Abstract
The use of naturally occurring microbial antagonists to suppress plant diseases offers a favorable alternative to classical methods of plant protection. The soybean epiphyte Pseudomonas syringae pv. syringae strain 22d/93 shows great potential for controlling P. syringae pv. glycinea, the causal agent of bacterial blight of soybean. Its activity against P. syringae pv. glycinea is highly reproducible even in field trials, and the suppression mechanisms involved are of special interest. In this work we demonstrated that P. syringae pv. syringae 22d/93 produced a significantly larger amount of siderophores than the pathogen P. syringae pv. glycinea produced. While P. syringae pv. syringae 22d/93 and P. syringae pv. glycinea produce the same siderophores, achromobactin and pyoverdin, the regulation of siderophore biosynthesis in the former organism is very different from that in the latter organism. The epiphytic fitness of P. syringae pv. syringae 22d/93 mutants defective in siderophore biosynthesis was determined following spray inoculation of soybean leaves. The population size of the siderophore-negative mutant P. syringae pv. syringae strain 22d/93ΔSid was 2 orders of magnitude lower than that of the wild type 10 days after inoculation. The growth deficiency was compensated for when wound inoculation was used, indicating the availability of iron in the presence of small lesions on the leaves. Our results suggest that siderophore production has an indirect effect on the biocontrol activity of P. syringae pv. syringae 22d/93. Although siderophore-defective mutants of P. syringae pv. syringae 22d/93 still suppressed development of bacterial blight caused by P. syringae pv. glycinea, siderophore production enhanced the epiphytic fitness and thus the competitiveness of the antagonist.
Application of epiphytic bacteria as control agents is considered a nonpolluting approach for alternative plant protection, and a number of potential antagonistic isolates have been described. However, only a few of these isolates have proven to be as effective under field conditions as they are in laboratory setups (1, 39). It has been proposed that several attributes contribute to biocontrol, including competition for nutrients, antibiosis, niche exclusion, and interference with cell signaling systems (13, 36).
Many potential antagonists have been selected from the fluorescent pseudomonad group, as this group includes various nonpathogenic species that are adapted to plant colonization and well known for their competitiveness (11, 19, 20). Pseudomonas fluorescens CHA0 has been proposed as biocontrol organism that can be used against several soilborne plant diseases (13). It has been suggested that secondary metabolites, such as 2,4-diacetylphloroglucinol, hydrogen cyanide, pyoverdin, and salicylate, are active principles in this isolate (11, 13).
P. fluorescens Pf-5 is a rhizosphere bacterium that suppresses seedling emergence diseases and produces a spectrum of antibiotics toxic to plant-pathogenic fungi (34). Pseudomonas putida WCS358, a plant growth-promoting rhizobacterium, is thought to protect its host plants by induction of induced systemic resistance (ISR) (46).
Screening for antagonistic epiphytes that can be used against Pseudomonas syringae plant pathogens identified P. syringae pv. syringae strain 22d/93 as a promising biocontrol agent. P. syringae pv. syringae 22d/93 was isolated from a soybean leaf that did not show any disease symptoms (48). It has a high level of antagonistic activity against the closely related pathogen P. syringae pv. glycinea, the causative agent of bacterial blight of soybean (48). P. syringae pv. syringae 22d/93 provided effective protection against P. syringae pv. glycinea in laboratory experiments, as well as in field trials (36, 47).
Supernatants of P. syringae pv. syringae 22d/93 had a direct inhibitory effect on the growth of P. syringae pv. glycinea isolate 1a/96, implying that antibiosis is involved in the antagonism (36). Production of the following three toxins by P. syringae pv. syringae 22d/93 has been demonstrated: the lipodepsipeptides syringomycin and syringopeptin and the amino acid derivative 3-methylarginine (5, 48). However, a comparison of different toxin-negative mutants with the wild type revealed that there were no differences in biocontrol activity against P. syringae pv. glycinea, suggesting that none of these toxins was essential for P. syringae pv. syringae 22d/93's antagonistic activity in planta (4).
Despite its great abundance, Fe3+ is considered a limiting nutrient in most microbial habitats due to its low solubility at neutral pH (33). The bioavailability of iron on leaf surfaces has been described as varying between low and limiting (32). Direct suppression of pathogen development by competition for iron has been proposed as a potential biocontrol trait (20, 31, 42). The hypothesis that iron uptake systems have an impact on biocontrol was proven for the control of Gaeumannomyces graminis by strains of P. fluorescens and for the control of Pythium-induced damping-off of tomato by Pseudomonas aeruginosa 7NSK2 (8, 21).
Recent studies have indicated that the iron uptake systems in P. syringae are more complex than previously supposed. In addition to the well-described peptide-type siderophore pyoverdin, some isolates produce a citrate-based siderophore called achromobactin (3). Other strains are able to produce yersiniabactin (6).
To determine the relevance of individual siderophore systems for the epiphytic fitness and biocontrol activity of P. syringae pv. syringae 22d/93, mutants defective in single or multiple siderophore biosynthesis systems were generated. The siderophore production of P. syringae pv. syringae 22d/93 and derivatives of this strain was analyzed and compared to that of the pathogen P. syringae pv. glycinea 1a/96.
MATERIALS AND METHODS
Bacterial strains, cultivation conditions, and plasmids.
Bacterial strains and plasmids used in this study are listed in Table 1. Pseudomonas strains were routinely grown in King's B (KB) medium at 28°C (28). Escherichia coli strains were cultivated at 37°C in Luria-Bertani (LB) medium (43). Antibiotics were used at final concentrations of 50 mg/liter for ampicillin and 25 mg/liter for chloramphenicol, kanamycin, and spectinomycin.
TABLE 1.
Strains, plasmids, and primer used in this study
| Strain, plasmid, or primer | Relevant characteristics | Reference or source |
|---|---|---|
| Escherichia coli strains | ||
| DH5α | supE44 ΔlacU169 (φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 43 |
| S17-1 λ-pir | λl-pir lysogen of S17-1 (thi pro hsdR hsdM+recA RP4:2− Tc::MU-Km::Tn7) Tcr Smr | 12 |
| Pseudomonas syringae strains | ||
| P. syringae pv. glycinea 1a/96 | Wild type isolated from soybean; Pvd+ Ach+ | 47 |
| P. syringae pv. syringae 22d/93 | Wild type isolated from soybean; Pvd+ Ach+ | 48 |
| P. syringae pv. syringae 22d/93ΔPvd | pvsA mutant carrying Kmr cassette in pvsA gene; Pvd− Ach+ Kmr | This study |
| P. syringae pv. syringae 22d/93ΔAch | acsD mutant carrying Kmr cassette in acsD gene; Pvd+ Ach− Kmr | This study |
| P. syringae pv. syringae 22d/93ΔSid | Tn5 mutant of P. syringae pv. syringae 22d/93ΔPvd carrying mini-Tn5 in acsD gene; Pvd− Ach− Kmr Spr | This study |
| Dickeya dadantii strains | ||
| 3937 cbsE-1 acsA-37 | cbsE::Ω acsA-37::MudII1734; chrysobactin− Ach− Spr Smr Kmr | 14 |
| 3937 acr-1 fct-34 | acr-1::Ω fct-34::lacZ; chrysobactin− Ach− Spr Smr Kmr | 16 |
| 3937 cbsE-1 tonB60 | cbsE::Ω tonB60::MudII1734; chrysobactin− Ach+ Spr Smr | 14 |
| Plasmids | ||
| pGEM-T Easy | TA cloning vector; Apr | Promega |
| pPVSA1 | 1.4-kb PCR fragment located in pvsA gene of P. syringae pv. syringae 22d/93, cloned in pGEM-T Easy; Apr | This study |
| pPVSA2 | 1.6-kb PCR fragment located in pvsA gene of P. syringae pv. syringae 22d/93, cloned in pGEM-T Easy; Apr | This study |
| pPVSA3 | 1.4-kb SpeI-KpnI fragment from pPVSA1 cloned in SpeI-KpnI-digested pPVSA2; Apr | This study |
| pPVSA4 | 1.8-kb KpnI Kmr cassette from pMKm cloned in KpnI-digested pPVSA3; Apr Kmr | This study |
| pACS1 | 1.3-kb PCR fragment located in acsF gene of P. syringae pv. syringae 22d/93, cloned in pGEM-T Easy; Apr | This study |
| pACS2 | 2-kb PCR fragment located in acsE and yhcA genes of P. syringae pv. syringae 22d/93, cloned in pGEM-T Easy; Apr | This study |
| pACS3 | 1.8-kb PstI Kmr cassette from pMKm blunt ended and cloned in SpeI-digested and blunt-ended pACS1; Apr Kmr | This study |
| pACS4 | 3-kb ScaI-EcoRI fragment from pACS3 blunt ended and cloned in EcoRV-digested pACS2; Apr Kmr | This study |
| pBBR1MCS | Broad-host-range cloning vector; Cmr | 30 |
| pBBR-11-Sid | 6-kb PstI fragment of P. syringae pv. syringae 22d/93ΔSid carrying Tn5 insertion site in pBBR1MCS; Cmr Spr | This study |
| pMKm | Donor of kanamycin cassette; Kmr | 41 |
| pUT/mini-Tn5 Sm/Sp | Mini-Tn5 encoding Smr/Spr on broad-host-range suicide plasmid pUT; Apr Smr Spr | 12 |
| Primers | ||
| pvsA_fwd1_Spe | 5′-AATACTAGTGGATCCTGATGCGACTGGCCTTCGATC-3′ | This study |
| pvsA_rev6_Kpn | 5′-TAAGGTACCACGTCGAGGCTGAGCGGATC-3′ | This study |
| pvsA_fwd7_Kpn | 5′-TTAGGTACCTCGAACTTGGCCTCGCGGCTG-3′ | This study |
| pvsA_rev4_Bam | 5′-TTTGGATCCGGCAGACCGTGGCTGAG-3′ | This study |
| Achr1_fwd | 5′-AGCGAGGACTCACAGATGTTG-3′ | This study |
| Achr2_KpnI_rev | 5′-GGTACCCAATGCTGCTGAATGGCAAC-3′ | This study |
| Achr5_EcoRV_fwd | 5′-GATATCAACTATGTGCGTCTTGCGTC-3′ | This study |
| Achr6_rev | 5′-ACGAATGCCACCAGACAGG-3′ | This study |
DNA manipulation techniques, plasmids, and primers.
Isolation and manipulation of DNA were performed using standard techniques (43). All chemicals and enzymes were commercial preparations and were used as specified by the supplier (Fermentas, St. Leon-Roth, Germany). All primers used in this study are listed in Table 1. Synthesis of oligonucleotides and DNA sequencing were performed by Eurofins MWG Operon (Ebersberg, Germany).
Growth under iron-limiting conditions and assays for siderophore detection.
Siderophore production was visualized on CAS agar plates (2). Bacterial concentrations were adjusted so that the optical density at 600 nm (OD600) was 1.0 in sterile water, and 5 μl of each suspension was spotted on a plate. Plates were incubated for 48 h at 28°C.
Different minimal media were used to investigate siderophore production by P. syringae pv. glycinea 1a/96 and P. syringae pv. syringae 22d/93 under iron-limiting conditions. The compositions of the media were as follows. Casamino Acids medium (CAA) (10) contained (per liter of demineralized water) 5 g of Difco Bacto Casamino Acids (nondesferrated), 0.9 g of K2HPO4·3H2O, and 0.25 g of MgSO4·7H2O. Succinate medium (SM) (37) contained (per liter of demineralized water) 4 g of succinic acid, 6 g of K2HPO4, 3 g of KH2PO4, 1 g of (NH4)2SO4, and 0.2 g of MgSO4·7H2O (pH 7.0). 5b medium contained (per liter of demineralized water) 2.6 g of KH2PO4, 5.5 g of Na2HPO4, 2.5 g of NH4Cl, and 1 g of Na2SO4 (solution A), as well as 10 g of glucose, 0.1 g of MgCl2·6H2O, and 0.01 g of MnSO4·4H2O (solution B). PIPES medium (44) contained (per liter of demineralized water) 30.24 g of piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES), 0.3 g of KH2PO4, 1 g of NH4Cl, and 1 g of Na2SO4 (pH 7.0) (solution A), as well as solution B of 5b medium. Solutions A and B of 5b and PIPES media were autoclaved separately and mixed 1:1 prior to use.
Siderophore content was quantified using the CAS assay (44). Briefly, 500 μl of CAS indicator solution containing 4 mM sulfosalicylic acid was mixed with the same volume of supernatant. The reaction mixtures were incubated for 60 min at room temperature to allow complex formation, and the siderophore-dependent color change was determined at a wavelength of 630 nm. For quantification, deferoxamin mesylate (DFOM) was used as the standard, and there was a linear relationship between decolorization and the DFOM concentration in the range from 0 to 20 μM.
Separation and identification of siderophores were performed by isoelectric focusing (IEF) using a Multiphore II electrophoresis unit (Amersham Pharmacia Biotech) as described by Koedam et al. (29). Purified siderophores of reference strains were used as standards. Siderophores were desalted and concentrated with XAD-4 resin (Sigma-Aldrich, Taufkirchen, Germany) prior to IEF separation (17). For binding of pyoverdin and achromobactin to XAD-4 resin, supernatants were acidified to pH 3.0, applied to water-equilibrated XAD-4 resin, washed twice with distilled water, and eluted with 50% methanol. The eluate was dried under a vacuum and resuspended in water.
Achromobactin cross-feeding assay.
The production of achromobactin by P. syringae pv. syringae 22d/93 was confirmed by performing cross-feeding assays under iron-depleted conditions as described previously (16). Briefly, 15 ml of L agar (38) supplemented with the iron chelator ethylenediamine-N,N′-bis(2-hydroxy-phenylacetic acid) (EDDHA) (40 μM; Sigma-Aldrich, Taufkirchen, Germany) was poured into plates, which were seeded with 10 μl of an overnight L broth culture of the indicator strain Dickeya dadantii 3937 cbsE-1 acsA-37 or 3937 acr-1 fct34. Under these conditions, the indicator strains were not able to grow unless an iron source, such as ferric siderophores, was provided. Sterile filter disks (diameter, 6 mm) were placed on the agar surface. Then 15-μl portions of filter-sterilized culture supernatants of the strains to be tested grown in KB medium for 24 h at 28°C were added to the filter disks. For reference, supernatant of the achromobactin producer D. dadantii 3937 cbsE-1 tonB60 grown overnight in Tris medium (16) was applied. Fe2+ (20 μM) was used as a control. The diameters of the zones of growth of the indicator strains were measured after 24 h.
Generation of siderophore-deficient mutants of P. syringae pv. syringae 22d/93.
For inactivation of pyoverdin biosynthesis, pvsA encoding a nonribosomal peptide synthase which is responsible for the formation of the pyoverdin chromophore was disrupted by marker exchange mutagenesis. Two fragments were PCR amplified using primer pairs pvsA_fwd1_Spe/pvsA_rev6_Kpn and pvsA_fwd7_Kpn/pvsA_rev4_Bam (Table 1). Primer sequences were derived from the genome sequence of P. syringae pv. syringae B728a. PCR products were cloned into pGEM-T Easy (Promega, Mannheim, Germany), yielding plasmids pPVSA1 and pPVSA2. A 1.4-kb KpnI-SpeI fragment cut from pPVSA1 was ligated into KpnI-SpeI-digested pPVSA2, yielding plasmid pPVSA3. A 1.8-kb KpnI kanamycin resistance cassette cut from pMKm (41) was ligated into KpnI-digested pPVSA3, yielding plasmid pPVSA4.
An achromobactin-negative mutant was constructed by disrupting the achromobactin biosynthesis gene acsD. A 1.3-kb fragment located in the acsF gene was PCR amplified using the primer pair Achr1_fwd/Achr2_KpnI_rev. A second 2-kb fragment located in the acsE and yhcA genes was amplified using the primer pair Achr5_EcoRV_fwd/Achr6_rev (Table 1). PCR products were ligated into pGEM-T Easy, yielding plasmids pACS1 and pACS2. A 1.8-kb PstI kanamycin resistance cassette cut from pMKm was blunt ended and ligated into SpeI-digested and blunt-ended pACS1, yielding pACS3. A 3-kb ScaI-EcoRI fragment cut from pACS3 was blunt ended and ligated into EcoRV-digested pACS2, yielding plasmid pACS4. Plasmids pPVSA4 and pACS4 were transformed into electrocompetent P. syringae pv. syringae 22d/93, and recombinants were selected on kanamycin plates. Putative mutants were screened for double homologous recombination events by PCR analysis. Mutants with the correct genotype were designated P. syringae pv. syringae 22d/93ΔPvd and P. syringae pv. syringae 22d/93ΔAch, respectively.
A siderophore-negative double mutant was constructed by Tn5 mutagenesis of P. syringae pv. syringae 22d/93ΔPvd. Plasmid pUT/mini-Tn5 Sm/Sp (12) was mobilized into P. syringae pv. syringae 22d/93ΔPvd by triparental mating. Mutants were selected on MG agar (27) with spectinomycin as the selection agent and screened for loss of siderophore production on CAS agar. For identification of the Tn5 insertion site, genomic DNA of the siderophore-negative strain P. syringae pv. syringae 22d/93ΔSid was cut with PstI and cloned into PstI-digested pBBR1MCS. Derived plasmids were transformed into electrocompetent E. coli and selected on spectinomycin plates. Sequencing of plasmid pBBR-11-Sid harboring an approximately 6-kb PstI fragment revealed insertion of Tn5 in the yhcA achromobactin biosynthesis gene.
Plant material and inoculation procedures.
Soybean plants (Glycine max cv. Maple Arrow) were grown on shelves equipped with fluorescent lamps at 22 to 24°C with 50% humidity and with supplemental light using a 14-h photoperiod (350 microeinsteins m−2 s−1).
For wound inoculation experiments, P. syringae strains grown on KB agar for 24 h at 28°C were suspended in distilled water, and the concentration was adjusted to 1.0 × 107 CFU per ml. For coinoculation experiments, cell suspensions of the pathogen P. syringae pv. glycinea 1a/96 were mixed with cell suspensions of P. syringae pv. syringae 22d/93 or mutants of this strain at a ratio of 1:2 (vol/vol). For single inoculations, the suspensions of P. syringae pv. syringae 22d/93, its mutants, or P. syringae pv. glycinea 1a/96 were mixed with sterile water to obtain the same number of cells that were used for coinoculation. Trifoliate leaves of 28-day-old soybean plants were inoculated by the wound inoculation technique described by May et al. (36). Leaves were pricked using a sterile needle, and each wound was inoculated with 5 μl of a bacterial suspension. Bacterial populations were monitored by removing 20 disks (diameter, 7 mm) surrounding the inoculation sites and homogenizing the leaf disks in 20 ml of isotonic NaCl. Bacterial counts (CFU per wound) were determined by plating dilutions of leaf homogenates onto KB agar.
For spray inoculation, P. syringae pv. syringae 22d/93 or siderophore mutants of this strain grown on KB agar for 24 h at 28°C were suspended in distilled water, the concentration was adjusted to 1.0 × 107 CFU per ml, and the strains were applied to leaves of 4-week-old soybean plants with an airbrush (∼8 lb/in2) until the leaf surface was uniformly wet. Growth of bacterial strains was monitored by removing random leaf samples 10 days after inoculation. Leaves were macerated in 10 ml isotonic NaCl per g (fresh weight). Bacterial counts (CFU per g [fresh weight]) were determined by plating dilutions of leaf homogenates onto KB agar.
RESULTS
Siderophore production by P. syringae pv. syringae 22d/93 in different media.
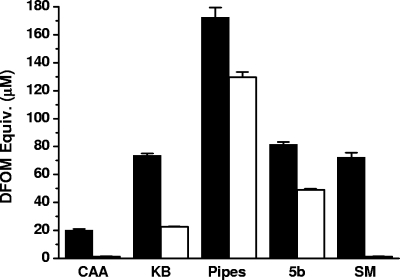
Screening for siderophore production on CAS agar plates showed that there was a remarkable difference between the antagonist P. syringae pv. syringae 22d/93 and the pathogen P. syringae pv. glycinea 1a/96; a significantly larger siderophore halo was produced by P. syringae pv. syringae 22d/93 than by P. syringae pv. glycinea 1a/96 (data not shown). The same tendency was observed in various low-iron liquid media when the siderophore activities of supernatants were determined by the CAS assay and normalized using cell density. In all media tested, the siderophore production by P. syringae pv. syringae 22d/93 was greater than that by P. syringae pv. glycinea 1a/96 (Fig. 1). The most obvious difference was observed with SM medium, while the greatest siderophore production by both strains was detected with PIPES medium. Thus, PIPES medium was used in subsequent experiments.
FIG. 1.
Influence of culture media on siderophore production. P. syringae pv. syringae 22d/93 (filled bars) and P. syringae pv. glycinea 1a/96 (open bars) were cultivated in various low-iron media at 28°C for 48 h. The siderophore activity of supernatants was determined by the CAS assay and normalized to an OD600 of 1.0. The data are the means and standard deviations of three independent experiments.
Production of pyoverdin and achromobactin by P. syringae pv. syringae 22d/93 and P. syringae pv. glycinea 1a/96.
Analysis of concentrated culture supernatants by IEF and detection of siderophore activity using CAS agar overlays revealed that P. syringae pv. syringae 22d/93 and P. syringae pv. glycinea 1a/96 produced identical siderophore patterns. The siderophores produced by P. syringae pv. syringae 22d/93 and P. syringae pv. glycinea 1a/96 were identified as pyoverdin and achromobactin by comparison with purified standards (Fig. 2). In addition to IEF analysis, cross-feeding experiments using achromobactin-deficient mutants of D. dadantii (formerly Erwinia chrysanthemi) were conducted to confirm the structural conformity of the achromobactin produced by P. syringae pv. syringae 22d/93 and that produced by D. dadantii (Table 2). Growth of the indicator strain D. dadantii 3937 cbsE-1 acs-37, which cannot produce endogenous siderophores, was restored by adding supernatant of P. syringae pv. syringae 22d/93, P. syringae pv. syringae B728a, or D. dadantii 3937 cbsE-1 tonB60, an achromobactin-producing D. dadantii strain. In contrast, supernatants of P. syringae pv. syringae 22d/93, P. syringae pv. syringae B728a, and D. dadantii 3937 cbsE-1 tonB60 could not restore growth of the achromobactin receptor mutant D. dadantii 3937 acr-1 fct34, which cannot produce or import achromobactin. Growth of both indicator strains was restored by adding the Fe2+ control, which excluded free iron in the supernatants tested.
FIG. 2.
Isoelectric focusing and CAS overlay for detection of siderophores in P. syringae. Siderophores were extracted from concentrated culture supernatants using XAD-4 resin at pH 3.0. After separation by isoelectric focusing, the polyacrylamide gel was overlaid with a thin layer of CAS agar to visualize siderophore activity. Lane 1, P. syringae pv. syringae 22d/93ΔSid; lane 2, purified pyoverdin of P. syringae; lane 3, P. syringae pv. syringae 22d/93; lane 4, P. syringae pv. glycinea 1a/96; lane 5, P. syringae pv. syringae 22d/93ΔPvd.
TABLE 2.
Cross-feeding assay using achromobactin biosynthesis and receptor mutants of D. dadantii as indicator strains
| Culture supernatant | Diam of indicator strain growth zone (mm)a |
|
|---|---|---|
| 3937 cbsE-1 acs-37b | 3937 acr-1 fct-34c | |
| No additive | 0 | 0 |
| 3937 cbsE-1 tonB60d | 11 | 0 |
| P. syringae pv. syringae 22d/93 | 11 | 0 |
| P. syringae pv. syringae B728a | 8 | 0 |
| Fe2+ controle | 5 | 5 |
The experiment was repeated three times. The data are data from a representative experiment.
Achromobactin and chrysobactin biosynthesis mutant of D. dadantii 3937.
Achromobactin and chrysobactin receptor mutant of D. dadantii 3937.
Achromobactin-producing and chrysobactin-negative mutant of D. dadantii 3937.
Fe2+ (20 μM) was used as a control.
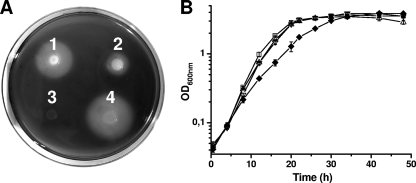
Further confirmation that achromobactin and pyoverdin are the only siderophores produced by P. syringae pv. syringae 22d/93 was obtained by performing a mutation analysis. Mutants defective for biosynthesis of either pyoverdin (P. syringae pv. syringae 22d/93ΔPvd) or achromobactin (P. syringae pv. syringae 22d/93ΔAch) still showed siderophore activity on CAS agar plates. The amounts of siderophores produced by P. syringae pv. syringae 22d/93ΔPvd and the wild type on CAS agar were similar, while P. syringae pv. syringae 22d/93ΔAch produced a significantly smaller siderophore halo (Fig. 3A). The pyoverdin- and achromobactin-negative double mutant P. syringae pv. syringae 22d/93ΔSid did not show any residual siderophore activity. To investigate the phenotypic difference between P. syringae pv. syringae 22d/93ΔPvd and P. syringae pv. syringae 22d/93ΔAch in more detail, the influence of siderophore production on in vitro growth was analyzed by culturing P. syringae pv. syringae 22d/93 and the siderophore mutants of this strain in PIPES medium. The optical densities of the cultures were monitored continuously until the cultures entered the late stationary growth phase (Fig. 3B). The doubling times of P. syringae pv. syringae 22d/93, P. syringae pv. syringae 22d/93ΔAch, and P. syringae pv. syringae 22d/93ΔPvd were comparable (2.4 to 2.8 h), while growth of P. syringae pv. syringae 22d/93ΔSid was significantly delayed under iron-limiting growth conditions (doubling time, about 3.8 h). Still, cultures of all of the strains reached similar optical densities in the stationary growth phase.
FIG. 3.
(A) Siderophore production by P. syringae pv. syringae 22d/93 and siderophore mutants of this strain on CAS agar. CAS agar plates were inoculated with 5-μl portions of suspensions (>106 CFU/ml) of the different strains and incubated at 28°C for 48 h. Siderophore production is indicated by the formation of haloes. 1, P. syringae pv. syringae 22d/93; 2, P. syringae pv. syringae 22d/93ΔAch; 3, P. syringae pv. syringae 22d/93ΔSid; 4, P. syringae pv. syringae 22d/93ΔPvd. (B) Growth of P. syringae pv. syringae 22d/93 and siderophore mutants of this strain in PIPES medium at 28°C, as determined by measurement of the OD600. The data are the means and standard deviations of three independent cultures. □, P. syringae pv. syringae 22d/93; ▿, P. syringae pv. syringae 22d/93ΔAch; ▵, P. syringae pv. syringae 22d/93ΔPvd; ⧫, P. syringae pv. syringae 22d/93ΔSid.
Regulation of siderophore biosynthesis in P. syringae pv. syringae 22d/93 and regulation of siderophore biosynthesis in P. syringae pv. glycinea 1a/96 are different.
Siderophore production by the pathogen P. syringae pv. glycinea 1a/96 during growth in PIPES medium and siderophore production by the antagonist P. syringae pv. syringae 22d/93 during growth in PIPES medium were compared. During the exponential growth phase, the siderophore production by both strains increased steadily, and the maximal values were reached in the early stationary phase. A slight decrease in the late stationary phase was observed. However, P. syringae pv. glycinea 1a/96 and P. syringae pv. syringae 22d/93 responded quite differently to changes in the growth conditions, indicating that they have different regulatory mechanisms for siderophore production. Incubation with a low ratio of culture volume to flask volume resulted in a growth delay for both strains. The doubling times of P. syringae pv. syringae 22d/93 and P. syringae pv. glycinea 1a/96 were 3.5 h and 5.0 h, respectively, at a ratio of 1:5. However, the doubling times of both strains increased to 9.5 h at a ratio of 1:10. Nevertheless, cultures of both strains reached similar cell densities under these growth conditions in late stationary phase.
The siderophore activity produced by the antagonist P. syringae pv. syringae 22d/93 was strongly dependent on the ratio of culture volume to flask volume. The maximal CAS activity was approximately 40 μM DFOM equivalents at a ratio of 1:5, compared to a CAS activity of approximately 1.2 mM DFOM equivalents at a 1:10 ratio. The siderophore activity of P. syringae pv. glycinea 1a/96 was not influenced by the ratio of culture volume to flask volume and reached values of approximately 500 μM DFOM equivalents in the stationary phase under both conditions.
Impact of siderophores produced by P. syringae pv. syringae 22d/93 on epiphytic fitness and biocontrol efficacy.
Development of bacterial populations on soybean leaves was observed for P. syringae pv. syringae 22d/93 and siderophore mutants of this strain 10 days after a single spray inoculation to evaluate the contribution of siderophore production to the epiphytic fitness of the antagonist (Table 3). P. syringae pv. syringae 22d/93 reached a population size of 6.7 × 104 CFU per g leaf tissue, whereas the population sizes of both single mutants, P. syringae pv. syringae 22d/93ΔAch and P. syringae pv. syringae 22d/93ΔPvd, were 1 order of magnitude lower (4.6 × 103 and 5.4 × 103 CFU per g leaf tissue, respectively). The greatest effect on in planta growth was observed for the siderophore-negative strain P. syringae pv. syringae 22d/93ΔSid, which reached a population size that was 2 orders of magnitude lower (4.0 × 102 CFU per g leaf tissue) than that of the wild type.
TABLE 3.
Population dynamics of P. syringae pv. syringae 22d/93 and siderophore mutants of this strain on soybean leaves after a single spray inoculation
| Strain | Concn (CFU per g leaf tissue)a |
|
|---|---|---|
| Day 1 | Day 10 | |
| P. syringae pv. syringae 22d/93 | 5.0 × 104 ± 2.2 × 104 | 6.7 × 104 ± 3.0 × 103 |
| P. syringae pv. syringae 22d/93ΔPvd | 1.3 × 104 ± 2.0 × 103 | 5.4 × 103 ± 2.0 × 103 |
| P. syringae pv. syringae 22d/93ΔAch | 8.9 × 104 ± 1.6 × 103 | 4.6 × 103 ± 2.0 × 103 |
| P. syringae pv. syringae 22d/93ΔSid | 9.2 × 104 ± 6.4 × 103 | 4.0 × 102 ± 5.0 × 101 |
The data are the means and standard deviations of three independent determinations.
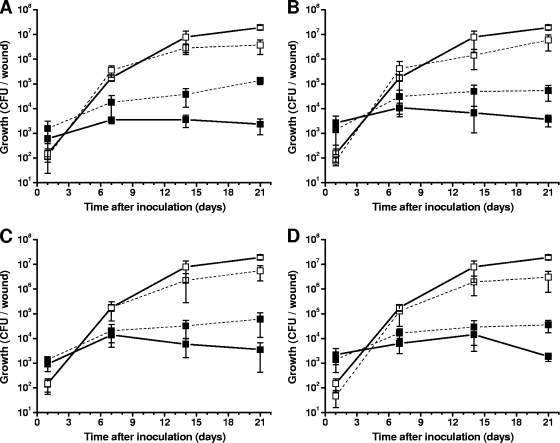
To examine if siderophores produced by P. syringae pv. syringae 22d/93 are responsible for the antagonistic activity against P. syringae pv. glycinea, the pathogen was coinoculated with either P. syringae pv. syringae 22d/93 or one of the siderophore mutants into wounds on pin-pricked soybean leaves. Single-inoculation experiments were conducted as a control. Following a single inoculation, P. syringae pv. glycinea 1a/96 caused typical symptoms (necrotic spots surrounded by chlorotic haloes) approximately 14 days after inoculation. At this time, the population size of P. syringae pv. glycinea 1a/96 was about 107 CFU per wound. P. syringae pv. syringae 22d/93 and the siderophore mutants developed stable populations whose sizes were about 104 CFU per wound 7 days after a single inoculation, and they did not cause any disease symptoms (Fig. 4).
FIG. 4.
Population dynamics of P. syringae pv. glycinea 1a/96, P. syringae pv. syringae 22d/93, and P. syringae pv. syringae 22d/93 siderophore mutants after single inoculation (solid lines) and coinoculation (dashed lines) into soybean leaves using wound inoculation. Single inoculations were used to evaluate the epiphytic fitness of the strains and as controls for comparison with the coinoculation experiment. (A) Coinoculation of P. syringae pv. glycinea 1a/96 (□) with P. syringae pv. syringae 22d/93 (▪) and controls; (B) coinoculation of P. syringae pv. glycinea 1a/96 (□) with P. syringae pv. syringae 22d/93ΔPvd (▪) and controls; (C) coinoculation of P. syringae pv. glycinea 1a/96 (□) with P. syringae pv. syringae 22d/93ΔAch (▪) and controls; (D) coinoculation of P. syringae pv. glycinea 1a/96 (□) with P. syringae pv. syringae 22d/93ΔSid (▪) and controls. The data are the means and standard deviations of four independent experiments.
In coinoculation experiments with P. syringae pv. glycinea 1a/96, the population sizes of P. syringae pv. syringae 22d/93 and mutants of this strain were approximately 1 order of magnitude higher than the population sizes in single-inoculation experiments, suggesting that the antagonist benefited from coinoculation with the pathogen. In contrast, the population size of the pathogen was about 1 order of magnitude lower in all coinoculation experiments than in the respective single-inoculation experiment. The smaller population was sufficient to completely suppress development of disease symptoms. Further, siderophore production by the antagonist had no effect on the population size of the pathogen, showing that siderophore production and uptake are not factors involved in the direct biocontrol activity of P. syringae pv. syringae 22d/93.
DISCUSSION
Siderophore production by P. syringae pv. syringae 22d/93.
It has been known for a long time that plant-associated P. syringae isolates produce pyoverdin, a peptide-type siderophore that causes the green fluorescence responsible for the term “fluorescent pseudomonads” (7, 9, 25). However, the hypothesis that one bacterial isolate produces one type of siderophore has been revised due to the increased availability of genome sequence information so that it is now acknowledged that there is great redundancy in iron uptake systems, which includes the presence of multiple of siderophore receptors in, and the production of several different siderophores by, a single bacterium (18). Thus, a recent study demonstrated that P. syringae pv. syringae B728a produces a second citrate-type siderophore, achromobactin, that was first isolated from D. dadantii (3, 40). In this study, we demonstrated that pyoverdin and achromobactin are also synthesized by P. syringae pv. syringae 22d/93 and P. syringae pv. glycinea 1a/96. However, a significant difference in the amounts of siderophores produced by these P. syringae isolates was found. Moreover, a strong correlation between siderophore production and growth conditions was observed for P. syringae pv. syringae 22d/93 but not for P. syringae pv. glycinea 1a/96. Since all other growth parameters were identical, the large amount of oxygen available was the most likely cause of the reduced growth rates of both organisms when the ratio of culture volume to flask volume was 1:10. There is an intimate relationship between iron metabolism and the oxygen concentration (22, 49). Generation of oxidative stress is an important plant defense response, and production of achromobactin has been linked to the survival of D. dadantii during an oxidative burst (15). The different responses of P. syringae pv. syringae 22d/93 and P. syringae pv. glycinea 1a/96 to high-oxygen conditions might be linked to adaptation to their epiphytic and pathogenic lifestyles, respectively.
Iron limitation during in planta growth.
Several studies have addressed the question of iron availability in the phyllosphere. Elaborative reporter gene analyses used ice nucleation activity and green fluorescent protein to determine iron resources in different habitats (24, 35). Both reporter constructs were based on an iron-regulated promoter derived from the pyoverdin biosynthesis gene cluster. Heterogeneous reporter activity responses were observed in the phyllosphere, indicating that only a small subset of the P. syringae population analyzed experienced low iron availability (24). However, the pathogen P. syringae pv. tabaci showed reduced virulence after mutagenesis of the pyoverdin biosynthesis system (45). Our analysis of siderophore mutants of the epiphyte P. syringae pv. syringae 22d/93 showed that loss of pyoverdin production results in only a slight reduction in in planta growth, while loss of all siderophore production had a more severe impact. Similarly, analysis of a yersiniabactin-negative mutant of P. syringae pv. tomato DC3000 that was still able to produce pyoverdin showed no impairment of growth on Arabidopsis (23). In contrast, we observed significantly reduced growth of the siderophore-negative mutant P. syringae pv. syringae 22d/93ΔSid after spray inoculation. However, no differences in growth between P. syringae pv. syringae wild-type strain 22d/93 and siderophore mutants of this strain were found in wound inoculation experiments, which might have been due to nutrient leakage and therefore improved iron availability. A similar effect of the inoculation method was observed for P. syringae pv. tabaci (45). While mutants affected in pyoverdin production showed only slightly reduced virulence when they were infiltrated into tobacco leaves, the phenotype was more severe when spray inoculation was used.
Another system in which siderophore production has been shown to influence plant colonization is the achromobactin-chrysobactin system in D. dadantii (15, 16). Mutational analysis resulted in the conclusion that achromobactin is required for initiation of plant colonization at the onset of infection, while chrysobactin is necessary for development of a systemic infection. The reduced population sizes of the P. syringae pv. syringae 22d/93 siderophore mutants indicate that achromobactin production had a similar influence on epiphytic colonization of soybean leaves. Studies of the relevance of the achromobactin-pyoverdin siderophore system in P. syringae might increase our understanding of the role of iron availability in host-pathogen interactions. Karamanoli and Lindow (26) demonstrated that leaf surface compounds had an iron-sequestering effect. The different results for the relevance of siderophore production for plant-associated bacteria undoubtedly reflect the natural gradient from iron sequestration by the host plant to leakage of iron from the plant tissue.
Impact of siderophore production on the biocontrol activity of P. syringae pv. syringae 22d/93.
There were no significant differences between disease suppression after coinoculation of P. syringae pv. syringae 22d/93 siderophore mutants and P. syringae pv. glycinea 1a/96 and disease suppression after coinoculation of P. syringae pv. syringae wild-type strain 22d/93 and P. syringae pv. glycinea 1a/96, suggesting that siderophores do not play an essential role in this antagonism. We demonstrated that P. syringae pv. syringae 22d/93 and P. syringae pv. glycinea 1a/96 produce the same siderophores, precluding the possibility of direct competition for iron between P. syringae pv. syringae 22d/93 and P. syringae pv. glycinea. Still, the foremost characteristic of a good biocontrol organism is efficient colonization of the host plant. Production of achromobactin and pyoverdin contributes significantly to the epiphytic fitness of P. syringae pv. syringae 22d/93, thus improving its biocontrol activity in an indirect way. Furthermore, the production of two high-affinity iron acquisition systems by P. syringae pv. syringae 22d/93 might increase its competitiveness with other epiphytes.
Acknowledgments
This study was financially supported by Deutsche Forschungsgemeinschaft (DFG) and Jacobs University Bremen.
We are grateful to J.-M. Meyer, Strasbourg, France, for kind provision of reference siderophores. We thank Yvonne Braun and Alexander Schenk for helpful discussions.
Footnotes
Published ahead of print on 5 March 2010.
REFERENCES
- 1.Agrios, G. N. 2005. Plant pathology, 5th ed. Elsevier Academic Press, San Diego, CA.
- 2.Alexander, D. B., and D. A. Zuberer. 1991. Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol. Fertil. Soil 12:39-45. [Google Scholar]
- 3.Berti, A. D., and M. G. Thomas. 2009. Analysis of achromobactin biosynthesis by Pseudomonas syringae pv. syringae B728a. J. Bacteriol. 191:4594-4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun, S. D., J. Hofmann, A. Wensing, H. Weingart, M. S. Ullrich, D. Spiteller, and B. Völksch. In vitro antibiosis by Pseudomonas syringae Pss22d, acting against the bacterial blight pathogen of soybean plants, does not influence in planta biocontrol. J. Phytopathol., in press.
- 5.Braun, S. D., B. Völksch, J. Nüske, and D. Spiteller. 2008. 3-Methylarginine from Pseudomonas syringae pv. syringae 22d/93 suppresses the bacterial blight caused by its close relative Pseudomonas syringae pv. glycinea. Chembiochem 9:1913-1920. [DOI] [PubMed] [Google Scholar]
- 6.Buell, C. R., V. Joardar, M. Lindeberg, J. Selengut, I. T. Paulsen, M. L. Gwinn, R. J. Dodson, R. T. Deboy, A. S. Durkin, J. F. Kolonay, R. Madupu, S. Daugherty, L. Brinkac, M. J. Beanan, D. H. Haft, W. C. Nelson, T. Davidsen, N. Zafar, L. Zhou, J. Liu, Q. Yuan, H. Khouri, N. Fedorova, B. Tran, D. Russell, K. Berry, T. Utterback, S. E. Van Aken, T. V. Feldblyum, M. D'Ascenzo, W. L. Deng, A. R. Ramos, J. R. Alfano, S. Cartinhour, A. K. Chatterjee, T. P. Delaney, S. G. Lazarowitz, G. B. Martin, D. J. Schneider, X. Tang, C. L. Bender, O. White, C. M. Fraser, and A. Collmer. 2003. The complete genome sequence of the Arabidopsis and tomato pathogen Pseudomonas syringae pv. tomato DC3000. Proc. Natl. Acad. Sci. U. S. A. 100:10181-10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bultreys, A., I. Gheysen, H. Maraite, and E. de Hoffmann. 2001. Characterization of fluorescent and nonfluorescent peptide siderophores produced by Pseudomonas syringae strains and their potential use in strain identification. Appl. Environ. Microbiol. 67:1718-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buysens, S., K. Huengens, J. Poppe, and M. Höfte. 1996. Involvement of pyochelin and pyoverdin in suppression of Pythium-induced damping-off of tomato by Pseudomonas aeruginosa 7NSK2. Appl. Environ. Microbiol. 62:865-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cody, Y. S., and D. C. Gross. 1987. Characterization of pyoverdinpss, the fluorescent siderophore produced by Pseudomonas syringae pv. syringae. Appl. Environ. Microbiol. 53:928-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornelis, P., V. Anjaiah, N. Koedam, P. Delfosse, P. Jacques, P. Thonart, and L. Neirinckx. 1992. Stability, frequency and multiplicity of transposon insertions in the pyoverdine region in the chromosomes of different fluorescent pseudomonads. J. Gen. Microbiol. 138:1337-1343. [DOI] [PubMed] [Google Scholar]
- 11.Couillerot, O., C. Prigent-Combaret, J. Caballero-Mellado, and Y. Moënne-Loccoz. 2009. Pseudomonas fluorescens and closely-related fluorescent pseudomonads as biocontrol agents of soil-borne phytopathogens. Lett. Appl. Microbiol. 48:505-512. [DOI] [PubMed] [Google Scholar]
- 12.de Lorenzo, V., M. Herrero, U. Jakubzik, and K. N. Timmis. 1990. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol. 172:6568-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duffy, B. K., and G. Défago. 1999. Environmental factors modulating antibiotic and siderophore biosynthesis by Pseudomonas fluorescens biocontrol strains. Appl. Environ. Microbiol. 65:2429-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enard, C., and D. Expert. 2000. Characterization of a tonB mutation in Erwinia chrysanthemi 3937: TonBEch is a member of the enterobacterial TonB family. Microbiology 146:2051-2058. [DOI] [PubMed] [Google Scholar]
- 15.Expert, D. 1999. Withholding and exchanging iron: interactions between Erwinia spp. and their plant hosts. Annu. Rev. Phytopathol. 37:307-334. [DOI] [PubMed] [Google Scholar]
- 16.Franza, T., B. Mahé, and D. Expert. 2005. Erwinia chrysanthemi requires a second iron transport route dependent of the siderophore achromobactin for extracellular growth and plant infection. Mol. Microbiol. 55:261-275. [DOI] [PubMed] [Google Scholar]
- 17.Georgias, H., K. Taraz, H. Budzikiewicz, V. Geoffroy, and J.-M. Meyer. 1999. The structure of the pyoverdin from Pseudomonas fluorescens 1.3. Structural and biological relationships of pyoverdins from different strains. Z. Naturforsch. C 54:301-308. [Google Scholar]
- 18.Grass, G. 2006. Iron transport in Escherichia coli: all has not been said and done. Biometals 19:159-172. [DOI] [PubMed] [Google Scholar]
- 19.Haas, D., C. Blumer, and C. Keel. 2000. Biocontrol ability of fluorescent pseudomonads genetically dissected: importance of positive feedback regulation. Curr. Opin. Biotechnol. 11:290-297. [DOI] [PubMed] [Google Scholar]
- 20.Haas, D., and G. Défago. 2005. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 3:307-319. [DOI] [PubMed] [Google Scholar]
- 21.Hamdan, H., D. M. Weller, and L. S. Thomashow. 1991. Relative importance of fluorescent siderophores and other factors in biological control of Gaeumannomyces graminis var. tritici by Pseudomonas fluorescens 2-79 and M4-80R. Appl. Environ. Microbiol. 57:3270-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hantke, K. 2001. Iron and metal regulation in bacteria. Curr. Opin. Microbiol. 4:172-177. [DOI] [PubMed] [Google Scholar]
- 23.Jones, A. M., S. E. Lindow, and M. C. Wildermuth. 2007. Salicylic acid, yersiniabactin, and pyoverdin production by the model phytopathogen Pseudomonas syringae pv. tomato DC3000: synthesis, regulation, and impact on tomato and Arabidopsis host plants. J. Bacteriol. 189:6773-6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joyner, D. C., and S. E. Lindow. 2000. Heterogeneity of iron bioavailability on plants assessed with a whole-cell GFP-based bacterial biosensor. Microbiology 146:2435-2445. [DOI] [PubMed] [Google Scholar]
- 25.Jülich, M., K. Taraz, H. Budzikiewicz, V. Geoffroy, J.-M. Meyer, and L. Gardan. 2001. The structure of the pyoverdin isolated from various Pseudomonas syringae pathovars. Z. Naturforsch. C 56:687-694. [DOI] [PubMed] [Google Scholar]
- 26.Karamanoli, K., and S. E. Lindow. 2006. Disruption of N-acyl homoserine lactone-mediated cell signaling and iron acquisition in epiphytic bacteria by leaf surface compounds. Appl. Environ. Microbiol. 72:7678-7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keane, P. J., A. Kerr, and P. B. New. 1970. Crown gall of stone fruit. II. Identification and nomenclature of Agrobacterium isolates. Aust. J. Biol. Sci. 23:585-595. [Google Scholar]
- 28.King, E. O., M. K. Ward, and D. E. Raney. 1954. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 44:301-307. [PubMed] [Google Scholar]
- 29.Koedam, N., E. Wittouck, A. Gaballa, A. Gillis, M. Höfte, and P. Cornelis. 1994. Detection and differentiation of microbial siderophores by isoelectric focusing and chrome azurol S overlay. Biometals 7:287-291. [DOI] [PubMed] [Google Scholar]
- 30.Kovach, M. E., R. W. Phillips, P. H. Elzer, R. M. Roop II, and K. M. Peterson. 1994. pBBR1MCS: a broad-host-range cloning vector. Biotechniques 16:800-802. [PubMed] [Google Scholar]
- 31.Leong, J. 1986. Siderophores: their biochemistry and possible role in the biocontrol of plant pathogens. Annu. Rev. Phytopathol. 24:187-209. [Google Scholar]
- 32.Lindow, S. E., and M. T. Brandl. 2003. Microbiology of the phyllosphere. Appl. Environ. Microbiol. 69:1875-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loper, J. E., and J. S. Buyer. 1991. Siderophores in microbial interactions on plant-surfaces. Mol. Plant-Microbe Interact. 4:5-13. [Google Scholar]
- 34.Loper, J. E., D. Y. Kobayashi, and I. T. Paulsen. 2007. The genomic sequence of Pseudomonas fluorescens Pf-5: insights into biological control. Phytopathology 97:233-238. [DOI] [PubMed] [Google Scholar]
- 35.Loper, J. E., and S. E. Lindow. 1994. A biological sensor for iron available to bacteria in their habitats on plant surfaces. Appl. Environ. Microbiol. 60:1934-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.May, R., B. Völksch, and G. Kampmann. 1997. Antagonistic activities of epiphytic bacteria from soybean leaves against Pseudomonas syringae pv. glycinea in vitro and in planta. Microb. Ecol. 34:118-124. [DOI] [PubMed] [Google Scholar]
- 37.Meyer, J.-M., A. Stintzi, D. De Vos, P. Cornelis, R. Tappe, K. Taraz, and H. Budzikiewicz. 1997. Use of siderophores to type pseudomonads: the three Pseudomonas aeruginosa pyoverdine systems. Microbiology 143:35-43. [DOI] [PubMed] [Google Scholar]
- 38.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 39.Montesinos, E. 2003. Development, registration and commercialization of microbial pesticides for plant protection. Int. Microbiol. 6:245-252. [DOI] [PubMed] [Google Scholar]
- 40.Münzinger, M., H. Budzikiewicz, D. Expert, C. Enard, and J. M. Meyer. 2000. Achromobactin, a new citrate siderophore of Erwinia chrysanthemi. Z. Naturforsch. C 55:328-332. [DOI] [PubMed] [Google Scholar]
- 41.Murillo, J., H. Shen, D. Gerhold, A. Sharma, D. A. Cooksey, and N. T. Keen. 1994. Characterization of pPT23B, the plasmid involved in syringolide production by Pseudomonas syringae pv. tomato PT23. Plasmid 31:275-287. [DOI] [PubMed] [Google Scholar]
- 42.O'Sullivan, D. J., and F. O'Gara. 1992. Traits of fluorescent Pseudomonas spp. involved in suppression of plant root pathogens. Microbiol. Rev. 56:662-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sambrook, J., E. F. Fritsch, and T. E. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 44.Schwyn, B., and J. B. Neilands. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160:47-56. [DOI] [PubMed] [Google Scholar]
- 45.Taguchi, F., T. Suzuki, Y. Inagaki, K. Toyoda, T. Shiraishi, and Y. Ichinose. 2009. The siderophore pyoverdine of Pseudomonas syringae pv. tabaci 6605 is intrinsic virulence factor in host tobacco infection. J. Bacteriol. 192:117-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Wees, S. C., C. M. Pieterse, A. Trijssenaar, Y. A. Van't Westende, F. Hartog, and L. C. Van Loon. 1997. Differential induction of systemic resistance in Arabidopsis by biocontrol bacteria. Mol. Plant-Microbe Interact. 10:716-724. [DOI] [PubMed] [Google Scholar]
- 47.Völksch, B., and R. May. 2001. Biological control of Pseudomonas syringae pv. glycinea by epiphytic bacteria under field conditions. Microb. Ecol. 41:132-139. [DOI] [PubMed] [Google Scholar]
- 48.Völksch, B., J. Nüske, and R. May. 1996. Characterization of two epiphytic bacteria from soybean leaves with antagonistic activities against Pseudomonas syringae pv. glycinea. J. Basic Microbiol. 36:453-462. [DOI] [PubMed] [Google Scholar]
- 49.Zheng, M., B. Doan, T. D. Schneider, and G. Storz. 1999. OxyR and SoxRS regulation of fur. J. Bacteriol. 181:4639-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]