Abstract
Although mycoplasmas are generally considered to be harmless commensals, some mycoplasma species are able to cause infections in pediatric, geriatric, or immunocompromised patients. Thus, accidental contamination of biologics with mycoplasmas represents a potential risk for the health of individuals who receive cell-derived biological and pharmaceutical products. To assess the efficiency of inactivation of mycoplasmas by the agents used in the manufacture of egg-derived influenza vaccines, we carried out a series of experiments aimed at monitoring the viability of mycoplasmas spiked into both chicken allantoic fluid and protein-rich microbiological media and then treated with beta-propiolactone, formalin, cetyltrimethylammonium bromide, Triton X-100, and sodium deoxycholate, which are agents that are commonly used for virus inactivation and disruption of viral particles during influenza vaccine production. Twenty-two mycoplasma species (with one to four strains of each species) were exposed to these inactivating agents at different concentrations. The most efficient inactivation of the mycoplasmas evaluated was observed with either 0.5% Triton X-100 or 0.5% sodium deoxycholate. Cetyltrimethylammonium bromide at concentrations of ≥0.08% was also able to rapidly inactivate (in less than 30 min) all mycoplasmas tested. In contrast, negligible reductions in mycoplasma titers were observed with 0.0125 to 0.025% formaldehyde. However, increasing the concentration of formaldehyde to 0.1 to 0.2% improved the mycoplasmacidal effect. Incubation of mycoplasmas with 0.1% beta-propiolactone for 1 to 24 h had a marked mycoplasmacidal effect. A comparison of the mycoplasma inactivation profiles showed that strains of selected species (Mycoplasma synoviae, Mycoplasma gallisepticum, Mycoplasma orale, Mycoplasma pneumoniae, and Acholeplasma laidlawii) represent a set of strains that can be utilized to validate the effectiveness of mycoplasma clearance obtained by inactivation and viral purification processes used for the manufacture of an inactivated egg-based vaccine.
The mycoplasmas (the trivial name for Mollicutes) are a class of broadly distributed wall-less bacteria with some of the smallest known genomes that support bacterial self-replication (43). While most mycoplasmas are naturally harmless commensals that colonize a wide range of plant, insect, reptilian, avian, mammalian, and human hosts (43), some of them are able to cause diseases whose severity varies in their natural hosts (1, 4, 10, 19, 43).
Mycoplasmas, particularly species of genera Mycoplasma and Acholeplasma, are also frequent contaminants of primary and continuous cell lines (45). This is a concern for research laboratories and commercial facilities involved in the development and production of cell-derived biological and pharmaceutical products. In addition, several natural mycoplasma infections of embryonated fowl eggs and primary chicken embryo cell cultures used for the production of viral vaccines have been reported (5-7, 16, 36, 46, 48, 55). Although only a few species (Mycoplasma synoviae, M. gallisepticum, and M. pullorum) (5-7, 39) have been isolated from infected embryonated fowl (chicken, turkey, or quail) eggs, the abilities of other nonavian mycoplasma species (M. fermentans, M. penetrans, and M. pneumoniae) to infect embryonated chicken eggs and to be pathogenic for chicken embryos have also been experimentally demonstrated (20, 24). Sporadic mycoplasma infections of immunocompetent and immunocompromised individuals that originated from domestic and wild animals have been reported (4, 37, 41). M. gallisepticum, M. synoviae, and M. iowae were found to survive on human nasal mucosa for 12 to 24 h after experimental inoculation (11). More recently, it has been reported that the avian organism M. lipofaciens can be transmitted to humans and cause mild symptoms of rhinitis (35). These findings raise serious concerns about the potential susceptibility of some humans, particularly children and persons with congenital or acquired immunodeficiencies, to nonhuman infectious mycoplasmas.
At present, primarily for cost-associated reasons, specific- or specified-pathogen-free (SPF) fowl eggs are usually used only for production of vaccine virus seeds, while production lots of egg-based vaccines are manufactured using embryonated eggs obtained from healthy flocks. Even though these flocks are normally tested for the absence of specific avian infectious agents that are horizontally and vertically transmissible (14, 18, 49), including the most frequent avian pathogens, M. synoviae and M. gallisepticum, this testing cannot guarantee that chickens and their eggs are also free of other avian-associated mycoplasmas (e.g., M. lipofaciens, M. gallinarum, M. gallinaceum, M. glycophilum, M. meleagridis, M. iowae, and Acholeplasma laidlawii) that are able to grow in ovo (5, 34). Because testing for these mycoplasma species is discretionary, there is always a risk of accidental contamination of embryonated eggs with these species (14).
Testing of cell lines and cell-derived biological products for mycoplasmas in the United States is currently regulated by Title 21 of the Code of Federal Regulations (21 CFR 610.30) for viral vaccines, and additional recommendations are described in the “Points to Consider (PTC) in the Characterization of Cell Lines Used to Produce Biologicals (1993)” (54a) and “Guidance for Industry: Characterization and Qualification of Cell Substrates and Other Biological Starting Materials Used in the Production of Viral Vaccines for the Prevention and Treatment of Infectious Diseases” (54). The analytical protocols recommended for mycoplasma testing include the use of broth and agar cultures and indicator cell tests (54, 57). Although in principle the PTC procedure can be used for highly efficient detection of mycoplasmas in egg-derived viral biologics, most manufacturers historically have used another approach to ensure the safety of viral vaccines. This approach is based on an evaluation of the efficiency of inactivation of any mycoplasmas potentially present in virus harvests using rigorous downstream manufacturing procedures for inactivation and purification of vaccine viruses. These procedures, which include the use of different chemicals and surfactants, are able to inactivate a large number of adventitious agents, including mycoplasmas (32). Validation of the efficiency of mycoplasma clearance during manufacture provides assurance of the safety of egg-derived viral vaccines, including inactivated influenza vaccine.
The main goal of the current study was to evaluate the rates of inactivation of different mycoplasma species that potentially are present in egg-derived virus harvests with the following reagents: beta-propiolactone (BPL), formalin, cetyltrimethylammonium bromide (CTAB), Triton X-100, and sodium deoxycholate (DOC). These agents are commonly used in the manufacture of inactivated viral vaccines, including inactivated influenza vaccine (21, 22, 27, 28, 31, 50).
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The following 48 strains of 22 Mollicutes species were obtained from the American Type Culture Collection (Manassas, VA) and the Mollicutes Collection at Purdue University (Purdue University, West Lafayette, IN): M. arginini ATCC 23838 and HT9; M. bovis ATCC 25523, ATCC 27368, and 70-213; M. fermentans ATCC 19989, ATCC 49891, and AOU; M. gallinaceum ATCC 33550 and SA; M. gallisepticum ATCC 19610; M. hominis ATCC 27545, ATCC 15056, and ATCC 33131; M. canis ATCC 19525; M. hyorhinis ATCC 23839 and ATCC 17981; M. orale ATCC 23714; M. salivarium ATCC 23064, MP1166, and AROZ; M. synoviae ATCC 25204, K3161C, K5664A, and K758; M. yeatsii ATCC 43094; M. arthritidis ATCC 14124 and ATCC 35943; M. iowae ATCC 33552 and PPAV; M. anatis ATCC 25524, K6193A, and K6193C; M. anatis-like strain 1220 (16); M. gallinarum ATCC 19708; M. edwardii ATCC 23462 and 04-3436 (47); M. capricolum subsp. capricolum ATCC 27343 (= California Kid) and Goat 6558; M. pneumoniae ATCC 15531 and ATCC 29343; Acholeplasma axanthum ATCC 27378, ATCC 25176, and 118; Acholeplasma equifetale ATCC 29724 and N93; and A. laidlawii ATCC 14089 and ATCC 23217. Selected species were grown using media and conditions recommended by the supplier (http://www.atcc.org) and described elsewhere (13, 23, 38). The authenticity of all mycoplasma species used in this study was confirmed by analyzing multiple mycoplasmal genetic markers as described elsewhere (56, 58).
Chemicals.
Beta-propiolactone (BPL) (grade II; ≥90% pure; catalog no. P5648), a formaldehyde solution (catalog no. F1268), cetyltrimethylammonium bromide (CTAB) (≥99% pure; catalog no. H9151), Triton X-100 (catalog no. T9284), and sodium deoxycholate (DOC) (≥99% pure; catalog no. D5670) were purchased from Sigma-Aldrich (St. Louis, MO). All stock solutions of the chemicals were prepared by dissolving or diluting the chemicals to obtain the required concentrations using UltraPure sterile water (Invitrogen, Carlsbad, CA). Five percent stock solutions of each of the surfactants (CTAB, DOC, and Triton X-100) were filter sterilized with 0.22-μm filters and stored at 4°C for no more than 1 month. Working solutions of BPL and formaldehyde were freshly prepared prior to each experiment.
Biological matrices.
Three biological matrices, minimum essential Eagle medium (MEM), allantoic fluid of chicken embryos, and mycoplasma broth containing 15 to 20% horse or swine serum, were used to assess the influence of different matrices on the mycoplasmacidal activities of the chemicals. To replace mycoplasma broth with MEM or allantoic fluid, 10-ml aliquots of a mycoplasma culture grown overnight were centrifuged at 5,204 × g for 20 min, and the cell pellets were carefully resuspended in the same volume (10 ml) of MEM, allantoic fluid, or fresh broth. The titers of mycoplasma cultures (in color-changing units [CCU]/ml and/or CFU/ml) were determined using SP4 medium (Remel, Lenexa, KS).
The total protein contents of different matrices were assessed using the Lowry protein assay (Thermo Fisher Scientific Inc., Rockford, IL).
Mycoplasma inactivation protocol.
The efficiency of mycoplasma inactivation was monitored using two methods: a 96-well microtiter plate method and a 10-ml tube macrodilution method. The same media and growth conditions were used for both methods. Mycoplasma stocks used for inactivation experiments were prepared by growing mycoplasma strains overnight in appropriate media at 36.5°C under either aerobic conditions (5% CO2) or anaerobic conditions (GasPak EZ anaerobe pouch system; BD, Franklin Lakes, NJ), depending on the biologic properties of the mycoplasma strain. To ensure a high level of viability of mycoplasma stocks, all cultures were grown to mid-logarithmic or early stationary phase, which corresponded to titers of 105 to 109 color-changing units per ml. The mycoplasmacidal activities of specified concentrations of each chemical agent, which were always near or above the critical micelle concentrations of the detergents (http://www.sigmaaldrich.com/img/assets/15402/Detergent_Selection_Table.pdf), were assessed by using the reductions in the mycoplasma titer determined at specific time points following incubation with the chemical. The titers of control (untreated) mycoplasma cultures were also monitored in each experiment to confirm viability and to assess any spontaneous death of microorganisms during the incubation period.
Growth of the mycoplasmas was monitored visually using changes in the color of the medium due to pH shifts as an indicator of the metabolic activity of living cells (i.e., acidification of the medium for glucose-utilizing species and alkalinization of the medium for species hydrolyzing arginine [23]). In general, the mycoplasmacidal activity of the chemical agents was assessed by determining mycoplasmal growth and viability in mycoplasma broth containing 0.5% glucose (pH 7.8) or 0.5% arginine (pH 7.3) and phenol red as a pH indicator. All broth media were also supplemented with 15 to 20% horse or swine serum (Sigma) and 100 IU/ml of benzylpenicillin (Sigma), which was used to prevent the growth of other bacterial contaminants.
Inactivation of mycoplasmas was tested using 10-ml aliquots of mycoplasma cultures supplemented with different amounts of each chemical (Table 1). Each mixture was thoroughly vortexed and incubated for up to 48 h at different temperatures (4°C, 37°C, and room temperature [RT] [22 to 25°C]) with intermittent manual mixing during the incubation period. To assess the residual titers of mycoplasmas after incubation, aliquots of the cultures taken at different time points (Table 1) were serially diluted 10-fold using Hanks' balanced salt solution, and the titers were determined using a microtiter plate method and/or a macrodilution tube method.
TABLE 1.
Chemical agents, concentrations, and inactivation conditions used in this study
| Chemical | Final concn with 2-fold dilution factor (%) | Inactivation time (h) | Inactivation temp (°C)a |
|---|---|---|---|
| Triton X-100 | 0.25-0.5 | 0.5-48 | 4, 37, 22-25 |
| Sodium deoxycholate | 0.25-0.5 | 0.5-48 | 4, 37, 22-25 |
| Cetyltrimethylammonium bromide | 0.01-0.08 | 0.5-48 | 4, 37, 22-25 |
| β-Propiolactone | 0.05-0.1 | 0.5-48 | 4, 37, 22-25 |
| Formaldehyde | 0.0125-0.2 | 0.5-48 | 4, 37, 22-25 |
RT was defined as 22 to 25°C.
Microtiter plate method.
The microtiter plate test was carried out as described previously (12, 23, 44), with some modifications. Serial 10-fold dilutions (up to a 10−12 dilution) of the mycoplasma cultures were prepared in 96-well plates using Hanks' balanced salt solution (dilution plate). After the dilutions were prepared, 20 μl of each dilution was transferred to another 96-well microtiter plate containing 180 μl of the appropriate broth (growth plates). The data for eight or four replicates of a single dilution of treated mycoplasma were used to calculate the residual titer.
The microtiter growth plates were closed with plastic lids and were incubated for 10 to 14 days under aerobic or anaerobic conditions, depending on the properties of individual species. The residual mycoplasma titers (in CCU/ml) were determined visually at each time point on the basis of changes in color compared with control uninoculated medium (negative control).
Macrodilution tube method.
The macrodilution tube procedure was performed as previously described (12). Briefly, 1 ml of a mycoplasma sample was added to 9 ml of Hanks' balanced salt solution, and serial 10-fold dilutions from 10−1 to 10−12 were prepared in tubes. Then 1 ml of each dilution was transferred to another tube containing 9 ml of the appropriate broth. The tubes were incubated for 10 to 14 days under aerobic or anaerobic conditions depending on the biological properties of the species tested. Titration endpoints (in CCU/ml) were determined visually at the end of the experiment on the basis of changes in color due to pH shifts compared with the tube containing control uninoculated medium (negative control). The mycoplasmacidal activity of each chemical was assessed in duplicate using this test.
RESULTS
Influence of biological matrices on mycoplasma inactivation.
To assess the potential influence of different biological matrices on the mycoplasmacidal activities of BPL, formaldehyde, Triton X-100, and DOC, we initially compared the rates of inactivation of five mycoplasmas (M. gallisepticum, M. arginini, M. iowae, A. laidlawii, and A. equifetale) at titers of 105 to 109 CCU/ml in MEM, allantoic fluid of chicken embryos, and mycoplasma broth supplemented with 15 to 20% horse or swine serum. This was done primarily to determine whether allantoic fluid can be replaced by other matrices without affecting the mycoplasma inactivation profiles during incubation with the chemicals tested. The total protein contents of the chicken embryo allantoic fluid and glucose- and arginine-containing broth media were 150 to 200 μg/ml and 15,640 to 18,570 μg/ml, respectively. However, despite the significant difference in protein content between allantoic fluid and mycoplasma broth media, we observed very minor difference (less than 1.0 to 1.5 log) in the mycoplasmacidal activities of the chemical agents in these matrices when five selected mycoplasma species were used. Figures 1A and 1B show the inactivation profiles obtained for M. gallisepticum ATCC 19610 using 0.05% BPL and for M. arginini ATCC 23838 using 0.25% DOC in different biological matrices. This test showed that incubating the mycoplasmas in broth media gave the same results as incubating the mycoplasmas in the allantoic fluid matrix. We therefore utilized the broth media for subsequent inactivation experiments with different concentrations of chemical agents and different temperatures.
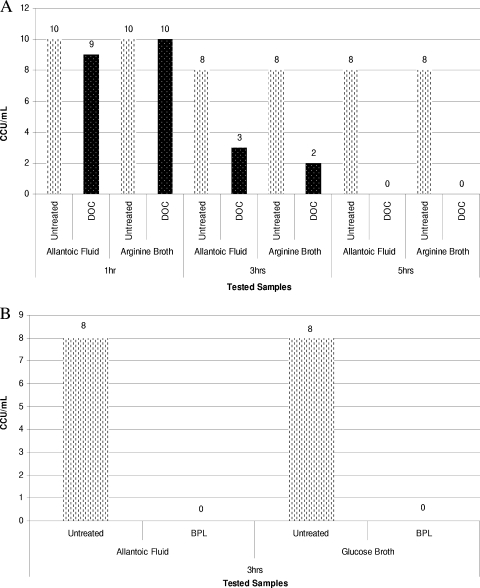
FIG. 1.
(A) Dynamics of inactivation of M. arginini ATCC 23838 using 0.25% sodium deoxycholate in two different biological matrices at RT. (B) Dynamics of inactivation of M. gallisepticum ATCC 19610 using 0.05% beta-propiolactone in two different biological matrices at RT. At the first time point (after 3 h of exposure) there was complete inactivation of M. gallisepticum ATCC 19610.
Mycoplasma inactivation using Triton X-100 and DOC.
The nonionic surfactant Triton X-100 and the anionic surfactant DOC are widely utilized to release immunogenic proteins (i.e., viral hemagglutinin and neuraminidase) from virions during the production of a split-virus influenza vaccine. Usually, the concentration of Triton X-100 and DOC used for disruption of virions is 0.5% or higher (15, 40, 52). In our experiments the concentrations of Triton X-100 and DOC were reduced to 0.25 to 0.5% because the higher concentrations of these chemical agents inactivated all mycoplasmas tested almost instantly and did not allow assessment of the relative resistance of different mycoplasma species to the chemicals.
At a lower concentration (0.25%), both surfactants completely inactivated almost all of the 22 mycoplasma species tested in 30 to 60 min. Two mycoplasma strains, M. hyorhinis GDL and M. synoviae WVU 1853, remained viable in the presence 0.25% DOC in the broth for up to 5 h (Fig. 2A and 2B). Increasing the concentration of DOC to 0.5% always resulted in rapid inactivation of these organisms. The inactivation experiments performed at different temperatures showed that there was not a significant dependence of inactivation of mycoplasmas by DOC and Triton X-100 on temperature (data not shown).
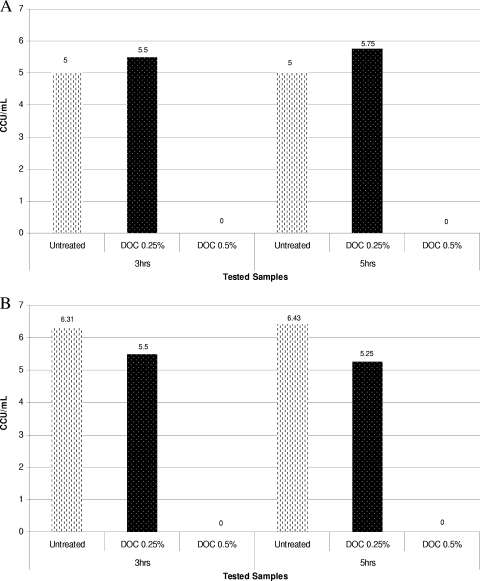
FIG. 2.
(A) Dynamics of inactivation of M. synoviae WVU 1853 using 0.25% sodium deoxycholate at RT. (B) Dynamics of inactivation of M. hyorhinis GDL using 0.25% sodium deoxycholate at RT.
Mycoplasma inactivation using beta-propiolactone and formaldehyde solutions.
BPL and formaldehyde are commonly used at concentrations of 0.05 to 0.2% as primary chemical agents for inactivation of vaccine viruses in cell- and egg-derived harvests. The inactivating effect of these reagents is based on their chemical reaction with nucleophilic groups of viral proteins and nucleic acids that results in irreversible changes in the structure of virions and the loss of viral infectivity (8, 9). The concentrations of chemicals, time, and temperature required for efficient virus inactivation may vary significantly depending on the type of virus and the protocol used by the vaccine manufacturer.
The results of the mycoplasma inactivation study with BPL at a concentration of 0.1% (13.87 mM) or higher showed that BPL efficiently inactivated most of the mycoplasma strains tested within 0.5 to 24 h (data not shown). Only one strain, M. arthritidis ATCC 35943, was still viable after 24 h of incubation at room temperature with 0.1% BPL (Fig. 3). Thus, even use of a relatively high concentration of BPL (0.1%), which generally results in complete inactivation of vaccine viruses, cannot always guarantee complete inactivation of mycoplasma contaminants that potentially are present in a virus harvest. Moreover, when the BPL concentration was reduced to 0.05%, a concentration that is also commonly used for vaccine virus inactivation, mycoplasmas such as M. salivarium AROZ, A. equifetale C112, M. anatis ATCC 25524, A. laidlawii ATCC 14089, M. arthritidis ATCC 35943, and M. synoviae 33 survived incubation for 24 h. Thus, our results showed that 0.05% BPL alone could not be considered an effective method for eliminating mycoplasma contaminants in protein-rich media.
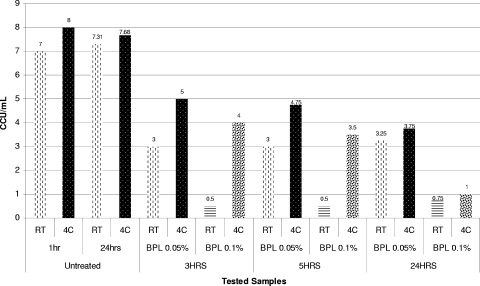
FIG. 3.
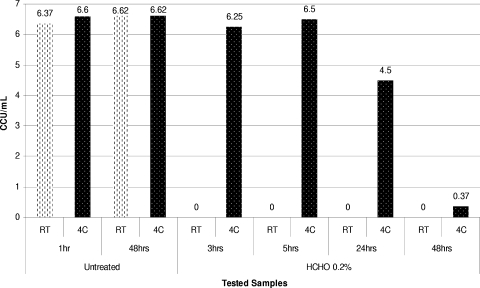
Dynamics of inactivation of M. arthritidis ATCC 35943 using 0.05 and 0.1% beta-propiolactone at RT and 4°C (4C).
In contrast to the observations with surfactants, inactivation of mycoplasmas with 0.05 to 0.1% BPL was found to be strongly dependent on the temperature of incubation. Reducing the incubation temperature to 4°C dramatically decreased the mycoplasmacidal activity of BPL and increased the mycoplasma survival time (Fig. 3 and 4).
FIG. 4.
Dynamics of inactivation of A. laidlawii ATCC 14089 using 0.05% beta-propiolactone at RT and 4°C (4C).
Formaldehyde at concentrations of 0.0125 to 0.025% was found to be the least efficient inactivating agent among the chemical agents tested. These concentrations of formaldehyde did not inactivate many of the mycoplasmas tested even when the time of incubation was increased to 48 h at both room temperature and 4°C. Increasing the formaldehyde concentration to 0.025% did not significantly affect the titers of the mycoplasma species tested and did not result in complete inactivation of mycoplasmas during 48 h of incubation. Increasing the formaldehyde concentration to 0.05% did result in efficient inactivation of most mycoplasma species after 24 h of incubation at RT. However, several mycoplasma strains, including M. salivarium AROZ, A. equifetale C112, M. bovis Madison, A. laidlawii ATCC 14089, M. arthritidis ATCC 35943, M. iowae ATCC 33552 and PPAV, and M. gallinaceum ATCC 33550, retained some viability (residual titers, ≤1.0 to 2.0 logs) during this inactivation experiment (data not shown).
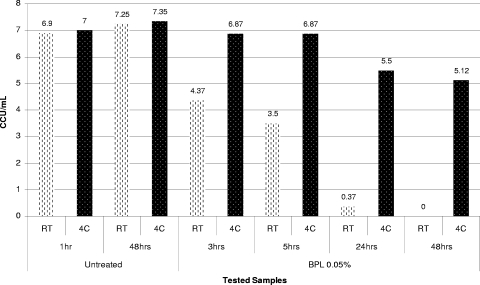
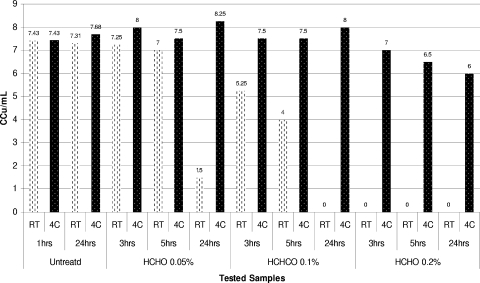
Increasing the formaldehyde concentration to 0.1% and then to 0.2% had an apparent germicidal effect on all mycoplasmas tested in inactivation experiments performed at room temperature. We observed that just a few species were able to survive for the first 5 h of incubation with 0.2% formaldehyde. Similar to the results for beta-propiolactone, inactivation of mycoplasmas with formaldehyde was strongly temperature dependent. Thus, when the temperature for inactivation of mycoplasmas using 0.2% formaldehyde was reduced to 4°C, some mycoplasma strains (e.g., A. laidlawii ATCC 14089, M. arthritidis ATCC 35943, and M. iowae PPAV) survived for more than 24 h of incubation (Fig. 5 and 6). In contrast, complete inactivation of these organisms was observed during the first 2 to 3 h of incubation at room temperature.
FIG. 5.
Dynamics of inactivation of A. laidlawii ATCC 14089 using 0.2% formaldehyde at RT and 4°C (4C).
FIG. 6.
Dynamics of inactivation of M. arthritidis ATCC 35943 using 0.05, 0.1, and 0.2% formaldehyde at RT and 4°C (4C).
Mycoplasma inactivation using CTAB.
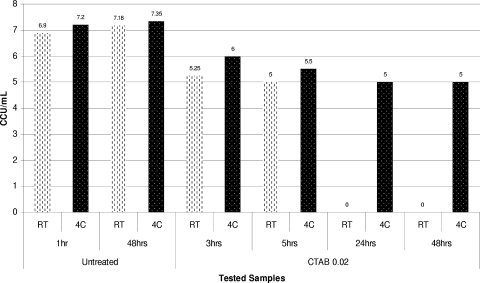
The cationic surfactant CTAB is generally used for the manufacture of inactivated viral vaccines at concentrations ranging from 0.075% to 0.15% or higher. In our experiments we were not able to explore the resistance of mycoplasmas at these concentrations of this surfactant due to very rapid inactivation of mycoplasmas. The results of a preliminary study showed that CTAB almost immediately (<30 min) inactivated all mycoplasmas tested at concentrations of ≥0.08% (≥2.2 mM). Therefore, we assessed the inactivating activities of concentrations of this surfactant ranging from 0.02 to 0.04%, which are considered to be a potential worst-case scenario for vaccine manufacture.
Of all the mycoplasma strains examined, only three, M. salivarium AROZ, M. synoviae WVU 1853, and A. equifetale C112, were able to survive for a few hours when they were exposed to 0.04% CTAB (data not shown). However, when the CTAB concentration was reduced to 0.02%, most of the mycoplasmas tested remained viable for the first 5 h of incubation at RT. Complete inactivation of some of these organisms required incubation times of 24 to 48 h. When the CTAB concentration was reduced to 0.01%, this surfactant did not completely inactivate most mycoplasmas within 24 h at any temperature tested.
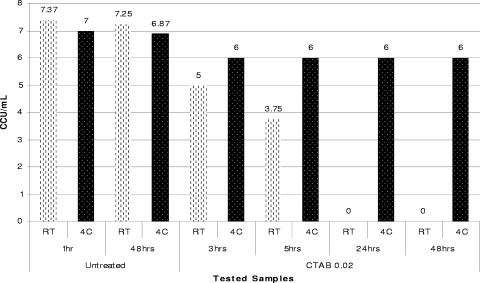
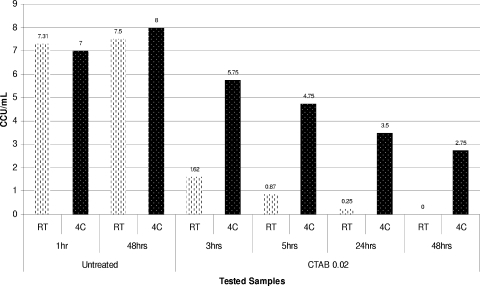
It is interesting that the mycoplasmacidal activity of the cationic surfactant CTAB, unlike the mycoplasmacidal activities of nonionic (Triton X-100) and anionic (sodium deoxycholate) detergents, was temperature dependent and was considerably lower at lower incubation temperatures. Thus, the reduction in temperature from room temperature to 4°C during inactivation of A. laidlawii ATCC 14089, M. iowae PPAV, A. equifetale C112, or M. synoviae WVU 1853 with 0.02% CTAB resulted in dramatic changes in the inactivation profiles obtained for these organisms at different temperatures (Fig. 7, 8, and 9).
FIG. 7.
Dynamics of inactivation of A. equifetale C112 using 0.02% CTAB at RT and 4°C (4C).
FIG. 8.
Dynamics of inactivation of M. iowae PPAV using 0.02% CTAB at RT and 4°C (4C).
FIG. 9.
Dynamics of inactivation of A. laidlawii ATCC 14089 using 0.02% CTAB at RT and 4°C (4C).
DISCUSSION
Contamination of biologics with mycoplasmas may represent a considerable risk to human health. While mycoplasmas are generally considered to be host-specific bacteria, a few sporadic cases of mycoplasmal infections of humans caused by animal and avian mycoplasmas have been reported (35, 37, 41). Severe cases of human infections with human and nonhuman mycoplasmas can occur, especially in immunocompromised individuals (19, 35, 37, 41). To ensure the purity and safety of parenterally administrated drugs and vaccines manufactured using different cell substrates, including embryonated chicken eggs, current FDA regulations (21 CFR 610.30 for viral vaccines) and FDA guidance in 1993 (PTC) and 2006 (54) recommend that a manufacturer show that cell substrates and unprocessed virus harvests are free of mycoplasma contamination and other adventitious agents. The use of embryonated eggs from healthy (but not SPF-grade) flocks for manufacture of virus vaccines can pose some risk of inadvertent mycoplasma contamination of vaccine products. Therefore, a risk assessment approach is used to ensure the safety of the egg-based vaccine products. To ensure that there is no mycoplasma contamination, the manufacturers perform a thorough validation showing that the downstream manufacturing processes (virus concentration, inactivation, and purification procedures) can completely inactivate mycoplasmas, if such contamination inadvertently occurs (27, 32). The influenza virus concentration, inactivation, and purification procedures include the use of several chemical reagents (beta-propiolactone, formalin, cetyltrimethylammonium bromide, Triton X-100, and sodium deoxycholate) which exhibit germicidal activity and efficiently inactivate any mycoplasmal or other bacterial contaminants.
The main objective of our study was to evaluate the efficiency of mycoplasma inactivation under conditions generally used for the production of inactivated virus vaccines, including inactivated influenza virus (split-virion) vaccine. To assess the contribution of each chemical agent used in the mycoplasma inactivation process, the agents were tested in separate experiments at concentrations that are commonly used or represent “worst-case scenarios” for flu vaccine production.
The first group of reagents included formaldehyde and BPL, which are commonly used for inactivation of vaccine viruses via chemical reaction with viral capsid proteins and nucleic acids (3, 8, 9). The second group consisted of surfactants, including cetyltrimethylammonium bromide, Triton X-100, and sodium deoxycholate, which are usually used to split virus particles into separate structural proteins.
The results obtained for BPL and formaldehyde demonstrated that all 22 mycoplasma species examined could be completely inactivated (no viable bacteria were detected by the microtiter and macrodilution methods) within 3 to 24 h at room temperature in mycoplasma media containing 0.2% (≥66.60 mM) formaldehyde and 0.1% (≥13.87 mM) BPL.
The results of our study using formaldehyde and BPL were consistent with previous data obtained for these reagents using only three mycoplasma species (M. gallisepticum, M. canis, and A. laidlawii) spiked into Vero and DK cell culture suspensions (30). Similar to the results described in the previous study, we observed incomplete inactivation of many of the species tested when the concentration of formaldehyde was 0.02% or lower. A significant improvement in inactivation of mycoplasmas was observed when the formaldehyde concentration was increased to 0.2% (30). Our data also showed that A. laidlawii exhibited enhanced stability in the presence of formaldehyde (30) and resistance to inactivation for the first 5 h of incubation with 0.2% formaldehyde. However, A laidlawii was not viable after 24 h of incubation with formaldehyde. A. laidlawii was also found to be more resistant to BPL than the other species examined. A. laidlawii remained viable for 2 h of incubation with 0.1% BPL; this result is consistent with the ability of this species to resist inactivation by BPL described previously (30).
In a majority of the protocols used for production of inactivated virus vaccines (2, 9, 17, 25, 27, 29, 33, 42, 51, 53), the concentration of BPL and formaldehyde exceeds the critical concentration (0.1% to 0.2%) required for complete inactivation of mycoplasmas. Our data showed that these conditions resulted in complete inactivation of many of the mycoplasmas evaluated. However, the results can change dramatically if a lower incubation temperature is used for inactivation by formaldehyde or BPL. We compared the inactivation profiles obtained at different temperatures (e.g., RT and 4°C) for selected species and found that a reduction in the temperature from RT to 4°C resulted in significantly increased survival of all mycoplasmas at all concentrations of formaldehyde and BPL tested, including 0.2% and 0.1%, respectively.
Among the chemical agents tested, cetyltrimethylammonium bromide at concentrations higher than 0.08% was the most efficient mycoplasma-inactivating agent. Complete inactivation of mycoplasmas by CTAB was observed in the first 30 min of incubation, regardless of the temperature and the protein composition of biological matrices (allantoic fluid or mycoplasma broth). The working concentration of CTAB during typical virus vaccine production varies from 0.075 to 0.15%. This concentration allows efficient disruption of virion structure and release of individual structural proteins. Our data showed that this concentration of CTAB is highly likely to kill any inadvertent mycoplasmas in the first 30 min of exposure.
The risk assessment approach used to ensure that mycoplasma contaminants are not present in the production of egg-derived viral biologics relies on data provided by special evaluation experiments aimed at assessing the efficiency of mycoplasma inactivation during the manufacturing processes (inactivation and split manufacturing steps). There are no standardized model mycoplasmas for this validation procedure, but M. gallisepticum, M. synoviae, M. orale, M. pneumoniae, and A. laidlawii are commonly used. These species were selected for testing based on the probability of contamination of eggs with avian species (M. synoviae, M. gallisepticum), differences in fermentation activity (M. orale, M. pneumoniae), and a wide distribution in natural environments (A. laidlawii). For example, A. laidlawii has been found as a mycoplasma contaminant in plant-derived peptones (26). To evaluate whether this set of mycoplasma species can be used as appropriate model species for evaluating mycoplasma clearance obtained with different chemical agents at different concentrations, we compared the sensitivities of 22 mycoplasmas (including the species mentioned above) that were of human, animal, and avian origin (see Materials and Methods). The results of this study did not reveal any significant intraspecies difference in sensitivity to the chemicals tested. However, the analysis of inactivation profiles obtained for different species showed that species could differ substantially in their sensitivities to formaldehyde, BPL, and CTAB, particularly when these reagents were used at the lowest concentrations tested. These differences were not apparent when the chemical concentrations were increased to the concentrations used during virus vaccine production.
A detailed evaluation of the inactivation profiles of the five mycoplasmas currently recommended for validation of mycoplasma inactivation during virus vaccine production, M. synoviae, M. gallisepticum, M. orale, M. pneumoniae, and A. laidlawii, showed that these species are appropriate for validation purposes. Thus, inactivation of M. synoviae and A. laidlawii usually required longer incubation times with lower and borderline inactivation doses of formaldehyde, beta-propiolactone, and CTAB than inactivation of other mycoplasmas, including M. gallisepticum, M. orale, and M. pneumoniae. However, differences were observed with formaldehyde, BPL, and CTAB only at concentrations less than 0.2%, 0.1%, and 0.04%, respectively. No differences in inactivation of these mycoplasmas were observed when Triton X-100 and DOC were used at a concentration of 0.5%, which is the concentration currently used for manufacture of split-virus influenza vaccines. Thus, M. synoviae, M. gallisepticum, M. orale, M. pneumoniae, and A. laidlawii represent a set of organisms acceptable for performing clearance validation procedures to ensure that there is no mycoplasma contamination in influenza vaccine products.
Moreover, a combination of different chemicals, such as formaldehyde or beta-propiolactone with surfactants (Triton X-100, sodium deoxycholate, and cetyltrimethylammonium bromide), during production provides additive safety, increasing the probability that any inadvertently introduced mycoplasmas are removed from egg-derived biologic products utilizing virus inactivation and detergent-based virion disruption procedures. However, it is important to emphasize that biologic manufacturing processes that utilize chemicals and conditions other than those included in our study should be carefully evaluated on a case-by-case basis to determine the efficiency of clearance of mycoplasmas by the specific production processes using appropriately selected strains.
Acknowledgments
We express our appreciation to Alfred Del-Grosso for his assistance with protein measurement, to Galina Vodeiko for her support during this study, and to Donna K. F. Chandler and Maureen K. Davidson for their critical reading of the manuscript and useful suggestions.
The findings and conclusions described in this paper have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any agency determination or policy.
Footnotes
Published ahead of print on 12 March 2010.
REFERENCES
- 1.Al Masalma, M., F. Armougom, W. M. Scheld, H. Dufour, P. H. Roche, M. Drancourt, and D. Raoult. 2009. The expansion of the microbiological spectrum of brain abscesses with use of multiple 16S ribosomal DNA sequencing. Clin. Infect. Dis. 48:1169-1178. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, E., S. Clouthier, W. Shewmaker, A. Weighall, and S. LaPatra. 2008. Inactivated infectious haematopoietic necrosis virus (IHNV) vaccines. J. Fish Dis. 31:729-745. [DOI] [PubMed] [Google Scholar]
- 3.Baczynska, A., H. F. Svenstrup, J. Fedder, S. Birkelund, and G. Christiansen. 2004. Development of real-time PCR for detection of Mycoplasma hominis. BMC Microbiol. 4:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker, A. S., K. L. Ruoff, and S. Madoff. 1998. Isolation of Mycoplasma species from a patient with seal finger. Clin. Infect. Dis. 27:1168-1170. [DOI] [PubMed] [Google Scholar]
- 5.Bencina, D., D. Dorrer, and T. Tadina. 1987. Mycoplasma species isolated from six avian species. Avian Pathol. 16:653-664. [DOI] [PubMed] [Google Scholar]
- 6.Bogomolova, N. N., I. A. Brudno, I. S. Kolianova, N. V. Izotova, and O. E. Orlova. 1981. Study of viral contamination of Japanese quail and cell cultures from their embryos. Vopr. Virusol. 1981:114-117. (In Russian.) [PubMed] [Google Scholar]
- 7.Bogomolova, N. N., N. V. Izotova, S. Boriskin Iu, V. D. Lotte, and I. S. Kolianova. 1978. Isolation and study of several properties of mycoplasma from Japanese quail embryos. Vopr. Virusol. 1978:560-564. (In Russian.) [PubMed] [Google Scholar]
- 8.Budowsky, E. I., E. A. Friedman, and N. V. Zheleznova. 1991. Principles of selective inactivation of viral genome. VII. Some peculiarities in determination of viral suspension infectivity during inactivation by chemical agents. Vaccine 9:473-476. [DOI] [PubMed] [Google Scholar]
- 9.Budowsky, E. I., E. A. Friedman, N. V. Zheleznova, and F. S. Noskov. 1991. Principles of selective inactivation of viral genome. VI. Inactivation of the infectivity of the influenza virus by the action of beta-propiolactone. Vaccine 9:398-402. [DOI] [PubMed] [Google Scholar]
- 10.Camara, B., M. Mouzin, D. Ribes, L. Esposito, J. Guitard, X. Game, D. Durand, L. Rostaing, and N. Kamar. 2007. Perihepatitis and perinephric abscess due to Mycoplasma hominis in a kidney transplant patient. Exp. Clin. Transplant. 5:708-709. [PubMed] [Google Scholar]
- 11.Christensen, N. H., C. A. Yavari, A. J. McBain, and J. M. Bradbury. 1994. Investigations into the survival of Mycoplasma gallisepticum, Mycoplasma synoviae and Mycoplasma iowae on materials found in the poultry house environment. Avian Pathol. 23:127-143. [DOI] [PubMed] [Google Scholar]
- 12.Christianson, G. G., J. R. Pemberton, and T. A. Koski. 1980. Inactivation of mycoplasma in serum with binary ethyleneimine. J. Clin. Microbiol. 11:377-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Council of Europe. 2010. Chapter 2.6.7, Mycoplasmas, p. 3317-3321. In European pharmacopoeia 6.1. Council of Europe, Strasbourg, France.
- 14.Council of Europe. 2010. Chapter 5.2.2, Chicken flocks free from specified pathogens for the production and quality control of vaccines, p. 5137-5139. In European pharmacopoeia 6.6. Council of Europe, Strasbourg, France.
- 15.Cox, J. H., B. Dietzschold, F. Weiland, and L. G. Schneider. 1980. Preparation and characterization of rabies virus hemagglutinin. Infect. Immun. 30:572-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobos-Kovacs, M., Z. Varga, G. Czifra, and L. Stipkovits. 2009. Salpingitis in geese associated with Mycoplasma sp. strain 1220. Avian Pathol. 38:239-243. [DOI] [PubMed] [Google Scholar]
- 17.Frazatti-Gallina, N. M., R. M. Mourao-Fuches, R. L. Paoli, M. L. Silva, C. Miyaki, E. J. Valentini, I. Raw, and H. G. Higashi. 2004. Vero-cell rabies vaccine produced using serum-free medium. Vaccine 23:511-517. [DOI] [PubMed] [Google Scholar]
- 18.Furuta, K., H. Ohashi, J. Obana, and S. Sato. 1980. Performance of 3 successive generations of specified-pathogenfree chickens maintained as a closed flock. Lab. Anim. 14:107-112. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-de-la-Fuente, C., E. Minambres, E. Ugalde, A. Saez, L. Martinez-Martinez, and M. C. Farinas. 2008. Post-operative mediastinitis, pleuritis and pericarditis due to Mycoplasma hominis and Ureaplasma urealyticum with a fatal outcome. J. Med. Microbiol. 57:656-657. [DOI] [PubMed] [Google Scholar]
- 20.Goodburn, G. M., and B. P. Marmion. 1962. A study of the properties of Eaton's primary atypical pneumonia organism. J. Gen. Microbiol. 29:271-290. [DOI] [PubMed] [Google Scholar]
- 21.Gross, P. A., F. A. Ennis, P. F. Gaerlan, C. R. Denning, U. Setia, W. J. Davis, and D. S. Bisberg. 1981. Comparison of new Triton X-100- and Tween-ether-treated split-treated vaccines in children. J. Clin. Microbiol. 14:534-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross, P. A., G. V. Quinnan, P. F. Gaerlan, C. R. Denning, M. Lazicki, and M. Bernius. 1983. Influenza vaccines in children. Comparison of new cetrimonium bromide and standard ether-treated vaccines. Am. J. Dis. Child. 137:26-28. [PubMed] [Google Scholar]
- 23.Hannan, P. C. 2000. Guidelines and recommendations for antimicrobial minimum inhibitory concentration (MIC) testing against veterinary mycoplasma species. International Research Programme on Comparative Mycoplasmology. Vet. Res. 31:373-395. [DOI] [PubMed] [Google Scholar]
- 24.Hayes, M. M., B. J. Li, D. J. Wear, and S. C. Lo. 1996. Pathogenicity of Mycoplasma fermentans and Mycoplasma penetrans in experimentally infected chicken embryos. Infect. Immun. 64:3419-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang, S. D., D. Pye, and J. C. Cox. 1986. Inactivation of poliovirus with beta-propiolactone. J. Biol. Stand. 14:103-109. [DOI] [PubMed] [Google Scholar]
- 26.Jornitz, M. W. 2009. Mycoplasma contamination in culture media. Genet. Eng. Biotechnol. News. http://www.genengnews.com/articles/chtitem.aspx?tid=2836&chid=3.
- 27.Kalbfuss, B., Y. Genzel, M. Wolff, A. Zimmermann, R. Morenweiser, and U. Reichl. 2007. Harvesting and concentration of human influenza A virus produced in serum-free mammalian cell culture for the production of vaccines. Biotechnol. Bioeng. 97:73-85. [DOI] [PubMed] [Google Scholar]
- 28.Kapteyn, J. C., A. M. Porre, E. J. de Rond, W. B. Hessels, M. A. Tijms, H. Kessen, A. M. Slotboom, M. A. Oerlemans, D. Smit, J. van der Linden, P. Schoen, and J. L. Thus. 2009. HPLC-based quantification of haemagglutinin in the production of egg- and MDCK cell-derived influenza virus seasonal and pandemic vaccines. Vaccine 27:1468-1477. [DOI] [PubMed] [Google Scholar]
- 29.Kistner, O., N. Barrett, A. Bruhmann, M. Reiter, W. Mundt, H. Savidis-Dacho, S. Schober-Bendixen, F. Dorner, and J. Aaskov. 2007. The preclinical testing of a formaldehyde inactivated Ross River virus vaccine designed for use in humans. Vaccine 25:4845-4852. [DOI] [PubMed] [Google Scholar]
- 30.Koski, T. A., G. G. Christianson, and F. L. Cole. 1976. Inactivation of mycoplasmas by use of phenol, formalin and beta-propiolactone. J. Biol. Stand. 4:151-154. [DOI] [PubMed] [Google Scholar]
- 31.Leroux-Roels, I., and G. Leroux-Roels. 2009. Current status and progress of prepandemic and pandemic influenza vaccine development. Expert Rev. Vaccines 8:401-423. [DOI] [PubMed] [Google Scholar]
- 32.Levandowski, R. A. 1999. Regulatory perspective in the United States on cell cultures for production of inactivated influenza virus vaccines. Dev. Biol. Stand. 98:171-175. (Discussion, 98:197.) [PubMed] [Google Scholar]
- 33.Liang, Y., S. Ma, Z. Yang, L. Liu, L. Wang, J. Wang, L. Jiang, C. Shi, C. Dong, and Q. Li. 2008. Immunogenicity and safety of a novel formalin-inactivated and alum-adjuvanted candidate subunit vaccine for mumps. Vaccine 26:4276-4283. [DOI] [PubMed] [Google Scholar]
- 34.Lierz, M., and H. M. Hafez. 2008. Time-dependent recovery of Mycoplasma lipofaciens (strain ML64) from incubated infertile chicken eggs and dead in shell chicken embryos. Avian Dis. 52:441-443. [DOI] [PubMed] [Google Scholar]
- 35.Lierz, M., A. Jansen, and H. M. Hafez. 2008. Avian Mycoplasma lipofaciens transmission to veterinarian. Emerg. Infect. Dis. 14:1161-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luginbuhl, R. E., R. Holdenried, and R. E. Stevenson. 1969. Establishment and maintenance of a specific pathogen free (SPF) flock of white leghorn chickens. Prog. Immunobiol Stand. 3:6-13. [PubMed] [Google Scholar]
- 37.Madoff, S., B. Q. Pixley, R. A. DelGiudice, and R. C. Moellering, Jr. 1979. Isolation of Mycoplasma bovis from a patient with systemic illness. J. Clin. Microbiol. 9:709-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milne, C., and A. Daas. 2006. Establishment of European pharmacopoeia Mycoplasma reference strains. Pharmeuropa Bio. 2006:57-72. [PubMed] [Google Scholar]
- 39.Moalic, P. Y., I. Kempf, F. Gesbert, and F. Laigret. 1997. Identification of two pathogenic avian mycoplasmas as strains of Mycoplasma pullorum. Int. J. Syst. Bacteriol. 47:171-174. [DOI] [PubMed] [Google Scholar]
- 40.Pickering, J. M., H. Smith, and C. Sweet. 1992. Influenza virus pyrogenicity: central role of structural orientation of virion components and involvement of viral lipid and glycoproteins. J. Gen. Virol. 73:1345-1354. [DOI] [PubMed] [Google Scholar]
- 41.Pitcher, D. G., and R. A. Nicholas. 2005. Mycoplasma host specificity: fact or fiction? Vet. J. 170:300-306. [DOI] [PubMed] [Google Scholar]
- 42.Poon, B., J. T. Safrit, H. McClure, C. Kitchen, J. F. Hsu, V. Gudeman, C. Petropoulos, T. Wrin, I. S. Chen, and K. Grovit-Ferbas. 2005. Induction of humoral immune responses following vaccination with envelope-containing, formaldehyde-treated, thermally inactivated human immunodeficiency virus type 1. J. Virol. 79:4927-4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Razin, S., D. Yogev, and Y. Naot. 1998. Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. 62:1094-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenbusch, R. F., J. M. Kinyon, M. Apley, N. D. Funk, S. Smith, and L. J. Hoffman. 2005. In vitro antimicrobial inhibition profiles of Mycoplasma bovis isolates recovered from various regions of the United States from 2002 to 2003. J. Vet. Diagn. Invest. 17:436-441. [DOI] [PubMed] [Google Scholar]
- 45.Rottem, S., and M. F. Barile. 1993. Beware of mycoplasmas. Trends Biotechnol. 11:143-151. [DOI] [PubMed] [Google Scholar]
- 46.Russell, R. G., and G. S. Cottew. 1972. Isolation of Mycoplasma gallisepticum from embryonated chicken eggs. Aust. Vet. J. 48:425-426. [DOI] [PubMed] [Google Scholar]
- 47.Stenske, K. A., D. A. Bemis, K. Hill, and D. J. Krahwinkel. 2005. Acute polyarthritis and septicemia from Mycoplasma edwardii after surgical removal of bilateral adrenal tumors in a dog. J. Vet. Intern. Med. 19:768-771. [DOI] [PubMed] [Google Scholar]
- 48.Stipkovits, L., and I. Kempf. 1996. Mycoplasmoses in poultry. Rev. Sci. Tech. 15:1495-1525. [DOI] [PubMed] [Google Scholar]
- 49.Takase, K., Y. Murakawa, R. Ariyoshi, S. Eriguchi, T. Sugimura, and H. Fujikawa. 2000. Serological monitoring on layer farms with specific pathogen-free chickens. J. Vet. Med. Sci. 62:1327-1329. [DOI] [PubMed] [Google Scholar]
- 50.Tannock, G. A., D. A. Bryce, M. J. Hensley, N. A. Saunders, R. S. Gillett, and W. S. Kennedy. 1984. Responses to one or two doses of a deoxycholate subunit influenza vaccine in a primed population. Vaccine 2:100-106. [DOI] [PubMed] [Google Scholar]
- 51.Tano, Y., H. Shimizu, J. Martin, Y. Nishimura, B. Simizu, and T. Miyamura. 2007. Antigenic characterization of a formalin-inactivated poliovirus vaccine derived from live-attenuated Sabin strains. Vaccine 25:7041-7046. [DOI] [PubMed] [Google Scholar]
- 52.Trudel, M., and F. Nadon. 1981. Virosome preparation: differences between influenza and rubella hemagglutinin adsorption. Can. J. Microbiol. 27:958-962. [DOI] [PubMed] [Google Scholar]
- 53.Twomey, T., J. Newman, T. Burrage, P. Piatti, J. Lubroth, and F. Brown. 1995. Structure and immunogenicity of experimental foot-and-mouth disease and poliomyelitis vaccines. Vaccine 13:1603-1610. [DOI] [PubMed] [Google Scholar]
- 54.U.S. Food and Drug Administration. 2010. Guidance for industry: characterization and qualification of cell substrates and other biological starting materials used in the production of viral vaccines for the prevention and treatment of infectious diseases. U.S. Food and Drug Administration, Rockville, MD. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/UCM202439.pdf.
- 54a.U.S. Food and Drug Administration. 1993. Points to consider in the characterization of cell lines used to produce biologicals (1993). U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research, Rockville, MD. http://www.fda.gov/downloads/BiologicsBloodVaccines/SafetyAvailability/UCM162863.pdf.
- 55.van Eck, J. H., N. van Kol, and B. Kouwenhoven. 1980. Egg production in relation to the results of a long term serological survey of 73 flocks of fowl. Tijdschr. Diergeneeskd. 105:15-24. [PubMed] [Google Scholar]
- 56.Volokhov, D. V., J. George, S. X. Liu, P. Ikonomi, C. Anderson, and V. Chizhikov. 2006. Sequencing of the intergenic 16S-23S rRNA spacer (ITS) region of Mollicutes species and their identification using microarray-based assay and DNA sequencing. Appl. Microbiol. Biotechnol. 71:680-698. [DOI] [PubMed] [Google Scholar]
- 57.Volokhov, D. V., H. Kong, J. George, C. Anderson, and V. E. Chizhikov. 2008. Biological enrichment of Mycoplasma agents by cocultivation with permissive cell cultures. Appl. Environ. Microbiol. 74:5383-5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Volokhov, D. V., A. A. Neverov, J. George, H. Kong, S. X. Liu, C. Anderson, M. K. Davidson, and V. Chizhikov. 2007. Genetic analysis of housekeeping genes of members of the genus Acholeplasma: phylogeny and complementary molecular markers to the 16S rRNA gene. Mol. Phylogenet. Evol. 44:699-710. [DOI] [PubMed] [Google Scholar]