Abstract
Although Neisseria gonorrhoeae is a prolific source of eight c-type cytochromes, little is known about how its electron transfer pathways to oxygen are organized. In this study, the roles in the respiratory chain to oxygen of cytochromes c2, c4, and c5, encoded by the genes cccA, cycA, and cycB, respectively, have been investigated. Single mutations in genes for either cytochrome c4 or c5 resulted in an increased sensitivity to growth inhibition by excess oxygen and small decreases in the respiratory capacity of the parent, which were complemented by the chromosomal integration of an ectopic, isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible copy of the cycA or cycB gene. In contrast, a cccA mutant reduced oxygen slightly more rapidly than the parent, suggesting that cccA is expressed but cytochrome c2 is not involved in electron transfer to cytochrome oxidase. The deletion of cccA increased the sensitivity of the cycB mutant to excess oxygen but decreased the sensitivity of the cycA mutant. Despite many attempts, a double mutant defective in both cytochromes c4 and c5 could not be isolated. However, a strain with the ectopically encoded, IPTG-inducible cycB gene with deletions in both cycA and cycB was constructed: the growth and survival of this strain were dependent upon the addition of IPTG, so gonococcal survival is dependent upon the synthesis of either cytochrome c4 or c5. These results define the gonococcal electron transfer chain to oxygen in which cytochromes c4 and c5, but not cytochrome c2, provide alternative pathways for electron transfer from the cytochrome bc1 complex to the terminal oxidase cytochrome cbb3.
Neisseria gonorrhoeae was originally described as an obligately aerobic bacterium that is moderately fastidious, requiring a rich culture medium and a microaerobic environment in which to grow (35, 54). Although the range of carbon and energy sources used by the gonococcus for growth is limited, it reduces oxygen rapidly with physiological substrates such as glucose, succinate, and lactate and especially with the artificial electron donor ascorbate-reduced tetramethyl-p-phenylenediamine (TMPD) (48). It is also a prolific source of multiple c-type cytochromes (53, 54), some of which have no functions assigned to them. Contrary to previous reports, gonococci can use nitrite or nitric oxide as an alternative electron acceptor during oxygen-deficient growth (2, 18).
Studies of the microbial physiology of the gonococcus have been limited by the facts that it is both a pathogen and rather difficult to grow in large-scale cultures. Results from early studies indicated the presence of cytochromes b, c, and o (26, 54). However, a spectroscopic study using ascorbate plus TMPD led to the conclusion that the gonococcal electron transfer chain includes cytochrome oxidases a and d (17). A subsequent, more detailed spectroscopic and kinetic study of electron transfer to oxygen in the closely related species Neisseria meningitidis led Yu and DeVoe (55) to conclude that there are branched electron transfer pathways to oxygen that diverge at the cytochrome bc1 complex and terminate with cytochrome oxidases o and a. Contrary to the conclusions from those early studies, the publication of the complete genome sequences of pathogenic and nonpathogenic Neisseria revealed that there is only one cytochrome oxidase that is closely related to cytochrome oxidase cbb3 of Bradyrhizobium japonicum, Rhodobacter capsulatus, and Azotobacter vinelandii (32, 34, 45). Consequently, little is known about how the electron transfer chains of the gonococcus are organized.
Cytochrome oxidase cbb3 from Bradyrhizobium japonicum has a very high affinity for oxygen, which is essential to reduce the oxygen concentration in root nodules to the nanomolar range, thus protecting nitrogenase from inactivation by oxygen (32, 33). In both B. japonicum and the Neisseriaceae, it is encoded by the ccoNOPQ operon (originally designated the fixNOPQ operon, for nitrogen fixation), in which the ccoO and ccoP genes encode c-type cytochromes. Genes for six other gonococcal c-type cytochromes include cycP, encoding cytochrome c′, which was implicated in protection against NO toxicity (48); ccp, encoding a cytochrome c peroxidase (47); petC, encoding the cytochrome c1 component of the cytochrome bc1 complex; and three genes, cccA, cycA, and cycB, encoding cytochromes of unknown function that have been designated cytochromes c2, c4, and c5, respectively. By staining proteins separated by SDS-PAGE for covalently bound heme, we and others have demonstrated the accumulation of six c-type cytochromes during aerobic growth, including both cytochromes c4 and c5 (43, 47, 48). A seventh c-type cytochrome, Ccp, is expressed at low levels during oxygen-limited growth (21, 47, 53). However, cytochrome c2 has not been visualized either in gonococci or in the closely related pathogen Neisseria meningitidis (4); consequently, its role in gonococcal electron transfer pathways remains to be defined.
Cytochromes c4 and c5 were first identified in the nitrogen-fixing bacterium Azotobacter vinelandii, where they were proposed to provide parallel pathways for electron transfer between the cytochrome bc1 complex and cytochrome oxidase (11). Many years later, it was tentatively suggested that they provide two independent pathways to a cytochrome cbb3 type of cytochrome oxidase (34). This offers a possible explanation for the conflicting previous proposals concerning the organization of the gonococcal respiratory chain, namely, that there are branched electron transfer pathways to oxygen that diverge at the cytochrome bc1 complex but terminate with the same cytochrome oxidase, cbb3. The current study was therefore designed to determine whether gonococcal cytochromes c4 and c5 provide alternative pathways for electron transfer between cytochrome bc1 and cytochrome oxidase cbb3 and whether cytochrome c2 also provides an alternative electron transfer pathway to cytochrome oxidase. The results obtained provide fascinating insights into why a pathogen with a microaerobic life-style maintains such a high respiratory capacity and is a prolific source of electron transfer components.
MATERIALS AND METHODS
Strains, media, and growth conditions.
Bacterial strains and plasmids used in this project are listed in Table 1, and oligonucleotide primers synthesized by Alta Bioscience, Birmingham, United Kingdom, are listed in Table 2.
TABLE 1.
Bacterial strains and plasmids used in this work
| Strain or plasmid | Genotype or characteristic(s) | Source or reference(s) |
|---|---|---|
| Strains | ||
| E. coli | ||
| JM109 | recA1 endA1 gyrA96 thi hsdR17(rK− mK+) relA1 supE44 Δ(lac-proAB) [F′ traD36 proAB lacIqZΔM15] | Promega |
| XL1-Blue supercompetent | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tetr)] | Stratagene |
| RV | Δlac | Laboratory stock |
| Neisseria gonorrhoeae | ||
| F62 | Parental strain | Laboratory stock |
| JCGC10 | ΔaspC-lctP::PlacI-cycA+ | This work |
| JCGC11 | ΔaspC-lctP::PlacI-cycB+ | This work |
| JCGC100 | fnr::erm | 21 |
| JCGC800 | cycA::erm | This work |
| JCGC802 | cycA::kan | This work |
| JCGC805 | cycA::FLAG | This work |
| JCGC810 | cycA::kan | This work |
| JCGC820 | cycA::kan ΔaspC-lctP::PlacI-cycA+ | This work |
| JCGC813 | cycA::FLAG fnr::erm | This work |
| JCGC850 | cycB::erm | This work |
| JCGC851 | cccA::kan | This work |
| JCGC852 | cycB::erm cccA::kan | This work |
| JCGC853 | cycA::erm cccA::kan | This work |
| JCGC855 | cycB::FLAG | This work |
| JCGC860 | cycB::chl | This work |
| JCGC870 | cycB::chl ΔaspC-lctP::PlacI-cycA+ | This work |
| JCGC880 | cycA::kan cycB::chl ΔaspC-lctP::PlacI-cycA+ | This work |
| Plasmids | ||
| pGEM T-Easy | Commercially available vector for cloning of PCR products | Promega |
| pET20bhc | Derivative of pET20 | GlaxoSmithKline |
| pET20bhc-c2 | pET20bhc containing the gonococcal cccA gene on an NdeI-BamHI fragment | This work |
| pSMT2 | ccmABCDEFGH NcoI-SalI fragment cloned into pACYC184 | 47 |
| pLES940 | Source of the cat gene | 40 |
| pSUB11 | Source of the kan gene | 3 |
| pAH105 | cat gene ligated into the AgeI site of pYL6 | This work |
| pAH106 | Kanr gene ligated into the AgeI site of NT100 | This work |
| pNT100 | Gonococcal cycA gene cloned into pGEM T-Easy | This work |
| pNT101 | ermC gene flanked by AgeI sites in pGEM T-Easy | This work |
| pNT102 | ermC gene ligated into the AgeI site of pNT100 | This work |
| pYL4 | kan gene flanked by AgeI sites in pGEM T-Easy | This work |
| pYL6 | 584 bp of DNA upstream of cycB linked by an AgeI site to 448 bp of DNA downstream of cycB in pGEM T-Easy | This work |
| pYL7 | ermC gene ligated into the AgeI site of pYL6 | This work |
| pYL12 | 430 bp of DNA upstream and 331 bp of DNA downstream of the cccA gene in a continuous sequence containing an AgeI restriction site at the end of the upstream DNA ligated into pGEM T-Easy | This work |
| pYL13 | kan gene ligated into the AgeI site of pYL600 | This work |
| pGCC4 | Neisserial insertional complementation system; ermC KanrlctP asp lacIqlacP | 23, 24, 41 |
| pGCC4_cycA | pGCC4 containing the cycA gene inserted into the PacI site | This work |
| pGCC4_cycB | pGCC4 containing the cycB gene inserted between the PacI and FseI sites | This work |
TABLE 2.
Oligonucleotide primers used in this work
| Primer | Sequence (5′-3′)a | Restriction site |
|---|---|---|
| NTCYC4 FOR | GACCCAATGTGCGCGTACCG | |
| NTCYC4 REV | GTGTGGGAGATATACGGGATTTACTC | |
| YLC5AUF | CAGGATCCGCGGCAGACTTGGCTACACGGGGTTG | |
| YLC5BUR | GTCAACAGCCGCTTTGACCGGTGTAGGCCTCGCCGTC | AgeI |
| YLC5CDR | GACTCGGATCCGTGATGGAGCGCCGG | |
| YLC5DDF | GCGATGAACCGGTCAAAGCGGCTGTTGAC | AgeI |
| YLc552AUF | GGCAAAATGGGTTACACCGAGCACGCAGG | |
| YLc552BUR2 | GGTGGCGGCAATATCACCGGTCGTAGCGGGTGAAAG | AgeI |
| YLc552DDF2 | CTACGACCGGTGATATTGCCGCCACC | AgeI |
| YLc552CDR | CGTGCTTCAGGCGGAGGATTTGGCG | |
| NTERY FOR | AGAAGACCGGTTAAGAGTGTGTTGATAGTGC | AgeI |
| NTERY REV | CAAATTACCGGTAGGCGCTAGGGACC | AgeI |
| YLKanF | CGTAACCGGTAAAGCCAGTCCGCAG | AgeI |
| YLKanR | CGATACCGGTGGAGGATCATCCAGC | AgeI |
| NCC4F | CTACGTTTAATTAACATTGTTGCGTTATCC | PacI |
| NCC4R | CTACGTTTAATTAACATCGATTCCAACGG | PacI |
| AHpGCC4PacIns | GCTGTGGTATGGCTGTG | |
| AHCptC5F | CTACGTTTAATTAACCTAACCTGACGGCG | PacI |
| AHCptC5R | CTACGTGGCCGGCCATTCATCCGATCGGGCAG | FseI |
| AHpGCC4_F | CCCGCATCAAACAGCTCGG | |
| AHpGCC4_R | CCATTGTTCGGGCGTAGGG | |
| CYC4 Flag FOR | CGTGGTACCTCCAAGCTCGAGTCTGCTTCAGAAGCCG | KpnI-XhoI |
| CYC4 Flag REV | AGACTCGAGCTTGGAGGTACCACGCAAACCCTGGATAAAG | KpnI-XhoI |
| NT C4 For TRUNK | GACGATTGACTTTATTGGCCTTTGTTTTGGC | |
| NT C5 For | CCGCGACAACAAAGCCCAAGGC | |
| C5 Flag FOR | TTCGGTACCTCCAAGCTCGAGAAAAACGGGGCAGG | KpnI-XhoI |
| C5 Flag REV | TTTCTCGAGCTTGGAGGTACCGAATTTTGCACCGG | KpnI-XhoI |
| AHCatAgeIF | ACTGACCGGTGGCAGGCCATGTCTGCC | AgeI |
| AHCatAgeIR | ACTGACCGGTGCTTACTCCCCATCCCC | AgeI |
Underlining indicates the location of the restriction site.
Bacteria were stored as glycerol stocks at −20°C (Escherichia coli strains) or in liquid nitrogen or at −80°C (N. gonorrhoeae strains). E. coli cells were grown at 37°C with aeration in 20 ml of Luria-Bertani broth (20 g tryptone, 10 g yeast extract, and 10 g of NaCl per liter of distilled water) in a 150-ml conical flask. Antibiotics used to maintain plasmids in transformants were 100 μg ml−1 ampicillin, 150 μg ml−1 erythromycin, and 25 or 100 μg ml−1 kanamycin. Transformants were recovered in SOC broth, which contained 2% tryptone, 0.5% yeast extract, 10 mM NaCl, 2.5 mM KCl, 10 mM MgCl2, 10 mM MgSO4, and 20 mM glucose.
N. gonorrhoeae inocula were cultured on gonococcal agar (GC agar) containing 18 g of GC medium base (BD, United Kingdom) and 3 g of bacteriological agar 1 (Oxoid) per 500 ml of distilled water supplemented after sterilization with 10 ml of Kellogg's supplement per liter (15). Antibiotics (5 μg ml−1 of erythromycin and 100 μg ml−1 of kanamycin) were added as required immediately before GC agar plates were poured. Plates were dried at 60°C for 10 min and used immediately or stored at 4°C and used within 7 days. GC agar plates were also supplemented with 3 mM isoleucine to induce piliation in preparation for transformation experiments (20).
Gonococcal liquid cultures were grown in GC broth (GCB) that contained, per liter of distilled water, 1 g soluble starch, 1 g KH2PO4, 4 g K2HPO4, 5 g NaCl, and 15 g protease peptone 3 (BD, United Kingdom). GC broth was supplemented with sodium nitrite at concentrations between 0.1 and 5 mM when relevant. Immediately prior to use, 10 ml of Kellogg's supplement was added per liter of GC broth. Unless stated otherwise, a standard protocol was used to generate inocula for gonococcal growth experiments. First, 2 μl of a liquid nitrogen stock of N. gonorrhoeae was plated onto a gonococcal agar plate and incubated in a candle jar at 37°C for 24 h. Bacteria from this plate were swabbed onto multiple plates and incubated in the same way for a further 20 h. The entire bacterial growth from these secondary plates was swabbed into a universal bottle containing 10 ml of GCB and incubated at 37°C in an orbital shaker at 100 rpm for 1 h. When more than 10 ml was required for an experiment, multiple 10-ml precultures were combined to provide a standardized inoculum. When the growth of different strains was to be compared, the starting cell density was adjusted to be the same in all the cultures.
To study the effects of different concentrations of oxygen on gonococcal growth, 10, 5, and 1.6 ml of the standardized inoculum were used to inoculate three 100-ml conical flasks containing 50, 25, and 8.4 ml of GCB, respectively, to ensure that the starting optical density at 650 nm (OD650) values of the cultures were comparable.
Construction of a gonococcal cycA mutant.
To obtain the cytochrome c4 mutant, the gonococcal cycA coding region and 480 bp of upstream DNA and 240 bp of downstream DNA were amplified by PCR from N. gonorrhoeae strain F62 chromosomal DNA using primers NTCYC4FOR and NTCYC4REV. Plasmid pNT100 was constructed by the ligation of the PCR fragment into the pGEM T-Easy vector. An AgeI restriction site was located approximately 120 bp downstream from codons for the heme attachment motif. The ermC gene was amplified from JCGC100 with NTERY FOR and NTERY REV, and the kanamycin resistance gene was amplified from pSUB11 using YLKanF and YLKanR. In both cases, the primers introduced AgeI sites at each end. The ermC gene and the kanamycin resistance genes were ligated into the AgeI site of pNT100 to create the constructs pNT102 and pAH106. Naturally competent, piliated N. gonorrhoeae F62 cells were then transformed with a linear DNA fragment amplified from pNT102 or pAH106 by using primers NTCYC4FOR and NTCYC4REV. The DNA fragment contained the ermC gene or kanamycin resistance gene flanked by DNA homologous to the coding region of the cycA gene and noncoding DNA upstream and downstream of the gene in addition to the gonococcal uptake sequence. Erythromycin- or kanamycin-resistant colonies were screened by PCR for the disruption of the cycA gene, and the PCR product was then sequenced.
Construction of a gonococcal cycB mutant.
To obtain the cytochrome c5 mutant, two fragments were amplified from N. gonorrhoeae strain F62 chromosomal DNA. Primers YLC5AUF and YLC5BUR were used to amplify 584 bp of noncoding DNA upstream of the cycB gene, and primers YLC5DDF and YLC5CDR were used to amplify 448 bp of noncoding DNA downstream of the cycB gene. The fragments contained regions of sequence identity at the 3′ end of the upstream fragment and at the 5′ end of the downstream fragment that included an AgeI restriction site. The fragments were able to anneal to one another during a subsequent PCR to form a larger fragment containing the upstream noncoding region immediately followed by the downstream noncoding region with an AgeI restriction site located at the crossover point. Plasmid pYL6 was constructed by the ligation of this enlarged PCR fragment into the pGEM T-Easy vector. The ermC gene was amplified from JCGC100 with NTERY FOR and NTERY REV, and the cat gene was amplified from pLES940 by using primers AHCatAgeIF and AHCatAgeIR. In both cases, the primers introduced AgeI sites at each end. The ermC gene and the cat gene were ligated into the AgeI site of pYL6 to create the constructs pYL7 and pAH105. Naturally competent, piliated N. gonorrhoeae strain F62 cells were then transformed with a linear DNA fragment amplified from pYL7 and pAH105 by using primers YLC5AUF and YLC5CDR. This DNA fragment contained either the ermC or cat gene flanked by DNA homologous to the noncoding regions of DNA directly up- and downstream of the cycB gene in addition to the gonococcal uptake sequence. After 3 days of growth on GC agar plates supplemented with erythromycin or chloramphenicol, purified colonies were screened for the disruption of the cycB gene by PCR. Candidate clones were confirmed first by sequencing and then by separating proteins from lysed bacteria by SDS-PAGE followed by staining for the presence of covalently bound heme.
Construction of a gonococcal cccA mutant.
To obtain the cytochrome c2 mutant, two fragments were amplified from N. gonorrhoeae strain F62 chromosomal DNA. Primers YLc552AUF and YLc552BUR2 were used to amplify 430 bp of noncoding DNA upstream of the cccA gene, and primers YLc552DDF2 and YLc552CDR were used to amplify 331 bp of noncoding DNA downstream of the cccA gene. The fragments contained regions of identity at the 3′ end of the upstream fragment and at the 5′ end of the downstream fragment and an AgeI restriction site and were combined by crossover PCR and ligated into the pGEM T-Easy vector to form plasmid pYL12. The kanamycin resistance gene was amplified from pSUB11 with YLKanF and YLKanR, which introduced AgeI sites at each end. The kanamycin resistance gene was ligated into the AgeI site of pYL12 to create the construct pYL13. Naturally competent, piliated N. gonorrhoeae F62 cells were then transformed with a linear DNA fragment amplified from pYL13 by using primers YLc552AUF and YLc552CDR. The DNA fragment contained the kan gene flanked by DNA homologous with the noncoding regions of DNA directly up- and downstream of the cccA gene in addition to the gonococcal uptake sequence. Kanamycin-resistant colonies were screened for the disruption of the cccA gene by PCR and sequencing.
Construction of gonococcal double mutants.
Double mutants were constructed by growing each of the single mutants on GC agar plates supplemented with isoleucine and transforming the resulting piliated bacteria with the PCR product carrying the disrupted gene to be introduced. The fragment containing the kanamycin resistance cassette and the upstream and downstream regions of cccA was amplified from pYL13 by using primers YLc552AUF and YLc552CDR and transformed into piliated N. gonorrhoeae cycB strain JCGC850. Kanamycin-resistant transformants were checked by digestion, PCR, and sequencing. The resulting double mutant that was defective in both cytochromes c2 and c5 is named JCGC852. The DNA fragment containing the cycA gene disrupted by the ermC gene was amplified from pNT102 by using primers NTCYC4FOR and NTCYC4REV and was transformed into piliated gonococcal strain JCGC851 to create strain JCGC853, which is defective in both cytochromes c2 and c4.
Construction of plasmids for ectopic complementation of cycA and cycB mutations.
To allow the expression of the cycA and cycB gene products at unlinked loci on the chromosome of N. gonorrhoeae F62 strains, the neisserial insertional complementation system (NICS) was used (23, 24, 41). This involved the construction of isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible copies of cycA and cycB by using plasmid pGCC4, which was kindly provided by Chris Tang. To construct the IPTG-inducible copy of the cycA gene, the gonococcal cycA coding region and 39 bp of upstream DNA and 103 bp of downstream DNA were amplified by PCR from N. gonorrhoeae strain F62 chromosomal DNA using primers NC_C4F and NC_C4R, respectively, which were flanked by PacI restriction sites. Plasmid pGCC4_cycA was constructed by the digestion of plasmid pGCC4 and the above-mentioned cycA insert with PacI and then the ligation of the insert into the plasmid. The correct orientation of the insert was verified by using primer pGCC4PacIns.
To construct the IPTG-inducible copy of the cycB gene, the gonococcal cycB coding region and 28 bp of upstream DNA and 149 bp of downstream DNA were amplified by PCR from N. gonorrhoeae strain F62 chromosomal DNA using primers AHCptC5F and AHCptC5R, which were flanked by PacI and FseI restriction sites, respectively. Plasmid pGCC4_cycB was constructed by the digestion of plasmid pGCC4 and the above-mentioned cycB insert with both PacI and FseI and then the ligation of the insert into the plasmid. The correct orientation of the insert was verified by using primer pGCC4PacIns.
In order to transfer the IPTG-inducible copies of cycA and cycB into the gonococcus, linear fragments were required. These included regions of homology to the chromosome at alternative loci to the original copies of the cycA and cycB genes as well as an erythromycin resistance cassette for the selection of transformants. These linear fragments were created either by digestion with the ClaI restriction enzyme or by PCR amplification of the required region using primers pGCC4_F and pGCC4_R. Relevant Neisseria gonorrhoeae F62 strains were transformed with these linear fragments and screened for erythromycin resistance. Candidates for mutants containing the IPTG-inducible cycA or cycB cassettes were screened by PCR and sequenced by using primer pGCC4PacIns.
Construction of strains accumulating FLAG-tagged cytochromes c4 and c5.
Strain JCGC520, encoding CycA fused in frame to a triple C-terminal FLAG epitope, was constructed in five steps. First, a fragment from downstream of the ATG translational start codon of cycA was amplified by using primers C4TRUNK and CYC4 FLAG REV and gonococcal strain F62 DNA as a template, yielding a 620-bp product with KpnI and XhoI restriction sites at its 3′ end. Primers CYC4 Flag FOR and NT CYC4 REV were used to amplify a 620-bp fragment of DNA directly downstream from the cycA gene. The forward primer introduced KpnI and XhoI restriction sites at its 5′ end. Both fragments were gel purified and used as templates for crossover PCR with primers C4TRUNK and NT CYC4 REV3. The 1.2-kb product with centrally located KpnI and XhoI restriction sites was ligated into pGEM T-Easy. This intermediate plasmid, pC4TKX, was cut with KpnI and XhoI and ligated with a 1.5-kb KpnI-XhoI fragment that contained the kanamycin resistance cassette and the triple FLAG sequence from plasmid pSUB11 (49). The structure of this plasmid, pC4TKX, was checked by restriction analysis and confirmed by sequencing. Primers C4TRUNK and NT CYC4 REV3 were used to generate a 2.8-kb PCR fragment that was transformed into N. gonorrhoeae strain F62 cells with selection for kanamycin resistance. Purified transformants were checked by PCR and restriction analysis for the integration of the FLAG-tagged cycA gene and the downstream kanamycin resistance cassette into the cycA locus on the gonococcal chromosome.
The same procedure was used to construct kanamycin-resistant derivatives of strain F62 with a chromosomal FLAG-tagged cycB gene. Primers NT C5 For and C5 FLAG REV were used to generate an 820-bp fragment of cycB DNA, and C5 FLAG FOR plus C5 REV C amplified 340 bp of DNA downstream from cycB. All subsequent steps were the same as those used for the construction of the cycA::FLAG strain.
Rates of oxygen reduction by gonococcal strains measured with an oxygen electrode.
To measure oxygen reduction rates, gonococcal strains were grown in 30 ml of GCB at 37°C and shaken at 100 rpm in an orbital shaker. Toward the end of exponential growth, the cultures were harvested by sedimentation at 10,000 × g in an MSE model 18 centrifuge. The bacterial pellet was washed with 50 mM potassium phosphate buffer (pH 7.4) and sedimented at 6,000 × g in an Eppendorf bench-top centrifuge. The pellet was resuspended in phosphate buffer.
Rates of oxygen reduction in the presence of either 50 mM lactate or 5 mM ascorbate plus 1 mM N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD) as a reductant were obtained by using an S1/Mini Clark-type oxygen electrode (Hansatech Instruments) in conjunction with an Oxytherm control unit. The rate of oxygen reduction was plotted by using the Oxygraph Plus program.
SDS-PAGE and heme staining.
The protein concentration of cell fractions was determined by using the Folin method (22). Proteins were resolved by Tris-Tricine SDS-PAGE using a 15% (wt/vol) polyacrylamide gel. Total protein was detected with 0.02% (wt/vol) Coomassie blue. Proteins containing covalently attached heme were detected with heme-dependent peroxidase activity (44).
Detection of AniA by Western blotting.
Proteins separated by SDS-PAGE were transferred electrophoretically onto a polyvinylidene difluoride (PVDF) membrane (Millipore) by using a Bio-Rad Trans-D semidry blotter at 0.25 A for 1.5 h in blotting buffer (28). The membrane was removed and gently agitated overnight in 50 ml of 5% (wt/vol) nonfat dry milk (Bio-Rad) in Tris-buffered saline (TBS) within a sealed bag at 4°C. The blocked membrane was washed three times for 5 min each with gentle agitation in TBS-Tween. The membrane was transferred into a plastic bag, which contained the primary antibody in 10 ml of 5% (wt/vol) nonfat dry milk in TBS-Tween, and was incubated with gentle agitation for 2 h at room temperature. The primary antibody used to detect AniA was anti-AniA (rabbit) antibody (obtained from J. Moir and M. Thomson) and was diluted 1:5,000. The membrane was transferred onto a tray and was washed in TBS-Tween three times for 5 min each with gentle agitation. The membrane was transferred into a plastic bag containing an anti-rabbit peroxidase-labeled secondary antibody (ECL Plus Western blotting detection reagents; Amersham Biosciences) diluted 1:5,000 in 10 ml of 5% nonfat dry milk-TBS-Tween and incubated with gentle agitation for 1 h at room temperature. The membrane was then transferred onto a tray and washed in TBS-Tween three times for 5 min each with gentle agitation. The membrane was then transferred into a plastic bag and treated with 5 ml of solution A and 125 μl of solution B (ECL Plus Western blotting detection reagents; Amersham Biosciences) for 5 min with gentle agitation. The membrane was then wrapped in cling film, exposed to film (Hyperfilm ECL) for 2 to 20 s, and developed on an X-ograph.
RESULTS
Mutagenesis of genes for gonococcal c-type cytochromes.
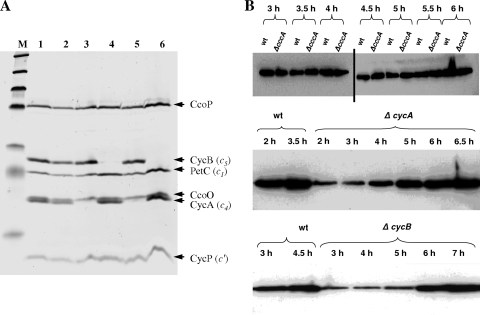
We have previously reported the isolation and phenotypes of gonococcal mutants defective in fnr, the gene for the transcription factor, FNR (regulator of fumarate and nitrate reduction), that is essential for the synthesis of AniA and Ccp during oxygen-limited growth; ccp, the gene encoding a cytochrome c peroxidase; and cycP, which encodes the NO-binding protein, cytochrome c′ (21, 47, 48). The same deletion-insertion mutagenesis approach was used to inactivate genes for other gonococcal c-type cytochromes. Gonococci are relatively fastidious organisms that are easily killed during laboratory manipulation, and successful mutagenesis depends upon an unstable trait, piliation (1, 8, 13, 36). To provide a positive control and to check for a loss of viability or competence, fnr mutants were reconstructed in every experiment designed to construct other mutants. This step routinely resulted in the isolation of 20 to 200 erythromycin-resistant colonies that were confirmed to have arisen from double recombination, resulting in deletion-insertion mutagenesis. Despite the success of these positive controls, five attempts to replace either the ccoP or the petC gene by a kanamycin resistance cassette were unsuccessful. This provided a strong indication that ccoP and petC are essential genes because gonococcal growth and respiration are totally dependent upon both a functional cytochrome bc1 complex and cytochrome oxidase cbb3. In contrast, the cccA, cycA, and cycB genes, encoding cytochromes c2, c4, and c5, respectively, were readily replaced by erythromycin or kanamycin resistance cassettes, suggesting that none of these genes encodes components essential for electron transfer to oxygen. The absence of cytochromes c4 and c5 from the cycA and cycB mutants, respectively, was confirmed by the staining of an SDS-PAGE gel for covalently bound heme (Fig. 1A, tracks 3 and 4). From tracks 3 and 5, it can be seen that the lower band of the closely spaced double band is missing from the cycA mutant, strain JCGC800, and from the cccA cycA double mutant, strain JCGC853. The finding that this band is cytochrome c4 was confirmed by constructing a derivative strain that accumulated a fully functional, C-terminal FLAG-tagged cytochrome c4 (see Fig. S1B, S1C, and S1D in the supplemental material). Similarly, the lack of heme-stained bands in tracks 4 and 6 (Fig. 1) together with the accumulation of a functional FLAG-tagged derivative (Fig. S1A, S1C, and S1E) confirmed the identity of cytochrome c5. In contrast, but as expected, cytochrome c2 was not detected in any of the samples tested, so the cytochrome c complement of the cccA mutant appeared to be the same as that of the parent strain (Fig. 1, tracks 1 and 2). In previous studies, no heme-stained band that could be attributed to cytochrome c2 was detected in proteins from a cycP mutant that lacks cytochrome c′, excluding the possibility that cytochrome c2 might comigrate with cytochrome c′ during SDS-PAGE (48). This conclusion was recently confirmed by the overexpression of cccA in E. coli (39): the recombinant cytochrome was clearly visible on heme-stained gels as a protein migrating less rapidly than cytochrome c′ with an apparent molecular mass of about 15 kDa. The combined data establish that very little cytochrome c2 accumulates in these strains, at least under any of the growth conditions so far tested (see also references 4, 29, 46, 47, and 52).
FIG. 1.
(A) SDS-PAGE gel of the cccA, cycA, and cycB mutants stained for covalently bound heme. N. gonorrhoeae strains F62 (lane 1), JCGC851 (cccA) (lane 2), JCGC800 (cycA) (lane 3), JCGC850 (cycB) (lane 4), JCGC853 (cccA cycA) (lane 5), and JCGC852 (cccA cycB) (lane 6) were grown on plates at 37°C in a candle jar overnight. Samples were collected, lysed, and separated by SDS-PAGE. The gel was then heme stained to detect c-type cytochromes. (B) Western analysis of the accumulation of AniA as cultures became oxygen limited. Equivalent amounts of bacterial biomass were loaded onto each track of all the gels shown. (Top) Comparison of the cccA mutant that lacks the cytochrome c2 gene with parental strain F62. Tracks on either side of the vertical black bar were from independent gels. (Middle) Comparison of strain F62 with the cycA mutant that lacks cytochrome c4. (Bottom) Comparison of F62 with the cycB mutant that lacks cytochrome c5.
Oxygen-sufficient and oxygen-limited growth of Neisseria gonorrhoeae strain F62.
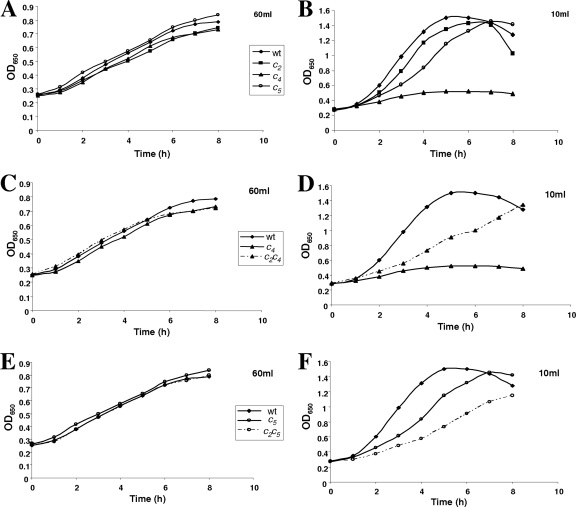
To define conditions under which mutations in genes for c-type cytochromes might reveal phenotypes that indicate gene function, the parental strain, N. gonorrhoeae strain F62, was grown with different levels of aeration, and growth rates and yields were determined. The most rapid growth and highest yields were obtained from oxygen-sufficient cultures in which 100-ml flasks shaken at 100 cycles per minute contained only 10 ml of GCB medium (Fig. 2). Yields and growth rates decreased with increasing culture volume, but oxygen-limited growth was readily achieved in flasks that were 60% full of medium. As previously reported (see Fig. 1 in reference 47), oxygen-limited growth was significantly stimulated by supplementing the medium with the alternative electron acceptor nitrite.
FIG. 2.
Effects of different levels of aeration on the growth of cccA, cycA, and cycB mutants compared with those of the parental strain together with double mutants defective in both cytochromes c2 and c4 or c2 and c5. Shown is a comparison of the effect of limiting or excess aeration on the growth of gonococcal strains F62 (parental strain) with single mutants defective in cytochrome c4 (strain JCGC800), cytochrome c5 (JCGC850), or cytochrome c2 (JCGC851) grown in either 60 ml (limiting aeration) (A) or 10 ml (oxygen sufficient) (B) of GC broth in 100-ml conical flasks. Cultures were shaken at 100 rpm and incubated at 37°C. The amount of the inoculum was adjusted so that each culture started with the same concentration of cells. (C and D) Growth of the cccA cycA double mutant defective in cytochromes c2 and c4 (strain JCGC 853) with the parent and the single mutant defective in only cytochrome c4 under oxygen-limited (C) and oxygen-sufficient (D) conditions. (E and F) Oxygen-limited (E) and oxygen-sufficient (F) growth of the cccA cycB double mutant defective in cytochromes c2 and c5 (strain JCGC852) compared with those of the parental strain and the cycB single mutant. An OD650 of 1.0 corresponds to a biomass of 0.4 mg ml−1.
Oxygen sensitivity of single mutants defective in cytochromes c2, c4, and c5.
The three single mutants defective in cytochromes c2, c4, and c5 were grown with different levels of aeration, and growth rates and cell yields were compared with those of the parent strain (Fig. 2A and B). The growths of both the cycA and cycB mutants were inhibited in highly aerated cultures (Fig. 2B), but oxygen-limited growth in flasks 60% filled with medium was similar to that of the parent strain (Fig. 2A). In contrast, the oxygen-sufficient growth of the cccA mutant was the same as that of strain F62. This suggests that cytochromes c4 and c5 might play similar roles in gonococcal electron transfer to oxygen but that cytochrome c2 is a component of one or more alternative electron transfer pathways.
Rates of oxygen reduction and myxothiazol inhibition by single mutants defective in cytochromes c2, c4, and c5.
Lactate is the preferred physiological electron donor for gonococcal respiration, with rates of oxygen reduction by parental strain F62 being approximately three times greater in the presence of lactate than in the presence of glucose (26, 53). The rate of lactate-dependent oxygen reduction by the cycA mutant that lacks cytochrome c4 was 16% lower than that of the parent, and the corresponding rate for the cycB mutant that lacks cytochrome c5 was 20% lower than that of the parent (Table 3). In contrast, the rate of oxygen reduction by the cccA mutant that lacks cytochrome c2 was almost 26% greater than that of the parent. These data were consistent with the proposal that cytochromes c4 and c5, but not cytochrome c2, are involved in electron transfer to the terminal cytochrome oxidase cbb3.
TABLE 3.
Rates of oxygen reduction of various cytochrome mutants of N. gonorrhoeaeb
| Strain or gene(s) deleted in mutant strain | Lactate rate (nmol O2 reduced·min−1·mg dry cell mass−1 ± SEM)a | % of WT lactate rate | Lactate + myxothiazol rate (nmol O2 reduced·min−1·mg dry cell mass−1 ± SEM)a | % inhibition by myxothiazol | Ascorbate + TMPD rate (nmol O2 reduced·min−1·mg dry cell mass−1 ± SEM)a | % of WT ascorbate + TMPD rate |
|---|---|---|---|---|---|---|
| F62 | 209 ± 1 | 100 | 152 ± 2 | 27 | 1,351 ± 11 | 100 |
| ΔcycA | 176 ± 2 | 84 | 89 ± 2 | 49 | 1,030 ± 31 | 76 |
| ΔcycB | 167 ± 2 | 80 | 65 ± 3 | 61 | 915 ± 29 | 68 |
| ΔcccA | 264 ± 5 | 126 | 199 ± 5 | 25 | 1,459 ± 16 | 108 |
| ΔcccA cycA | 224 ± 3 | 107 | 112 ± 3 | 50 | 1,144 ± 13 | 85 |
| ΔcccA cycB | 118 ± 2 | 57 | 51 ± 2 | 57 | 696 ± 13 | 52 |
Rates are in nmol O2 reduced·min−1·mg dry cell mass−1 ± standard errors of the means from at least five assays of samples from two to seven independent growth experiments.
WT, wild type.
The artificial electron donor TMPD donates electrons to the cytochrome c1 component of the cytochrome bc1 complex, saturating the terminal components of the respiratory chain with reducing equivalents (54). Mutations that result in the loss of the capacity for electron transfer between cytochrome bc1 and cytochrome oxidase should therefore also be reflected in lower rates of TMPD-dependent oxygen reduction. Furthermore, the deletion of the gene for one of two components of the electron transfer pathway would allow the capacity of the remaining component to be estimated. Oxygen reduction by ascorbate-TMPD was much more rapid than that by lactate for all four strains (Table 3), but rates for the cytochrome c4 and c5 mutants were again 24% and 32% lower, respectively, that that of the parent. This difference provided the first indication that the maximum rate of electron transfer via cytochrome c5 might be greater than that through cytochrome c4. The rate of ascorbate-TMPD oxidation by the cccA mutant was again slightly higher than that of the parent (Table 3).
Myxothiazol specifically inhibits electron transfer between ubiquinol and the cytochrome bc1 complex by binding competitively to the quinol-binding site of the cytochrome bc1 complex (30, 51). Deeudom et al. (4) previously showed that Neisseria meningitidis respiration is completely inhibited by 1 μM myxothiazol. The rates of oxygen reduction by the gonococcal cytochrome c4 and c5 mutants were strongly inhibited by 1 μM myxothiazol, with the cycB mutant defective in cytochrome c5 being slightly more sensitive than the cycA mutant defective in cytochrome c4 (Table 3). Lactate oxidation by the cccA mutant was also sensitive to myxothiazol but less sensitive than the parental strain or either mutant. Even 20 μM myxothiazol still only partially inhibited gonococcal respiration. Although 1 μM myxothiazol was sufficient to inhibit meningococcal electron transfer to oxygen completely (4), an isolated membrane fraction rather than intact bacteria was used for the meningococcal experiments, so the lack of complete inhibition in the current study is not surprising.
As in other bacteria, the gonococcal FNR protein is active only during oxygen-limited growth because oxygen destroys the [4Fe-4S] iron-sulfur center that is essential for FNR to dimerize and bind to its DNA target site (29). Active FNR is essential for the transcription of aniA, encoding the copper-containing nitrite reductase AniA (25). Mutations that result in decreased rates of oxygen reduction will also delay the development of oxygen-limited growth and, hence, delay the activation of FNR. This, in turn, would result in a delayed induction of AniA synthesis, but not the ultimate rate of nitrite reduction, once AniA had accumulated.
An AniA antiserum was used to detect the accumulation of the AniA protein during the growth cycle of parental strain F62 and mutants defective in cycA, cycB, and cccA by Western blotting. Although similar concentrations of the AniA antigen had accumulated in samples taken as all four strains entered the stationary phase of growth, during the middle of exponential growth, very little AniA had accumulated in the cytochrome c4 and c5 mutants compared with the amount for the parent strain (Fig. 1B). In contrast, AniA accumulated slightly more rapidly in the cccA mutant than in the parent, consistent with the higher respiratory rate of this strain. The combined data confirm that the primary effect of mutations in cycA or cycB, but not cccA, is to decrease the ability of gonococci to reduce oxygen. This results in a delay in the activation of FNR, which in turn leads to a delayed accumulation of the AniA protein.
Complementation of the cycA and cycB phenotypes by FLAG-tagged proteins and positive identification of cytochromes c4 and c5.
Both the cycA and cycB mutations were replaced by genes encoding cytochromes c4 and c5 fused in frame to triplicate FLAG epitopes to facilitate detection both by Western blotting and by altered mobility during SDS-PAGE. As these constructs also included a kanamycin resistance cassette downstream of the functional cyc gene, the restoration of function would confirm that the phenotypes of the mutants were due only to a disruption of the cyc gene and not to polarity effects on downstream genes. The replacement of both mutations by the cyc::FLAG constructs fully restored oxygen-sufficient growth (see Fig. S1A and S1B in the supplemental material), and rates of oxygen reduction by harvested bacteria in the presence of lactate were insignificantly different from those of the parent strain (Fig. S1C).
Cytochrome c4 migrates at almost the same rate as CcoO during SDS-PAGE, and the two cytochromes are not always easily distinguishable on gels stained for covalently bound heme (compare Fig. 1 with Fig. S1D and S1E in the supplemental material). However, cytochrome c4::FLAG was easily distinguished from CcoO, and both staining for covalently bound heme and Western analysis using a commercial anti-FLAG antibody confirmed that the FLAG-tagged and untagged proteins were equally abundant (Fig. S1D and data not shown). These experiments clearly established for the first time that cytochrome c4 migrates slightly faster than CcoO during SDS-PAGE and is seen as the lower band of the doublet on gels stained for covalently bound heme. In contrast, cytochrome c5 is readily detected as a well-isolated band on SDS-PAGE gels, and as expected, the FLAG-tagged protein migrated slightly more slowly during SDS-PAGE (Fig. S1E).
Complementation of the cycA and cycB mutations by ectopic expression of IPTG-inducible cycA and cycB genes.
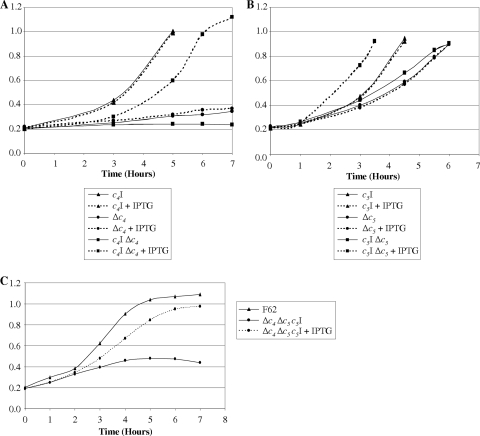
IPTG-inducible copies of either cycA or cycB were integrated between the lctP and aspC genes on the chromosomes of parental strain F62 and the cycA or cycB mutant, respectively. The presence or absence of IPTG had no effect on the oxygen-sufficient growth of cycA+ strain JCGC10, but the effect of the restoration of the cycA+ phenotype on the growth of complemented cycA mutant strain JCGC820 was dependent upon the addition of IPTG (Fig. 3A). The oxygen sensitivity of the original cycA mutant was unaffected by the addition of IPTG (Fig. 3A). Similar results were obtained with the corresponding cycB derivatives. IPTG stimulated the oxygen-sufficient growth of complemented cycB strain JCGC870 but had no effect on the growth of the original cycB mutant strain JCGC860 or cycB+ strain JCGC11 (Fig. 3B). Proteins from samples harvested at the late exponential phase of each of these cultures were separated by SDS-PAGE and stained for covalently bound heme (see Fig. S2 in the supplemental material). Ectopically expressed copies of cycA+ or cycB+ enhanced the staining intensity of the cytochrome c4 or cytochrome c5 band of the cycA+ or cycB+ strain and restored cycA+ or cycB+ synthesis to the corresponding mutant strains (Fig. S2 [only cycB data are shown]). Furthermore, rates of oxygen reduction by the complemented strains were identical to those of the parental strain following growth in the presence, but not in the absence, of IPTG (data not shown).
FIG. 3.
(A) Cultures of parent strain F62 containing a second, IPTG-inducible, ectopic copy of cycA (labeled c4I) (triangles), the cycA mutant (labeled Δc4) (circles), and the cycA mutant complemented with the IPTG-inducible copy of cycA (labeled c4I Δc4) (squares). (B) Cultures of parent strain F62 containing the IPTG-inducible copy of cycB (labeled c5I) (triangles), the cycB mutant (labeled Δc5) (circles), and the cycB mutant complemented with the IPTG-inducible copy of cycB (labeled c5I Δc5) (squares). For A and B, cells were grown at 37°C in 30 ml of GCB in 100-ml conical flasks with shaking at 100 rpm. Continuous lines indicate that no IPTG was added to cultures, whereas dotted lines indicate cultures where 1 mM IPTG was added at time zero. (C) Cultures of parental strain F62 (triangles) and the cycA cycB mutant containing the IPTG-inducible copy of cycB (circles). Growth conditions were the same as those described above (A and B). Continuous lines indicate that no IPTG was added to cultures, whereas dotted lines indicate cultures where 0.1 mM IPTG was added at time zero.
Phenotypes of double mutants defective in cytochromes c2 and c4 or cytochromes c2 and c5.
The results presented above implicated both cytochromes c4 and c5 in electron transfer between the cytochrome bc1 complex and cytochrome oxidase cbb3 but suggested that cytochrome c2 either is not involved in aerobic respiration or, at most, plays only a very minor role, at least under the growth conditions used in these experiments. Double mutants defective in both cytochromes c2 and c4 or c2 and c5 were constructed, and their growth phenotypes were compared with those of the parent strain and the single mutants. The growths of both strains were sensitive to oxygen inhibition; however, the cytochrome c2-cytochrome c4 double mutant was slightly less sensitive than the cytochrome c4 single mutant (Fig. 2C and D), but the cytochrome c2-cytochrome c5 double mutant was more sensitive than the cytochrome c5 single mutant (Fig. 2E and F).
The rate of oxygen reduction by the cytochrome c2-cytochrome c4 double mutant in the presence of lactate was similar to that of the parent strain, indicating that the capacity of the cytochrome c5 branch of the respiratory chain is sufficient to sustain a full rate of oxidation of physiological substrates (Table 3). Consistent with this conclusion, the rate of ascorbate and TMPD oxidation by this double mutant was only 15% less than that of the parent strain. In contrast, the rates of oxidation of both lactate and ascorbate plus TMPD by the double mutant defective in both cytochromes c2 and c5 were far lower than those of the other mutants, confirming that the capacity of the cytochrome c4 branch of electron transfer to oxygen is lower than that of the cytochrome c5 branch.
Failure to isolate a double mutant defective in both cytochromes c4 and c5.
Various methods were used in attempts to construct a double mutant defective in both cytochromes c4 and c5, by both transforming a piliated cytochrome c4 mutant with a cytochrome c5 deletion-insertion cassette and vice versa. In more than 20 such attempts, fnr or other single mutants were isolated in parallel control experiments with the same recipient cultures, but no double mutants were isolated. Furthermore, attempts to recover double-mutant transformants anaerobically in the presence of nitrite were also unsuccessful, even when the recipient had been preadapted to oxygen-limited but nitrite-supplemented growth. This extensive series of experiments provided strong evidence that either cytochrome c4 or cytochrome c5 is essential for gonococcal survival because it provides parallel pathways for electron transfer between the cytochrome bc1 complex and cytochrome oxidase cbb3.
IPTG-dependent survival of a double mutant defective in both cytochromes c4 and c5 complemented by an inducible copy of the ectopically located cycB gene.
A cycA mutation was transformed into strain JCGC860 in which the cycB mutation had been complemented by an ectopically expressed copy of cycB integrated at the aspC-lctP locus on the gonococcal chromosome. Transformants were created and maintained in the presence of IPTG and plated onto either unsupplemented GC agar or GC agar supplemented with 0.1 mM or 0.5 mM IPTG. Unlike strain JCGC860, which formed normal colonies on all three media, the survival of strain JCGC870 was totally dependent upon the presence of IPTG. Furthermore, the growth of this strain in GC broth stopped a few hours after subculture from the IPTG-supplemented inoculum as the cytochrome c5 complement of the inoculum was diluted out and had stopped completely after 5 h (Fig. 3C). In contrast, growth in the presence of IPTG was indistinguishable from that of the cycA single mutant. These experiments confirmed that gonococcal growth and survival are totally dependent upon the expression of either cytochrome c4 or cytochrome c5. Furthermore, at least under the conditions normally used to culture gonococci in the laboratory, cytochrome c2 cannot support growth or oxygen respiration.
DISCUSSION
The cytochrome cbb3 family of cytochrome oxidases is widely distributed among microaerobic bacteria, many of which also synthesize the diheme cytochromes c4 and c5 (reviewed in reference 31). Despite many studies of the distributions, assemblies, mechanisms of proton pumping, and biophysical properties of cytochrome cbb3 oxidases from various bacteria, no structure of any cbb3 oxidase has yet been published (5, 9, 10, 31, 32, 33, 50). Although soluble monoheme cytochromes transfer electrons between the cytochrome bc1 complex and cytochrome oxidase caa3, little is known about how electrons are transferred from the cytochrome bc1 complex to the cytochrome oxidase cbb3 family, and there is currently no evidence that small cytochromes are involved.
Essential roles of either cytochrome c4 or cytochrome c5 in electron transfer between the gonococcal cytochrome bc1 complex and cytochrome oxidase.
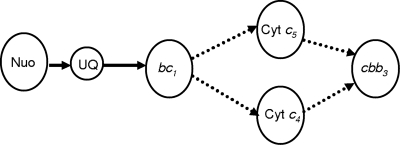
Downs and Jones (7) were among the first groups to propose that in Azotobacter vinelandii, the diheme cytochromes c4 and c5 might provide alternative but parallel pathways for electron transfer from the cytochrome bc1 complex to the terminal oxidase (reviewed by Haddock and Jones [11]). Much later, it was realized that there are multiple cytochrome oxidases in A. vinelandii and that one of them is a homologue of the B. japonicum high-affinity oxidase FixNOQP (6, 16, 19, 45). A. vinelandii single mutants defective in either cytochrome c4 or cytochrome c5 have been constructed, and as in the current gonococcal study, rates of ascorbate-TMPD oxidation were lower in each of the mutants than in the parent strain. However, unlike the gonococcus, the loss of one or the other of these cytochromes had little effect on oxygen-sufficient growth or on rates of respiration in the presence of physiological substrates (27, 34). Also unlike the gonococcus was the successful isolation of a double mutant defective in both cytochromes c4 and c5 (34). This is highly significant because it is consistent with the presence of alternative respiratory pathways in Azotobacter that are unavailable to pathogenic Neisseria. This alone provides strong evidence not only that gonococcal cytochromes c4 and c5 are involved in electron transfer to oxygen but also that there is no major third pathway for oxygen reduction. This finding suggests that one of these two cytochromes is essential for survival when the gene for the other has been mutated, as shown in Fig. 4. This conclusion is supported by the demonstration that both single mutants are more sensitive to high levels of aeration than the parent strain (Fig. 2A and B) and that the accumulation of AniA, the product of an FNR-dependent gene, is delayed during oxygen-limited growth because the cytochrome c4 and c5 mutants are less able to reduce oxygen than the parent (Fig. 2B). This results in the delayed activation of the FNR protein because oxygen prevents the incorporation of the iron-sulfur center of FNR, which in turn prevents dimerization and transcription activation (29). Our conclusion that cytochromes c4 and c5 provide the only physiologically significant pathways for electron transfer to oxygen was confirmed by the demonstration that the survival of a cycA cycB double mutant was totally dependent upon the IPTG-induced expression of an ectopic copy of cycB (Fig. 3).
FIG. 4.
Scheme for electron transfer from NADH to oxygen in the gonococcus. Cyt, cytochrome; Nuo, NADH dehydrogenase; UQ, ubiquinone.
Lack of a significant role of cytochrome c2 in electron transfer to cytochrome oxidase.
Gonococcal cytochrome c2 is clearly orthologous to cytochrome cx of N. meningitidis (4) but is only distantly related to any other c-type cytochromes currently in the databases. As previously noted (4), residues 30 to 132 are 34% similar to cytochrome c552 of Thermus thermophilus, which was proposed to transfer electrons between the cytochrome bc1 complex and cytochrome oxidase cbb3 in that organism. As small, soluble, c-type cytochromes are normally involved in electron transfer between the cytochrome bc1 complex and cytochrome oxidases of both mammals and many types of bacteria, cytochrome c2 was a possible candidate to fulfill such a role in the gonococcus. However, in contrast to cytochromes c4 and c5, no evidence was found to show that cytochrome c2 is involved directly in electron transfer to oxygen; on the contrary, oxygen reduction by the cccA mutant was more rapid than that of the parent strain, and this was supported by Western blotting data that showed a more rapid accumulation of AniA in oxygen-limited cultures of the cccA mutant than in cultures of the parent strain. Although the predicted cytochrome has never been seen on polyacrylamide gels stained for proteins with covalently bound heme, the evidence presented above indicates that the cccA gene is expressed. First, the cccA mutant reduced oxygen slightly more rapidly than the parent strain (Table 3). Second, clear differences in the effects of a secondary cccA mutation on mutants defective in cycA or cycB were detected (Table 3 and Fig. 2). The cccA mutation decreased the respiration rate of the cycB mutant, in which respiration was totally dependent upon cytochrome c4, but increased the rate of oxygen reduction by the cycA mutant, in which oxygen reduction was dependent upon cytochrome c5 (Fig. 2 and Table 3). Furthermore, the cccA cycA double mutant was less sensitive to full aeration than the cycA single mutant; in contrast, the cccA cycB mutant was more sensitive to growth inhibition by full aeration than the cycB single mutant. These observations suggest that cytochrome c2 might regulate electron flow between the cytochrome c4 and cytochrome c5 branches of the respiratory chain. However, other explanations need to be investigated. We are currently investigating whether cytochrome c2, like the CcoP subunit of cytochrome oxidase itself, plays a physiologically significant role in transferring electrons to outer membrane redox proteins, such as the lipid-associated azurin Laz and the cytochrome c peroxidase Ccp, that protect gonococci against hydrogen peroxide (14, 37, 46, 47).
Why is a pathogen with a microaerobic life-style such a prolific source of electron transfer components?
To establish an infection, pathogens must be able to protect themselves against reactive oxygen and nitrogen species generated externally as part of the host defense mechanisms and by other bacteria that coinhabit their environment. We suggested previously that the outer membrane lipoproteins Ccp, cytochrome c′, Laz, and AniA provide pathogenic neisseria with a first line of defense against reactive oxygen and reactive nitrogen species generated by their human host (47, 48). Results from the current study with the single mutants defective in either cytochrome c4 or c5 have provided both insight into the rate-determining steps in gonococcal respiration and an explanation for the following long-standing paradox: why should an obligate human pathogen with a microaerobic life-style maintain such high rates of respiration and be such a prolific source of c-type cytochromes?
Rates of ascorbate-plus-TMPD-dependent respiration by washed bacterial suspensions of all of the strains studied were far higher (5.1- to 6.5-fold) than rates in the presence of the physiological substrate lactate (Table 3). This finding implies that the capacity of electron transfer between lactate and the cytochrome bc1 complex is less than that of the terminal components of the respiratory chain, a conclusion supported by the fact that the sum of the rates of the TMPD oxidation of the cycA and cycB mutants was greater than that of the parent strain. Despite the small loss of the respiratory capacity, the cycA and cycB single mutants were far more sensitive to excess oxygen than the parent, indicating that this excess capacity for oxygen reduction protects the gonococcus from reactive oxygen species generated during aerobic growth. One possible explanation for this finding is that one or both of these cytochromes are required to protect gonococci against reactive oxygen species generated during aerobic growth. It is more likely, however, that the increased sensitivity to oxygen is due to the increased generation of reactive oxygen species by the mutants, for example, by the chemical interaction of oxygen with reduced flavoproteins or the quinone pool (12, 42), which will be more reduced in the cycA and cycB mutants than in the parent strain due to their lower rates of electron transfer from the cytochrome bc1 complex to oxygen. If this is correct, the high respiratory capacity of gonococci prevents the accumulation of oxygen, which would generate toxic oxygen species such as hydrogen peroxide, superoxide, and hydroxyl radicals. We therefore propose that the excess capacity for oxygen reduction provides a defense against reactive oxygen species generated as by-products of their own metabolism, which supplements the many other defense mechanisms documented previously (38).
Supplementary Material
Acknowledgments
We gratefully acknowledge J. W. T. Moir for drawing our attention to the presence of the cccA gene encoding cytochrome c2 in Neisseria gonorrhoeae and for providing the anti-AniA antibody.
Y.L. was funded by a scholarship from the Darwin Trust of Edinburgh, and N.T., D.J.P.S., and A.H. were funded by UK Biotechnology and Biological Sciences Research Council postgraduate training grants.
Footnotes
Published ahead of print on 12 February 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Biswas, G. D., T. Sox, E. Blackman, and P. F. Sparling. 1977. Factors affecting genetic transformation of Neisseria gonorrhoeae. J. Bacteriol. 129:983-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark, V., L. A. Campbell, D. A. Palermo, T. M. Evans, and K. W. Klimpel. 1987. Induction and repression of outer membrane proteins by anaerobic growth of Neisseria gonorrhoeae. Infect. Immun. 55:1359-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deeudom, M., J. Rock, and J. Moir. 2006. Organization of the respiratory chain of Neisseria meningitidis. Biochem. Soc. Trans. 34:139-142. [DOI] [PubMed] [Google Scholar]
- 5.de Gier, J. W. L., M. Schepper, W. N. M. Reijnders, S. J. van Dyke, D. J. Slotboom, A. Warne, M. Saraste, K. Krab, M. Finel, A. H. Stouthamer, R. J. M. van Spanning, and J. van der Oost. 1996. Structural and functional analysis of aa3-type and cbb3-type cytochrome c oxidases of Paracoccus denitrificans reveals significant differences in proton-pump design. Mol. Microbiol. 20:1247-1260. [DOI] [PubMed] [Google Scholar]
- 6.D'Mello, R., D. Purchase, R. K. Poole, and S. Hill. 1997. Expression and content of terminal oxidases in Azotobacter vinelandii grown with excess NH4+ are modulated by oxygen supply. Microbiology 143:231-237. [DOI] [PubMed] [Google Scholar]
- 7.Downs, A. J., and C. W. Jones. 1975. Respiration-linked proton translocation in Azotobacter vinelandii. FEBS Lett. 60:42-46. [DOI] [PubMed] [Google Scholar]
- 8.Dupuy, B., and A. P. Pugsley. 1994. Type IV prepilin peptidase gene of Neisseria gonorrhoeae MS11: presence of a related gene in other piliated and nonpiliated Neisseria strains. J. Bacteriol. 176:1323-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Horsman, J. A., E. Berry, J. P. Shapleigh, J. O. Alben, and R. B. Gennis. 1994. A novel cytochrome c oxidase from Rhodobacter sphaeroides that lacks CuA. Biochemistry 33:3113-3119. [DOI] [PubMed] [Google Scholar]
- 10.Gray, K. A., M. Grooms, H. Myllykallio, C. Moomaw, C. Slaughter, and F. Daldal. 1994. Rhodobacter capsulatus contains a novel cb-type cytochrome c oxidase without a CuA centre. Biochemistry 33:3120-3127. [DOI] [PubMed] [Google Scholar]
- 11.Haddock, B. A., and C. W. Jones. 1977. Bacterial respiration. Bacteriol. Rev. 41:47-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hägerhäll, C., S. Magnitsky, V. D. Sled, I. Schröder, R. P. Gunsalus, G. Cecchini, and T. Ohnishi. 1999. An Escherichia coli mutant quinol:fumarate reductase contains an EPR-detectable semiquinone stabilized at the proximal quinone-binding site. J. Biol. Chem. 274:26157-26164. [DOI] [PubMed] [Google Scholar]
- 13.Hill, S. A., T. Woodward, A. Reger, R. Baker, and T. Dinse. 2007. Role of the RecBCD recombination pathway for pilE gene variation in repair-proficient Neisseria gonorrhoeae. J. Bacteriol. 189:7983-7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hopper, A., N. Tovell, and J. Cole. 2009. A physiologically significant role in nitrite reduction of the CcoP subunit of the cytochrome oxidase cbb3 from Neisseria gonorrhoeae. FEMS Lett. 301:232-240. [DOI] [PubMed] [Google Scholar]
- 15.Kellogg, S., W. L. Peacock, W. E. Deacon, L. Brown, and D. I. Pirkle. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 85:1274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly, M. J., R. K. Poole, M. G. Yates, and C. Kennedy. 1990. Cloning and mutagenesis of genes encoding the cytochrome bd terminal oxidase complex in Azotobacter vinelandii: mutants deficient in the cytochrome d complex are unable to fix nitrogen in air. J. Bacteriol. 172:6010-6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenimer, E. A., and D. F. Lapp. 1978. Effects of selected inhibitors on electron transport in Neisseria gonorrhoeae. J. Bacteriol. 134:537-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knapp, J. S., and V. Clark. 1984. Anaerobic growth of Neisseria gonorrhoeae coupled to nitrite reduction. Infect. Immun. 46:176-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolonay, J. F., Jr., F. Moshiri, R. B. Gennis, T. M. Kaysser, and R. J. Maier. 1994. Purification and characterization of the cytochrome bd complex from Azotobacter vinelandii: comparison to the complex from Escherichia coli. J. Bacteriol. 176:4177-4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larribe, M., M. K. Taha, A. Topilko, and C. Marchal. 1997. Control of Neisseria gonorrhoeae pilin gene expression by environmental factors: involvement of the pilA/pilB regulatory genes. Microbiology 145:1757-1764. [DOI] [PubMed] [Google Scholar]
- 21.Lissenden, S., S. Mohan, T. Overton, T. Regan, H. Crooke, J. A. Cardinale, T. C. Householder, P. Adams, C. D. O'Connor, V. L. Clark, H. Smith, and J. A. Cole. 2000. Identification of transcription activators which regulate gonococcal adaptation from aerobic to anaerobic or oxygen-limited growth. Mol. Microbiol. 37:839-855. [DOI] [PubMed] [Google Scholar]
- 22.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 23.Mehr, I. J., and H. S. Seifert. 1998. Differential roles of homologous recombination pathways in Neisseria gonorrhoeae pilin antigenic variation, DNA transformation, and DNA repair. Mol. Microbiol. 30:697-710. [DOI] [PubMed] [Google Scholar]
- 24.Mehr, I. J., C. D. Long, C. D. Serkin, and H. S. Seifert. 2000. A homologue of the recombination-dependent growth gene, rdgC, is involved in gonococcal pilin antigenic variation. Genetics 154:523-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mellies, J., J. Jose, and T. F. Meyer. 1997. The Neisseria gonorrhoeae gene aniA encodes an inducible nitrite reductase. Mol. Gen. Genet. 256:532-537. [DOI] [PubMed] [Google Scholar]
- 26.Morse, S. A., A. F. Cacciapuoti, and P. G. Lysko. 1979. Physiology of Neisseria gonorrhoeae. Adv. Microb. Physiol. 20:251-320. [DOI] [PubMed] [Google Scholar]
- 27.Ng, T. C. N., A. N. Laheri, and R. J. Maier. 1995. Cloning, sequencing and mutagenesis of the cytochrome c4 gene from Azotobacter vinelandii: characterisation of the mutant strain and a proposed new branch in the respiratory chain. Biochim. Biophys. Acta 1230:119-129. [DOI] [PubMed] [Google Scholar]
- 28.Nilavongse, A., T. H. C. Brondijk, T. W. Overton, D. J. Richardson, E. R. Leach, and J. A. Cole. 2006. The NapF protein of the Escherichia coli periplasmic nitrate reductase system: demonstration of a cytoplasmic location and interaction with the catalytic subunit, NapA. Microbiology 152:3227-3237. [DOI] [PubMed] [Google Scholar]
- 29.Overton, T., E. G. F. Reid, R. Foxall, H. Smith, S. J. W. Busby, and J. A. Cole. 2003. Transcription activation at E. coli FNR-dependent promoters by the gonococcal FNR protein: effects of a novel S18F substitution, and comparison with the corresponding substitution in E. coli FNR. J. Bacteriol. 185:4734-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pember, S. O., L. C. Fleck, W. K. Moberg, and M. P. Walker. 2005. Mechanistic differences in inhibition of ubiquinol cytochrome c reductase by the proximal Qo-site inhibitors famoxadone and methoxyacrylate stilbene. Arch. Biochem. Biophys. 435:280-290. [DOI] [PubMed] [Google Scholar]
- 31.Pitcher, R. S., and N. J. Watmough. 2004. The bacterial cytochrome cbb3 oxidases. Biochim. Biophys. Acta 1655:388-399. [DOI] [PubMed] [Google Scholar]
- 32.Preisig, O., D. Anthamatten, and H. Hennecke. 1993. Genes for a microaerobically induced oxidase complex in Bradyrhizobium japonicum are essential for nitrogen-fixing endosymbiosis. Proc. Natl. Acad. Sci. U. S. A. 90:3309-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Preisig, O., R. Zufferey, L. Thöny-Meyer, C. A. Appleby, and H. Hennecke. 1996. A high-affinity cbb3-type cytochrome oxidase terminates the symbiosis-specific respiratory chain of Bradyrhizobium japonicum. J. Bacteriol. 178:1532-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rey, L., and R. J. Maier. 1997. Cytochrome c terminal oxidase pathways of Azotobacter vinelandii: analysis of cytochrome c4 and c5 mutants and up-regulation of cytochrome c-dependent pathways with N2 fixation. J. Bacteriol. 179:7191-7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reyn, A. 1974. Gram negative cocci and coccibacilli, p. 427-433. In R. E. Buchanan and N. E. Gibbons (ed.), Bergey's manual of determinative bacteriology. Williams & Wilkins, Baltimore, MD.
- 36.Rudel, T., D. Facius, R. Barten, I. Scheuerpflug, E. Nonnenmacher, and T. F. Meyer. 1995. Role of pili and the phase-variable PilC protein in natural competence for transformation of Neisseria gonorrhoeae. Proc. Natl. Acad. Sci. U. S. A. 92:7986-7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seib, K. L., H.-J. Tseng, A. G. McEwan, M. A. Apicella, and M. P. Jennings. 2004. Defenses against oxidative stress in Neisseria gonorrhoeae and Neisseria meningitidis: distinctive systems for different lifestyles. J. Infect. Dis. 190:136-147. [DOI] [PubMed] [Google Scholar]
- 38.Seib, K. L., H.-J. Wu, S. P. Kidd, M. A. Apicella, M. P. Jennings, and A. G. McEwan. 2006. Defenses against oxidative stress in Neisseria gonorrhoeae: a system tailored for a challenging environment. Microbiol. Mol. Biol. Rev. 70:344-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sevastsyanovich, Y., S. Alfasi, T. Overton, R. Hall, J. Jones, C. Hewitt, and J. Cole. 2009. Exploitation of GFP fusion proteins and stress avoidance as a generic strategy for the production of high quality recombinant proteins. FEMS Microbiol. Lett. 299:86-94. [DOI] [PubMed] [Google Scholar]
- 40.Silver, L. E., and V. L. Clark. 1995. Construction of a translational lacZ fusion system to study gene regulation in Neisseria gonorrhoeae. Gene 166:101-104. [DOI] [PubMed] [Google Scholar]
- 41.Stohl, E. A., A. K. Criss, and H. S. Seifert. 2005. The transcriptome response of Neisseria gonorrhoeae to hydrogen peroxide reveals genes with previously uncharacterised roles in oxidative damage protection. Mol. Microbiol. 58:520-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Storz, G., and J. A. Imlay. 1999. Oxidative stress. Curr. Opin. Microbiol. 2:188-194. [DOI] [PubMed] [Google Scholar]
- 43.Strange, J. C., and R. C. Judd. 1994. Identification of heme containing proteins of Neisseria gonorrhoeae, p. 417-423. In C. J. Conde-Glez, S. Morse, P. Rice, F. Sparling, and E. Calderón (ed.), Pathobiology and immunology of Neisseriaceae. Instituto Nacional de Salud Pública Press, Morelos, Mexico.
- 44.Thomas, P. E., D. Ryan, and W. Ledwin. 1976. An improved staining procedure for the detection of peroxidase activity of cytochrome P-450 on sodium dodecyl sulphate polyacrylamide gels. Anal. Biochem. 75:168-176. [DOI] [PubMed] [Google Scholar]
- 45.Thöny-Meyer, L., C. Beck, O. Preisig, and H. Hennecke. 1994. The ccoNOQP gene cluster codes for a cb-type cytochrome oxidase that functions in aerobic respiration of Rhodobacter capsulatus. Mol. Microbiol. 14:705-716. [DOI] [PubMed] [Google Scholar]
- 46.Trees, D. L., and S. M. Spinola. 1990. Localization of and immune response to the lipid modified azurin of pathogenic Neisseria. J. Infect. Dis. 161:336-339. [DOI] [PubMed] [Google Scholar]
- 47.Turner, S., E. Reid, H. Smith, and J. Cole. 2003. A novel cytochrome c peroxidase from Neisseria gonorrhoeae: a lipoprotein from a Gram-negative bacterium. Biochem. J. 373:865-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turner, S. M., J. W. B. Moir, L. Griffiths, T. W. Overton, H. Smith, and J. A. Cole. 2005. Mutational and biochemical analysis of cytochrome c′, a nitric oxide-binding lipoprotein important for adaptation of Neisseria gonorrhoeae to oxygen-limited growth. Biochem. J. 388:545-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uzzau, S., N. Figueroa-Bossi, S. Rubino, and L. Bossi. 2001. Epitope tagging of chromosomal genes in Salmonella. Proc. Natl. Acad. Sci. U. S. A. 98:15264-15269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Visser, J. M., G. A. H. De Jong, S. De Vries, L. A. Robertson, and J. G. Kuenen. 2006. cbb3-type cytochrome oxidase in the obligately chemolithotrophic Thiobacillus sp. W5. FEMS Microbiol. Lett. 147:127-132. [Google Scholar]
- 51.Von Jagow, G., P. O. Ljungdahl, P. Graft, T. Ohnishi, and B. L. Trumpower. 1984. An inhibitor of mitochondrial respiration which binds to cytochrome b and displaces quinone from the iron-sulfur protein of the cytochrome bc1 complex. J. Biol. Chem. 259:6318-6323. [PubMed] [Google Scholar]
- 52.West, P. A., R. M. Daniel, C. J. Knowles, and J. V. Lee. 1978. Tetramethyl-p-phenylenediamine (TMPD) oxidase activity and cytochrome distribution in the genus Vibrio. FEMS Microbiol. Lett. 4:339-342. [Google Scholar]
- 53.Whitehead, R. N., T. W. Overton, L. A. S. Snyder, S. J. McGowan, H. Smith, J. A. Cole, and N. J. Saunders. 2007. The small FNR regulon of Neisseria gonorrhoeae: comparison with the larger Escherichia coli FNR regulon and interaction with the NarQ-NarP regulon. BMC Genomics 8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winter, D. B., and S. A. Morse. 1975. Physiology and metabolism of pathogenic Neisseria: partial characterisation of the respiratory chain of Neisseria gonorrhoeae. J. Bacteriol. 123:631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu, S. K. C., and I. W. DeVoe. 1980. Terminal branching of the respiratory electron transport chain in Neisseria meningitidis. J. Bacteriol. 142:879-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.