Abstract
Understanding signaling pathways that modulate conidiation of mitosporic fungi is of both practical and theoretical importance. The enzymatic origin of nitric oxide (NO) and its roles in conidiation by the sclerotial parasite Coniothyrium minitans were investigated. The activity of a nitric oxide synthase-like (NOS-like) enzyme was detected in C. minitans as evidenced by the conversion of l-arginine to l-citrulline. Guanylate cyclase (GC) activity was also detected indirectly in C. minitans with the GC-specific inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), which significantly reduced production of cyclic GMP (cGMP). The dynamics of NOS activity were closely mirrored by the cGMP levels during pycnidial development, with the highest levels of both occurring at the pycnidial initiation stage of C. minitans. Furthermore, the NO donor, sodium nitroprusside (SNP), stimulated the accumulation of cGMP almost instantly in mycelium during the hyphal growth stage. When the activity of NOS or GC was inhibited with Nω-nitro-l-arginine or ODQ, conidial production of C. minitans was suppressed or completely eliminated; however, the suppression of conidiation by ODQ could be reversed by exogenous cGMP. The results also showed that conidiation of an l-arginine auxotroph could be restored by the NO donor SNP, but not by cGMP. Thus, NO-mediated conidiation has more than one signal pathway, including the cGMP signal pathway and another yet-unknown pathway, and both are essential for conidiation in C. minitans.
Coniothyrium minitans is a sclerotial parasite of the notorious plant pathogen Sclerotinia sclerotiorum, and its potential for biological control of Sclerotinia diseases has been well demonstrated in several countries (13, 17, 21, 28, 33, 34, 36, 37). Efficient production of conidia will further enhance the potential of C. minitans as a biological control agent. Understanding signaling pathways that modulate conidiation of C. minitans will not only facilitate manipulation of the biocontrol agent for commercial use but also advance our understanding of fungal biology.
Nitric oxide (NO) is a widespread signaling molecule involved in regulation of a wide range of cellular functions in animals and plants (7). NO synthesis and signaling have been well studied in animals and plants. In mammals, NO plays roles in relaxation of smooth muscle, inhibition of platelet aggregation, neural communication and immune regulation, while in plants NO is involved in disease resistance, abiotic stress, cell death, respiration, senescence, root development, seed germination, and other functions (reviewed in references 6 and 32). NO is also involved in the development of several members of the mycetozoa, such as, Dictyostelium discoideum (10) and Physarum polycephalum (27). The wide variety of effects reflects the basic signaling mechanism that is used by mammals, plants, and virtually all organisms (2). Despite of the extensive research on NO synthesis and signaling processes in animals and plants, our knowledge about NO in fungi is very limited.
Our understanding of NO synthesis and signaling in fungi is based mainly on pharmacological studies using NO donors, NOS inhibitors and NO scavengers. Both NO function and nitric oxide synthase (NOS) activity have been identified in fungi. NO plays roles in asexual spore development in the ascomycete Neurospora crassa (19), the zygomycete Phycomyces blakesleeanus (15), and the blastocladiomycete Blastocladiella emersonii (29), as well as C. minitans (11). NO stimulates the formation of sexual fruiting bodies in the basidiomycete Flammulina velutipes (26). It is also involved in other fungal physiological processes, such as suppression of pseudomycelial formation in the yeast Candida tropicalis (35), and delay in conidial germination in Colletotrichum coccodes (30). In addition, NO formation was detected in the mycobiont of the lichen Ramalina lacera during transitions between desiccation and rehydration (31). Recently, NO signaling and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) S nitrosylation are linked with H2O2-induced apoptotic cell death and also mediate cell death during chronological life span pointing in Saccharomyces cerevisiae (1). Despite the significance of NO in a large variety of physiological processes, the origin of NO in fungi is not clearly understood, and how this molecule interacts with upstream receptors and downstream response elements in fungi are still unknown.
It is suggested that NO in fungi is derived from l-arginine under the catalysis of NOS-like enzymes. Recently, NO levels and NOS activity have been confirmed in S. cerevisiae by measuring NO production through monitoring nitrate and nitrite formation, by direct measurement using a NO-selective electrode (AmiNO-700) and by measuring the formation of l-[3H]citrulline from l-[3H]arginine (1). Another source of NO may be from cytochrome c oxidase, since this mitochondrial enzyme reduces NO2 to NO at low-oxygen concentrations (3).
In animals and plants, NO and NO-derived species function through chemical modification of targets. These signaling molecules mostly act through binding to transition metals of metalloproteins (metal nitrosylation), and covalent modification of cysteine (Cys; S nitrosylation) and tyrosine (Tyr; tyrosine nitration) residues (2). One function of NO is as an activator of soluble guanylate cyclase (GC) by binding to the heme iron, resulting in a transient increase in the second messenger cyclic GMP (cGMP). cGMP is a well-established signaling molecule in many prokaryotes and eukaryotes (14). In fungi, cGMP has been detected in N. crassa (23), P. blakesleeanus (12), and B. emersonii (25).
Previously, we reported that l-arginine is required for conidiation of C. minitans, and the highest amount of nitric oxide (NO) in mycelial mass in primordial formation stage compared to that in hyphal growth stage was observed (11). It was suspected that NO is involved in conidiation of C. minitans. However, the pathway of NO regulation for conidiation is not understood, and the origin of NO in C. minitans is still unknown. The objectives of this investigation were to ascertain the enzymatic origin of NO and to determine the possible NO signal pathways in modulating conidiation of C. minitans.
MATERIALS AND METHODS
Fungal strains and maintenance.
Wild-type strain ZS-1 (CCAM 041057) of C. minitans produces pycnidia and conidia normally on potato dextrose agar (PDA) dishes and produces abundant conidia in liquid shake culture (4). Mutant ZS-1T2029 (CCAM 041058) derived from strain ZS-1 is an l-arginine auxotroph, whose gene coding for l-arginine-specific carbamoyl-phosphate synthase was disrupted by a T-DNA insertion. It can grow, but it cannot produce conidia on PDA petri dishes due to l-arginine deficiency (11). The wild-type and the mutant strains were cultured on PDA or potato dextrose broth (PDB) at 20 to 22°C and stored on PDA slants at 4°C.
Culture conditions and pharmacological studies.
To collect conidia, the wild-type strain ZS-1 was grown on PDA slants for 20 days at 20 to 22°C, whereas the mutant ZS-1T2029 was grown on l-arginine-amended PDA slants under the same conditions. Conidia were harvested with sterile distilled water and passed through two layers of cheesecloth to remove debris and pycnidia. The conidial suspension was adjusted to a concentration of 105 conidia/ml with a hemacytometer for experimentation.
To study pycnidial development of C. minitans, 100 μl of inoculum was mixed carefully with 20 ml of molten PDA (∼50°C) and poured into sterile 90-mm-diameter dishes. Dishes were incubated at 20°C, and colonies were monitored under a microscope every 12 h.
To obtain fungal mycelia, 100 μl of inoculum of the wild-type strain ZS-1 and the mutant ZS-1T2029 were placed onto cellophane membranes overlaying PDA in 90-mm-diameter dishes or on PDA with different amendments as described below and incubated at 20 to 22°C. After incubation of 48, 60, 72, 84, and 96 h, mycelia were collected and used to detect NO production and cGMP levels.
To test the effect of 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one (ODQ), an inhibitor of the soluble GC, on conidiation, 5 mg of ODQ (Sigma-Aldrich, Germany) were dissolved in 2 ml of sterilized ddH2O to make a stock concentration of 10 mmol/liter, and this stock was diluted to different concentrations before use. The inoculum (100 μl) of the wild-type strain ZS-1 was carefully mixed with 10 ml of molten PDA and poured into sterile petri dishes. After 24 h of incubation at 20°C, 200-μl portions of different concentrations of ODQ were spread onto the surface of dishes to arrive at final approximate concentrations ranging from 10 to 100 μmol/liter, assuming that the chemical diffused evenly throughout the medium. Colonies were checked by microscopy at ×100 at 48, 60, 72, 84, and 96 h after ODQ amendment. Images were captured with a Digital Sight DS-5 M digital camera (Nikon, Japan) using the NIS-elements F Package version 3.0 (Nikon, Japan). Final conidial production of each dish was determined 7 days after incubation as described below.
To compare the effect of ODQ and the NOS specific inhibitor Nω-nitro-l-arginine (l-NNA; Sigma-Aldrich, Germany) on conidiation, conidia (100 μl) were inoculated into PDA medium and incubated for 24 h. Then, 200 μl of l-NNA (50 mmol/liter) was amended into PDA medium with a final concentration of 1 mmol/liter, and colonies were checked under microscope as described above.
To test potential interactions between exogenous cGMP and ODQ on conidiation of C. minitans, the inoculated PDA dishes were preincubated at 20°C for 24 h, and then the dishes were amended simultaneously with ODQ at 50 μmol/liter and with cGMP (at 0.1 to 10 mmol/liter). The treated dishes were incubated at 20 to 22°C for 7 days, and the conidial production was determined as described below.
To determine whether exogenous cGMP could restore the conidiation of mutant ZS-1T2029, two experiments were attempted. In the first experiment, this mutant was cultured on PDA amended with different concentrations of cGMP (final concentration from 10 μmol to 10 mmol/liter). In the second experiment, a conidial suspension (100 μl) of the mutant strain ZS-1T2029 was mixed with 10 ml of molten PDA and poured into petri dishes. Inoculated dishes were incubated at 20°C for 24, 36, 48, 60, or 72 h, and then cGMP was spread onto the surface of dishes to arrive at final concentrations ranging from 10 μmol/liter to 10 mmol/liter approximately. The cGMP-treated dishes were further incubated for 10 days, and then the conidiation and conidial production of mutant were determined as described below.
To determine conidial production, two mycelial agar plugs (5.0 mm in diameter) from each dish were transferred into a microfuge tube containing 1 ml of sterile H2O and vortexed for 1 min. Conidia released from pycnidia were counted with a hemacytometer and calculated as conidia per square centimeter. Experiments were performed at least three times, and all colony morphology images were captured with a digital camera (Sony T10′Japan).
NOS activity assay.
To confirm whether some or all of the NO is derived from l-arginine catalyzed by NOS-like enzyme, an NOS assay kit (Calbiochem) was used. Formation of NO was determined by the conversion of l-[14C]arginine (Perkin-Elmer) to l-[14C]citrulline. Extraction of protein samples from mycelia collected at different stages was performed according to the manufacturer's instructions. Ten microliters mycelial extract was incubated with 2 μl of l-[14C]arginine (50 μCi/μl), 5 mmol of NADPH/liter, 0.6 mmol of CaCl2/liter, and 10 μg of calmodulin/ml for 60 min at 37°C. Control reactions contained 5 μl of rat cerebellum provided in the kit. After the removal of residual l-[14C]arginine by passage through the resin supplied in the kit, converted l-[14C]citrulline was measured in a liquid scintillation counter (Beckman, Germany). Protein concentrations in the mycelial extracts were determined with a Coomassie brilliant blue staining kit (Beyotime Institute Biotech, Nanjing, China). Values were expressed as pmol/min/mg of protein. This experiment was repeated four times.
Nitric oxide assay.
The levels of nitric oxide in mycelia were determined with the Griess reagent kit (Beyotime Institute Biotech). The optical density at 550 nm of the reaction product was measured with a UV mini-1240 spectrophotometer (Shimadzu, Japan). The total protein in mycelia of wild-type strain ZS-1 or mutant ZS-1T2029 was determined with a Coomassie brilliant blue staining kit. The production of NO (μmol/g of total protein) was calculated with a formula supplied in the kit. There were three replicates in each treatment, and the experiment was repeated three times.
cGMP assay.
Cytoplasmic cGMP was assayed by using a 125I-cGMP radioimmunoassay kit (Shanghai University of Traditional Chinese Medicine, China). About 100 mg of fresh mycelial mass was frozen with liquid nitrogen and homogenized on the ice in 2 ml of 50 mmol/liter acetate buffer (pH 4.75). Cellular debris was removed by centrifugation (3,500 rpm, 15 min), and the supernatant was extracted two times with an equal volume of ethanol. The aqueous layer was lyophilized and redissolved in 1 ml of 50-mmol/liter acetate buffer (pH 4.75), and samples were acetylated prior to radioimmunoassay. Preparation of standard curves and assay procedures were as described in the supplier's manual, and radioactivity was measured by using an SN-697 gamma counter (Shanghai Institute of Applied Physics, China). The data are expressed as pmol of cGMP per gram of fresh mycelial weight (pmol/g FW). All measurements were performed in duplicate, and the experiment was repeated three times.
Data analyses.
Data from the experiments were analyzed by using an analysis of variance carried out using SAS version 8.1 (SAS Institute, Inc., Cary, NC). When significant treatment differences were found, treatment means were separated using the protected least significant difference test at P = 0.05.
RESULTS
Pycnidial development of C. minitans.
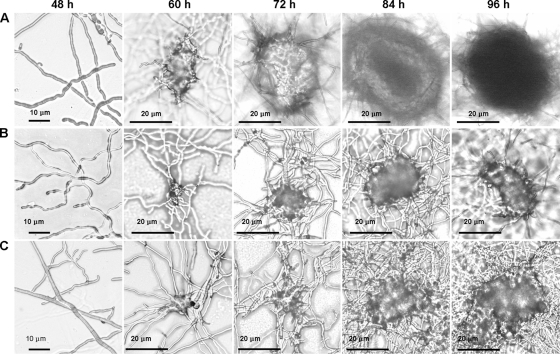
The pycnidial development of C. minitans could be divided into five growth stages morphologically (Fig. 1). The first is the hyphal growth stage, occurring during the first 48 h postinoculation (hpi). The second stage is the primordial formation stage, with hyphal intertwisting and the formation of small knots, but with no conidiophore formation in the knots. The best time to observe primordial formation is at 60 hpi. The third stage is the pycnidial initiation stage, with conidiophore formation with a few conidia, a clear outline of the pycnidium, and thin-walled pycnidia, allowing light passage under a microscope. This stage is very short and usually occurs at 72 hpi. The fourth stage is the pycnidial formation stage. Here, the outlines of the pycnidia are clear, the pycnidial wall turns thick, and light cannot pass through the center and the margin of the structure. However, there is little melanin accumulation, and the pycnidia are white, but full with conidia. This stage usually occurs at 84 hpi. The fifth and final stage is the pycnidial maturation stage, the last stage of C. minitans. The most distinct characteristic at this stage is the accumulation of melanin; thus, mature pycnidia are dark. Mature pycnidia could be observed at 96 hpi (Fig. 1A).
FIG. 1.
Five stages of pycnidial development and their morphological features in Coniothyrium minitans wild-type strain ZS-1 on PDA alone (A) and on PDA amended with l-NNA (B) or with ODQ (C). The stages are as follows: hyphal growth stage (48 hpi), primordial formation stage (60 hpi), pycnidial initiation stage (72 hpi), pycnidial formation stage (84 hpi), and pycnidial maturation stage (96 hpi). Each dish contained 105 conidia/ml in 10 ml of medium. ODQ and l-NNA were added at 24 hpi to the medium for final concentrations of 50 μmol/liter and 1 mmol/liter, respectively. Magnification, ×200.
NOS activity.
NOS activity in C. minitans was measured by the formation of l-[14C]citrulline from l-[14C]arginine. NOS activity was detected from the hyphal growth stage to the pycnidial maturation stage. A low level of NOS activity was detected at 48 hpi on PDA. The activity increased at 60 hpi, peaked at 72 hpi, and then declined. At 48 hpi the l-[14C]citrulline production rate was approximate 1.5 pmol/min/mg, whereas at 72 hpi the rate was approximately 20 pmol/min/mg (Fig. 2A). Thus, NOS-like activity was detected in C. minitans and is tightly associated with conidiation of C. minitans.
FIG. 2.
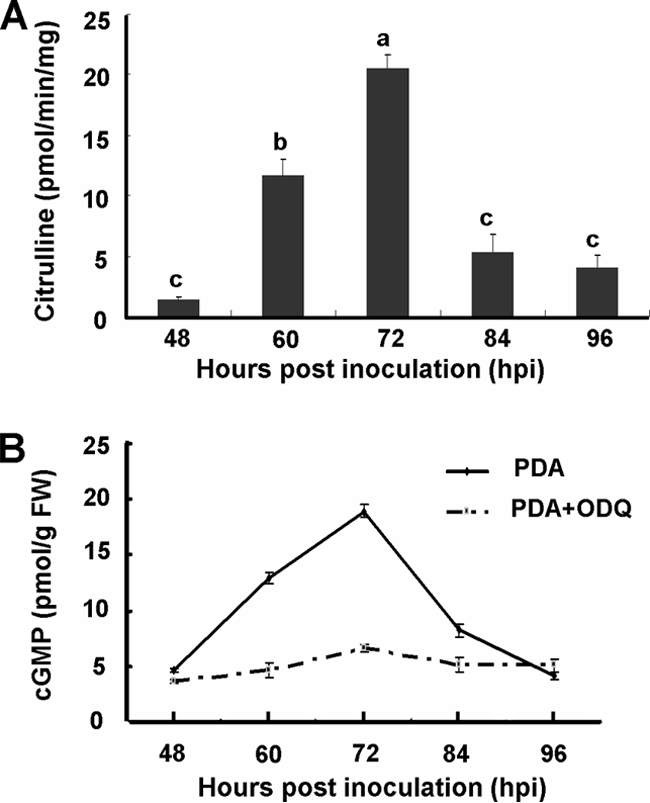
NOS-like activities (A) and intracellular cGMP levels (B) in mycelial extracts from five pycnidial development stages of C. minitans wild-type strain ZS-1. (A) Putative NOS-like activity was detected by measuring the conversion rate of l-[14C]citrulline from l-[14C]arginine. Values are means ± the standard error of four independent experiments. Bars with the same letter are not significantly different (P < 0.05). (B) Intracellular cGMP levels on PDA and on PDA amended with ODQ were estimated by using a 125I-based radioimmunoassay. Values are means of four independent experiments. Bars represent the standard deviation from the mean (n = 4).
Intracellular cGMP levels and GC activity.
Intracellular cGMP in C. minitans was detected at different developmental stages. During the hyphal growth stages, cGMP was maintained at a relatively low level at about 4 to 5 pmol/g FW. The intracellular cGMP level increased to 12.8 pmol/g FW in the primordial formation stage (at 60 hpi). The cGMP level was 18.9 pmol/g FW and reached the highest level at the pycnidial initiation stage, and then the cGMP level dropped in the pycnidial formation stage and the pycnidial maturation stage (Fig. 2B).
The activity of GC in C. minitans was confirmed indirectly using the soluble GC inhibitor ODQ. At 50 μmol of ODQ/liter, the cGMP level in mycelia remained at low levels at all sampling points tested. The cytosolic cGMP concentrations were about 4 to 5 pmol/g FW (Fig. 2B).
Inhibition of conidiation by ODQ.
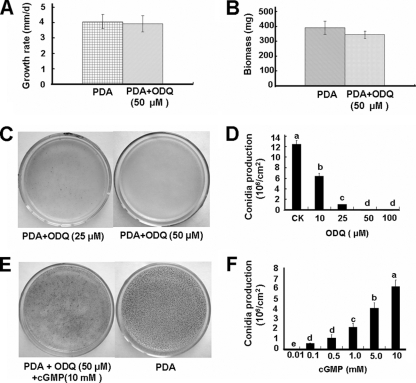
The production of conidia was significantly suppressed by addition of ODQ at all four concentrations tested (Fig. 3C and D). At 10 μmol of ODQ/liter, a few pycnidia were produced, and at 50 and 100 μmol of ODQ/liter, the conidiation of C. minitans was completely inhibited (Fig. 3C and D). Except for inhibiting conidiation, ODQ had no observable effect on hyphal growth. At 50 μmol of ODQ/liter, hyphal growth appeared to be normal (Fig. 3A and B), and primordial development was observed, but these primordia did not develop into pycnidia (Fig. 1C). This phenomenon was also observed in dishes treated with the NOS inhibitor l-NNA (Fig. 1B).
FIG. 3.
Effect of ODQ on the mycelial growth (A and B) and conidiation (C, D, E, and F) of C. minitans without or with exogenous cGMP. (A and B) ODQ does not suppress hyphal growth and biomass. (C) ODQ inhibits conidiation. (D) Conidial production was affected by ODQ. (E) Reversal of ODQ inhibition by cGMP. (F) Conidial production as affected by cGMP in the presence of 50 μM ODQ. The solid bars represent means of four independent experiments, and error bars represent standard deviation from the mean (n = 6). Bars labeled with different lowercase letters are statistically different from one another (P < 0.05).
The suppression of conidiation by ODQ could be reversed by the addition of exogenous cGMP (Fig. 3E and F). After cell-permeable cGMP was applied at the same time as ODQ, mature pycnidia could be observed later in dishes, and the inhibitory effect of ODQ was reduced or even eliminated, depending on the relative concentrations of ODQ and cGMP in the media (Fig. 3F).
Endogenous NO increases cellular cGMP.
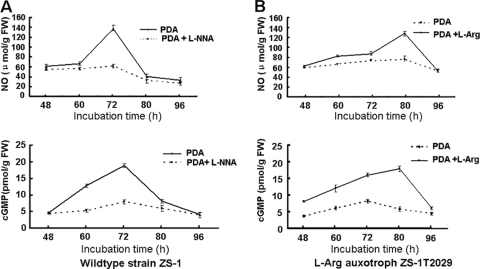
When wild-type strain ZS-1 was treated with l-NNA, an NOS inhibitor, the NO accumulation in C. minitans was reduced, and conidiation was blocked. Interestingly, the cGMP level in l-NNA-treated C. minitans was 7.95 pmol/g FW, which was much lower than that in untreated wild-type strain ZS-1 (Fig. 4A). Thus, the NOS-inhibitor reducing NO accumulation may also reduce the accumulation of cytosolic cGMP.
FIG. 4.
Time course relationship of endogenous nitric oxide and intracellular cGMP in C. minitans wild-type strain ZS-1 on PDA and PDA amended with l-NNA (A) and the l-Arg auxotroph mutant ZS-1T2029 on PDA and PDA amended with l-Arg (B). Values are the means of three independent experiments, and bars represent the standard deviation from the mean (n = 3).
The cGMP level in l-arginine auxotroph mutant ZS-1T2029 was examined; the results showed that the cGMP level in the mutant ZS-1T2029 was maintained at a relatively low level, and there were no significant differences between the time points sampled (Fig. 4B). However, on media amended with l-arginine (2.5 mmol/liter), the accumulation of cGMP in the mutant ZS-1T2029 increased to 17.95 pmol/g FW during the pycnidial formation stage (Fig. 4B), which was as much as in wild-type strain ZS-1. The levels of cGMP in the mycelial mass of the mutant ZS-1T2029 in media with or without l-arginine are consistent with the production of NO and pycnidial development. The NO production pattern of the mutant in l-arginine-amended medium was similar to that of wild strain ZS-1, although the time of the highest level of NO observed occurred ∼12 h later than that in the wild-type strain ZS-1 (Fig. 4).
Cytoplasmic cGMP level increased instantly by NO donor.
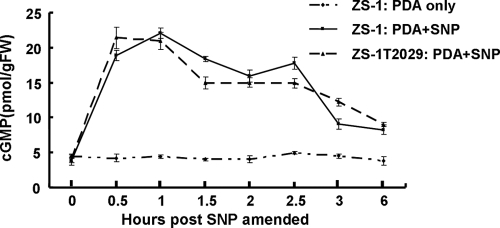
Addition of sodium nitroprusside (SNP), a nitric oxide donor, almost instantly increased cGMP levels during the hyphal growth stage of C. minitans (Fig. 5). SNP was applied to both the wild-type strain ZS-1 and the mutant ZS-1T2029 after 48 h in PDB. The cellular cGMP levels were increased to 23.3 pmol/g FW 1 h after SNP treatment, which was about five times higher than that in the control. The high levels of cGMP persisted for ∼2.5 h and then declined (Fig. 5).
FIG. 5.
Effect of NO-donor sodium nitroprusside (SNP) on cytoplasmic cGMP levels in C. minitans during the hyphal growth stage. The data points are means of three independent experiments, and the bars represent the standard deviation from the mean (n = 3).
Exogenous cGMP did not restore conidiation of a l-arginine auxotroph mutant.
All treatments with cGMP to restore the conidiation of mutant ZS-1T2029 failed. However, our previous work showed that both l-arginine and nitric oxide donor SNP restored conidiation of the mutant ZS-1T2029 (11). This indicated that the second messenger cGMP is only one of the signal molecules that NO activates for pycnidial development and conidiation in C. minitans. We speculate that the NO-mediated conidiation should have more than one signal pathway and that other signal pathways which are also regulated by NO should exist and parallel cGMP signaling.
DISCUSSION
Nitric oxide has been detected in many fungi, including C. minitans (15, 19, 20, 29, 30), but its origin in fungi is still unknown. In animals the biosynthesis of NO is primarily catalyzed by the enzyme nitric oxide synthase (18), and in plants the production of NO is catalyzed by nitrate reductase, xanthine oxidoreductase, certain plasma membrane-bound enzymes, and NOS-like enzymes (5). NOS activities have been found in several other fungi (19, 26, 29, 35). Our results confirmed NOS activity in C. minitans as evidenced by the conversion of l-[14C]arginine to l-[14C]citrulline. Different levels of NOS activity have been observed in various fungi, ranging from <10 pmol/mg/min in P. blakesleeanus mycelia (19) to >500 pmol/mg/min in F. velutipes fruiting bodies (26). In C. minitans, the highest level of NOS activity reached 20 pmol/min/mg of protein during conidiation, and the timing of the enzyme activity coincided with NO production in mycelia of C. minitans during conidiation. NOS genes have been identified and cloned from animals but not from plants nor fungi. Recently, DNA sequences coding for hypothetical NOS-like proteins have been annotated in some fungal genomes, such as P. blakesleeanus and Magnaporthe grisea (29); however, the putative NOS-like gene remains to be identified.
The signal molecular cGMP is derived from GTP catalyzed by GC (14), and ODQ is an irreversible inhibitor of GC (24). We found that ODQ suppressed conidiation in C. minitans, and interestingly, this suppression could be reversed by exogenous cGMP, indicating that a cGMP-mediated signal is involved in conidiation of C. minitans. cGMP has been detected in several model fungi, including N. crassa (22, 23), P. blakesleeanus (12), and S. cerevisiae (8). With 125I radioactive labeling and a radioimmunoassay technique, we found that the concentration of cGMP in mycelia was similar to those reported in other fungi. The highest level of cGMP in C. minitans occurred during the primordial formation stage. Similarly, cGMP levels in B. emersonii changed during cell differentiation, and the highest level was found during sporulation (25). In S. cerevisiae, the levels of intracellular cGMP depended on the metabolic conditions, and the highest level was during the exponential growth stage (8), which is an asexual reproductive stage in yeast. Recently, a NO-cGMP signaling pathway was reported to modulate zoospore biogenesis in the aquatic fungus B. emersonii (29). However, when ODQ was used to treat several other fungi, including Aspergillus nidulans, A. oryzae, A. niger, and Cryphonectria parasitica, ODQ reduced pycnidial formation significantly only in Cryphonectria parasitica but did not have any observable effect on sporulation of the Aspergillus species (B. Li et al., unpublished data). This indicates that the cGMP signal in conidiation may not be universal for fungi.
In N. crassa exogenous cGMP could stimulate mycelial elongation in cAMP-treated adenylate cyclase-deficient mutants (23). In addition, cGMP caused significant increases in both hyphal extension rate and hyphal growth unit length for both a wild-type strain and a highly branched, “colonial” mutant strain of Fusarium graminearum (9). Our work showed that cGMP is involved in conidiation by C. minitans. Thus, cGMP may be involved in a variety of biological processes.
Our results presented here demonstrated that NO, even exogenous NO, stimulates the accumulation of cGMP in C. minitans. This phenomenon is consistent with the model that NO may function through a cGMP-dependent pathway, posttranslationally activating GC, and GC leads to a transient increase in the second messenger cGMP (16). Thus, it is likely that cGMP acts as a second messenger in NO-mediated conidiation of C. minitans.
However, genes for GC or other functionally similar enzymes responsible for cGMP synthesis have not been cloned from fungi. The gene coding for GC was not annotated in any fungal genome sequences. Recently, three putative BeGC genes with high similarity to guanylyl cyclase catalytic domains have been found in B. emersonii (29), which is distantly related to C. minitans phylogenetically. The transcriptional expression profiles of these putative BeGC genes were consistent with the guanylyl cyclase activity determined during B. emersonii sporulation (29). However, we could not find any homologous genes in C. minitans and other fungi belonging to the Ascomycota. The function of these genes needs to be further studied.
In summary, NOS activity and cGMP were detected in C. minitans, and the NOS activity and cGMP levels were consistent with conidiation of C. minitans. Both endogenous and exogenous NO could stimulate the accumulation of cGMP. The GC inhibitor ODQ suppressed conidiation of C. minitans, but the suppression could be reversed by exogenous cGMP. Although the NO donor SNP restored conidiation in the l-arginine auxotroph mutant ZS-1T2029, exogenous cGMP did not. Therefore, the NO signal transduction-regulated conidiation in C. minitans must have an additional pathway besides the second messenger cGMP. Further investigation will be necessary to determine the other NO signal pathway and signaling transmission downstream of the second messenger cGMP in C. minitans.
Acknowledgments
The research was financially supported in part by the National Natural Science Foundation of China (grant 30771438), the Commonweal Specialized Research Fund of China Agriculture (grant 3-21), and the Hi-Tech Research and Development Program of China (863 Program; 2006AA10A211).
We thank Weidong Chen of the USDA-ARS (Washington State University) and Tom Hsiang of School of Environmental Sciences, University of Guelph, for their editorial assistance.
Footnotes
Published ahead of print on 5 March 2010.
REFERENCES
- 1.Almeida, B., F. Madeo, and P. Ludovico. 2007. NO-mediated apoptosis in yeast. J. Cell Sci. 120:3279-3288. [DOI] [PubMed] [Google Scholar]
- 2.Besson-Bard, A., A. Pugin, and D. Wendehenne. 2008. New insights into nitric oxide signaling in plants. Annu. Rev. Plant Biol. 59:21-39. [DOI] [PubMed] [Google Scholar]
- 3.Castello, P. R., P. S. David, T. McClure, Z. Crook, and R. O. Poyton. 2006. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab. 3:277-287. [DOI] [PubMed] [Google Scholar]
- 4.Cheng, J., D. H. Jiang, X. H. Yi, Y. P. Fu, G. Q. Li, and J. M. Whipps. 2003. Production, survival and efficacy of Coniothyrium minitans conidia produced in shaken liquid culture. FEMS Microbiol. Lett. 227:127-131. [DOI] [PubMed] [Google Scholar]
- 5.Corpas, F. J., J. B. Barroso, and L. A. del Río. 2004. Enzymatic sources of nitric oxide in plant cells—beyond one protein-one function. New Phytol. 162:243-251. [Google Scholar]
- 6.Crawford, N. M., and F. Q. Guo. 2005. New insights into nitric oxide metabolism and regulatory functions. Trends Plant Sci. 10:195-200. [DOI] [PubMed] [Google Scholar]
- 7.Durner, J., J. G. Andrew, S. S. Jonathan, and J. Glazebrook. 1999. Ancient origins of nitric oxide signaling in biological systems. Proc. Natl. Acad. Sci. U. S. A. 96:14206-14207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckstein, H. 1988. 3′,5′-Cyclic GMP in the yeast Saccharomyces cerevisiae at different metabolic conditions. FEBS Lett. 232:121-124. [DOI] [PubMed] [Google Scholar]
- 9.Geoffredy, D. R., G. W. Marilyn, and P. T. Anthony. 1991. Exogenous cAMP and cGMP modulate branching in Fusarium graminearum. J. Gen. Microbiol. 137:963-969. [DOI] [PubMed] [Google Scholar]
- 10.Georg, G., E. R. Werner, S. Leitner, P. Gröbner, and G. Werner-Felmayer. 2003. Nitric oxide synthase is induced in sporulation of Physarum polycephalum. Genes Dev. 15:1299-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong, X. Y., Y. P. Fu, D. H. Jiang, G. Q. Li, X. H. Yi, and Y. L. Peng. 2007. l-Arginine is essential for conidiation in the filamentous fungus Coniothyrium minitans. Fungal Genet. Biol. 44:1368-1379. [DOI] [PubMed] [Google Scholar]
- 12.Leutwiler, L. S., and M. Brandt. 1983. Absence of significant light-induced changes in cAMP levels in sporangiophores of Phycomyces blakesleeanus. J. Bacteriol. 153:555-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li, G. Q., H. C. Huang, H. J. Miao, R. S. Erickson, D. H. Jiang, and Y. N. Xiao. 2006. Biological control of sclerotinia diseases of rapeseed by aerial applications of the mycoparasite Coniothyrium minitans. Eur. J. Plant Pathol. 114:345-355. [Google Scholar]
- 14.Lucas, K. A., G. M. Pitari, S. Kazerounian, I. Ruiz-Stewart, J. Park, S. Schulz, K. P. Chepenik, and S. A. Waldman. 2000. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol. Rev. 52:375-414. [PubMed] [Google Scholar]
- 15.Maier, J., R. Hecker, P. Rockel, and H. Ninnemann. 2001. Role of nitric oxide synthase in the light-induced development of sporangiophores in Phycomyces blakesleeanus. Plant Physiol. 126:1323-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald, L. J., and F. Murad. 1995. Nitric oxide and cGMP signaling. Adv. Pharmacol. 34:263-275. [DOI] [PubMed] [Google Scholar]
- 17.McQuilken, M. P., J. Gemmell, R. A. Hill, and J. M. Whipps. 2003. Production of macrosphelide A by the mycoparasite Coniothyrium minitans. FEMS Microbiol. Lett. 219:27-31. [DOI] [PubMed] [Google Scholar]
- 18.Nathan, C., and Q. W. Xie. 1994. Nitric oxide synthases: roles, tolls, and controls. Cell 78:915-918. [DOI] [PubMed] [Google Scholar]
- 19.Ninnemann, H., and J. Maier. 1996. Indications for the occurrence of nitric oxide synthases in fungi and plants and the involvement in photoconidiation of Neurospora crassa. Photochem. Photobiol. 61:393-398. [DOI] [PubMed] [Google Scholar]
- 20.Prats, E., T. L. Carver, and L. A. Mur. 2008. Pathogen-derived nitric oxide influences formation of the appressorium infection structure in the phytopathogenic fungus Blumeria graminis. Res. Microbiol. 159:476-480. [DOI] [PubMed] [Google Scholar]
- 21.Ren, L., G. Q. Li, Y. C. Han, D. H. Jiang, and H. C. Huang. 2007. Degradation of oxalic acid by Coniothyrium minitans and its effects on production and activity of β-1,3-glucanase of this mycoparasite. Biol. Control 43:1-11. [Google Scholar]
- 22.Rosenberg, G., and M. L. Pall. 1978. Cyclic AMP and cyclic GMP in germinating conidia of Neurospora crassa. Arch. Microbiol. 118:87-90. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg, G., and M. L. Pall. 1979. Properties of two cyclic nucleotide-deficient mutants of Neurospora crassa. J. Bacteriol. 137:1140-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schrammel, A., S. Behrends, K. Schmidt, D. Koesling, and B. Maye. 1996. Characterization of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one as a heme-site inhibitor of nitric oxide-sensitive guanylyl cyclase. Mol. Pharmacol. 50:1-5. [PubMed] [Google Scholar]
- 25.Silverman, P. M., and P. M. Epstein. 1975. Cyclic nucleotide metabolism coupled to cytodifferentiation of Blastocladiella emersonii. Proc. Natl. Acad. Sci. U. S. A. 72:442-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song, N. K., C. S. Jeong, and H. S. Choi. 2000. Identification of nitric oxide synthase in Flammulina velutipes. Mycologia 92:1027-1032. [Google Scholar]
- 27.Tao, Y. P., T. P. Misko, A. C. Howlett, and C. Klein. 1997. Nitric oxide, an endogenous regulator of Dictyostelium discoideum differentiation. Development 124:3587-3595. [DOI] [PubMed] [Google Scholar]
- 28.Tomprefa, N., M. P. McQuilken, R. A. Hill, and J. M. Whipps. 2009. Antimicrobial activity of Coniothyrium minitans and its macrolide antibiotic macrosphelide A. J. Appl. Microbiol. 106:2048-2056. [DOI] [PubMed] [Google Scholar]
- 29.Vieira, L. G., E. Linares, O. Augusto, and S. L. Gomes. 2009. Evidence of a Ca2+-NO-cGMP signaling pathway controlling zoospore biogenesis in the aquatic fungus Blastocladiella emersonii. Fungal Genet. Biol. 46:575-584. [DOI] [PubMed] [Google Scholar]
- 30.Wang, J., and V. J. Higgins. 2005. Nitric oxide has a regulatory effect in the germination of conidia of Colletotrichum coccodes. Fungal Genet. Biol. 42:284-292. [DOI] [PubMed] [Google Scholar]
- 31.Weissman, L., J. Garty, and A. Hochman. 2005. Rehydration of the lichen Ramalina lacera results in production of reactive oxygen species and nitric oxide and a decrease in antioxidants. Appl. Environ. Microb. 71:2121-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wendehenne, D., A. Pugin, D. F. Klessig, and J. Durner. 2001. Nitric oxide: comparative synthesis and signaling in animal and plant cells. Trends Plant Sci. 6:177-183. [DOI] [PubMed] [Google Scholar]
- 33.Whipps, J. M., and M. Gerlagh. 1992. Biology of Coniothyrium minitans and its potential for use in disease biocontrol. Mycol. Res. 96:897-907. [Google Scholar]
- 34.Whipps, J. M., S. Sreenivasaprasad, S. Muthumeenakshi, C. W. Rogers, and M. P. Challen. 2008. Use of Coniothyrium minitans as a biocontrol agent and some molecular aspects of sclerotial mycoparasitism. Eur. J. Plant Pathol. 121:323-330. [Google Scholar]
- 35.Wilken, M., and B. Huchzermeyer. 1999. Suppression of mycelia formation by NO produced endogenously in Candida tropicalis. Eur. J. Cell Biol. 78:209-213. [DOI] [PubMed] [Google Scholar]
- 36.Yang, R., Y. C. Han, G. Q. Li, D. H. Jiang, and H. C. Huang. 2007. Suppression of Sclerotinia sclerotiorum by antifungal substances produced by the mycoparasite Coniothyrium minitans. Eur. J. Plant Pathol. 119:411-420. [Google Scholar]
- 37.Yang, R., Y. C. Han, G. Q. Li, D. H. Jiang, and H. C. Huang. 2008. Effects of ambient pH and nutritional factors on antifungal activity of the mycoparasite Coniothyrium minitans. Biol. Control 44:116-127. [Google Scholar]