Abstract
Free chlorine is an effective disinfectant for controlling adenoviruses in drinking water, but little is known about the underlying inactivation mechanisms. The objective of this study was to elucidate the molecular components of adenovirus type 2 (Ad2) targeted by free chlorine during the inactivation process. The effects of free chlorine treatment on several Ad2 molecular components and associated life cycle events were compared to its effect on the ability of adenovirus to complete its life cycle, i.e., viability. Free chlorine treatment of Ad2 virions did not impair their ability to interact with monoclonal antibodies specific for hexon and fiber proteins of the Ad2 capsid, as measured by enzyme-linked immunosorbent assays, nor did it impair their interaction with recombinant, purified Coxsackie-adenovirus receptor (CAR) proteins in vitro. Free chlorine-treated Ad2 virions also retained their ability to bind to CAR receptors on A549 cell monolayers, despite being unable to form plaques, suggesting that free chlorine inactivates Ad2 by inhibiting a postbinding event of the Ad2 life cycle. DNA isolated from Ad2 virions that had been inactivated by free chlorine was able to be amplified by PCR, indicating that genome damage was not the cause of inactivation. However, inactivated Ad2 virions were unable to express E1A viral proteins during infection of A549 host cells, as measured by using immunoblotting. Collectively, these results indicate that free chlorine inactivates adenovirus by damaging proteins that govern life cycle processes occurring after host cell attachment, such as endocytosis, endosomal lysis, or nuclear delivery.
The adequate detection and control of viruses remains a challenge in modern water treatment. Because viruses can be infective at low concentrations and require host cells to replicate, they are difficult to detect in a timely manner in most water treatment settings. The application of advanced molecular approaches for more rapid virus detection is limited by their inability to distinguish between inactivated and viable viruses (22). In addition to being difficult to detect, many viruses are relatively resistant to UV light and monochloramine, two disinfectants that are increasingly utilized at water treatment plants in industrialized regions, particularly in the United States. As a result, the development of alternative or complementary primary disinfection technologies with greater efficacy against viruses is important for sustainable water supplies (25). Elucidating the molecular mechanisms by which disinfectants elicit virus inactivation will assist in the identification of novel approaches to detect and control viruses in water.
The current understanding of the fundamental mechanisms governing virus inactivation during the disinfection of water is limited. Free chlorine, for example, is a disinfectant that has been applied in municipal water treatment for over 100 years. The high rates of virus inactivation achieved with free chlorine have been characterized for many viruses that are considered potentially problematic in drinking water supplies, including coxsackievirus B5 (5, 29), Norwalk virus (9, 27), hepatitis A virus (HAV) (29), and adenovirus (Ad) (1, 19, 31). However, the molecular mechanism by which free chlorine inactivates viruses is not well understood (16).
In the present study, adenovirus serotype 2 (Ad2) was used as a model system for studying the mechanisms of virus inactivation with free chlorine. There are over 50 types of adenoviruses, and they are a concern in water treatment due to their high resistance to UV light and monochloramine (see references 2, 27, and 38). Ad2 is primarily associated with respiratory infections, but transmission of nongastrointestinal adenoviruses can occur via water (38). Furthermore, Ad2 is an attractive model system for laboratory studies since it is more easily assayed in cell culture-based systems than adenovirus types 40 or 41, two adenoviruses that induce gastrointestinal tract infections.
Free chlorine treatment was shown previously to inactivate adenovirus types 2, 5, 40, and 41 at similar rates (1, 19, 31). Based on the high rate of Ad2 inactivation observed over a range of pH and temperature conditions, we (19) hypothesized that free chlorine diminished adenovirus viability by damaging proteins that comprise the viral capsid. Because the rate of inactivation was not constant for a given pH value and the shape of the inactivation profile changed at different pH values (19), it was further hypothesized that several unique, rate-determining inactivation pathways may exist for each condition and that free chlorine may affect different portions of the virus life cycle for each pathway.
The Ad2 virion structure and associated life cycle steps are well characterized (2, 3, 8, 17, 23, 33). The main structural components of adenovirus are its genome and capsid. The linear, double-stranded DNA (dsDNA) genome is 35,000 base pairs (bp) in length. The capsid is comprised mostly of hexon, penton, and fiber proteins, each with important roles in replication. To replicate, adenovirus fiber must first bind to a Coxsackie-adenovirus receptor (CAR) protein on the surface of its host cell. Subsequent events trigger endocytosis, after which the virus is temporarily retained in an endosome. Upon translocation to the nucleus, adenovirus DNA is transcribed and translated into viral proteins that induce genome replication and, ultimately, morphogenesis of mature virions that are released after cell lysis.
To elucidate the mechanisms of Ad2 inactivation with free chlorine, a series of molecular approaches was employed to assess the effects of free chlorine on several attributes of Ad2. The structural integrity of the capsid proteins and the ability of the virions to attach to host cells were measured by performing enzyme-linked immunosorbent assays (ELISAs). The integrity and processivity of the viral genome were measured by using comparative PCR (cPCR). The synthesis of the Ad2 E1A protein, as a measure of viral genome processivity in infected host cells, was measured by using immunoblotting. The results from each assay were compared to the replication competency of free chlorine-treated virions, as measured by plaque assay. The mechanisms of inactivation were explored at both near-neutral and high pH values to determine whether unique inactivation mechanisms dominated at each pH.
MATERIALS AND METHODS
Adenovirus preparation and quantification.
The methods used for adenovirus propagation and viability assessment have been described in detail (27). Briefly, adenovirus serotype 2 (VR-846) was propagated in human lung A549 carcinoma cells (CCL-185). High-titer, purified adenovirus stock suspensions were produced using a freeze-thaw method followed by micro- and ultrafiltration. Adenovirus viability was assessed by plaque assay on confluent A549 cell monolayers with soft agar overlays.
Disinfectant exposure.
Free chlorine disinfection experiments were performed in 1 mM sodium phosphate buffer solution (PBS) prepared in distilled, deionized (DDI) water. Inactivation of adenovirus in this buffering system was determined to be representative of that in natural waters in a previous study (19). The pH was adjusted to 7.4 or 10.0 using sodium hydroxide. All chemicals utilized were reagent grade or better. The temperature of the solution was monitored continuously and maintained at 1.0 ± 0.5°C for all experiments.
Controlled free chlorine doses were delivered to adenovirus solutions using batch and flowthrough reactors as described previously (19). Batch reactors were used primarily for the pH 10.0 experiments, when the chlorine exposure (i.e., the integral of free chlorine concentration over time of exposure, also referred to as CT) required was in excess of 0.05 mg per min/liter. A flowthrough reactor system was used at pH 7.4 to deliver lower chlorine exposures, which were not achievable when using a batch system. In general, free chlorine exposures were applied so as to achieve target inactivation levels of 50 and 90%. Free chlorine concentrations were measured over a representative time course for each experiment by the DPD (N,N-diethyl-p-phenylenediamine) colorimetric method (4). Samples with concentrations higher than 1.5 mg/liter as Cl2 were diluted in DDI prior to analysis. All virus samples were quenched in 0.1% (wt/vol) sodium thiosulfate to stop the action of free chlorine.
The resulting untreated or treated Ad2 samples were then analyzed in parallel (i) for viability (as described above in “Adenovirus preparation and quantification”) and (ii) for the effect of free chlorine on several molecular attributes of adenovirus as described in the following sections.
Capsid protein integrity measurement by ELISA.
Untreated or free chlorine-treated Ad2 samples were incubated overnight in an Immulon ELISA 96-well plate (Thermo Scientific, Middletown, VA) in 20 mM phosphate buffer solution (PBS; pH 7.2) at 4°C. Wells were washed three times with PBST (PBS, pH 7.2, 0.05% [vol/vol] Tween 20). Next, wells were incubated with PBST blocking solution containing 0.1% (wt/vol) bovine serum albumin (BSA) and 0.1% (wt/vol) nonfat milk (PBSTM) for 1 h and then incubated in a solution containing mouse monoclonal antihexon antibody (1:300) (1E11; Santa Cruz Biotechnology, Santa Cruz, CA) or mouse monoclonal anti-fiber knob antibody (3C9; J. Chroboczek, Institute de Biologie Structurale, Grenoble, France) (6) for 1 h. After being rinsed 3 times, wells were incubated in PBSTM containing horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (1:1,000; Thermo Scientific Pierce, Rockford, IL) for 30 min. Wells were rinsed three times for 5 min each in PBSTM, followed by an additional 3 rinses in PBST. Bound secondary antibody was detected by incubating wells with tetramethylbenzidine (TMB) substrate solution (Thermo Scientific Pierce, Rockford, IL). The signal was quenched by adding sulfuric acid to each well, and colorimetric signals were detected at a wavelength of 570 nm by using a microplate reader (Bio-Rad Laboratories, Hercules, CA). To ensure linear signal resolution over one order of magnitude and to provide a standard curve from which normalized concentrations were calculated, internal standard signals were generated in parallel, using serially diluted untreated Ad2 virions. A separate set of wells received no viruses, no primary antibodies, or no secondary antibodies to ensure signal specificity and to assess background levels. Background levels were similar in each sample and were always less than the signal from the 10-times diluted, untreated viral control. Background values were not subtracted from sample values since the internal standards used to calculate each sample value were equally affected by the background values.
CAR affinity measurement by sandwich ELISA.
A sandwich ELISA was utilized to assess the effect of free chlorine treatment on the ability of adenovirus particles to bind to immobilized CAR proteins. Purified CAR was produced in XL1Blue Escherichia coli using methods described previously (14). His-tagged CAR proteins were purified by using nickel-nitrilotriacetic acid (Ni-NTA) chromatography according to the instructions provided by the manufacturer (Qiagen, Valencia, CA). The presence of 6×His-tagged CAR and the absence of other contaminating proteins were verified by separating a portion of each eluent sample with sodium dodecyl sulfate-12% (wt/vol) polyacrylamide gel electrophoresis (SDS-12% PAGE) and detecting proteins by using Coomassie staining. In an additional experiment, the immunoblotting of eluates using monoclonal mouse anti-His antibodies (1:1,000; GE Healthcare, Piscataway, NJ) verified the presence of 6×His-tagged CAR.
An excess of purified CAR protein (200 μl, 0.1 mg/ml) was immobilized in each well of an Immulon ELISA 96-well plate by incubation overnight at 4°C. Wells were incubated in PBST containing 0.1% BSA for 2 h and then aspirated and incubated with 100 μl of untreated or free chlorine-treated Ad2 virions in PBS containing 0.1% BSA for 90 min. The wells were rinsed three times with PBST and subsequently incubated in PBST containing 0.1% milk for 30 min. A PBST solution containing 0.1% milk and mouse monoclonal antihexon antibodies (1:300; Santa Cruz Biotechnologies, Santa Cruz, CA) was added to each well. Next, the wells were rinsed three times in PBST prior to filling them with a solution containing HRP-conjugated goat anti-mouse IgG (1:1,000; Thermo Scientific, Waltham, MA). Bound secondary antibody was detected by incubating wells with TMB substrate solution (Thermo Scientific Pierce, Rockford, IL). The signal was quenched by adding sulfuric acid to each well, and colorimetric signals were detected by using a microplate reader (model 680; Bio-Rad Laboratories, Hercules, CA). For each assay, control wells that received no recombinant CAR proteins, Ad2, antihexon, or anti-mouse antibodies were assayed in parallel. ELISA signals were determined as described above in “Capsid protein integrity measurement by ELISA,” based on values from internal standards generated by dilution of the untreated Ad2 control.
Viral genome integrity measurement by cPCR.
The integrity of the adenovirus genome was assessed by using cPCR, an approach similar to those developed by other researchers (15, 27). Genomes were extracted from untreated or free chlorine-treated Ad2 virus samples by using a DNeasy blood kit (Qiagen, Valencia, CA) according to the manufacturer's procedures. A portion of each of the genome extracts was subjected to PCR using the GoTaq system (Promega, Madison, WI). Different primer sets (Table 1) were used to amplify different sections of the Ad2 genome. For PCRs in which primer set 4 (PS4) was present, the amplification cycle consisted of denaturing at 95°C for 45 s, annealing at 61°C for 45 s, and extension at 72°C for 2 min. For all other PCRs, the amplification cycle consisted of denaturing at 95°C for 45 s, annealing at 61°C for 45 s, and extension at 72°C for 45 s. For all PCRs, a total of 20 amplification cycles was determined to be optimal for achieving linear signal resolution ranging at least one order of magnitude below that observed when amplifying genomic DNA from untreated Ad2. In addition, for all experiments, additional PCR amplifications were performed with genomes extracted from untreated virions that were diluted 1:2 or 1:10, which served as internal standards. After PCR amplification, 10 μl of each reaction mixture was separated by electrophoresis in a 1% (wt/vol) agarose gel containing ethidium bromide (0.2 μg/ml). Bands were visualized by using a UV transilluminator system (Bio-Rad Laboratories, Hercules, CA). Images were inverted, and band densities were quantified by using the GelPlot2 macro in Scion Image (Scion Corporation, Frederick, MD) as described previously (27). In calculating relative concentrations, the signals obtained from PCRs using genomes (diluted and undiluted) from untreated Ad2 served as internal standards to confirm linear signal resolutions ranging at least one order of magnitude below the signal of the untreated virus control. Integrated band densities were calculated based on the standard curve fitting, and samples were then normalized with respect to the integrated band density of the untreated virus control sample.
TABLE 1.
Primer sets used in cPCR analysis of Ad2 genome extracts
| Primer set | Forward sequence (5′-3′) | Reverse sequence (5′-3′) | Region of genome amplified (bp) | Amplicon length (bp) |
|---|---|---|---|---|
| PS1 | GGATTGAAGCCAATATGATAATGAGGGGG | GGCCCTAGACAAATATTACGCGCTAT | 25-355 | 330 |
| PS2 | GGCCCGCTGCCCTGG | GCCTCCGGAGCGCGG | 27435-27760 | 325 |
| PS3 | TTGGGTCCGGTTTCTATGCCAAAC | GTTCAGACACAGGACCTTTTAAAAAATCAC | 936-1240 | 304 |
| PS4 | GGATTGAAGCCAATATGATAATGAGGGGG | GTTCAGACACAGGACCTTTTAAAAAATCAC | 25-1240 | 1,215 |
Determination of Ad2 attachment to host cells using cPCR.
The ability of Ad2 to attach to its host in a cell culture setting was quantified by using a cPCR approach similar to those applied previously (18, 37). Wells containing 106 A549 cells were mock infected or infected with treated or untreated virus samples at a multiplicity of infection (MOI) of 2 and incubated for 90 min at room temperature to facilitate attachment. Immediately thereafter, supernatants were removed and cellular monolayers were washed three times in Ham's F12K medium to remove unbound virions. Cells were dislodged from the plates by scraping into 1 ml of medium. Total DNA was extracted from each sample, and PCR amplification, electrophoresis, detection, and quantification were conducted as described above in “Viral genome integrity measurement by cPCR.” Controls to demonstrate CAR binding specificity included preincubation of A549 cells with coxsackievirus B5 (VR-185; ATCC, Manassas, VA) prior to Ad2 infection, preincubation of Ad2 samples with mouse monoclonal antifiber antibody (1:1,000) (6), or infection of CAR-negative rabbit kidney epithelial RK13 cells with Ad2.
Detection of Ad2 E1A protein production by using immunoblotting.
Quantification of the synthesis of the adenovirus E1A early protein in A549 host cells was described previously (27). Briefly, untreated or treated virus samples were used to infect 106 A549 cells at an MOI of 5. As a control, a separate set of A549 cells was instead mock infected. Cells were harvested at 12 h postinfection and subjected to protein extraction. Protein extracts were separated by using SDS-12% PAGE and electrophoretically transferred to a polyvinylidene fluoride (PVDF) membrane (Thermo Scientific). E1A proteins were detected by probing membranes with mouse monoclonal anti-E1A antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA) followed by HRP-conjugated goat anti-mouse IgG antibody (1:100,000; Thermo Scientific Pierce, Rockford, IL). To confirm that equal amounts of cellular material were analyzed in each sample, immunoblots were reprobed with rabbit polyclonal antiactin antibodies diluted at 1:1,000 (Sigma Aldrich, St. Louis, MO), followed by HRP-conjugated goat anti-rabbit antibodies (1:10,000 dilution; Fisher Scientific, Waltham, MA). Blots were incubated with SuperSignal West Pico reagents (Thermo Scientific Pierce, Rockford, IL), and chemiluminescent signals were captured and quantified as described above.
Data analysis.
The mean values of Ad2 viability were compared to the mean values obtained from each molecular analysis using the fixed-effect, one-way analysis of variance (ANOVA) function in Microsoft Excel. For two data sets, this equates to a paired t test assuming unequal variances. Resulting P values of less than 0.05 were interpreted as representing a significant difference between adenovirus viability and the given molecular parameter of interest.
Values for raw data are provided in the supplemental material.
RESULTS
Inactivation kinetics.
The chlorine exposures (CT) and the resulting adenovirus survival ratios (N/N0, where N is the number of viable viruses after treatment and N0 is the initial Ad2 concentration) for all disinfection experiments performed in neutral (pH 7.4) or basic (pH 10.0) PBS solutions at 1°C in this study are presented in Fig. 1. These results are consistent with the predictions from a model (continuous and dashed lines in Fig. 1) that we developed previously (19) for the effect of pH on the rate of Ad2 inactivation with free chlorine. In each experiment, N/N0 values of 1.0, 0.5, and 0.1 were targeted to allow for at least one order of magnitude resolution. The average N/N0 values achieved are presented with corresponding standard error values (depicted as error bars representing 95% confidence intervals in remaining figures) in Table 3. The CT values were calculated taking into consideration the chlorine decay that occurred within the timescale of the disinfectant exposure as described previously (19). The initial free chlorine concentration (c0) and corresponding chlorine decay rate constant (kd) and the N0 for each data set shown in Fig. 1 are presented in Table 2. A portion of the free chlorine underwent relatively fast decay when using relatively high N0 values. In such cases, CT values were calculated using a modified initial free chlorine concentration (c0*) obtained by extrapolation of the chlorine data to time zero using a first-order decay model as described previously (19). Chlorine decayed too fast for the two experiments performed at the highest N0 values (experiments 13 and 18) and had to be dosed once or twice after the initial addition to achieve target CT values.
FIG. 1.
Inactivation of Ad2 with free chlorine at pH 7.4 or pH 10.0 and 1°C, as observed in the current study. Solid and dashed lines represent predictions generated from a kinetic model developed in a previous study (19). Note the break in the x axis. Exp, experiment; L, liter.
TABLE 3.
Comparisons of normalized signals from molecular parameters of interest to overall adenovirus viability as a function of disinfectant exposure and pH
| Molecular parameter (X/X0) | pH | CT (mg Cl2 per min/liter [avg ± SE])a | Adenovirus viability (N/N0 [avg ± SE])a | No. of data pointsb | Molecular parameter (X/X0 [avg ± SE])a | No. of data pointsb | P valuec |
|---|---|---|---|---|---|---|---|
| Hexon integrity (H/H0) | 7.4 | 0 | 1 | 4 | 1 | 4 | |
| 0.0090 ± 0.0043 | 0.43 ± 0.05 | 3 | 0.78 ± 0.06 | 3 | 0.019 | ||
| 0.0134 ± 0.0018 | 0.12 ± 0.01 | 5 | 0.74 ± 0.04 | 5 | <0.0001 | ||
| 0.0154 ± 0.0007 | 0.05 ± 0.01 | 5 | 0.63 ± 0.01 | 5 | <0.0001 | ||
| 10.0 | 0 | 1 | 1 | 1 | 1 | ||
| 1.08 | 0.44 | 1 | 0.73 | 1 | |||
| 1.35 | 0.054 | 1 | 0.87 | 1 | |||
| Fiber integrity (F/F0) | 7.4 | 0 | 1 | 4 | 1 | 3 | |
| 0.0090 ± 0.0043 | 0.43 ± 0.05 | 3 | 0.87 ± 0.04 | 3 | 0.003 | ||
| 0.0134 ± 0.0018 | 0.12 ± 0.01 | 5 | 0.88 ± 0.08 | 3 | <0.0001 | ||
| 0.0154 ± 0.0007 | 0.05 ± 0.01 | 5 | 0.98 ± 0.05 | 3 | <0.0001 | ||
| 10.0 | 0 | 1 | 1 | 1 | 1 | ||
| 1.08 | 0.44 | 1 | 0.68 | 1 | |||
| 1.35 | 0.054 | 1 | 0.63 | 1 | |||
| CAR affinity (C/C0) | 7.4 | 0 | 1 | 4 | 1 | 3 | |
| 0.0090 ± 0.0043 | 0.43 ± 0.05 | 3 | 0.66 ± 0.10 | 3 | 0.158 | ||
| 0.0134 ± 0.0018 | 0.12 ± 0.01 | 5 | 0.68 ± 0.06 | 3 | <0.0001 | ||
| 0.0154 ± 0.0007 | 0.05 ± 0.01 | 5 | 0.73 ± 0.14 | 3 | 0.002 | ||
| 10.0 | 0 | 1 | 2 | 1 | 3 | ||
| 0.94 ± 0.07 | 0.43 ± 0.05 | 3 | 0.77 ± 0.04 | 3 | 0.016 | ||
| 1.36 ± 0.01 | 0.07 ± 0.01 | 2 | 0.66 ± 0.08 | 2 | |||
| Attachment ability in | 7.4 | 0 | 1 | 3 | 1 | 3 | |
| cell culture (A/A0) | 0.0061 ± 0.0003 | 0.53 ± 0.07 | 3 | 0.64 ± 0.07 | 3 | 0.27 | |
| 0.0120 ± 0.0008 | 0.09 ± 0.03 | 3 | 0.46 ± 0.07 | 3 | 0.0007 | ||
| 10.0 | 0 | 1 | 3 | 1 | 3 | ||
| 0.85 ± 0.04 | 0.49 ± 0.04 | 3 | 0.70 ± 0.10 | 2 | 0.21 | ||
| 1.44 ± 0.09 | 0.12 ± 0.02 | 3 | 0.63 ± 0.05 | 3 | 0.00003 | ||
| DNA integrity (D/D0) | 7.4 | 0 | 1 | 3 | 1 | 3 | |
| 0.0061 ± 0.0003 | 0.53 ± 0.07 | 3 | 0.49 ± 0.02 | 3 | 0.44 | ||
| 0.0120 ± 0.0008 | 0.09 ± 0.03 | 3 | 0.43 ± 0.03 | 3 | 0.02 | ||
| 10.0 | 0 | 1 | 3 | 1 | 3 | ||
| 0.85 ± 0.04 | 0.49 ± 0.04 | 3 | 0.59 ± 0.04 | 3 | 0.26 | ||
| 1.44 ± 0.09 | 0.12 ± 0.02 | 3 | 0.62 ± 0.09 | 3 | 0.02 | ||
| Early protein synthesis | 7.4 | 0 | 1.00 ± 0.00 | 4 | 1 | 4 | |
| (E/E0) | 0.0074 ± 0.0003 | 0.46 ± 0.03 | 4 | 0.43 ± 0.02 | 3 | 0.47 | |
| 0.0130 ± 0.0006 | 0.08 ± 0.02 | 6 | 0.06 ± 0.02 | 7 | 0.59 | ||
| 10.0 | 0 | 1.00 ± 0.00 | 4 | 1 | 4 | ||
| 0.53 ± 0.05 | 0.81 ± 0.02 | 4 | 0.78 ± 0.10 | 4 | 0.86 | ||
| 0.87 ± 0.04 | 0.52 ± 0.03 | 3 | 0.42 ± 0.06 | 3 | 0.33 | ||
| 1.53 ± 0.08 | 0.10 ± 0.02 | 7 | 0.06 ± 0.02 | 7 | 0.23 |
Where no standard error is shown, the actual value is given.
Values are the number of unique data points analyzed for the given parameter.
P, values reflect statistical significance based on t test comparison of the given molecular parameter to adenovirus viability (N/N0).
TABLE 2.
Summary of experimental conditions and disinfectant decay parameters used for assessing the inactivation of adenovirus serotype 2 with free chlorinea
| Expt | pH | N0 (PFU/ml) | c0 (mg Cl2/liter) | c0*b (mg Cl2/liter) | kd (min−1) | Capsid protein integrity (H/H0, F/F0) | CAR affinity (C/C0) | Attachment in cell culture (A/A0) | Genome/DNA integrity (D/D0) | E1A synthesis ability (E/E0) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7.4 | 4.6 × 106 | 1.48 | 1.48 | 114 | X | X | |||
| 2 | 7.4 | 1.4 × 106 | 1.75 | 1.75 | 111 | X | X | |||
| 3 | 7.4 | 1.6 × 106 | 1.90 | 1.90 | 108 | X | X | |||
| 4 | 7.4 | 4.2 × 106 | 1.90 | 1.82 | 101 | X | ||||
| 5 | 7.4 | 6.7 × 106 | 4.00 | 4.00 | 276 | X | X | |||
| 6 | 7.4 | 2.2 × 107 | 4.08 | 3.69 | 235 | X | ||||
| 7 | 7.4 | 3.4 × 107 | 3.67 | 3.07 | 356 | X | ||||
| 8 | 7.4 | 1.1 × 108 | 6.37 | 5.21 | 271 | X | X | |||
| 9 | 7.4 | 1.3 × 108 | 5.16 | 4.48 | 266 | X | X | |||
| 10 | 7.4 | 1.5 × 108 | 5.16 | 3.88 | 255 | X | X | |||
| 11 | 7.4 | 1.0 × 107 | 1.25 | 0.73 | 0.919 | X | ||||
| 12 | 10.0 | 3.9 × 106 | 1.31 | 0.85 | 0.087 | X | X | |||
| 13c | 10.0 | 9.0 × 108 | 2.11 | 1.24 | 2.17 | X | ||||
| 0.25 | 0.24 | 1.82 | ||||||||
| 0.50 | 0.51 | 0.260 | ||||||||
| 14 | 10.0 | 1.1 × 108 | 3.70 | 1.54 | 0.687 | X | ||||
| 15 | 10.0 | 1.2 × 108 | 4.24 | 2.04 | 0.713 | X | ||||
| 16 | 10.0 | 4.2 × 107 | 3.94 | 1.15 | 0.633 | X | X | X | X | X |
| 17 | 10.0 | 8.0 × 107 | 3.12 | 1.30 | 0.461 | X | X | X | ||
| 18d | 10.0 | 1.4 × 109 | 12.3 | 5.46 | 4.04 | X | X | |||
| 6.50 | 6.44 | 0.957 | ||||||||
| 19 | 10.0 | 1.0 × 107 | 5.32 | 1.81 | 0.272 | X |
The experimental conditions used for the inactivation of adenovirus serotype 2 with free chlorine were the pH and the initial concentrations of viable virions (N0) and disinfectant (c0), and the disinfectant decay parameters were the decomposition rate constant (kd) and the extrapolated initial disinfectant concentration (c0*). X, virus samples from the given experiment were subjected to the corresponding molecular analysis (D/D0, E/E0, A/A0, H/H0, F/F0, and C/C0 are normalized parameters for the various molecular signals defined in Results).
c0*, modified initial free chlorine concentration obtained by extrapolation of the chlorine data to 0 min (or the time of midexperiment dosing for experiments 13 and 18) using a first-order decay model as described previously (19).
Chlorine was dosed three times during the performance of experiment 13, i.e., c1,0 = 2.11 mg Cl2/liter at 0 min, c2,0 = 0.25 mg Cl2/liter at 2 min, and c3,0 = 0.50 mg Cl2/liter at 3 min.
Chlorine was dosed twice during the performance of experiment 18, i.e., c1,0 = 12.3 mg Cl2/liter at 0 min and c2,0 = 6.50 mg Cl2/liter at 1.5 min.
Effect of free chlorine on capsid-antibody interactions.
To determine if free chlorine treatment of Ad2 damages the hexon protein (potentially causing a loss of structural integrity of the virion) or the fiber knob (potentially affecting virion interactions with CAR [11]), free chlorine-treated Ad2 virus particles were tested for their ability to bind to antibodies specific for either the hexon protein or the fiber knob protein. Interactions were quantified by performing an ELISA.
Hexon protein structural integrity, as measured by hexon-antihexon antibody interactions, was assessed as a function of free chlorine exposure at pH 7.4 (Fig. 2a) or pH 10.0 (Fig. 2b). Hexon protein integrity values (H/H0) were calculated by normalizing the ELISA signal values obtained from treated virions (H) to the ELISA signal values from untreated viruses (H0). Hexon protein integrity values decreased by about 22 to 28% when Ad2 virions were exposed to free chlorine at CT values of 0.009 to 0.015 mg Cl2 per min/liter at pH 7.4 (Fig. 2a and Table 3), but there was no significant trend with increasing free chlorine exposure within the range investigated (Fig. 2a and Table 3). A similar effect was observed when studying Ad2 inactivation in solutions of pH 10.0 (Fig. 2b and Table 3). If the reaction mixtures contained neither virus nor primary or secondary antibodies, the values were lower than those observed in normal reaction mixtures containing 10-fold fewer untreated Ad2 virions (data not shown). A significant difference between adenovirus viability (N/N0) and hexon protein integrity (H/H0) was observed for virions treated with the chlorine exposures investigated (Fig. 2 and Table 3), regardless of pH, indicating that the integrity of the hexon protein structure components that interact with the antibodies was relatively stable during Ad2 inactivation by free chlorine.
FIG. 2.
Effects of free chlorine on adenovirus hexon coat protein integrity (H/H0), fiber knob attachment protein integrity (F/F0), Coxsackie-adenovirus receptor (CAR) protein binding affinity (C/C0), and viability (N/N0). Prior to each analysis, mature Ad2 virions were exposed to the indicated levels of free chlorine at pH 7.4 (a) or pH 10.0 (b) and 1°C. Hexon and fiber knob protein integrity was measured by performing an ELISA in which untreated or treated virions were immobilized and then incubated with antibodies recognizing either hexon or fiber proteins. CAR-Ad2 interactions were assessed by performing a sandwich ELISA in which treated or untreated virions were incubated with immobilized CAR protein and bound virions were detected using an antihexon antibody. Error bars represent 95% confidence intervals. L, liter.
Similar trends were observed when the integrity of the adenovirus fiber proteins of mature virions was analyzed by incubating virions with antifiber antibodies (F/F0, where F is the ELISA signal from treated viruses and F0 is the ELISA signal from untreated viruses) (Fig. 2). While the antifiber antibody interacted readily with untreated Ad2 virions, free chlorine treatment of Ad2 resulted in a 2 to 13% decrease in antibody-virion interactions at pH 7.4 (Fig. 2a and Table 3). When virions were treated in solutions of pH 10.0, the decrease in antibody-antigen interactions was higher, with signals decreasing about 35 to 40% (Fig. 2b and Table 3). However, significant differences between adenovirus viability (N/N0) and fiber protein integrity (F/F0) were observed at each pH (Table 3). These results indicate that the region of the fiber knob responsible for binding to the antifiber antibody, which is critical for binding CAR (6), is relatively stable during free chlorine exposures that result in adenovirus inactivation.
Effect of free chlorine on CAR-adenovirus interactions.
To more closely mimic virus-host cell interactions in vivo, the effect of free chlorine on the interactions between Ad2 virions and CAR was measured by performing a sandwich ELISA. The effect of chlorine exposure on the ability of Ad2 virions to bind to immobilized CAR protein (C/C0, where C is the ELISA signal from treated viruses and C0 is the ELISA signal from untreated viruses) is also depicted in Fig. 2. Similar to trends described above, there was an initial decrease in Ad2-CAR interactions when viruses were treated at the lowest exposures of free chlorine studied. However, no further decrease in C/C0 was observed with virions that had received higher free chlorine exposures. As a result, the signals associated with Ad2 bound to CAR (C/C0) were not consistent with the level of Ad2 viability (N/N0) at pH 7.4 or 10.0 (Fig. 2 and Table 3). The similarities between the hexon protein integrity (H/H0) and CAR binding affinity (C/C0) values indicate that the C/C0 reductions were likely associated with reductions in antihexon recognition of bound virions rather than CAR affinity.
Effect of free chlorine on Ad2-CAR interactions in cell culture.
The effect of free chlorine on Ad2-CAR interactions was further examined by using cPCR to measure the viruses associated with A549 cell monolayers at 90 min postinfection. The dissociation constant for the interaction between the Ad2 attachment fiber and the CAR protein is reported to be 1 nM (14). Therefore, at the virus concentrations used in the cPCR attachment experiments (N0 of 1.4 × 106 to 4.6 × 106 PFU/ml), the number of viruses bound to host cells would be directly dependent on the number of viruses present in the infecting solution. This assertion was confirmed by infecting cells with decreasing concentrations of the untreated virus control (data not shown).
The effects of free chlorine exposure on Ad2 attachment to A549 cells at pH 7.4 and 10.0 are presented in Fig. 3a and b, respectively. At both pH values, the normalized PCR signals obtained from cells possessing attached viruses (A/A0) decreased as a result of free chlorine exposure. However, the reduction in signals did not increase with increasing CT. Consistent with the ELISA results, these findings provide further evidence that virion-CAR interactions do not decrease at the same rate as viability is lost.
FIG. 3.
Effects of free chlorine on adenovirus attachment ability (A/A0) and viability (N/N0). Prior to host cell infection, mature Ad2 virions were subjected to the indicated exposures of free chlorine at pH 7.4 (a) or pH 10.0 (b) and 1°C. Attachment ability was measured by washing and harvesting cells 90 min after infection and subjecting the resulting suspension to PCR amplification using primer set 1. Ad2 genome integrity (D/D0) is presented to account for PCR signal reduction caused by exposure of the viruses to free chlorine prior to infection. Error bars represent 95% confidence intervals for experiments conducted in triplicate. L, liter.
As a control, a rabbit kidney epithelial RK13 cell line, lacking human CAR proteins and therefore unable to bind Ad2 efficiently, was tested in parallel to identify the percentage of Ad2 virions that nonspecifically bound to cells. Using this approach, it was determined that approximately 5% of the viruses bound nonspecifically to cells (data not shown), consistent with nonspecific binding levels observed previously for adenovirus type 5 (37), which has a fiber similar to that of Ad2 (24). In other control experiments (data not shown), Ad2-CAR interactions were inhibited when A549 cell monolayers were pretreated with either coxsackievirus B5 or an antifiber antibody (6), two reagents known to prevent Ad2-CAR interactions. Thus, the Ad2-CAR interactions observed in these assays were specific.
To ensure that the initial PCR signal reductions observed were associated with chlorination of the viruses before attachment, genomes extracted from the same untreated or treated Ad2 samples prior to incubation with A549 cells were also PCR amplified. As shown in Fig. 3, the normalized cPCR values obtained from cell-associated virions (A/A0) were similar to those obtained from virions not incubated with cell monolayers (D/D0). The PCR signals generated from genomes extracted from treated viruses were lower than those from untreated viruses, indicating that exposure of viruses to free chlorine results in some viral genomic degradation. Therefore, the reduction in PCR signals obtained from cells infected with treated viruses (A/A0) was likely not the result of fewer treated Ad2 virions binding to the cells. Rather, the signal reduction was likely due to a reduced signal from the viruses themselves.
Effect of free chlorine on viral genome integrity.
Free chlorine has been reported to react with the amine functional groups present on nucleotides (20, 21). Thus, we assessed whether free chlorine would inactivate Ad2 virions by reacting with the nucleotides comprising its genome. Genomes were isolated from untreated or treated virions and then PCR amplified using primer sets specific for different regions of the Ad2 genome (Table 1). For average CT values of 0.0061 and 0.0120 mg Cl2 per min/liter, the average signal from the PCR amplicon was reduced by approximately 50% compared to that of the PCR product obtained when viral DNA from untreated virions was amplified (Fig. 4, primer set 1 [PS1]). However, the genome integrity did not decrease to the same extent as viability when greater free chlorine exposure was applied (Fig. 4, PS1). Since similar results were obtained when using primer sets that targeted different, nonoverlapping regions of the viral genome (PS2 or PS3 in Table 1 and Fig. 4), the effect of free chlorine was likely consistent across the entire genome. The reduction in PCR product signal was also independent of the length of the target amplicon. As depicted by the results in Fig. 4, the apparent loss of genome integrity when using primers that amplified a 1,215-bp region (PS4) was similar to the loss seen when amplifying a 400-bp region (PS1, PS2, and PS3). A similar trend was observed if nonencapsidated (naked) Ad2 genomes (NG) were instead exposed to free chlorine (Fig. 4, PS1, NG), indicating that the Ad2 genome is not protected from chlorine by its capsid.
FIG. 4.

Effects of free chlorine (pH 7.4, 1°C) on Ad2 genome integrity (D/D0) and viability (N/N0). Mature Ad2 virions or naked Ad2 genomes (NG) were treated with the indicated exposures of free chlorine. Genomes were isolated from virions and subjected to PCR amplification using the primer sets indicated. A portion of each PCR product was subjected to agarose gel electrophoresis, and the intensities of ethidium bromide-stained bands from free chlorine-treated samples were compared to those obtained from untreated samples. Treated and untreated Ad2 virions were also incubated with A549 cell monolayers, and virus viability was assessed by using plaque assays. Error bars represent 95% confidence intervals for experiments conducted in triplicate. L, liter.
At higher CT values (up to 0.2 mg Cl2 per min/liter), which inactivated Ad2 at levels as high as 99.95%, the apparent genome integrity decreased by only 70% (data not shown). Collectively, the results of the cPCR analyses suggest that free chlorine may affect genome integrity and virus viability similarly at relatively low disinfectant exposures. However, genome integrity is relatively stable at higher levels of free chlorine treatment.
Effect of free chlorine on early protein synthesis.
Next, the ability of free chlorine treatment to inhibit the function of viral DNA during the host cell infection process was examined by using immunoblotting. Since a portion of the E1A open reading frame (ORF) was affected by free chlorine (Fig. 4, PS4), the transcription and translation of the E1A gene were examined. The E1A product is the first viral gene product expressed during infection, and as such, its expression is not dependent on the integrity or expression of other genes.
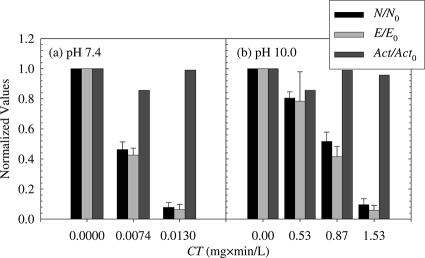
The normalized quantities of E1A protein synthesized in A549 cells are presented in Fig. 5 (E/E0). At either low (Fig. 5a) or high (Fig. 5b) pH values, the normalized quantity of E1A proteins (E/E0) decreased if the infecting viruses were first exposed to free chlorine. Interestingly, E1A protein levels continued to decrease as viruses were treated with increasing free chlorine doses. In fact, a comparison of E1A protein levels (E/E0) to adenovirus viability (N/N0) as a function of free chlorine exposure (Fig. 5) revealed that early protein synthesis was inhibited to an extent similar to the decrease in overall adenovirus viability. Significant differences were not observed at target adenovirus inactivation levels of 50% and 90% at pH 7.4 (Fig. 5a) and 20%, 50%, and 90% at pH 10.0 (Fig. 5b) (Table 3). This observation indicated that free chlorine treatment of virions resulted in a diminution of the transcription of the E1 ORF or affected earlier steps in the adenovirus life cycle.
FIG. 5.
Effects of free chlorine on the synthesis of E1A early proteins in A549 host cells (E/E0) and on Ad2 viability (N/N0). Prior to host cell infection, Ad2 virions were treated with the indicated exposures of free chlorine at pH 7.4 (a) or pH 10.0 (b) and 1°C. E1A protein synthesis was quantified by harvesting cells at 12 h postinfection, extracting the proteins, and separating them by SDS-12% PAGE, followed by immunoblotting with primary antibody specific for E1A protein. Normalized actin levels (Act/Act0), also measured by immunoblotting, served as a cellular loading control. Error bars represent 95% confidence intervals for experiments conducted in triplicate. L, liter.
DISCUSSION
While several important molecular structures and steps of the Ad2 life cycle were analyzed, only E1A protein synthesis continually decreased at a rate similar to that of viability loss. In contrast, other early steps in the virus life cycle, notably virus-CAR interactions, were stable relative to viability loss.
Mechanistic aspects of Ad2 inactivation with free chlorine.
As depicted in Fig. 6, the results from this study indicate that free chlorine neutralizes Ad2 virions by acting on the viral proteins necessary for postbinding events (e.g., endocytosis). While E1A protein levels decreased at a rate similar to the rate of Ad2 viability loss, free chlorine damage to viral DNA is likely not the dominant mechanism of adenovirus inactivation or of reductions in E1A protein levels, for the following reasons. First, increasing doses of free chlorine, which increasingly affected virus viability, did not result in increased viral DNA damage as detected by the ability of the genome to be a template for cPCR. A similar extent of irreversible loss of DNA integrity (∼50%) was reported previously for hypochlorite treatment of native dsDNA (21), suggesting that free chlorine may have a limited ability to damage DNA. Second, the apparent DNA damage did not continue to decrease with greater chlorine exposure relative to E1A protein levels. Therefore, it is unlikely that the reduction in E1A synthesis observed by immunoblotting was solely due to the reduction in E1A gene integrity. Rather, it may be that the genome damage observed by PCR has little bearing on the Ad2 life cycle and that proteins governing endocytosis, endosomal lysis, or nuclear delivery are targeted by free chlorine. That proteins are targeted is consistent with findings from several mechanistic studies of pathogen inactivation with free chlorine (18, 27, 36).
FIG. 6.
Graphical summary of the Ad2 life cycle. The larger arrow indicates the observed lack of inhibition of the step by free chlorine. The box with dashed lines contains the step(s) thought to be targeted by free chlorine.
Endocytosis, which occurs after Ad2-CAR interactions, is mediated upon the interaction of an adenovirus penton base protein with integrin receptors. The interaction of host cell integrin receptors with an RGD (arginine-glycine-aspartic acid) motif on the penton base is required for triggering endocytosis (30, 34). While arginine, glycine, and aspartic acid are not very reactive with free chlorine (7), their interactions with the host cell may be affected by damage to other residues proximal to the RGD motif. Indeed, chlorine-sensitive residues, namely, methionine and histidine, are located within five residues of the RGD motif (39). It is also thought that flexibility of the adenovirus fiber shaft is required to facilitate endocytosis (37). An additional possibility, then, is that free chlorine damages the fiber shaft, making it incapable of triggering endocytosis.
The endosomal lysis and nuclear delivery steps of the adenovirus life cycle have not been characterized as extensively as earlier steps (17). Endosomal lysis is thought to be triggered by the low endosomal pH and subsequent release of the penton bases and protein VI, which disrupt the endosome by an unknown mechanism (35). After escaping the endosome, a loosened viral capsid, still comprised mostly of hexon proteins and containing the dsDNA, localizes to the outer nuclear membrane of the host cell, after which viral DNA is transferred through nucleopore complexes onto histones within the nucleus. Thus, disruption of either the penton or hexon protein structure by chlorine could have an impact on overall viability by inhibiting these steps that take place within the cell.
Multiplicity of inactivation mechanisms.
A kinetic model developed in a previous study (19) to predict the inactivation of adenovirus as a function of the free chlorine dose (CT) was based on the hypothesis that different rate-limiting inactivation mechanisms may dominate at different pH values. Based on the results of the current study, the various effects assessed do not appear to be pH dependent. The different rates or pathways of inactivation observed in the previous kinetic study may be due to variable reaction pathways and rates within a unique molecular target or, perhaps, to different targets that have yet to be resolved within the endocytosis, endosome lysis, and nuclear delivery pathways.
Comparison to other viruses and disinfectants.
Previous studies on the mechanisms of virus inactivation with free chlorine have focused primarily on positive-sense, single-stranded RNA (ssRNA) enteroviruses, such as hepatitis A virus (HAV), poliovirus type 1(PV-1), and norovirus (12, 18, 28). In a study of viral genome stability as a function of disinfectant exposure (28), it was concluded that the ssRNA genome is relatively stable at the free chlorine exposures required for virus inactivation. This low rate of ssRNA genome degradation by free chlorine is consistent with the low secondary rate of adenovirus genome integrity reduction observed in the current study at higher chlorine exposures. It should be noted that the chlorine exposures required for the inactivation of PV-1 and some of the other ssRNA viruses studied previously are generally orders of magnitude higher than the doses required for comparable levels of adenovirus inactivation.
A more recent study examined the effects of free chlorine on capsid functionality for PV-1, HAV, and feline calicivirus (18). The study concluded that free chlorine inactivates these viruses by inhibiting their ability to bind to their host cells, in contrast to the findings presented here for Ad2. It is unknown whether these differences are due to different binding mechanisms of the viruses, different protein compositions of capsids, or reactions associated with the higher chlorine exposures required for inactivating ssRNA viruses.
Another recent study on the mechanisms of Ad2 inactivation by monochloramine shows similar findings; as with free chlorine, monochloramine resulted in an inhibition of E1A protein synthesis (27). Whether monochloramine produced this effect by inhibiting an earlier step in the adenovirus life cycle, such as attachment, endocytosis, endosomal lysis, or nuclear delivery, is unknown.
Practical implications.
The mechanistic insights obtained in this study are relevant to current challenges in the control and monitoring of viruses in water. From the perspective of the development of disinfection technology, the results support the idea that disinfectants capable of efficiently transforming proteins should be effective against viruses. This conclusion is consistent with the results of kinetic studies that have demonstrated the high virucidal efficacy of bulk phase oxidants like free chlorine and ozone (3, 19, 26, 31, 32). Unfortunately, as currently applied in bulk solution, such oxidants often generate undesirable disinfection by-products. However, if viral capsid proteins are found to be key molecular targets for inactivation, heterogeneous disinfection technologies, such as photocatalysts with high affinity for viruses (13), with the potential to inactivate viruses selectively at interfaces while controlling the formation of disinfection by-products, could be developed.
The mechanistic findings in this study also have potential implications for the monitoring and detection of viruses in water. Data obtained in the current study and results reported for other viruses (15, 27) indicate that PCR signals decay slowly during exposure to free chlorine, generally requiring disinfectant exposures at least an order of magnitude greater than those required for similar levels of inactivation. The stability of adenovirus capsid proteins, as measured by ELISA, indicates that immunocapture methods might also fail to discriminate some types of viruses that have been inactivated by free chlorine. Further studies are needed to identify molecular markers that can be probed easily and that relate directly to the overall viability after treatment with free chlorine and other common disinfectants.
Supplementary Material
Acknowledgments
This work was funded by the Center of Advanced Materials for the Purification of Water with Systems (WaterCAMPWS), a National Science Foundation Science and Technology Center, under award no. CTS-0120978.
We thank Jadwiga Chroboczek of L'Institut de Biologie Structurale (France) and Mark van Raaij of Universidad de Santiago de Compostela (Spain) for providing materials used in this research.
Footnotes
Published ahead of print on 19 March 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Baxter, C. S., R. Hofmann, M. R. Templeton, M. Brown, and R. C. Andrews. 2007. Inactivation of adenovirus types 2, 5, and 41 in drinking water by UV light, free chlorine, and monochloramine. J. Environ. Eng. 133:95-103. [Google Scholar]
- 2.Berk, A. J. 2007. Adenoviridae: the viruses and their replication, p. 2355-2394. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 3.Chroboczek, J., E. Gout, A. Favier, and R. Galinier. 2003. Novel partner proteins of adenovirus penton, p. 37-56. In W. Doerfler and P. Bohm (ed.), Adenoviruses: model and vectors in virus-host interactions. Springer-Verlag, Berlin, Germany. [DOI] [PubMed]
- 4.Eaton, A. D., L. S. Clesceri, E. W. Rice, and A. E. Greenberg (ed.). 2005. Standard methods for the examination of water and wastewater, 21st ed. American Public Health Association, Washington, DC.
- 5.Engelbrecht, R. S., M. J. Weber, B. L. Salter, and C. A. Schmidt. 1980. Comparative inactivation of viruses by chlorine. Appl. Environ. Microbiol. 40:249-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fender, P., A. H. Kidd, R. Brebant, M. Öberg, E. Drouet, and J. Chroboczek. 1995. Antigenic sites on the receptor-binding domain of human adenovirus type 2 fiber. Virology 214:110-117. [DOI] [PubMed] [Google Scholar]
- 7.Hawkins, C. L., D. I. Pattison, and M. J. Davies. 2003. Hypochlorite-induced oxidation of amino acids, peptides, and proteins. Amino Acids 25:259-274. [DOI] [PubMed] [Google Scholar]
- 8.Huang, S., T. Kamata, Y. Takada, Z. M. Ruggeri, and G. R. Nemerow. 1996. Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J. Virol. 70:4502-4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keswick, B. H., T. K. Satterwhite, and P. C. Johnson. 1985. Inactivation of Norwalk virus in drinking water by chlorine. Appl. Environ. Microbiol. 50:261-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reference deleted.
- 11.Kirby, I., E. Davison, A. J. Beavil, C. P. C. Soh, T. J. Wickham, P. W. Roelvink, I. Kovesdi, B. J. Sutton, and G. Santis. 1999. Mutations in the DG loop of adenovirus type 5 fiber knob protein abolish high-affinity binding to its cellular receptor CAR. J. Virol. 73:9508-9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li, J. W., Z. T. Xin, X. W. Wang, J. L. Zheng, and F. H. Chao. 2002. Mechanisms of inactivation of hepatitis A virus by chlorine. Appl. Environ. Microbiol. 68:4951-4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li, Q., M. A. Page, B. J. Mariñas, and K. S. Jian. 2008. Treatment of coliphage MS2 with palladium-modified nitrogen-doped titanium oxide photocatalyst illuminated by visible light. Environ. Sci. Technol. 42:6148-6153. [DOI] [PubMed] [Google Scholar]
- 14.Lortat-Jacob, H., E. Chouin, S. Cusack, and M. J. van Raaij. 2001. Kinetic analysis of adenovirus fiber binding to its receptor reveals an avidity mechanism for trimeric receptor-ligand interactions. J. Biol. Chem. 276:9009-9015. [DOI] [PubMed] [Google Scholar]
- 15.Ma, J. F., T. M. Straub, I. L. Pepper, and C. P. Gerba. 1994. Cell culture and PCR determination of poliovirus inactivation by disinfectants. Appl. Environ. Microbiol. 60:4203-4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maillard, J. Y. 1999. Viricidal activity of biocides. D. Mechanisms of viricidal action, p. 272-323. In A. D. Russell, W. B. Hugo, and G. A. J. Ayliffe (ed.), Principles and practice of disinfection, preservation, and sterilization. Blackwell Science, Oxford, England.
- 17.Nemerow, G. R., L. Pache, V. Reddy, and P. L. Stewart. 2009. Insights into adenovirus host cell interactions from structural studies. Virology 384:380-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nuanualsuwan, S., and D. O. Cliver. 2003. Capsid functions of inactivated human picornaviruses and feline calicivirus. Appl. Environ. Microbiol. 69:350-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page, M. A., J. L. Shisler, and B. J. Mariñas. 2009. Kinetics of adenovirus type 2 inactivation with free chlorine. Water Res. 43:2916-2926. [DOI] [PubMed] [Google Scholar]
- 20.Prutz, W. A. 1996. Hypochlorous acid interactions with thiols, nucleotides, DNA, and other biological substrates. Arch. Biochem. Biophys. 332:110-120. [DOI] [PubMed] [Google Scholar]
- 21.Prutz, W. A. 1998. Interactions of hypochlorous acid with pyrimidine nucleotides, and secondary reactions of chlorinated pyrimidines with GSH, NADH, and other biological substrates. Arch. Biochem. Biophys. 349:183-191. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez, R. A., I. L. Pepper, and C. P. Gerba. 2009. Application of PCR-based methods to assess the infectivity of enteric viruses in environmental samples. Appl. Environ. Microbiol. 75:297-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roelvink, P. W., I. Kovesdi, and T. J. Wickham. 1996. Comparative analysis of adenovirus fiber-cell interaction: adenovirus type 2 (Ad2) and Ad9 utilize the same cellular fiber receptor but use different binding strategies for attachment. J. Virol. 70:7614-7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roelvink, P. W., G. M. Lee, D. A. Einfeld, I. Kovesdi, and T. J. Wickham. 1999. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science 286:1568-1571. [DOI] [PubMed] [Google Scholar]
- 25.Shannon, M. A., P. W. Bonn, M. Elimelech, J. G. Georgiadis, B. J. Mariñas, and A. M. Mayes. 2008. Science and technology for water purification in the coming decades. Nature 452:301-310. [DOI] [PubMed] [Google Scholar]
- 26.Shin, G. A., and M. D. Sobsey. 2003. Reduction of Norwalk virus, poliovirus 1, and bacteriophage MS2 by ozone disinfection of water. Appl. Environ. Microbiol. 69:3975-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sirikanchana, K., J. L. Shisler, and B. J. Marinas. 2008. Effect of exposure to UV-C irradiation and monochloramine on adenovirus serotype 2 early protein expression and DNA replication. Appl. Environ. Microbiol. 74:3774-3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sobsey, M. D., D. A. Battigelli, G.-A. Shin, and S. Newland. 1998. RT-PCR amplification detects inactivated viruses in water and wastewater. Water Sci. Technol. 38(12):91-94. [Google Scholar]
- 29.Sobsey, M. D., T. Fuji, and P. A. Shields. 1988. Inactivation of hepatitis A virus and model viruses in water by free chorine and monochloramine. Water Sci. Technol. 20(11-12):385-391. [Google Scholar]
- 30.Stewart, P. L., C. Y. Chiu, S. Huang, T. Muir, Y. Zhao, B. Chait, P. Mathias, and G. R. Nemerow. 1997. Cryo-EM visualization of an exposed RGD epitope on adenovirus that escapes antibody neutralization. EMBO J. 16:1189-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thurston-Enriquez, J. A., C. N. Haas, J. Jacangelo, and C. P. Gerba. 2003. Chlorine inactivation of adenovirus type 40 and feline calicivirus. Appl. Environ. Microbiol. 69:3979-3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thurston-Enriquez, J. A., C. N. Haas, J. Jacangelo, and C. P. Gerba. 2005. Inactivation of enteric adenovirus and feline calicivirus by ozone. Water Res. 39:3650-3656. [DOI] [PubMed] [Google Scholar]
- 33.van Raaij, M. J., N. Louis, J. Chroboczek, and S. Cusack. 1999. Structure of the human adenovirus serotype 2 fiber head domain at 1.5 Å resolution. Virology 262:333-343. [DOI] [PubMed] [Google Scholar]
- 34.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins αv β3 and αv β5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]
- 35.Wiethoff, C. M., H. Wodrich, L. Gerace, and G. R. Nemerow. 2005. Adenovirus protein VI mediates membrane disruption following capsid disassembly. J. Virol. 79:1992-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winter, J., M. Ilbert, P. C. F. Graf, D. Ozcelik, and U. Jakob. 2008. Bleach activates a redox-regulated chaperone by oxidative protein unfolding. Cell 135:691-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu, E., L. Pache, D. J. VonSeggern, T. M. Mullen, Y. Mikyas, P. L. Stewart, and G. R. Nemerow. 2003. Flexibility of the adenovirus fiber is required for efficient receptor (CAR) interaction. J. Virol. 77:7225-7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yates, M. V., J. Malley, P. Rochelle, and R. Hoffman. 2006. Effect of adenovirus resistance on UV disinfection requirements: a report on the state of adenovirus science. J. Am. Water Works Assoc. 98:93-106. [Google Scholar]
- 39.Zubieta, C., G. Schoehn, J. Chroboczek, and S. Cusack. 2005. The structure of the human adenovirus 2 penton. Mol. Cell 17:121-135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.