Abstract
Wild animals are well-known reservoirs of Campylobacter and Salmonella. We investigated the influence of insalubrious diets on the prevalence of both enterobacteria in seagulls. Campylobacter occurrence in gull chicks sampled along the northeastern Iberian coast was directly related to the degree of refuse consumption. High Salmonella values from the sampling sites did not reflect any dietary relationship.
Campylobacter and Salmonella spp. are the leading causes of zoonotic enteric infections in developed and developing countries, and their incidence is increasing even in countries with adequate public health surveillance (14, 27, 44). Despite the health impact of these enteropathogenic bacteria, their epidemiology remains poorly understood, and the full epidemiological pathways leading to infection in humans have not yet been elucidated.
Well-known modes of transmission to humans include physical contact with domestic animals, person-to-person spread, and consumption of contaminated food and water (44). In addition, wild animals might play a significant role in the epidemiology of enterobacteria (3, 34). For instance, the role of wild birds in the bacteriological deterioration of drinking and recreational water reservoirs by fecal contamination is well documented (2, 19). Furthermore, due to their ability to fly freely and to cover long distances during annual movements, wild-living birds are suspected of functioning as effective dispersers of disease via the aforementioned fecal contamination of pastures and surface waters throughout the world (33).
Seagulls in particular, due to their scavenging feeding habits, are one of the most documented carriers of Campylobacter and Salmonella (8, 11, 18, 23). The increasing number of studies concerning seagulls and environmental public health are also partially due to the fact that populations of several species of gulls (Larus spp.) have increased dramatically throughout Australia, North America, and Europe during the past several decades (36, 41). Although feeding habits related to garbage and sewage have been largely assumed to increase the risk of microbiological infection on wildlife (8, 19), to our knowledge no studies prove this assumption by combining dietary analysis and microbiological carriage determination.
During the late chick-rearing period of 2005, 182 yellow-legged gull chicks were sampled in three colonies along the northeastern Iberian coast (Medes Islands, n = 75; Ebro Delta, n = 36; and Columbretes Islands, n = 71) (see Table 1 for colony details). A single fledgling from each brood was captured, sampled, measured, weighed, and marked. Avian health status was evaluated through a body condition index computed from residuals from a regression of body size (principal-component analysis based on head-bill, tarsus, and wing lengths) against mass (39, 43). We collected 6 to 8 growing scapular feathers from each bird as well as some food samples spontaneously regurgitated (kept frozen at −20°C) for stable isotope analysis of carbon (C), nitrogen (N), and sulfur (S). Once regurgitated items were identified and classified according to their origin (marine, brackish, and freshwaters; crops and terrestrial environments; or refuse sites), we followed standard procedures (7, 13, 32), weighing and placing into tin capsules subsamples of powdered feathers and powdered food for final combustion in a stable isotope mass spectrometer (ThermoFinnigan, Bremen, Germany). Stable isotope ratios were expressed in the standard δ notation relative to Pee Dee Belemnite (δ13C), atmospheric nitrogen (δ15N), and troilite of the Canyon Diablo meteorite (δ34S) (32). Relative indexes of the different food sources were estimated for every individual, using a triple-isotope, four-endpoint (marine and freshwaters, terrestrial environments, and refuse sites) mixing model (30). Finally, for bacterial isolation, duplicate cloacal swabs from each chick were taken and placed in Amies charcoal medium (Deltalab, Barcelona, Spain), stored under refrigeration, and cultured within 2 to 4 days after sampling. Salmonella isolation was performed according to ISO 6579:2002 (1), and presumptive colonies on MacConkey agar (Oxoid, United Kingdom) were confirmed with the Mucap test kit (Biolife, Italy) and API 20E system (bioMérieux, France). Salmonella species isolates were serotyped at the National Reference Centre for Animal Salmonellosis (Algete, Madrid, Spain). Campylobacter isolation was performed as described previously (42). Identification of Campylobacter jejuni, Campylobacter coli, and Campylobacter lari were carried out with a species-specific PCR (10, 37).
TABLE 1.
Main informative parameters of studied breeding sites
| Location (coordinates) | Breeding site | Distance from human settlements (km) | No. of breeding pairs | Fishing vessel activity around each area (2007)a |
Reference | ||
|---|---|---|---|---|---|---|---|
| Relative estimation | No. of vessels | Gross tonnage | |||||
| Medes Islands (42°0′N, 3°13′E) | Protected islands off the coast of a tourist resort | 0.9 | 6,500b | Moderate-high | 392 | 8,956 | 6 |
| Ebro Delta (40°40′N, 0°45′E) | Isolated peninsula in a natural park | 7.5 | 6,000 | High | 358 | 11,195 | 28 |
| Columbretes Islands (39°54′N, 0°41′E) | Isolated archipelago in a marine reserve | 55 | 450 | High | 245 | 9,248 | 28 |
Fishing vessel information for each area was taken from http://ec.europa.eu/fisheries.
After several cullings.
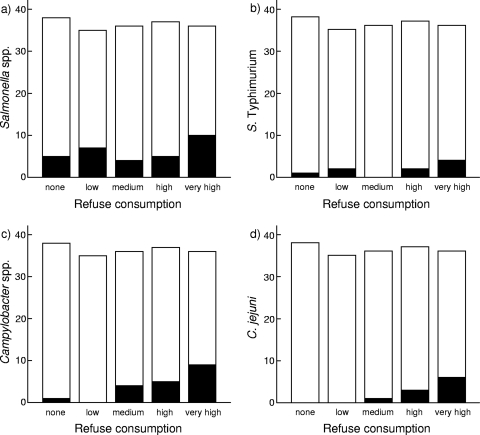
Salmonella spp. were isolated from 31 birds (17.0%; 95% confidence interval [CI], 11.9 to 23.1%), which generated an overall incidence similar to those described from other studies within the same area (5) as well as from the Atlantic Iberian Peninsula (12), the British Islands (23), Fennoscandia (34), and Central Europe (11, 16). However, Campylobacter spp. were recovered only from 19 (10.4%; 95% CI, 6.6 to 15.7%) of all the samples collected (Table 2), which, compared to the results of other studies, represented a relatively low carrier rate (8, 16, 24). The highest values of Salmonella species prevalence among fledging gulls were observed in the Medes Islands (Table 2), although differences among localities were not statistically significant (χ2 = 1.99, P = 0.39). Similarly, the prevalence of Salmonella enterica serovar Typhimurium did not show any significant geographical variation (χ2 = 2.53, Monte Carlo P = 0.36). A conditional logistic model using locality as the stratum did not find any reliable model to fit Salmonella species and S. Typhimurium prevalences using the consumption of refuse, the body condition index of gulls, and their possible interaction as dependent variables (likelihood ratio [LR] test = 3.29, Monte Carlo P = 0.19, and LR test = 0.8, Monte Carlo P = 0.71, respectively). On the other hand, Campylobacter prevalence was significantly greater in gulls from the Medes Islands (18.7%) than in gulls from the Columbretes Islands, where only three birds (4.2%) were found positive for Campylobacter (χ2 = 9.28, P = 0.0007) (Table 2). A conditional logistic model using locality as the stratum showed that Campylobacter prevalence was positively related to refuse consumption (parameter ± standard error, 7.19 ± 3.31; P = 0.029) (see Fig. 2), whereas the body condition index did not show a significant relationship. Similarly, C. jejuni carriage was more probable in gulls from the Medes Islands than in gulls from the other localities (χ2 = 10.5, Monte Carlo P = 0.001), although we failed to find any relationship with refuse consumption or body condition using conditional logistic regression (LR test = 2.41, Monte Carlo P = 0.29).
TABLE 2.
Campylobacter species and Salmonella serovar carriage rates of fecal samples from yellow-legged gull chicks sampled throughout three western Mediterranean colonies
| Species or serovar | No. (%) of infected gull chicks froma: |
||
|---|---|---|---|
| Medes Islands (n = 75) | Ebro Delta (n = 36) | Columbretes Islands (n = 71) | |
| Campylobacter species | |||
| C. jejuni | 8 | 0 | 1 |
| C. coli | 0 | 0 | 0 |
| C. lari | 0 | 0 | 0 |
| Not determined | 6 | 2 | 2 |
| Total carriers | 14 (18.67) | 2 (5.56) | 3 (4.23) |
| S. enterica subsp. salamae serovar Sofia (4,12:b:-) | 1 (1.33) | 0 (0) | 0 (0) |
| S. enterica subsp. enterica serovar | |||
| Azteca | 1 | 0 | 0 |
| Bardo | 1 | 0 | 0 |
| Brandenburg | 0 | 0 | 1 |
| Bredeney | 2 | 2 | 1 |
| Corvallis | 0 | 0 | 2 |
| Derby | 1 | 0 | 2 |
| Enteritidis | 0 | 0 | 1 |
| Hadar | 0 | 2 | 2 |
| Ituri | 1 | 0 | 0 |
| Lexington | 1 | 0 | 0 |
| Newport | 2 | 0 | 0 |
| Paratyphi B | 1 | 0 | 0 |
| Rissen | 1 | 0 | 0 |
| Typhimurium | 6 | 1 | 2 |
| Virchow | 0 | 0 | 1 |
| 1,4,5,12:i:- | 1 | 0 | 0 |
| 1,4,12:i:- | 1 | 0 | 0 |
| 4,12:i:- | 1 | 0 | 0 |
| 4,5,12:i:- | 1 | 0 | 0 |
| Total carriers | 16 (21.33) | 4 (11.11) | 11 (15.49) |
Bacterial prevalences are in parentheses.
FIG. 2.
Enterobacterial prevalence on yellow-legged gull chicks according to refuse consumption on the Iberian Mediterranean coast. The numbers of birds positive for Salmonella spp. (a), Salmonella enterica subsp. enterica serovar Typhimurium (b), Campylobacter spp. (c), and Campylobacter jejuni (d) are shown in black according to refuse consumption. The numbers of gulls negative for enterobacteria are shown in white. x axis categories represent the quintiles (n = 182; none = 38, low = 35, medium = 36, high = 37, very high = 36) according to the individual refuse consumption percentages estimated from isotopic mixing models.
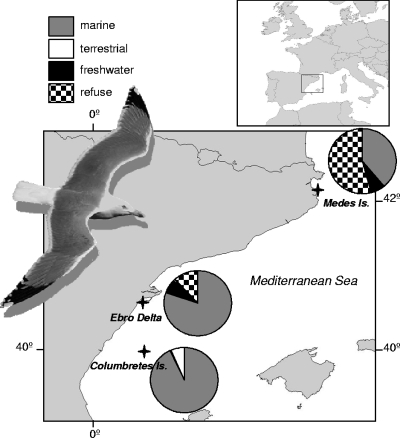
Campylobacter and Salmonella have been isolated from a variety of ecological sources, although in sealife both pathogens are thought to cause little or no disease (22). In addition, several extensive studies on Antarctic seabirds found them all to be Salmonella and Campylobacter negative (4, 29), suggesting that seabirds, in general, acquire both bacteria after exposure to human-altered environments, particularly to those related to garbage and sewage (40). In agreement with this, birds from the Medes Islands feeding abundantly on refuse waste showed greater Campylobacter bacterial prevalence than those from the Columbretes Islands, where chicks fed almost exclusively on fish (Fig. 1). However, chicks from the Columbretes Islands showed a relatively high prevalence of Salmonella but a low prevalence of Campylobacter, which might be due to differential ecological behaviors between these bacteria. Salmonella can persist in the environment for long periods (20) and probably survives in the soil of the breeding colony between reproductive periods. On the other hand, Campylobacter infection may be restricted to direct transmission, since some abiotic variables, such as temperature and aerobic atmosphere (26, 35), particularly dehydration, negatively affect the survival of Campylobacter in the environment (25). In support of these different ecological behaviors in the infection pathways of Salmonella and Campylobacter, we found no associative relationship between both bacteria within each individual (Fisher's exact test, P = 0.25; only one bird from the Medes Islands was positive for both bacteria). In addition, constant values of Salmonella incidence in gulls throughout Europe (from 10% to 20%) may represent a stable level of carriage compared to the variable Campylobacter prevalence (ranging from 1% to 62%), further corroborating the difference in persistence between these bacteria in the environment.
FIG. 1.
Map locations and foraging habitat exploitation of the yellow-legged gull colonies sampled in the study along the western Mediterranean coast. Foraging habitat exploitation percentages estimated by individual isotopic mixing models are represented as colony means in the circle diagrams.
Two of the most threatening enterobacteria for human health, S. Typhimurium and C. jejuni (38), were the most frequently isolated bacteria in the studied area. However, other bacteria more related to wild avifauna, such as C. lari, were surprisingly not detected. S. Typhimurium and C. jejuni were more abundant in the Medes Islands (Table 2), where yellow-legged gulls extensively exploited refuse sites. Although we failed in detecting a specific significant relationship of S. Typhimurium and C. jejuni with gulls' feeding habits, the results presented here provide additional insights into this issue (Fig. 2). An unusual serovar, S. enterica subsp. salamae serovar Sofia (4,12:b:-), was also isolated; its habitat is believed to be cold-blooded animals and the environment (31), although it has also been isolated from other sources (9, 12, 17). On the other hand, S. enterica serotype Paratyphi B (isolated from one chick from the Medes Islands) is able to cause both enteric fever and gastroenteritis and is recovered mainly from humans (21). The presence of this virulent human pathogen in seagulls is also of notable public health concern because of the potential risk that these birds may pose for the transmission of enteric fevers.
The dispersal ranges of infectious pathogens are linked to the movement capacity of their infected hosts as well as of their animal reservoirs (15). In spite of that fact, there are few published epidemiological studies focusing on the potential effect of bacterial carriage on the health status of wildlife. Presumably, birds with enterobacterial infections or with food limitations are in poorer body condition than noninfected birds and they might be negatively affected, especially during the sensitive chick development stage (39). However, our results suggested that Campylobacter and Salmonella did not affect the body condition of chick gulls, providing some evidence that gulls may merely act as nonaffected carriers of these enterobacteria rather than show clinical signs of disease. Therefore, as subclinical carriers, there would be no health limitations imposed on yellow-legged gulls by infection. In turn, the fledgling and adult movement capacity is not hampered, and therefore there is potential for the dispersal of pathogenic Campylobacter and Salmonella over large geographical areas. This could, in part, contribute to the nearly worldwide distribution of both enteropathogens (44).
Acknowledgments
We dedicate this article to the memory of Xavier Ruiz, who unexpectedly died on 27 April 2008 when we were writing the manuscript of this article. For his constant effort in proposing brilliant ideas which improved this and other papers and for his encouragement and general support, we will always be in his debt.
We thank J. González-Solís for his critical reading of the manuscript and both J. Dent and S. Kurtz for helping in the English correction.
Footnotes
Published ahead of print on 5 March 2010.
REFERENCES
- 1.Anonymous. 2002. Microbiology of food and animal feeding stuffs—horizontal method for the detection of Salmonella (EN ISO 6579:2002). International Organization for Standardization, Geneva, Switzerland.
- 2.Benton, C., F. Khan, P. Monaghan, W. N. Richards, and C. B. Shedden. 1983. Contamination of a major water supply by gulls (Larus sp.): a study of the problem and remedial action taken. Water Res. 17:789-798. [Google Scholar]
- 3.Bogomolni, A. L., R. J. Gast, J. C. Ellis, M. Dennett, K. R. Pugliares, B. J. Lentell, and M. J. Moore. 2008. Victims or vectors: a survey of marine vertebrate zoonoses from coastal waters of the Northwest Atlantic. Dis. Aquat. Org. 81:13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnedahl, J., T. Broman, J. Waldenström, H. Palgrem, T. Niskanen, and B. Olsen. 2005. In search of human-associated bacterial pathogens in Antarctic wildlife: report from six penguin colonies regularly visited by tourists. Ambio 34:430-432. [PubMed] [Google Scholar]
- 5.Bosch, M., and M. Muniesa. 1996. Las gaviotas patiamarillas (Larus cachinnans) de la colonia de las islas medes (NE de España) como posibles agentes transmisores de contaminación microbiana. Doñana Acta Vert. 23(1):75-81. [Google Scholar]
- 6.Bosch, M., D. Oro, F. J. Cantos, and M. Zabala. 2000. Short-term effects of culling on the ecology and population dynamics of the yellow-legged gull. J. Appl. Ecol. 37:369-385. [Google Scholar]
- 7.Bosch, M., D. Oro, and X. Ruiz. 1994. Dependence of yellow-legged gulls (Larus cachinnans) on food from human activity in two western Mediterranean colonies. Avocetta 18:135-139. [Google Scholar]
- 8.Broman, T., H. Palmgren, S. Bergström, M. Sellin, J. Waldenström, M.-L. Danielsson-Tham, and B. Olsen. 2002. Campylobacter jejuni in black-headed gulls (Larus ridibundus): prevalence, genotypes, and influence on C. jejuni epidemiology. J. Clin. Microbiol. 40:4594-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carramiñana, J. J., J. Yangüela, D. Blanco, C. Rota, A. I. Agustín, A. Ariño, and A. Herrera. 1997. Salmonella incidence and distribution of serotypes throughout processing in a Spanish poultry slaughterhouse. J. Food Prot. 160:1312-1317. [DOI] [PubMed] [Google Scholar]
- 10.Chuma, T., S. Hashimoto, and K. Okamoto. 2000. Detection of thermophilic Campylobacter from sparrows by multiplex PCR: the role of sparrows as a source of contamination of broilers with Campylobacter. J. Vet. Med. Sci. 62:1291-1295. [DOI] [PubMed] [Google Scholar]
- 11.Cízek, A., I. Literák, K. Hejlícek, F. Treml, and J. Smola. 1994. Salmonella contamination of the environment and its incidence in wild birds. Zentralbl. Veterinarmed. B 41:320-327. [DOI] [PubMed] [Google Scholar]
- 12.Duarte, E. L., M. M. Guerra, and F. M. Bernardo. 2002. Salmonella and Listeria spp. carriage by gulls (larids). Rev. Port. Ciênc. Vet. 97:181-187. [Google Scholar]
- 13.Folch, J., M. Lees, and G. H. Sloane-Stanley. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226:497-509. [PubMed] [Google Scholar]
- 14.Friedman, C. R., J. Neimann, H. C. Wegener, and R. V. Tauxe. 2001. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations, p. 121-138. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, DC.
- 15.Frost, J. A. 2001. Current epidemiological issues in human campylobacteriosis. J. Appl. Microbiol. 30:85S-95S. [DOI] [PubMed] [Google Scholar]
- 16.Glunder, G., U. Neumann, S. Braune, J. Pruter, S. Petersen, and G. Vauk. 1991. The occurrence of Campylobacter spp. and Salmonella spp. in gulls in northern Germany. Dtsch. Tierarztl. Wochenschr. 98:152-155. [PubMed] [Google Scholar]
- 17.Harrington, C. S., J. A. Lanser, P. A. Manning, and C. J. Murray. 1991. Epidemiology of Salmonella sofia in Australia. Appl. Environ. Microbiol. 57:223-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapperud, G., and O. Rosef. 1983. Avian wildlife reservoir of Campylobacter fetus subsp. jejuni, Yersinia spp., and Salmonella spp. in Norway. Appl. Environ. Microbiol. 45:375-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lévesque, B., P. Brousseau, F. Bernier, É. Dewailly, and J. Joly. 2000. Study of the bacterial content of ring-billed gull droppings in relation to recreational water quality. Water Res. 34:1089-1096. [Google Scholar]
- 20.Literák, I., A. Cízek, and J. Smola. 1996. Survival of salmonellas in a colony of common black-headed gulls Larus ridibundus between two nesting periods. Colon. Waterbirds 19:268-269. [Google Scholar]
- 21.Martínez-Urtaza, J., A. Echeita, and E. Liebana. 2006. Phenotypic and genotypic characterization of Salmonella enterica serotype paratyphi B isolates from environmental and human sources in Galicia, Spain. J. Food Prot. 169:1280-1285. [DOI] [PubMed] [Google Scholar]
- 22.Minette, H. P. 1986. Salmonellosis in the marine environment. A review and commentary. Int. J. Zoonoses 13:71-75. [PubMed] [Google Scholar]
- 23.Monaghan, P., C. D. Shedden, K. Ensor, C. R. Fricker, and R. W. A. Girdwood. 1985. Salmonella carriage by herring gulls in the Clyde area of Scotland in relation to their feeding ecology. J. Appl. Ecol. 22:669-680. [Google Scholar]
- 24.Moore, J. E., D. Gilpin, E. Crothers, A. Canney, A. Kaneko, and M. Matsuda. 2002. Occurrence of Campylobacter spp. and Cryptosporidium spp. in seagulls (Larus spp.). Vector Borne Zoonotic Dis. 2:111-114. [DOI] [PubMed] [Google Scholar]
- 25.Murphy, C., C. Carroll, and K. N. Jordan. 2006. Environmental survival mechanisms of the foodborne pathogen Campylobacter jejuni. J. Appl. Microbiol. 100:623-632. [DOI] [PubMed] [Google Scholar]
- 26.Newell, D. G., and C. Fearnley. 2003. Sources of Campylobacter colonization in broiler chickens. Appl. Environ. Microbiol. 69:4343-4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oberhelman, R. A., and D. N. Taylor. 2000. Campylobacter infections in developing countries, p. 139-154. In I. Nachamikin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, DC.
- 28.Oro, D., A. Martinez-Abrain, M. Paracuellos, J. C. Nevado, and M. Genovart. 2006. Influence of density dependence on predator-prey seabird interactions at large spatio-temporal scales. Proc. Biol. Sci. 273:379-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmgrem, H., D. J. McCafferty, A. Aspán, T. Broman, M. Sellin, R. Wollin, S. Bergström, and B. Olsen. 2000. Salmonella in sub-Antarctica: low heterogeneity in salmonella serotypes in South Georgian seals and birds. Epidemiol. Infect. 125:257-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips, D. L., and P. L. Koch. 2002. Incorporating concentration dependence in stable isotope mixing models. Oecologia 130:114-125. [DOI] [PubMed] [Google Scholar]
- 31.Popoff, M. Y., and L. E. Le Minor. 2005. Salmonella. Springer Science, New York, NY.
- 32.Ramos, R., F. Ramírez, C. Sanpera, L. Jover, and X. Ruiz. 2009. Feeding ecology of yellow-legged gulls Larus michahellis in the western Mediterranean: a comparative assessment using conventional and isotopic methods. Mar. Ecol. Prog. Ser. 377:289-297. [Google Scholar]
- 33.Reed, K. D., J. K. Meece, J. S. Henkel, and S. K. Shukla. 2003. Birds, migration and emerging zoonoses: West Nile virus, Lyme disease, influenza A and enteropathogens. Clin. Med. Res. 1:5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Refsum, T., K. Handeland, D. L. Baggesen, G. Holstad, and G. Kapperud. 2002. Salmonellae in avian wildlife in Norway from 1969 to 2000. Appl. Environ. Microbiol. 68:5595-5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinton, L. W., R. R. Braithwaite, C. H. Hall, and M. L. Mackenzie. 2007. Survival of indicator and pathogenic bacteria in bovine feces on pasture. Appl. Environ. Microbiol. 73:7917-7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith, G. C., and N. Carlile. 1993. Methods for population control within a silver gull colony. Wildl. Res. 20:219-226. [Google Scholar]
- 37.Stonnet, V., L. Sicinschi, F. Megraud, and J. L. Guesdon. 1995. Rapid detection of Campylobacter jejuni and Campylobacter coli isolated from clinical specimens using the polymerase chain reaction. Eur. J. Clin. Microbiol. Infect. Dis. 14:355-359. [DOI] [PubMed] [Google Scholar]
- 38.Tauxe, R. V. 1997. Emerging foodborne diseases: an evolving public health challenge. Emerg. Infect. Dis. 3:425-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tella, J. L., M. G. Forero, M. Bertelotti, J. A. Donázar, G. Blanco, and O. Ceballos. 2001. Offspring body condition and immunocompetence are negatively affected by high breeding densities in a colonial seabird: a multi-scale approach. Proc. Biol. Sci. 268:1455-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tizard, I. 2004. Salmonellosis in wild birds. J. Exot. Pet Med. 13:50-66. [Google Scholar]
- 41.Vidal, E., F. Medail, and T. Tatoni. 1998. Is the yellow-legged gull a superabundant bird species in the Mediterranean? Impact on fauna and flora, conservation measures and research priorities. Biodivers. Conserv. 7:1013-1026. [Google Scholar]
- 42.Waldenström, J., T. Broman, I. Carlsson, D. Hasselquist, R. P. Achterberg, J. A. Wagenaar, and B. Olsen. 2002. Prevalence of Campylobacter jejuni, Campylobacter lari, and Campylobacter coli in different ecological guilds and taxa of migrating birds. Appl. Environ. Microbiol. 68:5911-5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Werschkul, D. F. 1979. Nestling mortality and the adaptive significance of early locomotion in the little blue Heron. Auk 96:116-130. [Google Scholar]
- 44.WHO Scientific Working Group. 1980. Enteric infections due to Campylobacter, Yersinia, Salmonella, and Shigella. Bull. World Health Organ. 58:519-537. [PMC free article] [PubMed] [Google Scholar]