Abstract
A bacterium producing an extracellular bioflocculant was isolated from contaminated LB medium and identified as Bacillus licheniformis by 16S rRNA gene sequencing and its biochemical/physiological characteristics. The optimum culture conditions for flocculant production were an initial medium pH of 7.2 and an inoculum size of 4% (vol/vol). The maximum flocculating activity (700 U/ml) was obtained after cultivation at 37°C for 48 h. Chemical analyses of the purified bioflocculant revealed that it was a proteoglycan composed of 89% carbohydrate and 11% protein (wt/wt). The mass ratio of neutral sugar, amino sugar, and uronic acid was measured at 7.9:4:1. Infrared spectrometry further indicated the presence of carboxyl, hydroxyl, and amino groups, typical of heteropolysaccharide. The average mass of the bioflocculant was calculated to be 1.76 × 106 Da. Scanning electron microscopy (SEM) images of the bioflocculant showed an irregular structure with netted texture. Its efficient flocculation capabilities suggest potential applications in industry.
Flocculants have been widely used in a variety of industrial processes, such as wastewater treatment, the food and fermentation industries, drinking water purification, and industrial downstream processes (25, 30). In general, flocculants are divided into chemically synthesized flocculants (organic and inorganic flocculants) and natural flocculants (chitosan, algin, and microbial flocculants) (26). Although chemical high-polymer flocculants, such as polyacrylamide (PAM), are frequently used because of their low cost and marked effectiveness, most of them are difficult to degrade, and many of the monomers derived from synthetic polymers are harmful to humans (4, 5, 13). Presently, several countries have banned or limited the use of such flocculants.
Bioflocculants, special macromolecules secreted by microorganisms, induce solid particles, bacteria, cells, and colloidal particles in a liquid suspension to flocculate and sediment. So far, microorganisms including algae, bacteria, actinomyces, and fungi have been reported to produce bioflocculants. Phormidium sp. strain J-1, isolated from a drainage channel, had excellent flocculation activity for bentonite particles in liquid suspension (9). A protein bioflocculant produced by Rhodococcus erythropolis could efficiently flocculate suspended solids, which easily lose their flocculating activity by enzymatic digestion (16, 28). Klebsiella sp. strain S11, screened from activated sludge, was unable to flocculate without the addition of Ca2+ (7). Shih et al. (25) first reported that a culture broth of Bacillus licheniformis CCRC 12816 displayed marked flocculating properties when cultivated in a medium containing citric acid, glutamic acid, and glycerol. The approximate mass of the bioflocculant from B. licheniformis CCTCC AB 208260 was determined to be 6.89 × 104 Da (18). In spite of these studies, bioflocculants have not yet been produced industrially because of their comparatively low flocculating capability and high cost. Thus, the search for microorganisms with better flocculant-producing abilities and reduced production costs continues among researchers in this field (10, 14).
In this study, a new strain, B. licheniformis CGMCC 2876, with a high flocculant-producing capability was isolated from contaminated LB medium in our laboratory. This paper reports the isolation of the microorganism and the identification of a novel proteoglycan bioflocculant.
MATERIALS AND METHODS
Strain.
A bioflocculant-producing strain was isolated in our laboratory and deposited in the China General Microbiological Culture Collection Centre (CGMCC) (Beijing, China) with the accession number 2876.
Media and cultivation conditions.
A medium used to select for bioflocculant-producing strains contained (per liter) glucose, 5 g; yeast extract, 1 g; urea, 1 g; KH2PO4, 0.1 g; K2HPO4, 0.1 g; NaCl, 0.1 g; MgSO4·7H2O, 0.2 g; and agar, 20 g. The medium for a slant contained (per liter) yeast extract, 1 g; beef extract, 1 g; tryptone, 2 g; glucose, 10 g; FeSO4, 0.02 g; and agar, 20 g. The seed medium contained (per liter) glucose, 10 g; yeast extract, 0.5 g; urea, 0.5 g; KH2PO4, 0.1 g; NaCl, 0.1 g; and MgSO4·7H2O, 0.2 g. The fermentation medium contained (per liter) sucrose, 10 g; yeast extract, 1 g; urea, 1 g; KH2PO4, 0.1 g; K2HPO4, 0.1 g; NaCl, 0.1 g; and MgSO4·7H2O, 0.2 g. LB medium contained (per liter) tryptone, 10 g; yeast extract, 5 g; and NaCl, 10 g. The initial pH of all media was adjusted to 7.2 to 7.5 with NaOH (1 M) and HCl (0.5 M). All the media were prepared with distilled water and sterilized at 121°C for 20 min. All cultivations were done at 37°C.
Isolation and culture of the bioflocculant-producing bacteria.
The contaminated LB medium was diluted and then plated on the selective medium described above. Strains with different colony morphologies were selected and inoculated in 250-ml flasks containing 50 ml fermentation medium. The strains were incubated for 48 h at 37°C with shaking at 200 rpm. The flocculating activities of the culture broths were observed. The strain with the highest flocculating activity (CGMCC 2876) was selected, examined for genetic stability, and then stored on slant medium at 4°C for further research.
Identification of strain CGMCC 2876.
Strain CGMCC 2876 was incubated in 250-ml flasks containing 50 ml fresh LB medium for 16 h at 37°C with shaking at 200 rpm. The genomic DNA of the strain was then extracted using the E.Z.N.A. Bacterial DNA Kit (Omega Bio-Tek). PCR amplification was carried out to determine the partial 16S rRNA gene. The PCR program was 30 cycles of 94°C (1 min), 55°C (30 s), and 72°C (1.5 min) (7). The PCR primers were 5′-AGAGTTTGATC(C/A)TGGCTCAG-3′ (forward) and 5′-TACGG(C/T)TACCTTGTTACGACTT-3′ (reverse). Purification of the PCR products and the determination of sequences were performed by GeneCore BioTechnologies Co., Ltd. (Shanghai, China). The 16S rRNA gene sequence of strain CGMCC 2876 obtained was compared with the NCBI database (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
The biochemical and physiological characteristics of strain CGMCC 2876 were identified according to Bergey's Manual of Systematic Bacteriology (2).
Production of the bioflocculant.
Strain CGMCC 2876 from a slant medium was inoculated into a 250-ml flask containing 100 ml seed medium and cultivated at 37°C for 14 h at 200 rpm. Four milliliters of the culture was then transferred into another 250-ml flask containing 100 ml fermentation medium. The bioflocculant was produced by shaking the flask at 37°C and 200 rpm for 48 h. Cell-free supernatant was obtained by centrifugation at 5,000 × g for 30 min. The cells were washed twice with sterile water and finally suspended in 100 ml of distilled water. The flocculating activities of the fermentation broth, cell-free supernatant, and cell suspension were each determined. Uninoculated medium was used as a control.
Purification of the bioflocculant.
The bioflocculant produced by strain CGMCC 2876 was purified according to the method described by Baror and Shilo (1) and Salehizadeh et al. (22). The fermentation broth was centrifuged at 5,000 × g for 30 min. The supernatant was then mixed with 2 volumes of chilled ethanol and left to stand at 4°C overnight. The resultant precipitate was collected by centrifugation at 5,000 × g for 30 min, and the crude bioflocculant was obtained. The crude bioflocculant was redissolved in distilled water, followed by the addition of 2% hexadecyl trimethyl ammonium bromide (HTAB) with stirring at 100 rpm. After 3 h, the HTAB suspension was separated by centrifugation (5,000 × g; 15 min) and redissolved in NaCl (0.5 M). Two volumes of cold ethanol was then added. The resulting precipitate was then washed three times with ethanol and finally lyophilized to collect the purified bioflocculant.
Physical and chemical analyses of the bioflocculant.
The total sugar content of the purified bioflocculant was determined by the phenol-sulfuric acid method using glucose as the standard solution (32). The total protein content was measured by the Lowery method using bovine serum albumin as the standard solution (32). The component sugars of the purified bioflocculant were formed by hydrolysis of the bioflocculant with trifluoroacetic acid at 121°C for 2 h. The resultant amino sugars were determined according to the Elson-Morgan method (3). The neutral-sugar content was determined by the anthrone reaction (32). The uronic acid content was assayed using the carbazole-sulfuric acid method (11).
The purified bioflocculant was analyzed with a Fourier transform infrared (FT-IR) spectrophotometer (Nicolet IR200; Thermo Electron Corporation) over a wave number range of 4,000 to 500 cm−1. The scanning electron microscopy (SEM) image of the bioflocculant was obtained using an FEI XL30 (FEI; Netherlands) and LEO 1530 (LEO; Germany).
Molecular mass determination of the bioflocculant.
The molecular mass of the bioflocculant was determined by high-performance gel permeation chromatography (HPGPC) coupled to refractive index (RI) detection. The samples were dissolved in distilled water and filtered through a 0.45-μm membrane, and then 50 μl of sample was injected into a high-performance liquid chromatography (HPLC) system with a TSK G4000PWxl column (300 mm by 7.8 mm [inside diameter]; 13 μm; 500 A; Tosoh, Japan). NaN3 (0.001%) was pumped into the HPLC system at a flow rate of 0.5 ml/min. The column temperature and pressure were maintained at 30°C and 1.3 MPa, respectively. A series of dextran T standards (Pharmacia, Sweden) were used to make a calibration curve for molecular mass determination. The following regression equation was obtained: log(mass) = K1T + K2, where mass (Da) and T (min) are the molecular mass and retention time of the samples, respectively, and K1 and K2 are constants.
Determination of cell dry weight.
Culture broth (10 ml) was centrifuged at 5,000 × g for 10 min, and the supernatant was discarded. The cell precipitate was washed twice with 10 ml distilled water and then dried at 105°C overnight to a constant weight.
Determination of the flocculating activity.
The flocculating activity was measured according to the method described by Kurane et al. (16). One milliliter of sample and 2.5 ml of CaCl2 solution (10 g liter−1) were mixed with 40 ml of 1% (wt/vol) kaolin solution, gently shaken, and left to stand for 5 min at room temperature. By measuring the decrease of turbidity in the upper phase, flocculating activity was expressed as the flocculating rate (FR): FR (U/ml) = (A − B)/A × 100 × D, where A and B are the optical densities of the control and the sample at 550 nm, respectively, and D is the dilution time of the culture broth free of cells. Each sample was measured in triplicate.
pH stability of the bioflocculant.
The pH values of 8 groups of sample solutions were adjusted to 2.0, 3.0, 4.0, 5.0, 6.0, 8.0, 10.0, and 12.0 with NaOH (1 M) and HCl (0.5 M), respectively, and then left at room temperature for 24 h, and the remaining flocculating activity of each sample was assayed.
Temperature stability of the bioflocculant.
The pH values of 6 groups of sample solutions were adjusted to 3.0, 4.0, 5.0, 6.0, 8.0, and 10.0. Each sample was then treated at 4°C, 35°C, 60°C, 80°C, and 100°C for 30 min. The remaining flocculating activity of each sample treated at different temperatures was determined.
Nucleotide sequence accession number.
A 1,457-bp fragment of the 16S rRNA gene was obtained and registered in GenBank with the accession number GQ148817.
RESULTS AND DISCUSSION
Isolation and identification of the bioflocculant-producing strain.
In total, 45 mucoid strains were screened from the contaminated LB medium. Strain CGMCC 2876 showed the highest flocculating activity in kaolin suspension and was thus chosen for further research.
Strain CGMCC 2876 was a long, rod-shaped, Gram-positive facultative anaerobe that formed spores. When grown on selective medium, the colonies appeared to be circular, transparent, slimy, viscous, and creamy in color. Some of the biochemical and physiological characteristics of the strain were as follows: arabinose (positive), xylose (positive), glucose (positive), sucrose (positive), phenylalanine (negative), urea (positive), citrate (negative), arginine (positive), starch (positive), hippurate hydrolysis (negative), indole test (negative), nitrate (positive), and motile (positive).
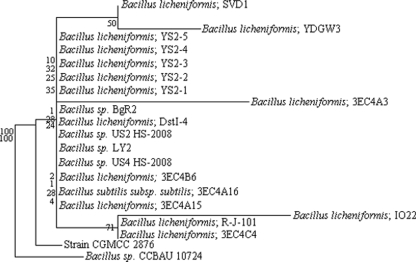
A BLAST search against GenBank indicated that the 16S rRNA gene nucleotide sequence of strain CGMCC 2876 was 99% similar to that of the B. licheniformis type strain ATCC 14580 (GenBank accession number CP000002.3). A phylogenetic tree was constructed between it and similar sequences found in GenBank (Fig. 1). Strain CGMCC 2876 was identified as B. licheniformis by both its morphological/physiological and phylogenetic characteristics.
FIG. 1.
Phylogenetic tree based on the nucleotide sequences of the 16S rRNA gene.
Growth profiles of B. licheniformis CGMCC 2876.
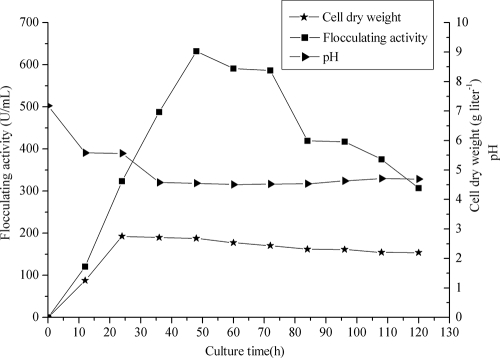
Time courses of the growth, pH, and flocculating activity of culture broth of B. licheniformis are given in Fig. 2. The flocculating activity of the culture broth increased in parallel with cell growth during the exponential phase. We observed a sharp decline in pH levels from 7.18 to 4.58 in the first 36 h, which might have been due to the production of organic acids from glucose or the presence of organic acid components of the flocculant polymer (7). Beyond that point, the pH was nearly stable for the following 80 h. The biomass reached its maximum in the first 24 h, and the flocculating activity started to decrease after 48 h because of cell lysis and enzymatic activity (19, 24). A yield of 2.93 g liter−1 purified bioflocculant was obtained when the flocculating activity reached the maximum.
FIG. 2.
Time courses of the growth, pH, and flocculating activity of culture broth of B. licheniformis cultivated on a rotary shaker at 200 rpm and 37°C for 130 h.
Some explicit comparisons of flocculant yields from different strains are provided in Table 1. Obviously, the yield of bioflocculant from B. licheniformis CGMCC 2876 was high and the fermentation period was relatively short. Both advantages may decrease the production cost of the bioflocculant and thus lay a foundation for further industrial application of the bioflocculant.
TABLE 1.
Comparison of the purified bioflocculant yields from different strains
| Strain | Fermentation conditions | Fermentation period (days) | Purified-bioflocculant yield (g liter−1) | Reference |
|---|---|---|---|---|
| Alcaligenes latus | 300 rpm, 30°C | 2-5 | 1.944 | 29 |
| Paecilomyces sp. | 200 rpm, 25°C | 5 | 0.5 | 27 |
| Rhodococcus erythropolis | No report | 4-5 | 0.205 | 28 |
| Bacillus mucilaginosus | 150 rpm, 30°C | 3-4 | 1 | 6 |
| Vagococcus sp. strain W31 | 120 rpm, 25°C | 2-3 | 2.3 | 10 |
| Bacillus firmus | 150 rpm, 35°C | 3 | 1.36 | 24 |
| Bacillus sp. strain F19 | 200 rpm, 30°C | 2 | 1.47 | 33 |
| Aeromonas sp. | 170 rpm, 30°C | 3 | 2.25 | 17 |
| Klebsiella sp. | 200 rpm, 30°C | 5 | 0.973 | 7 |
| Proteus mirabilis TJ-1 | 130 rpm, 25°C | 2 | 1.33 | 31 |
| Bacillus licheniformis X14 | 160 rpm, 37°C | 2 | No report | 18 |
| Bacillus licheniformis CGMCC 2876 | 200 rpm, 37°C | 2 | 2.93 | Our study |
Effects of carbon and nitrogen sources on bioflocculant production by B. licheniformis CGMCC 2876.
Different carbon and nitrogen sources in the medium were very important for cell growth and bioflocculant production (23). Glucose, soluble starch, and sucrose produced high flocculating activities of the culture broth (FR > 600 U/ml), among which sucrose was the most effective and economical substrate for the secretion of bioflocculant from B. licheniformis. In contrast, there was no distinct impact on bioflocculant synthesis when lactose, trisodium citrate dehydrate, and glycerol were used (FR < 250 U/ml).
B. licheniformis CGMCC 2876 could effectively use urea as the nitrogen source to lead to a high yield of bioflocculant (FR > 600 U/ml). In contrast with the report of Shih et al. on B. licheniformis CCRC 12826 (25), glutamic acid, citric acid, glycerol, and ammonium chloride were unfavorable carbon and nitrogen sources for B. licheniformis CGMCC 2876 to produce bioflocculant (FR < 200 U/ml).
Effect of the initial pH of the fermentation medium on bioflocculant production by B. licheniformis CGMCC 2876.
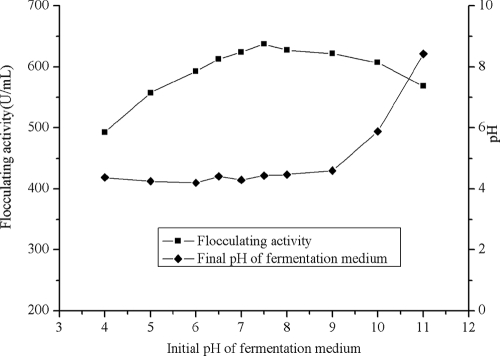
The initial pH of the fermentation medium affected bioflocculant synthesis (Fig. 3). The flocculating activity of the culture broth reached a maximum at pH 7.5 and then gradually decreased with the increase of the initial pH. Perhaps the high electric charge on the cells or the nutrient absorption potentials affected the enzymatic reactions (21, 31). As noted in previous studies, the optimum pH for bioflocculant accumulation varied with different strains. In the case of Corynebacterium xerosis, the flocculant was produced at relatively low pH (8), whereas alkaline medium was much more favorable for bioflocculant production by Aspergillus sojae (20).
FIG. 3.
Effect of initial pH on flocculant production by B. licheniformis CGMCC 2876.
Effects of cultivation conditions on bioflocculant production by B. licheniformis CGMCC 2876.
The effects of the culture temperature and seed culture inoculum size on bioflocculant production were also determined. The maximum flocculating activity was 650 U/ml, which was reached at 37°C. The activity dropped drastically when the cultivation temperature fell below 35°C or increased above 40°C, which suggested that the cultivation temperature strongly influenced flocculant biosynthesis.
When the inoculum size of the fermentation medium was 4% (vol/vol), the final flocculating activity of the culture broth reached 700 U/ml. A small inoculum size prolonged the stagnant phase, whereas a large inoculum size made the niche of B. licheniformis strain CGMCC 2876 overlap excessively and inhibited bioflocculant production (23).
Composition and molecular mass analysis.
The bioflocculant from B. licheniformis CGMCC 2876 was a proteoglycan comprised of 89% (wt/wt) carbohydrate and 11% (wt/wt) protein. Further analysis of the hydrolyzed bioflocculant revealed that the mass proportion of neutral sugar, amino sugar, and uronic acid was 7.9:4:1. Sufficient content of uronic acid in a bioflocculant molecule can provide carboxyl groups to the molecular chain, increasing the number of effective sites for adsorption of particles (10).
The HPGPC spectrum of the purified bioflocculant revealed only a single symmetrical peak with a retention time of 10.556 min, indicating the purity of the bioflocculant (data not shown). The molecular mass-retention time equation developed by calibration curve was as follows: log(molecular mass) = −0.2743T + 9.1327. The average mass of the bioflocculant was calculated to be 1.76 × 106 Da.
Spectroscopic characterization.
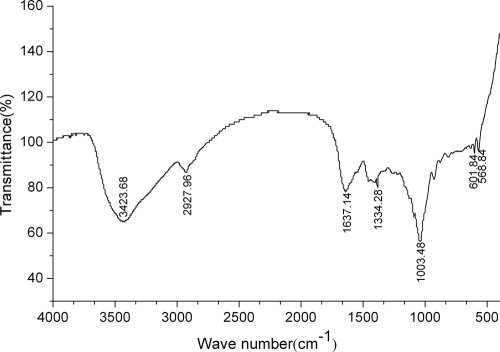
FT-IR spectroscopy was performed on the purified bioflocculant (Fig. 4). The spectrum showed a broad, intense absorption peak at 3,423 cm−1, characteristic of a hydroxyl group, which could be caused by the vibration of —OH or —NH in the sugar ring. A weak peak at 2,927 cm−1, known to be typical of carbohydrates, indicated C—H asymmetrical stretching vibration. An asymmetrical stretching peak observed at 1,637 cm−1 was characteristic of C=O stretching vibration in —NHCOCH3. The weak peak at 1,384 cm−1 may be assigned to be C=O symmetrical and asymmetrical stretching of a carboxylate group in the bioflocculant (6, 15). In addition, the strong absorption band at 1,063 cm−1 indicated asymmetrical stretching vibration of a C—O—C ester linkage. The small absorption band at about 895 cm−1 could be associated with β-glycosidic linkages between the sugar monomers, suggested by the study of Gupta et al. (12). The infrared spectrum showed characteristic peaks for carbohydrates and amides. Therefore, it can be inferred that the bioflocculant is a β-type heteropolysaccharide containing some protein.
FIG. 4.
Infrared spectra of purified bioflocculant from B. licheniformis CGMCC 2876.
Analysis of the morphology structure of the bioflocculant.
SEM observations were carried out to determine the surface structure of the purified bioflocculant. The results showed that the polymer had an irregular, coarse-grained structure connected in netted textures (see Fig. S1 in the supplemental material). The configuration of the bioflocculant molecule may contribute to its high flocculating efficiency.
Rapid flocculation was observed when 5.8 mg liter−1 bioflocculant was added to a kaolin clay suspension, which gave more convincing proof for the high flocculating capability of the molecule through comparison with SEM of a naked kaolin clay particle (see Fig. S1 in the supplemental material).
Distribution of bioflocculant from B. licheniformis CGMCC 2876.
The distribution of the flocculating activity in the culture broth was measured. The cell-free supernatant had high flocculating activity (675 U/ml), while cells displayed very low flocculation capability (25 U/ml), indicating that the bioflocculant produced by B. licheniformis CGMCC 2876 was an extracellular polymer. Similar results were reported by Salehizadeh and Shojaosadati (23).
Physicochemical properties and preliminary application of bioflocculant from B. licheniformis CGMCC 2876.
An investigation of the stability of the bioflocculant showed that the proteoglycan flocculant was extremely stable at a wide range of temperatures (below 80°C) and pH (3.0 to 8.0). For example, over 90% of the flocculating activity was maintained after heat treatment at 80°C for 30 min when the pH ranged from 3.0 to 8.0. The application potential of the bioflocculant was also studied in our laboratory. In kaolin clay suspension, the dosage of bioflocculant from B. licheniformis CGMCC 2876 was 5.8 mg liter−1, comparable to those of some chemically synthesized flocculants, such as Al2(SO4)3 (5 mg liter−1) and PAM (4.5 mg liter−1). The dosage of PAC was 7 mg liter−1, and the total concentration of PAM (polyaluminum chloride) plus Al2(SO4)3 mixed in equal weights was 4.4 mg liter−1. Also, the flocculating efficiency in sugarcane juice clarification and its decoloration activity in molasses wastewater were significant (data not shown). So far, there has been no similar research reported in the field of bioflocculants. Thus, sugar refinery operations may be a promising market for the bioflocculant from B. licheniformis CGMCC 2876.
Conclusion.
A bioflocculant-producing strain was isolated from contaminated LB medium and identified as B. licheniformis. The optimal conditions for bioflocculant production were an initial pH of 7.2 in fermentation medium, a temperature of 37°C, an inoculum concentration of 4%, and a fermentation period of 48 h. Chemical analyses revealed a novel extracellular proteoglycan composed of 89% carbohydrate and 11% protein (wt/wt). The carbohydrate was composed of a neutral sugar, an amino sugar, and uronic acid, with a mass proportion of 7.9:4:1. The advantageous properties of the bioflocculant from B. licheniformis CGMCC 2876, such as stable thermal and pH characteristics, a dosing rate comparable to those of chemical flocculants to flocculate suspended solids, and flocculation efficiency in the sugar refinery process, suggest its potential industrial utility. Further studies on the genes responsible for flocculation and industrial application are in progress in our laboratory.
Supplementary Material
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (no. 30700020 and 40801195), the Xiamen Science and Technology Committee (no. 3502Z20093006), and the Ministry of Science and Technology of the People's Republic of China (no. 2009EG111023).
We also gratefully acknowledge William Reznikoff, University of Wisconsin—Madison, for his technical instructions.
Footnotes
Published ahead of print on 5 March 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Baror, Y., and M. Shilo. 1987. Characterization of macromolecular flocculants produced by Phormidium sp. strain J-1 and by Anabaenopsis circularis PCC 6720. Appl. Environ. Microbiol. 53:2226-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchanan, R. E., and N. E. Gibbons. 1984. Bergey's manual of systematic bacteriology, 8th ed. Science Press, Beijing, China.
- 3.Cai, W. C., and H. J. Yuan. 1982. Chemical analysis methods of biological substance. Science Press, Beijing, China.
- 4.Crapper, D. R., S. S. Krishnan, and S. Quittkat. 1976. Aluminium, neurofibrillary degeneration and Alzheimer's disease. Brain 99:67-80. [DOI] [PubMed] [Google Scholar]
- 5.Dearfield, K. L., and C. O. Abermathy. 1988. Acrylamide: its metabolism developmental and reproductive effects, genotoxicity, and carcinogenicity. Mutat. Res. 195:45-77. [DOI] [PubMed] [Google Scholar]
- 6.Deng, S. B., R. B. Bai, X. M. Hu, and Q. Luo. 2003. Characteristics of a bioflocculant produced by Bacillus mucilaginosus and its use in starch wastewater treatment. Appl. Microbiol. Biotechnol. 60:588-593. [DOI] [PubMed] [Google Scholar]
- 7.Dermlim, W., P. Prasertsan, and H. Doelle. 1999. Screening and characterization of bioflocculant produced by isolated Klebsiella sp. Appl. Microbiol. Biotechnol. 52:698-703. [DOI] [PubMed] [Google Scholar]
- 8.Esser, K., and U. Kues. 1983. Flocculation and its implication for biotechnology. Process Biochem. 18:21-23. [Google Scholar]
- 9.Fattom, A., and M. Shilo. 1984. Phormidium J-1 bioflocculant—production and activity. Arch. Microbiol. 139:421-426. [Google Scholar]
- 10.Gao, J., H. Y. Bao, M. X. Xin, Y. X. Liu, Q. Li, and Y. F. Zhang. 2006. Characterization of a bioflocculant from a newly isolated Vagococcus sp. W31. J. Zhejiang Univ. Sci. B 7:186-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo, X., X. D. Gao, and X. B. Yang. 2004. Determination of content glucuronic acid and neutral sugar of acidic polysaccharide. Chin. J. Biochem. Pharm. 25:100-101. [Google Scholar]
- 12.Gupta, S., R. N. Madan, and M. C. Bansal. 1987. Chemical composition of Pinus caribaea hemicellulose. TAPPI J. 70:113-114. [Google Scholar]
- 13.Haruhiko, Y., N. Osamu, H. Jun, H. Sachio, and T. Yoshiyuki. 1995. Characteristics of a biopolymer flocculant produced by Bacillus sp. PY-90. J. Ferment. Bioeng. 79:378-380. [Google Scholar]
- 14.He, N., Y. Li, and J. Chen. 2004. Production of a polygalacturonic acid bioflocculant REA-11 by Corynebacterium glutamicum. Bioresour. Technol. 94:99-105. [DOI] [PubMed] [Google Scholar]
- 15.Kumar, C., J. H. Ganesh, R. Kavali, J. W. Choi, and C. S. Chang. 2004. Characterization of an extracellular biopolymer flocculant from a haloalkalophilic Bacillus isolate. World J. Microbiol. Biotechnol. 20:837-843. [Google Scholar]
- 16.Kurane, R., K. Hatamochi, T. Kakuno, M. Kiyohara, M. Hirano, and Y. Taniguchi. 1994. Production of a bioflocculant by Rhodococcus erythropolis S-1 grown on alcohols. Biosci. Biotechnol. Biochem. 58:428-429. [Google Scholar]
- 17.Li, X. M., Q. Yang, K. Huang, G. M. Zeng, D. X. Xiao, J. J. Liu, and W. F. Long. 2007. Screening and characterization of a bioflocculant produced by Aeromonas sp. Biomed. Environ. Sci. 20:274-278. [PubMed] [Google Scholar]
- 18.Li, Z., S. Zhong, H. Y. Lei, R. W. Chen, Q. Yu, and H. L. Li. 2009. Production of a novel bioflocculant by Bacillus licheniformis X14 and its application to low temperature drinking water treatment. Bioresour. Technol. 100:3650-3656. [DOI] [PubMed] [Google Scholar]
- 19.Lu, W. Y., T. Zhang, D. Y. Zhang, C. H. Li, J. P. Wen, and L. X. Du. 2005. A bioflocculant produced by Enterobacter aerogenes and its use in defecating the trona suspension. Biochem. Eng. J. 27:1-7. [Google Scholar]
- 20.Nakamura, J., S. Miyashiro, and Y. Hirose. 1976. Conditions of production of microbial cell flocculant by Aspergillus sojae AJ-7002. Agric. Biol. Chem. 40:1341-1347. [Google Scholar]
- 21.Nakata, K., and R. Kurane. 1999. Production of an extracellular polysaccharide bioflocculant by Klebsiella pneumoniae. Biosci. Biotechnol. Biochem. 63:2064-2068. [DOI] [PubMed] [Google Scholar]
- 22.Salehizadeh, H., M. Vossoughi, and I. Alemzadeh. 2000. Some investigations on bioflocculant producing bacteria. Biochem. Eng. J. 5:39-44. [Google Scholar]
- 23.Salehizadeh, H., and S. A. Shojaosadati. 2001. Extracellular biopolymeric flocculants: recent trends and biotechnological importance. Biotechnol. Adv. 19:371-385. [DOI] [PubMed] [Google Scholar]
- 24.Salehizadeh, H., and S. A. Shojaosadati. 2002. Isolation and characterisation of a bioflocculant produced by Bacillus firmus. Biotechnol. Lett. 24:35-40. [Google Scholar]
- 25.Shih, I. L., Y. T. Van, L. C. Yeh, H. G. Lin, and Y. N. Chang. 2001. Production of a biopolymer flocculant from Bacillus licheniformis and its flocculation properties. Bioresour. Technol. 78:267-372. [DOI] [PubMed] [Google Scholar]
- 26.Suh, H. H., G. S. Kwon, C. H. Lee, H. S. Kim, H. M. Oh, and B. D. Yoon. 1997. Characterization of bioflocculant produced by Bacillus sp. DP-152. J. Ferment. Bioeng. 84:108-112. [Google Scholar]
- 27.Takagi, H., and K. Kadowaki. 1985. Flocculant production by Paecilomyces sp. Taxonomic studies and culture conditions for production. Agric. Biol. Chem. 49:3151-3157, 3159-3164. [Google Scholar]
- 28.Takeda, M., R. Kurane, J. I. Koizumi, and I. Nakamura. 1991. A protein bioflocculant produced by Rhodococcus erythropolis. Agric. Biol. Chem. 5:2663-2664. [Google Scholar]
- 29.Toeda, K., and R. Kurane. 1991. Microbial flocculant from Alcaligenes cupidus KT201. Agric. Biol. Chem. 55:2793-2799. [Google Scholar]
- 30.Wu, J. Y., and H. F. Ye. 2007. Characterization and flocculating properties of an extracellular biopolymer produced from a Bacillus subtilis DYU1 isolate. Process Biochem. 42:1114-1123. [Google Scholar]
- 31.Xia, S. Q., Z. Q. Zhang, X. J. Wang, A. M. Yang, L. Chen, J. F. Zhao, L. Didier, and J. R. Nicole. 2008. Production and characterization of a bioflocculant by Proteus mirabilis TJ-1. Bioresour. Technol. 99:6520-6527. [DOI] [PubMed] [Google Scholar]
- 32.Zhang, W. 2003. Biological-chemical analysis of glycoconjugates, 2nd ed. Zhejiang University Press, Zhejiang, China.
- 33.Zheng, Y., Z. L. Ye, X. L. Fang, Y. H. Li, and W. M. Cai. 2008. Production and characteristics of a bioflocculant produced by Bacillus sp. F19. Bioresour. Technol. 99:7686-7691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.