coast tick) had garnered little attention compared to other species of human-biting ticks in the United States. A. maculatum is now recognized as the principal vector of Rickettsia parkeri, a pathogenic spotted fever group rickettsia (SFGR) that causes an eschar-associated illness in humans that resembles Rocky Mountain spotted fever. A novel SFGR, distinct from other recognized Rickettsia spp., has also been detected recently in A. maculatum specimens collected in several regions of the southeastern United States. In this study, 198 questing adult Gulf Coast ticks were collected at 4 locations in Florida and Mississippi; 28% of these ticks were infected with R. parkeri, and 2% of these were infected with a novel SFGR. Seventeen isolates of R. parkeri from individual specimens of A. maculatum were cultivated in Vero E6 cells; however, all attempts to isolate the novel SFGR were unsuccessful. Partial genetic characterization of the novel SFGR revealed identity with several recently described, incompletely characterized, and noncultivated SFGR, including “Candidatus Rickettsia andeanae” and Rickettsia sp. Argentina detected in several species of Neotropical ticks from Argentina and Peru. These findings suggest that each of these “novel” rickettsiae represent the same species. This study considerably expanded the number of low-passage, A. maculatum-derived isolates of R. parkeri and characterized a second, sympatric Rickettsia sp. found in Gulf Coast ticks.
Amblyomma maculatum (the Gulf Coast tick) is an aggressive, human- and animal-biting ixodid tick that is distributed widely across the southeastern United States (5, 19). Until recently, A. maculatum had never been associated directly with any known tick-borne infection of humans; however, A. maculatum is now recognized as a vector of Rickettsia parkeri, a spotted fever group rickettsia (SFGR) that causes a disease similar to, but milder than, Rocky Mountain spotted fever (RMSF). Since the recognition of the index patient in 2002 (37), more than 20 cases of R. parkeri rickettsiosis have been identified in Alabama, Florida, Kentucky, Maryland, Mississippi, North Carolina, South Carolina, Virginia, and Texas (12, 38, 57; CDC, unpublished data). In 1923, Cowdry (11) described minute intracellular bacteria, apparently rickettsiae, in the tissues and eggs of female A. maculatum ticks collected in Jackson County, MS. Parker et al. (41) subsequently isolated R. parkeri from Gulf Coast ticks collected in southeastern Texas in 1937. Since that report, spotted fever group (SFG) rickettsiae have been detected in A. maculatum specimens collected in at least 8 states (27, 42, 44, 55), but the only characterized Rickettsia sp. associated with the Gulf Coast tick is R. parkeri and the only extant strain of R. parkeri obtained from A. maculatum was isolated in 1948 (4).
A novel and incompletely characterized SFGR, distinct from other recognized Rickettsia spp., was detected recently in A. maculatum specimens collected in Georgia, Florida, and Mississippi (55). In this report, we describe further genetic characterization of this novel rickettsia and cultivation of 17 contemporary strains of R. parkeri obtained from Gulf Coast ticks collected in Florida and Mississippi.
MATERIALS AND METHODS
Tick collection and processing.
Questing adult Gulf Coast ticks, collected from vegetation by using flannel cloth flags, were obtained from August 2005 to August 2007 from Tate's Hell State Forest (THSF), Franklin County, FL; Sopchoppy, Wakulla County, FL; Starkville, Oktibbeha County, MS; and Grand Bay National Wildlife Refuge (GBNWR), Jackson County, MS (see Table 1). The collection sites were characterized by palustrine flatwood or savannah habitats containing predominantly slash pine (Pinus elliottii), longleaf pine (Pinus palustris), saw palmetto (Serenoa repens), wiregrass (Aristida stricta), and mesic woody shrubs. Ticks were transported as live specimens to the laboratory and washed sequentially in 3 solutions, 2% Micro-Chem Plus (National Chemical Laboratories of PA, Inc., Philadelphia, PA), 10.5% sodium hypochlorite, and 3% hydrogen peroxide. The ticks were agitated gently in each solution for ∼5 min and rinsed in sterile distilled water after the final wash. Individual specimens were blotted lightly on sterile filter paper and bisected longitudinally; one half of each tick was placed in 0.5 ml of Eagle's minimum essential medium with Earle's salts (MEM) and frozen at −80°C, and the other half was processed for DNA extraction.
TABLE 1.
Collection sites for questing adult Gulf Coast ticks (A. maculatum) obtained in Florida and Mississippi from 2005 to 2007 and percentages of ticks infected with spotted fever group rickettsiae
| State | County | Year collected | No. of adult ticks tested |
No. positive (% of total) for: |
||
|---|---|---|---|---|---|---|
| Males | Females | R. parkeri | Novel Rickettsia sp.a | |||
| Florida | Franklin | 2005 | 11 | 16 | 3 (11) | 1 (4) |
| 2007 | 45 | 47 | 24 (26) | 1 (1) | ||
| Wakulla | 2006 | 8 | 1 | 1 (11) | 0 | |
| Mississippi | Oktibbeha | 2007 | 2 | 6 | 2 (25) | 0 |
| Jackson | 2007 | 28 | 34 | 25 (40) | 3 (5) | |
| Total | 94 | 104 | 55 (28) | 5 (2) | ||
Molecular detection of SFG rickettsiae in ticks.
DNA was extracted from one half of each bisected tick, as described previously (31), and eluted in 100 μl (final volume). DNA extracts were screened by using a direct PCR assay with primers 190-70 and 190-701 (47) to amplify a 632-bp segment of the rickettsial outer membrane protein A gene (ompA). All tick extracts that produced an amplicon of the expected size were tested subsequently by using a real-time SYBR green PCR assay designed to specifically amplify a 408-bp segment of the ompA gene of the novel SFGR. Five microliters of tick extract was combined with core reagents of a SYBR brilliant green kit (Stratagene, La Jolla, CA) in a 50-μl reaction mixture containing primers Rx-190-F (5′-GTGATGTTGCTGAGTTCG) and Rx-190-R (5′-TTATCTTTGCCGGGGTTA), each at a final concentration of 300 nM. The thermal cycler parameters were activation at 95°C for 10 min, followed by 40 cycles of 95°C for 30 s, 55°C for 60 s, and 72°C for 90 s. The parameters for dissociation curves included denaturation at 95°C for 60 s, followed by 55°C for 30 s and then a ramp from 55°C to 95°C at a rate of 0.2°C/s with continuous collection of data. DNA extracts of previously identified, novel SFGR-infected A. maculatum ticks (55) were used as positive-control samples to calibrate the assay. The negative controls included DNA samples of A. maculatum ticks infected with R. parkeri and a Vero E6 cell culture infected with R. rickettsii.
Amplicons from ompA-positive ticks were also evaluated by using restriction fragment length polymorphism (RFLP) analysis (49). For each sample, 5.0 μl of PCR product obtained from the ompA screening assay was incubated with 5 U of AluI endonuclease (New England BioLabs, Inc., Ipswich, MA) and 1.0 μl of 10× enzyme buffer for 6 h at 37°C. Digested products were separated by using a 4% agarose gel in Tris-acetate-EDTA buffer and stained with ethidium bromide.
Cell culture isolation.
All specimens that tested positive for the novel SFGR by the SYBR green assay and a subset of R. parkeri-positive ticks were evaluated by using cell culture. The remaining half of each identified tick was thawed and triturated with a sterile scalpel blade in 0.5 ml of MEM. Each triturate was inoculated onto a semiconfluent monolayer of Vero E6 cells in a T25 tissue culture flask containing 5 ml of MEM with 500 mmol l-glutamine, 5% tetracycline-free fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin sulfate, and 0.25 μg/ml amphotericin B. Cell cultures were incubated at 34.5°C in an air atmosphere containing 5% CO2. The medium and tick triturate were removed after ∼24 h and replaced with 5.0 ml of fresh cell culture medium containing no antibiotics. The medium was changed approximately once a week thereafter. Cultures were monitored for evidence of infection by examining cytospin preparations of ∼0.1 ml of cell culture medium fixed in absolute methanol and stained with 0.01% acridine orange. Cell cultures were examined weekly for a minimum of 4 weeks. The identity of each isolate was confirmed by ompA PCR and RFLP analysis or sequencing of the amplicon.
Electron microscopy of R. parkeri.
Vero E6 cells infected with a tick-derived isolate of R. parkeri were scraped from a cell culture flask, pelleted by centrifugation, and fixed in 2.5% glutaraldehyde at room temperature for 1 h. The fixed pellet was embedded in 2% agarose and placed in buffered 1% osmium tetroxide at 4°C for 30 min. The specimen was then dehydrated using series of a graded ethanol concentrations and propylene oxide and embedded in an Epon substitute-Araldite mixture. Sections were stained with 4% uranyl acetate and Reynold's lead citrate.
Phylogenetic analysis of the novel SFGR.
Additional genetic characterization of the novel SFGR was performed by using PCR assays targeting segments of multiple genes. A nested PCR assay was used to amplify a 535-bp segment of ompA, as described previously (55). An 811-bp segment of the ompB gene was amplified directly by using primers 120-2788 and 120-3599 (52). For the citrate synthase (gltA) gene, direct PCR amplification using three different primer sets was used to assemble a nearly complete, 1,191-bp sequence of the gene. Primers CS-78 and CS-323 (23) were used to amplify a 401-bp segment at the 5′ end; primers CS-239 and CS-1069 (24) were used to amplify 830 bp of sequence that overlapped the 3′ end of the leading segment and the 5′ end of the terminal segment; and primers Rp877p and CS1273f (50) were used to amplify a 476-bp segment at the 3′ end of gltA. Primers rpoB-Fav (5′-CGTGTTGAAGGCGGTAATT-3′) and rpoB-Rav (5′-AAGAAAGCCACAAGCACGTT-3′) were used to amplify directly a 512-bp segment of the RNA polymerase β-subunit (rpoB) gene. The 25-μl reaction mixtures for the rpoB assay contained primers at a final concentration of 1 μM, deoxynucleoside triphosphates (dNTPs) at a concentration of 200 μM, 1.5 mM MgCl2, 0.625 U of GoTaq polymerase (Promega, Madison, WI), and 5 μl of template. The thermal cycler program for the rpoB assay consisted of an initial denaturing step at 94°C for 3 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 1 min. The program ended with a 5-min elongation step at 72°C.
For each of these assays, distilled water was used as a negative-control sample, and DNA extracts of R. parkeri, R. akari, or R. amblyommii were used as positive-control samples. All amplified gene segments, excluding primers, were compared to sequences in the GenBank database by using the Basic Local Alignment Search Tool (National Center for Biotechnology Information; www.ncbi.nlm.nih.gov). Gene sequences were aligned by using CLUSTAL W software (26), and phylogenetic relationships were inferred from alignments of ompB and gltA DNA sequences using MEGA 4 software (21) and the parsimony and neighbor-joining methods.
RESULTS
Frequency of SFGR infections in A. maculatum.
Molecular evidence of infection with SFGR was obtained for 60 (30%) of 198 adult A. maculatum ticks collected at 4 sites in northwest Florida and southeast Mississippi in 2005 to 2007 (Table 1). A novel SFGR was detected using the SYBR green assay in 2 (2%) of 119 ticks collected at THSF and in 3 (5%) of 62 ticks collected at GBNWR (cycle threshold [CT] values, 19.0 to 21.9). Three (60%) of the 5 ticks containing DNA of the novel SFGR were female. AluI digests of the novel SFGR ompA amplicons produced one large and slightly blurred band, consistent with the predicted RFLP pattern for this rickettsia, comprising three closely spaced fragments of 205, 210, and 214 bp.
DNA of R. parkeri was detected in each of the 55 remaining ticks that were negative as determined by the SYBR green assay (Table 1). AluI digests of the ompA amplicons consistently produced an RFLP pattern unique to R. parkeri comprising five distinct bands (55, 86, 124, 153, and 214 bp) (49). Three (11%) of 27 ticks collected at the High Bluff tract in THSF in 2005 and 24 (26%) of 92 ticks collected at the same site in 2007 were infected with R. parkeri; 13 (54%) of the infected ticks from this site were female. Twenty-five (40%) of 62 ticks collected at GBNWR in 2007 were infected with R. parkeri; 14 (56%) of the infected ticks from this site were female. R. parkeri was also detected in smaller collections of ticks from Sopchoppy, FL, in 2006 (11%) and Starkville, MS, in 2007 (25%). No RFLP pattern for the R. parkeri-positive or novel SFGR-positive samples suggested that there was simultaneous infection with both Rickettsia spp.
Cultivation of R. parkeri in Vero E6 cells.
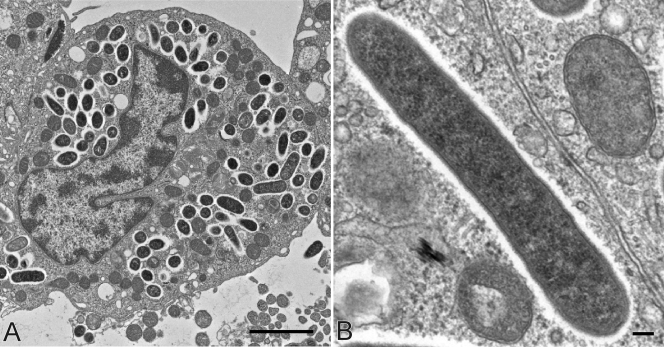
Seventeen stable isolates of R. parkeri were obtained from individual ticks collected in Florida (Tate's Hell, High Bluff, Sandbank Creek, Longleaf, SR-65, Apalachicola, Cash Bayou, and TH07-94) and Mississippi (Oktibbeha, Moss Point, Bayou Heron, MS07-44, I-10, Grand Bay, Franklin Creek, Escatawpa, and MS07-22). In most of the primary cultures small rods were detected by acridine orange staining within 4 to 7 days after inoculation of the tick triturate. In cultured cells, R. parkeri rickettsiae were distributed freely in the cytoplasm and appeared to be coccoid or rod-shaped bacteria that were 1.0 μm to 2.7 μm long (mean, 1.5 μm; median, 1.4 μm) and 0.2 μm to 0.4 μm wide (mean and median, 0.4 μm) (Fig. 1A) and had an electron-lucent halo or “slime” layer adjacent to the central beaded microcapsular layer and an internal trilaminar cell wall (Fig. 1B). No intranuclear rickettsiae were identified in any of the >500 infected cells examined by electron microscopy.
FIG. 1.
Electron microscopy of the R. parkeri Moss Point isolate in Vero E6 cells stained with uranyl acetate and lead citrate. (A) Multiple intracellular rickettsiae free in the cytoplasm of an infected cell. Bar = 2 μm. (B) Bacillary form of R. parkeri, showing an outer electron-lucent halo or “slime” layer adjacent to the central beaded microcapsular layer and the internal trilaminar cell wall. Bar = 100 nm.
All attempts to isolate the novel SFGR in Vero E6 cells incubated at 34.5°C were unsuccessful. Similar attempts to cultivate this Rickettsia sp. at 32°C using Vero E6 cells and the arthropod cell lines ISE6 and C6/36 were also unsuccessful (data not shown). No isolates of R. parkeri were obtained in any cell culture inoculated with a tick infected with the novel SFGR.
Phylogenetic analyses of the novel SFGR.
Extracts of ticks containing DNA of the novel SFGR as determined by the SYBR green assay were tested by performing additional PCR assays. Partial sequences of each of the genes evaluated were identical for tick specimens collected in Florida and Mississippi. Complete identity was observed with segments of several other partial ompA sequences available from published sources, including sequences amplified from field-collected A. maculatum (GenBank accession no. EF372578) reported in our previous study (55), and with an overlapping 402-bp segment of the Rickettsia sp. Argentina sequence (GenBank accession no. EF451004) amplified from Amblyomma parvum ticks collected in Córdoba Province, Argentina (36). Complete identity was also observed with partial sequences from several unpublished sources, including sequences amplified from colony-reared A. maculatum (GenBank accession no. EF524203) and from a field-collected A. maculatum tick from Texas (GenBank accession no. EF689729), and with an overlapping 424-bp ompA sequence (GenBank accession no. EU826513) amplified from an Amblyomma pseudoconcolor specimen collected in northern Argentina. The highest levels of identity of the amplified segment of the ompA sequence with corresponding sequences of previously described species of SFG rickettsiae were <95%; these sequences included sequences of R. massiliae (GenBank accession no. DQ212707), R. rhipicephali (GenBank accession no. U43803), R. raoultii (GenBank accession no. DQ365801), R. montanensis (GenBank accession no. U43801), R. aeschlimannii (GenBank accession no. DQ235777), and “Candidatus Rickettsia amblyommii” (GenBank accession no. EF68973). Analysis of the partial ompB sequence (GenBank accession no. GU131157) revealed complete identity with the overlapping 725-bp segment of the “Candidatus Rickettsia andeanae” sequence (GenBank accession no. AY652981), a sequence detected previously in A. maculatum and Ixodes boliviensis ticks collected in Peru (6), and ∼96% to 97% identity with the overlapping segments of the sequences of several other SFG rickettsiae, including R. massiliae (GenBank accession no. CP000683), R. rhipicephali (GenBank accession no. AF123719), R. raoultii (DQ365798), R. aeschlimannii (GenBank accession no. AF123705), and “Candidatus Rickettsia amblyommii” (GenBank accession no. FJ455415).
The concatenated sequence of the gltA gene (GenBank accession no. GU131156) showed complete identity with an overlapping 1,064-bp segment of the Rickettsia sp. Argentina gltA sequence (GenBank accession no. EF451001) (36) and an overlapping 1,065-bp segment of the “Candidatus Rickettsia andeanae” gltA sequence (GenBank accession no. GU169050) and 99% identity with corresponding segments of the sequences of several other SFG rickettsiae, including R. raoultii (GenBank accession no. DQ365804), R. aeschlimannii (GenBank accession no. DQ235776), and R. massiliae (GenBank accession no. CP000683). A 427-bp segment of the rpoB gene (GenBank accession no. GU131158) showed 99% identity with overlapping segments of the rpoB sequences of many SFG rickettsiae, including R. massiliae (GenBank accession no. CP000683) and R. rhipicephali (GenBank accession no. AF440728).
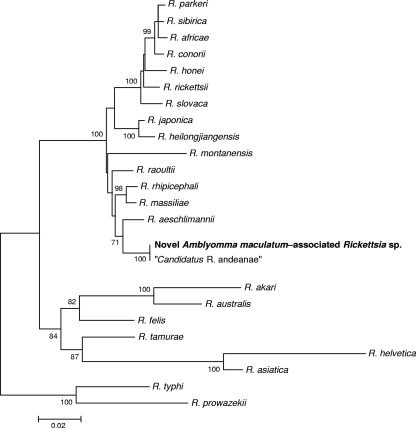
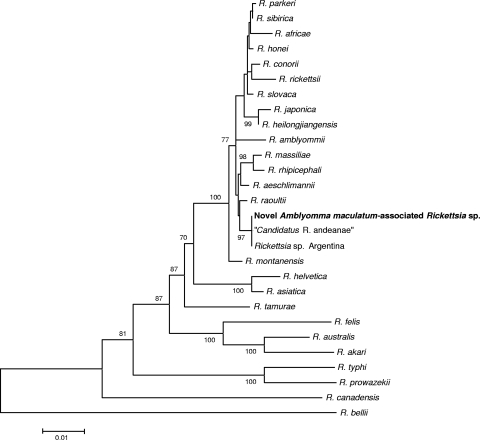
Phylogenetic analyses of ompB (Fig. 2) and gltA (Fig. 3) sequences of the novel SFGR placed this bacterium in a clade of rifampin-resistant Rickettsia spp. comprising the R. massiliae subgroup (51). This new rickettsia appears to be a unique species (14) and to be identical to three other partially characterized SFG rickettsiae detected previously in at least 4 species of Neotropical ticks collected in Peru and Argentina (6, 20, 36).
FIG. 2.
Unrooted dendrogram showing the phylogenetic position of a novel spotted fever group Rickettsia sp. detected in A. maculatum ticks, inferred from comparison of 725 nucleotides in the outer membrane protein B gene (ompB) sequence aligned with complete or partial ompB sequences available in the GenBank database by using the neighbor-joining method. Bootstrap values of >70% for 1,000 replicates are indicated at the nodes. Bar = 2% nucleotide sequence divergence.
FIG. 3.
Unrooted dendrogram showing the phylogenetic position of a novel spotted fever group Rickettsia sp. detected in A. maculatum ticks, inferred from comparison of 1,095 nucleotides of the citrate synthase gene (gltA) sequence aligned with complete or partial gltA sequences available in GenBank by using the neighbor-joining method. Bootstrap values of >70% for 1,000 replicates are indicated at the nodes. Bar = 1% nucleotide sequence divergence.
DISCUSSION
In this investigation, we obtained 17 isolates of R. parkeri from Gulf Coast ticks collected in Florida and Mississippi and identified a second, phylogenetically distinct and as-yet-uncultivable SFGR that resides in A. maculatum ticks in the United States and in several other species of ixodid ticks in South America. From 1937 to 1974, investigators cultivated at least 4 stable isolates of R. parkeri from Gulf Coast ticks (strains 6-1, C, 62-♀1, and Maculatum 20), which were often obtained from pooled samples comprising multiple specimens collected in Alabama, Mississippi, and Texas (4, 43-45, 48); of these historical isolates, only the extensively passaged Maculatum 20 strain remains in reference collections around the world. The isolates of R. parkeri described in this report originated from individual specimens of A. maculatum and represent the first strains obtained from Gulf Coast ticks in >35 years; our study greatly increased the number of low-passage, A. maculatum-derived isolates of R. parkeri recovered from single tick specimens.
Some isolates of other Rickettsia species, following serial passage in cell culture or animals, exhibit changes in the genotype or virulence and differ markedly from the original wild-type SFGR (1, 2, 13, 15, 41). The availability of a large panel of minimally passaged isolates of R. parkeri that includes South American strains obtained from Amblyomma triste (35, 53) and R. parkeri-like rickettsiae obtained from Amblyomma dubitatum and Amblyomma nodosum (23, 34) provides investigators with opportunities to more accurately evaluate the molecular and phenotypic characteristics of this SFGR (2, 46).
The morphological appearance and intracellular location of the tick-derived Moss Point isolate of R. parkeri examined in this study were identical to the morphological appearance and intracellular location of the Portsmouth strain, which was isolated from a human skin biopsy specimen, determined previously by electron microscopy (37); however, in neither evaluation were rickettsiae identified in nuclei of Vero E6 cells, despite close inspection of hundreds of infected cells. Previously, investigators using light microscopy reported finding intranuclear R. parkeri rickettsiae in yolk sacs of infected chicken embryos (25); nonetheless, we have not found these rickettsiae in the nuclei of infected Vero E6 cells. The capacity of SFG rickettsiae to invade and replicate within host cell nuclei has been documented by electron microscopy for many species, including R. rickettsii, R. japonica, and R. africae (7, 16, 56), and this is considered a defining feature of this group; however, electron microscopy studies of cultured cells infected with other SFG rickettsiae, including “Candidatus Rickettsia amblyommii,” R. peacockii, and R. raoultii, have not found intranuclear localization of these rickettsiae (29, 30, 54). The ability of SFG rickettsiae to directionally polymerize host cell actin has been suggested to be a mechanism that is used by these bacteria to penetrate host cell nuclei (18). Nonetheless, R. parkeri directionally polymerizes F-actin in Vero cells (18) and exhibits robust intracellular motility in several different cell lines (M. Welch and G. Baldridge, personal communications). Invasion of host cell nuclei by SFG rickettsiae might also depend on the stage of infection or the type of cell infected (3, 30). Additional studies that examine the kinetics and physiology of host cell infection by R. parkeri are needed to resolve this paradox.
We identified infections with R. parkeri in approximately 10% to 40% of questing adult A. maculatum ticks collected at 4 locations in 2 states during a 3-year period. Although precise estimates of the prevalence of infection cannot be obtained from the data, it is evident from this investigation and from our previous survey (55) that R. parkeri occurs commonly in widely separated populations of Gulf Coast ticks. Indeed, the consistently high frequency with which this agent is detected in A. maculatum ticks is remarkable when it is compared with the occurrence of other pathogenic SFG rickettsiae found in the United States. For example, R. rickettsii (the agent of RMSF) is rarely found in >1% of ticks surveyed for this rickettsia, and generally the prevalence is considerably lower (33, 39). In 1955, Philip and White (44) were surprised to discover that no isolates of R. rickettsii were obtained from any of >3,200 Amblyomma, Dermacentor, and Ixodes spp. tick specimens collected in Jackson County, MS, despite multiple reports of serologically confirmed “RMSF” in this county during the previous 10 years. In contrast, these investigators repeatedly isolated R. parkeri from pooled specimens of A. maculatum collected at several sites in the same county.
During a previous survey (55), we detected a unique ompA sequence in specimens of A. maculatum collected in several southeastern states that suggested that there is a Rickettsia species that was not recognized previously in the United States. During the present investigation, we identified the same unique ompA sequence in Gulf Coast tick specimens collected at additional sites, and analyses of other genes of this Rickettsia sp. revealed identity to several recently deposited sequences amplified from various Amblyomma tick species in South America, including sequences of “Candidatus Rickettsia andeanae” from A. maculatum (6, 20), Rickettsia sp. Argentina from A. parvum (36), and an unidentified SFGR from A. pseudoconcolor. By evaluating multiple gene targets and comparing identical regions of the gene of interest, identities could be established in order to collectively link these sequences to a common SFGR; however, full species status for this novel SFGR requires cultivation and more thorough genomic characterization.
Other Rickettsia spp. and rickettsia-like bacteria detected in ticks have proven to be difficult to cultivate. For many years, attempts to isolate R. peacockii, an endosymbiont associated with Dermacentor andersoni, were unsuccessful; these attempts included inoculation of guinea pigs, meadow voles, mice, embryonated chicken eggs, and at least 7 different cell lines (8, 32). R. peacockii was eventually cultivated when investigators established a cell line from embryonic tissues of laboratory-reared D. andersoni (DAE100) that was persistently infected with R. peacockii (54). Cell-free R. peacockii obtained from persistently infected DAE100 cell cultures can be propagated in many other cell lines of arthropod origin (22); however, a primary isolate of R. peacockii has not been obtained by using any cell line other than DAE100. Our efforts to isolate the novel SFGR in broadly permissive cell lines of primate, mosquito, or tick origin were unsuccessful; cultivation of this novel SFGR may eventually require a strategy similar to that used to obtain R. peacockii in continuous culture. It is also possible that the disinfectants used to treat the tick exoskeleton penetrated the integument and inactivated the novel SFGR. The disinfection protocol was required to prevent fungal overgrowth, which consistently hampered our previous attempts to isolate SFG rickettsiae from A. maculatum ticks when lower concentrations of the antimicrobial solutions were used. Nonetheless, we were able to obtain 17 consecutive isolates of R. parkeri from other ticks prepared in the same way, suggesting that our disinfectant protocol did not affect the viability of rickettsiae within the tissues of these ticks.
R. parkeri and the novel SFGR occur sympatrically in populations of Gulf Coast ticks in the southeastern United States (55; this study); however, we did not detect simultaneous infections with both of these rickettsiae in any of ∼200 ticks evaluated in our study. Naturally acquired coinfection of ticks with multiple Rickettsia spp. has been described infrequently (9, 58). Laboratory studies have suggested that Dermacentor variabilis and D. andersoni ticks are unable to transovarially maintain infections with more than one SFGR; i.e., ovarian infection with the primary infecting species blocks subsequent vertical transmission of a second infecting Rickettsia sp. (8, 28). However, it may be possible for a transovarially infected tick to acquire a different Rickettsia sp. during feeding and maintain both agents horizontally through the nymphal and adult stages (8). These data and the apparent infrequency with which the novel SFGR occurs in A. maculatum specimens in several southeastern states compared to the frequency of infection with R. parkeri (55; this study) suggest that R. parkeri establishes residence in Gulf Coast ticks more successfully and may limit the number of infections with the novel SFGR by transovarial interference; nonetheless, there are currently no experimental data that demonstrate that either Rickettsia sp. is able to exclude infection by the other organism. Similarly, nothing is known about the impact of either Rickettsia sp. on the fitness of infected A. maculatum ticks. Considerable work to examine these interactions and the pathogenic potential, if any, of the novel SFGR remains.
For almost 100 years, A. maculatum has been recognized as an aggressive human-biting tick (19). Surprisingly, the only direct association between Gulf Coast ticks and human health prior to 2004 was an isolated case of tick paralysis (40). It now appears that R. parkeri and a newly recognized SFGR are distributed widely in A. maculatum and many other Amblyomma sp. ticks in the Western Hemisphere (6, 10, 17, 20, 31, 35, 36, 53, 55). Studies that better characterize the phenotypic characteristics, microbiology, and ecology of R. parkeri and this novel SFGR will undoubtedly provide greater perspective to these initial discoveries.
Acknowledgments
We thank Kristine Edwards (Mississippi State University) for assistance with collecting ticks; Uli Munderloh (University of Minnesota) for providing ISE6 cells; Allen Richards and Ju Jiang (Naval Medical Research Center) for providing complete gltA sequence data for “Candidatus Rickettsia andeanae”; Sherif Zaki (CDC) and Didier Raoult (Unité des Rickettsies) for their support and encouragement during this investigation; and two anonymous reviewers for their constructive comments.
The findings and conclusions presented in this work are those of the authors and do not necessarily represent the views of the U.S. Department of Health and Human Services.
Footnotes
Published ahead of print on 5 March 2010.
REFERENCES
- 1.Baldridge, G. D., N. Y. Burkhardt, R. F. Felsheim, T. J. Kurtti, and U. G. Munderloh. 2008. Plasmids of the pRH/pRF family occur in diverse Rickettsia species. App. Environ. Microbiol. 74:645-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldridge, G. D., N. Y. Burkhardt, M. B. Labruna, R. C. Pacheco, C. D. Paddock, P. C. Williamson, P. M. Billingsley, R. F. Felsheim, T. J. Kurtti, and U. G. Munderloh. 2010. Wide dispersal and possible multiple origins of low-copy-number plasmids in Rickettsia species associated with blood-feeding arthropods. Appl. Environ. Microbiol. 76:1718-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balraj, P., K. El Karkouri, G. Vestris, L. Espinosa, D. Raoult, and P. Renesto. 2008. RickA expression is not sufficient to promote actin-based motility of Rickettsia raoultii. PLoS One 3:e2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell, E. J., and E. G. Pickens. 1953. A toxic substance associated with the rickettsias of the spotted fever group. J. Immunol. 70:461-472. [PubMed] [Google Scholar]
- 5.Bishopp, F. C., and H. Hixson. 1936. Biology and economic importance of the Gulf Coast tick. J. Econ. Entomol. 29:1068-1076. [Google Scholar]
- 6.Blair, P. J., J. Jiang, G. B. Schoeler, C. Moron, E. Anaya, M. Cespedes, C. Cruz, V. Felices, C. Guevara, L. Mendoza, P. Villaseca, J. W. Sumner, A. L. Richards, and J. G. Olson. 2004. Characterization of spotted fever group rickettsiae in flea and tick specimens from northern Peru. J. Clin. Microbiol. 42:4961-4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgdorfer, W., R. L. Anacker, R. G. Bird, and D. S. Bertram. 1968. Intranuclear growth of Rickettsia rickettsii. J. Bacteriol. 96:1415-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgdorfer, W., S. F. Hayes, and A. J. Mavros. 1981. Nonpathogenic rickettsiae in Dermacentor andersoni: a limiting factor for the distributon of Rickettsia rickettsii, p. 585-594. In W. Burgdorfer and R. L. Anacker (ed.), Rickettsiae and rickettsial diseases. Academic Press, New York, NY.
- 9.Carmichael, J. R., and P. A. Fuerst. 2006. A rickettsial mixed infection in a Dermacentor variabilis tick from Ohio. Ann. N. Y. Acad. Sci. 1078:334-337. [DOI] [PubMed] [Google Scholar]
- 10.Cohen, S. B., M. J. Yabsley, L. E. Garrison, J. D. Freye, B. G. Dunlap, J. R. Dunn, D. G. Mead, T. F. Jones, and A. C. Moncayo. 2009. Rickettsia parkeri in Amblyomma americanum ticks, Tennessee and Georgia, U. S. A. Emerg. Infect. Dis. 15:1471-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowdry, E. V. 1923. The distribution of rickettsia in the tissues of insects and arachnids. J. Exp. Med. 37:431-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cragun, W. C., B. L. Bartlett, M. W. Ellis, A. Z. Hoover, S. K. Tyring, N. Mendoza, T. J. Vento, W. L. Nicholson, M. E. Eremeeva, J. P. Olano, R. P. Rapini, and C. D. Paddock. The expanding spectrum of eschar-associated rickettsioses in the United States. Arch. Dermatol., in press. [DOI] [PubMed]
- 13.Ellison, D. W., T. R. Clark, D. E. Sturdevant, K. Virtaneva, S. F. Porcella, and T. Hackstadt. 2008. Genomic comparison of virulent Rickettsia rickettsii Sheila Smith and avirulent Rickettsia rickettsii Iowa. Infect. Immun. 76:542-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fournier, P. E., J. S. Dumler, G. Greub, J. Zhang, Y. Wu, and D. Raoult. 2003. Gene sequence-based criteria for identification of new Rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J. Clin. Microbiol. 41:5456-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fournier, P. E., L. Belghazi, C. Robert, K. Elkarkouri, A. L. Richards, G. Greub, F. Collyn, M. Ogawa, A. Portillo, J. A. Oteo, A. Psaroulaki, I. Bitam, and D. Raoult. 2008. Variations in plasmid content in Rickettsia felis. PLoS One 3:e2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fournier, P. E., K. El Karkouri, Q. Leroy, C. Robert, B. Giumelli, P. Renesto, C. Socolovschi, P. Parola, S. Audic, and D. Raoult. 2009. Analysis of the Rickettsia africae genome reveals that virulence acquisition in Rickettsia species may be explained by genome reduction. BMC Genomics 10:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goddard, J., and B. R. Norment. 1986. Spotted fever group rickettsiae in the lone star tick, Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 23:465-472. [DOI] [PubMed] [Google Scholar]
- 18.Heinzen, R. A., S. F. Hayes, M. G. Peacock, and T. Hackstadt. 1993. Directional actin polymerization associated with spotted fever group rickettsia infection of Vero cells. Infect. Immun. 61:1926-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter, W. D., and F. C. Bishopp. 1911. Some of the more important ticks of the United States, p. 219-230. In J. A. Arnold (ed.), Yearbook of the United States Department of Agriculture, 1910. U. S. Department of Agriculture, Washington, DC.
- 20.Jiang, J., P. J. Blair, V. Felices, C. Moron, M. Cespedes, E. Anaya, G. B. Schoeler, J. W. Sumner, J. G. Olson, and A. L. Richards. 2005. Phylogenetic analysis of a novel molecular isolate of spotted fever group rickettsiae from northern Peru. Ann. N. Y. Acad. Sci. 1063:337-342. [DOI] [PubMed] [Google Scholar]
- 21.Kumars, S., J. Dudley, M. Nei, and K. Tamura. 2008. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinform. 9:299-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtti, T. J., J. A. Simser, G. D. Baldridge, A. T. Palmer, and U. G. Munderloh. 2005. Factors influencing in vitro growth of Rickettsia peacockii (Rickettsiales: Rickettsiaceae), an endosymbiont of the Rocky Mountain wood tick, Dermacentor andersoni (Acari, Ixodidae). J. Invertebr. Pathol. 90:177-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labruna, M. B., T. Whitworth, M. C. Horta, D. H. Bouyer, J. W. McBride, J. W. Pinter, A. Popov, S. M. Gennari, and D. H. Walker. 2004. Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of Sao Paulo, Brazil, where Brazilian spotted fever is endemic. J. Clin. Microbiol. 42:90-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labruna, M. B., D. H. Bouyer, J. W. McBride, L. M. A. Camargo, E. P. Camargo, and D. H. Walker. 2004. Molecular evidence for a spotted fever group Rickettsia species in the tick Amblyomma longirostre in Brazil. J. Med. Entomol. 41:533-537. [DOI] [PubMed] [Google Scholar]
- 25.Lackman, D. B., E. J. Bell, H. G. Stoenner, and E. G. Pickens. 1965. The Rocky Mountain spotted fever group of rickettsias. Health Lab. Sci. 2:135-141. [PubMed] [Google Scholar]
- 26.Larkin, M. A., G. Blackshields, N. P. Brown, R. Chenna, P. A. McGettigan, H. McWilliam, F. Valentine, I. M. Wallace, A. Wilm, R. Lopez, J. D. Thompson, T. J. Gibson, and D. G. Higgins. 2007. Clustal W and Clustal X version 2. Bioinformatics 23:2947-2948. [DOI] [PubMed] [Google Scholar]
- 27.Loving, S. M., A. B. Smith, A. F. DiSalvo, and W. Burgdorfer. 1978. Distribution of spotted fever group rickettsiae in ticks from South Carolina, with an epidemiological survey of persons bitten by infected ticks. Am. J. Trop. Med. Hyg. 27:1255-1260. [DOI] [PubMed] [Google Scholar]
- 28.Macaluso, K. R., D. E. Sonenshine, S. M. Ceraul, and A. F. Azad. 2002. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. J. Med. Entomol. 39:809-813. [DOI] [PubMed] [Google Scholar]
- 29.Mediannikov, O., K. Matsumoto, I. Samoylenko, M. Drancourt, V. Roux, E. Rydkina, B. Davoust, I. Tarasevich, P. Broqui, and P. E. Fournier. 2008. Rickettsia raoultii sp. nov., a spotted fever group rickettsia associated with Dermacentor ticks in Europe and Russia. Int. J. Sys. Evol. Microbiol. 58:1635-1639. [DOI] [PubMed] [Google Scholar]
- 30.Munderloh, U. G., S. F. Hayes, J. Cummings, and T. J. Kurtti. 1998. Microscopy of spotted fever rickettsia movement through tick cells. Microsc. Microanal. 4:115-121. [Google Scholar]
- 31.Nava, S., Y. Elshenawy, M. E. Eremeeva, J. W. Sumner, M. Mastropaulo, and C. D. Paddock. 2008. Rickettsia parkeri in Argentina. Emerg. Infect. Dis. 14:1894-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niebylski, M. K., M. E. Schrumpf, W. Burgdorfer, E. R. Fischer, K. L. Gage, and T. G. Schwan. 1997. Rickettsia peacockii sp. nov., a new species infecting wood ticks, Dermacentor andersoni, in western Montana. Int. J. Syst. Bacteriol. 47:446-452. [DOI] [PubMed] [Google Scholar]
- 33.Niebylski, M. K., M. G. Peacock, and T. G. Schwan. 1999. Lethal effect of Rickettsia rickettsii on its tick vector (Dermacentor andersoni). Appl. Environ. Microbiol. 65:773-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogrzewalska, M., R. C. Pacheco, A. Uezu, L. J. Richtzenhain, F. Ferreira, and M. B. Labruna. 2009. Rickettsial infection in Amblyomma nodosum ticks (Acari: Ixodidae) from Brazil. Ann. Trop. Med. Parasitol. 103:413-425. [DOI] [PubMed] [Google Scholar]
- 35.Pacheco, R. C., J. M. Venzal, L. J. Richtzenhain, and M. B. Labruna. 2006. Rickettsia parkeri in Uruguay. Emerg. Infect. Dis. 12:1804-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pacheco, R. C., J. Morares-Filho, S. Nava, P. E. Brandão, L. J. Richtzenhain, and M. B. Labruna. 2007. Detection of a novel spotted fever group rickettsia in Amblyomma parvum ticks (Acari: Ixodidae) from Argentina. Exp. Appl. Acarol. 43:63-71. [DOI] [PubMed] [Google Scholar]
- 37.Paddock, C. D., J. W. Sumner, J. A. Comer, S. R. Zaki, C. S. Goldsmith, J. Goddard, S. L. F. McLellan, C. L. Tamminga, and C. A. Ohl. 2004. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin. Infect. Dis. 38:805-811. [DOI] [PubMed] [Google Scholar]
- 38.Paddock, C. D., R. W. Finley, C. S. Wright, H. R. Robinson, B. J. Schrodt, C. C. Lane, O. Ekenna, M. A. Blass, C. L. Tamminga, C. A. Ohl, S. L. F. McLellan, J. Goddard, R. C. Holman, J. J. Openshaw, J. W. Sumner, S. R. Zaki, and M. E. Eremeeva. 2008. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin. Infect. Dis. 47:1188-1196. [DOI] [PubMed] [Google Scholar]
- 39.Paddock, C. D. 2009. The science and fiction of emerging rickettsioses. Ann. N. Y. Acad. Sci. 1166:133-143. [DOI] [PubMed] [Google Scholar]
- 40.Paffenbarger, R. S. 1951. Tick paralysis: implicating Amblyomma maculatum. New Orleans Med. Surg. J. 103:329-332. [PubMed] [Google Scholar]
- 41.Parker, R. R., G. M. Kohls, G. W. Cox, and G. E. Davis. 1939. Observations on an infectious agent from Amblyomma maculatum. Public Health Rep. 54:1482-1484. [Google Scholar]
- 42.Parker, R. R. 1940. A pathogenic rickettsia from the Gulf Coast tick, Amblyomma maculatum, p. 390-391. In Report of the Proceedings of the Third International Congress for Microbiology, New York, September 2-9, 1939. International Association of Microbiologists, New York, NY.
- 43.Parker, R. R., J. F. Bell, W. S. Chalgren, F. B. Thrailkill, and M. T. McKee. 1952. The recovery of strains of Rocky Mountain spotted fever and tularemia from ticks of the eastern United States. J. Infect. Dis. 91:231-237. [DOI] [PubMed] [Google Scholar]
- 44.Philip, C. B., and J. S. White. 1955. Disease agents recovered incidental to a tick survey of the Mississippi Gulf Coast. J. Econ. Entomol. 48:396-400. [Google Scholar]
- 45.Philip, R. N., E. A. Casper, W. Burgdorfer, R. K. Gerloff, L. E. Hughes, and E. J. Bell. 1978. Serologic typing of rickettsiae of the spotted fever group by microimmuofluorescence. J. Immunol. 121:1961-1968. [PubMed] [Google Scholar]
- 46.Pornwiroon, W., A. Bourchookarn, C. D. Paddock, and K. R. Macaluso. 2009. Proteomic analysis of Rickettsia parkeri strain Portsmouth. Infect. Immun. 77:5262-5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Regnery, R. L., C. L. Spruill, and B. D. Plikaytis. 1991. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 173:1576-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robertson, R. G., and C. L. Wisseman. 1973. Tick-borne rickettsiae of the spotted fever group in West Pakistan. II. Serological classification of isolates from West Pakistan and Thailand: evidence for two new species. Am. J. Epidemiol. 97:55-64. [DOI] [PubMed] [Google Scholar]
- 49.Roux, V., P. E. Fournier, and D. Raoult. 1996. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J. Clin. Microbiol. 34:2058-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roux, V., E. Rydkina, M. E. Eremeeva, and D. Raoult. 1997. Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the rickettsiae. Int. J. Sys. Bacteriol. 47:252-261. [DOI] [PubMed] [Google Scholar]
- 51.Roux, V., and D. Raoult. 1999. Phylogenetic analysis and taxonomic relationships among the genus Rickettsia, p. 52-66. In D. Raoult and P. Brouqui (ed.), Rickettsiae and rickettsial diseases at the turn of the third millennium. Elsevier, Paris, France.
- 52.Roux, V., and D. Raoult. 2000. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB). Int. J. Syst. Evol. Microbiol. 50:1449-1455. [DOI] [PubMed] [Google Scholar]
- 53.Silveira, I., R. C. Pacheco, M. P. Szabó, H. G. Ramos, and M. B. Labruna. 2007. Rickettsia parkeri in Brazil. Emerg. Infect. Dis. 13:1111-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simser, J. A., A. T. Palmer, U. G. Munderloh, and T. J. Kurtti. 2001. Isolation of a spotted fever group rickettsia, Rickettsia peacockii, in a Rocky Mountain wood tick, Dermacentor andersoni, cell line. Appl. Environ. Microbiol. 67:546-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sumner, J. W., L. A. Durden, J. Goddard, E. Y. Strohmdahl, K. L. Clark, W. K. Reeves, and C. D. Paddock. 2007. Gulf Coast ticks (Amblyomma maculatum) and Rickettsia parkeri, United States. Emerg. Infect. Dis. 13:751-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uchida, T., T. Uchiyama, K. Kumano, and D. H. Walker. 1992. Rickettsia japonica sp. nov., the etiological agent of spotted fever group rickettsiosis in Japan. Int. J. Sys. Bacteriol. 42:303-305. [DOI] [PubMed] [Google Scholar]
- 57.Whitman, T. J., A. L. Richards, C. D. Paddock, et al. 2007. Rickettsia parkeri infection after tick bite, Virginia. Emerg. Infect. Dis. 13:334-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wikswo, M. E., R. Hu, G. A. Dasch, L. Krueger, A. Arugay, K. Jones, B. Hess, S. Bennett, V. Kramer, and M. E. Eremeeva. 2008. Detection and identification of spotted fever group rickettsiae in Dermacentor species from southern California. J. Med. Entomol. 45:509-516. [DOI] [PubMed] [Google Scholar]