Abstract
Abundant in milk and other dairy products, lactose is considered to have an important role in oral microbial ecology and can contribute to caries development in both adults and young children. To better understand the metabolism of lactose and galactose by Streptococcus mutans, the major etiological agent of human tooth decay, a genetic analysis of the tagatose-6-phosphate (lac) and Leloir (gal) pathways was performed in strain UA159. Deletion of each gene in the lac operon caused various alterations in expression of a PlacA-cat promoter fusion and defects in growth on either lactose (lacA, lacB, lacF, lacE, and lacG), galactose (lacA, lacB, lacD, and lacG) or both sugars (lacA, lacB, and lacG). Failure to grow in the presence of galactose or lactose by certain lac mutants appeared to arise from the accumulation of intermediates of galactose metabolism, particularly galatose-6-phosphate. The glucose- and lactose-PTS permeases, EIIMan and EIILac, respectively, were shown to be the only effective transporters of galactose in S. mutans. Furthermore, disruption of manL, encoding EIIABMan, led to increased resistance to glucose-mediated CCR when lactose was used to induce the lac operon, but resulted in reduced lac gene expression in cells growing on galactose. Collectively, the results reveal a remarkably high degree of complexity in the regulation of lactose/galactose catabolism.
Lactose, a β1,4-linked disaccharide of β-d-galactose and α/β-d-glucose, is commonly found in the dairy-rich diets of most industrialized nations. Lactose is rapidly fermented by streptococci, including the cariogenic oral bacterium Streptococcus mutans (21), as well as by a variety of industrially important lactic acid bacteria (LAB) (19). Multiple pathways have been identified in bacteria for the utilization of lactose encountered in the environment. For example, Streptococcus salivarius strain 25975 (26) secretes a β-galactosidase that hydrolyzes extracellular lactose into galactose and glucose, although it is more common for lactose to be transported before cleavage (18). Most efficiently, and almost exclusively in Gram-positive bacteria, lactose is internalized by the phosphoenolpyruvate (PEP)-dependent sugar-phosphotransferase system (PTS), yielding lactose-6-phosphate (Lac-6-P) (36). The Lac-6-P is hydrolyzed to glucose and galactose-6-phosphate (Gal-6-P) by a cytoplasmic phospho-β-galactosidase (LacG), and the Gal-6-P can be catabolized by the tagatose-6-phosphate pathway (18) (Fig. 1). Many bacteria, including Escherichia coli, Lactococcus lactis strain 7962, and S. salivarius strain 57.I, can internalize lactose through non-PTS transporters. Intracellular lactose is cleaved by a β-galactosidase enzyme and the d-galactose can directly enter the Leloir pathway (Fig. 1) (18, 20).
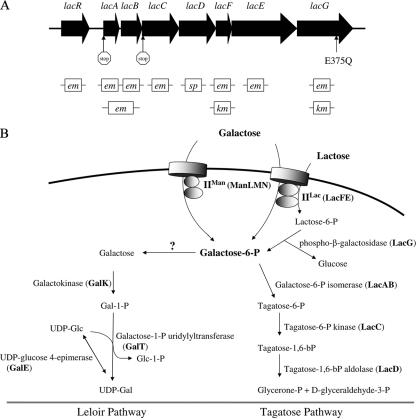
FIG. 1.
Genetic organization of the lac operon in S. mutans UA159 (A) and the pathways for metabolism of galactose and lactose (B). (A) The encoding sequences of all eight genes are depicted as filled arrows: lacR, the DeoR-like negative transcriptional regulator; lacA and lacB, the A and B subunits of the galactose-6-P isomerase; lacC, the tagatose-6-P kinase; lacD, tagatose-1,6-bP adolase; lacF and lacE, the A and BC components of the lactose-PTS enzyme II; and lacG, the phospho-β-galactosidase. Below the genes are the antibiotic-resistance-encoding elements used in the allelic exchange mutagenesis of each open reading frame, with em, km and sp. representing the erythromycin, kanamycin, and spectinomycin resistance cassette, respectively. Locations of three point mutations are indicated by vertical arrows. (B) Schematics of the Leloir (left) and tagatose-6-phosphate (right) pathways. Gal-6-P, galactose-6-phosphate; Gal-1-P, galactose-1-phosphate; UDP-Gal, UDP-galactose; UDP-Glc, UDP-glucose; Glc-1-P, glucose-1-phosphate.
S. mutans has a functional lactose-specific PTS (14, 26) encoded by the lacF (EIIA) and lacE (EIIBC) genes (40). A phospho-β-galactosidase (lacG) and the enzymes of the tagatose-6-phosphate pathway (Fig. 1B), including the two subunits of the heteromeric galactose-6-phosphate isomerase (lacAB), a tagatose-6-phosphate kinase (lacC), and a tagatose-1,6-bisphosphate aldolase (lacD), are encoded in the same operon (Fig. 1A) as lacFE (9, 27, 40, 43). The tagatose pathway is responsible for catabolism of Gal-6-P, and the glucose liberated from Lac-6-P can be phosphorylated by a glucokinase before entering glycolysis (25, 26). S. mutans also possesses a Leloir pathway (Fig. 1B) encoded in an operon that includes galK (galactokinase), galT (galactose-1-phosphate uridylyltransferase), and galE (UDP-glucose 4-epimerase) (9). Inactivation of the galK gene was shown to severely impair the ability of S. mutans to grow with galactose as the sole carbohydrate (3). Of note, some galactose-containing carbohydrates appear to be transported by the multiple sugar metabolism (msm) system (41), the permease responsible for transporting galactose has not yet been identified (3).
Expression of the tagatose pathway in S. mutans is inducible by lactose (25, 26), whereas expression of the Leloir pathway can be induced by galactose in the medium or by intracellular galactose released from internalized α-galactosides, such as melibiose (9). Repression of the gal operon is mediated by GalR, which is believed to have its DNA-binding activity to an operator site(s) located in the galR-galK intergenic region modified by intracellular galactose (8). A more recent study by our group suggested that the Leloir and tagatose-6-phosphate pathways are both important for the utilization of galactose and that the transcript levels of the galK and lac genes were elevated during growth on galactose (3).
Carbon catabolite repression (CCR) is a mechanism regulating energy metabolism in most known bacterial species. CCR enables a bacterium to selectively utilize more rapidly metabolizable carbon sources in the presence of nonpreferred sources, thus helping the organisms to maintain energy efficiency and competitiveness (22). In most low-G+C Gram-positive bacteria, CCR is primarily controlled by the phosphocarrier protein HPr of the PTS and by catabolite control protein A, CcpA, which binds to catabolite response elements (CRE) found in promoter regions of CCR-sensitive genes. Binding of CcpA to it target sites is strongly stimulated by Ser46-phosphorylated HPr (17). Interestingly, despite essential roles for CcpA in control of metabolism and virulence (4), CcpA does not appear to play a dominant role in CCR in S. mutans (23, 42, 48). Instead, three proteins of the enzyme II complexes of the PTS (EIIABMan, FruI, and EIILev) have dominant roles in CCR of nonpreferred carbohydrate utilization genes (1, 51). Also, we recently determined that there is an apparent direct involvement of HPr-Ser46-PO4 and the glucose/mannose-PTS EIIABMan (ManL) in CCR of the fructan hydrolase (fruAB) and the levDEFGX operons (53, 54). Lactose and galactose metabolism are also sensitive to CCR, but the molecular mechanisms have not been explored. To better understand how the catabolism of galactose and lactose is controlled by S. mutans, we examined the properties of various mutant strains lacking certain PTS components, regulatory proteins, and constituents of the catabolic pathways for lactose and galactose. The results shed new light on the regulation of carbohydrate catabolism in this important pathogen and reveal unexpected mechanisms for control of galactose and lactose catabolism by EIIMan and the enzymes of the lactose utilization pathway.
MATERIALS AND METHODS
Bacterial strains and culture condition.
Wild-type S. mutans strain UA159 and its derivatives were maintained on brain heart infusion (BHI; Difco, Detroit, MI) agar at 37°C in a 5% CO2 incubator. Antibiotics were added to BHI agar, when necessary, at the following concentrations: kanamycin (Km), 1 mg ml−1; erythromycin (Em), 10 μg ml−1; and spectinomycin (Sp), 1 mg ml−1. Half the amount of each antibiotic was used in BHI broth cultures, if necessary. For chloramphenicol acetyltransferase (CAT) assays and growth tests, bacterial cells were cultivated in tryptone-vitamin (TV) base medium (12) supplemented with 0.5% of each carbohydrate, unless otherwise specified. The growth phenotype of various strains was monitored by using a Bioscreen C reader (Oy Growth Curves Ab, Ltd., Helsinki, Finland) at 37°C with 50 μl of mineral oil overlay in each well, with the optical density at 600 nm (OD600) of each sample recorded every 30 min.
Construction of lac and gal mutants.
The entire coding sequences of lacA, lacB, or both genes were replaced by a nonpolar erythromycin resistance (em) marker (51) via allelic exchange, as described previously (31, 52). The same em marker was used to construct deletion mutants of lacC, lacF, lacE, and lacG. To accommodate multiple antibiotic resistance markers, additional lacF and lacG mutants were engineered by using a nonpolar kanamycin resistance (km) cassette. Similarly, a lacD mutant was constructed by using a nonpolar spectinomycin resistance marker (51) (Fig. 1A). Also engineered was a plasmid, designated pLacA-cat, with the lacA promoter fused to a promoterless chloramphenicol acetyltransferase (cat) gene from the staphylococcal plasmid pC194 in pJL84 (53), which allows single-copy integration of the reporter fusion at a remote site (phnA-mtlA) of the genome. Plasmid pLacA-cat was used to transform all lac mutants to help assess the impact of these mutations on transcriptional regulation of the lac operon.
We also engineered three additional site-directed point mutants into the chromosome of S. mutans (54). Briefly, a mutator DNA containing an otherwise wild-type sequence of lacA with a point mutation that substituted a stop codon (TAG) for the translational start codon was used to transform strain UA159, along with plasmid pLacA-cat. The km marker on the plasmid allowed for selection of Km-resistant colonies that were subsequently screened for the presence of the desired lacA(stop) point mutation using an allele-specific MAMA (mismatch amplification mutational analysis) PCR (15). A lacC(M7stop) mutant strain that has its Met7 (instead of Met1, to avoid potential alternative start of translation) of lacC replaced with a stop codon, and a lacG(E375Q) mutant were created in the same fashion. The desired mutants were confirmed by PCR and sequencing.
CAT, PTS, and real-time quantitative RT-PCR assays.
CAT (53) and PEP-dependent sugar-phosphotransferase assays (32) were performed as previously described. RNA extraction and real-time reverse transcription-PCR (RT-PCR) were carried out as described elsewhere (6). The following primers were used in PCRs: lacC, 5′-GCT GGA ATT ACA TCG GCT CTT GC-3′ (forward) and 5′-CCT CCG CTA CCT CAA TTT GTT GG-3′ (reverse); lacD, 5′-TCT TCT CAG ACG AGC GTT TTG G-3′ (forward) and 5′-GCG GTG TTG CTT GAT CTT GTT G-3′ (reverse); lacF, 5′-GAA GCG ACT CTT TTG GGG TTT G-3′ (forward) and 5′-CTT CTG CCC TAT CGT ACT CAC C-3′ (reverse); lacE, 5′-ATG TGG CTC AGT CAA TTG GAA CC-3′ (forward) and 5′-ACA AAC CAG AAC AAG GCG TAA GC-3′ (reverse); lacG, 5′-ATT GGA TGC GTG CTT TTG ATG G-3′ (forward) and 5′-CGA CCG ACA CCC TTA ATC TGG-3′ (reverse); and galK, 5′-CTT GAC ACG CTG GCT CAT ACC-3′ (forward) and 5′-AGG CTG CAA CCT TAT CTT TGG C-3′ (reverse).
RESULTS
Effects of mutations in lac genes on lacA expression and utilization of lactose and galactose.
A series of nonpolar mutations were constructed in all eight genes in the lac operon (Fig. 1A). Subsequently, a PlacA-cat reporter fusion was integrated into the chromosome of strain UA159 and into the various mutant strains for monitoring expression of the lac operon. Strains were cultivated in TV medium with 0.5% glucose or fructose, in 0.5% lactose or galactose when possible, or in combinations of these carbohydrates (Table 1). Strains able to grow on the provided carbohydrates were harvested at mid-exponential phase (OD600 = 0.3 to 0.4) and subjected to CAT assays. Loss of lacR, the putative negative regulator of the operon, led to constitutive expression from the lacA promoter and relief of CCR under all tested conditions. Little reduction in the rate of growth on galactose was noted in the LacR-deficient strain, although the final yield was slightly lower than that of the wild-type strain. Significantly higher levels of lacA expression were also noted in the lacR mutant when the medium contained galactose alone or a combination of glucose and galactose.
TABLE 1.
Expression of lacA promoter:cat fusion as represented by the CAT specific activities in the wild-type strain UA159 and various mutants
| Strain | Avg CAT sp act (nmol of chloramphenicol acetylated mg of protein−1 min−1) ± SDa on various growth carbohydrates |
|||||||
|---|---|---|---|---|---|---|---|---|
| Glc | Glc + Gal | Glc + Lac | Fru | Fru + Gal | Fru + Lac | Gal | Lac | |
| UA159 | 84 ± 9 | 140 ± 7 | 593 ± 57 | 99 ± 4 | 873 ± 61 | 1,253 ± 37 | 2,729 ± 531 | 2,968 ± 180 |
| manL mutant | 53 ± 7 | 106 ± 1 | 1,189 ± 51 | 44 ± 1 | 70 ± 1 | 1,396 ± 9 | 932 ± 64 | 1,767 ± 297 |
| fruI fruCD levD mutant | 54 ± 4 | 93 ± 9 | 1,142 ± 24 | 52 ± 3 | 507 ± 18 | 676 ± 20 | 1,498 ± 247 | 1,894 ± 145 |
| lacR mutant | 6,532 ± 367 | 11,742 ± 267 | 7,194 ± 217 | 6,274 ± 250 | 6,706 ± 881 | 6,705 ± 226 | 10,915 ± 818 | 6,652 ± 482 |
| lacA mutant | 584 ± 14 | 1,187 ± 80 | 603 ± 5 | 178 ± 29 | 8,013 ± 371 | 1,567 ± 50 | NG | NG |
| lacB mutant | 404 ± 25 | 371 ± 26 | 234 ± 11 | 146 ± 20 | 9,597 ± 204 | 575 ± 64 | NG | NG |
| lacC mutant | 184 ± 14 | 98 ± 11 | 770 ± 78 | 136 ± 21 | 799 ± 71 | 486 ± 52 | 2,287 ± 252 | 2,102 ± 76 |
| lacD mutant | 9 ± 1 | 10 ± 1 | 11 ± 0 | 10 ± 0 | 372 ± 56 | 9 ± 1 | LG | LG |
| lacF mutant | 33 ± 7 | 33 ± 4 | 34 ± 5 | 28 ± 6 | 2,002 ± 384 | 54 ± 8 | 4,547 ± 389 | NG |
| lacG mutant | 19 ± 2 | 14 ± 1 | 19 ± 2 | 11 ± 0 | 596 ± 75 | 10 ± 0 | NG | NG |
The data are presented as the average results from at least three independent cultures. Cells were cultured in 0.5% concentrations of the indicated sugars, and assays were performed as detailed in Materials and Methods. Glc, glucose; Gal, galactose; Lac, lactose; Fru, fructose. NG, no growth; LG, little growth.
Introduction of lacA, lacB, and lacAB mutations, created by replacing the genes for the Gal-6-P isomerase subunits with a nonpolar Em resistance marker, enhanced expression from the lacA promoter, particularly in the absence of lactose (Table 1). These mutants were unable to grow on galactose (Table 2), probably because a functional Gal-6-P isomerase is required for converting Gal-6-P to tagatose-6-P. Interestingly, the lacA, lacB, and lacAB mutants also could not grow in TV containing 0.5% lactose, even in the presence of a functional LacG enzyme, which would release both Gal-6-P and free glucose within the cell. To exclude the possibility that polarity of the mutations in lacAB decreased expression of downstream genes, we also constructed a point mutation that replaced the start codon of lacA with a stop codon (TAG), without introducing any additional modifications in the lac operon or elsewhere in the genome (see Materials and Methods for detail). The resultant strain, LacAstop, showed a nearly identical pattern of PlacA-cat expression (data not shown) and loss of growth on either galactose or lactose. Therefore, for simplicity, only data from the Em-resistant mutants are reported in the remainder of the results.
TABLE 2.
Results of overnight growth tests performed in TV containing various carbohydrates, each added at a concentration of 0.5%
| Strain | Overnight growth test result in TV supplemented with: |
||||
|---|---|---|---|---|---|
| Gal | Lac | Sorbitol | Sorbitol + Gal | Sorbitol + Lac | |
| UA159 | + | + | + | + | + |
| lacA mutant | - | - | + | - | - |
| lacB mutant | - | - | + | - | - |
| lacAB mutant | - | - | + | - | - |
| lacC mutant | + | + | + | + | + |
| lacD mutant | +/- | +/- | + | +/- | + |
| lacF mutant | + | - | + | + | + |
| lacE mutant | + | - | + | + | + |
| lacG mutant | - | - | + | - | + |
| lacA lacF mutant | - | - | + | - | + |
| lacD manL mutant | +/- | + | + | + | + |
| lacAB manL mutant | + | - | + | + | + |
| lacG manL mutant | - | - | + | + | + |
| lacG lacR mutant | +/- | - | + | +/- | + |
| lacG galR mutant | - | - | + | - | + |
| lacG galR lacR mutant | +/- | - | + | +/- | + |
Mutation of the gene for tagatose-6-P kinase (lacC), either through replacement with an em marker or by substituting a stop codon for the Met7 codon, had little impact on lacA promoter activity (Table 1), and both mutants grew as well as the wild-type strain on galactose or lactose. In contrast, inactivation of lacD, encoding the tagatose-1,6-bP aldolase, led to the most severe reduction in PlacA-cat expression of all strains tested. Consistent with the gene expression data, the lacD mutant displayed only marginal growth on either galactose or lactose (see Fig. S1 in the supplemental material). The growth defect was rescued by a lacD-containing fragment introduced into the chromosome via the integration vector pBGE, which allowed for expression of lacD to be driven from the gtfA promoter (52; data not shown). Mutation of lacF and lacE, encoding the EIIALac and EIIBCLac of the lactose PTS permease, respectively, led to a complete loss of growth on lactose (Table 2) and no induction of the lacA promoter by lactose (Table 1, data for lacE not shown). However, neither mutation affected growth on galactose or the ability of galactose to induce expression of the lacA promoter (Tables 1 and 2).
Disruption of lacG (phospho-β-galactosidase) led to markedly reduced induction of the lacA promoter by lactose. The lacG mutant was also unable to grow on either lactose or galactose. However, induction of the operon by galactose was evident when cells were cultivated in a combination of galactose and fructose (Table 1). Collectively, the data provide evidence that Gal-6-P, the cleavage product of Lac-6-P generated by LacG, is required for induction of the lac operon. This idea is consistent with the observation that lacAB mutations resulted in higher lac expression than in the wild-type strain (Table 1), since Gal-6-P should accumulate in these mutants.
Transport of galactose via the PTS.
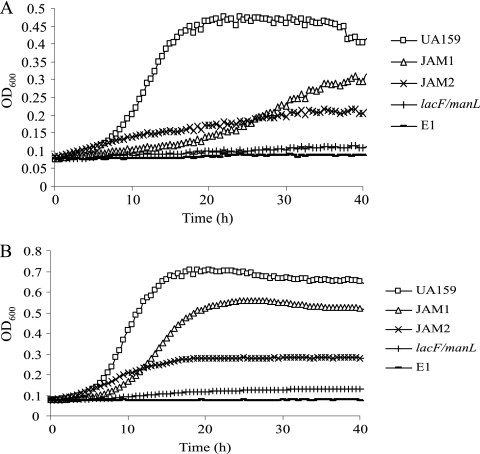
As indicated by the phenotype of the lacF and lacE mutants, lactose is transported via EIILac. It was previously suggested that EIILac could also transport galactose, albeit at a very slow rate (3). Since disruption of lacF or lacE had little impact on galactose utilization, additional transporters that can internalize galactose are apparently present in S. mutans. During our characterization of a manL mutant (JAM1) derived from strain UA159 (2), a significant reduction in the growth rate of the mutant strain on galactose was evident (J. Abranches and R. A. Burne, unpublished data). To test the possibility that both EIIMan and EIILac could transport galactose, a lacF manL double mutant was constructed. This strain showed consistently poor growth in TV containing 0.5 or 2% galactose, with the OD600 in 2% galactose only increasing from 0.07 to 0.13 after >45 h of incubation (Fig. 2). Similar results were obtained when a lacE manL double mutant was tested (data not shown). Notably, a mutation in manL alone (strain JAM1) caused a significant reduction in growth on 0.5% galactose, but this effect was partially reversed by increasing the galactose concentration to 2% (Fig. 2). A null mutant (ptsI) lacking enzyme I (EI) of the PTS (16), which is deficient in all PTS activity, also failed to grow on galactose at either concentration (Fig. 2).
FIG. 2.
Growth curves generated using a Bioscreen C while monitoring the growth of strains UA159, JAM1 (manL), JAM2 (galK), lacF/manL double mutant, and the deletion mutant of ptsI (strain EI). Cells were incubated in TV medium supplemented with 0.5% (A) or 2% (B) of galactose.
To further probe the role of EIIMan and EIILac in galactose utilization, galactose PTS activity in the lacE, lacF, and manL mutants was compared to the wild-type strain. Using permeabilized cells of S. mutans UA159 that had been grown in TV-galactose, a significant increase in the rate of PEP-dependent PTS activity was noted as the concentration of galactose used in the assay was increased from 10 to 50 mM (data not shown). Since most PTS permeases have a Km in the low μM range for their cognate substrates (39), the affinity of the S. mutans PTS for galactose appears substantially lower than for other PTS sugars, e.g., glucose or fructose (39). To ensure that the substrate was present in excess for PTS assays, 50 mM galactose was utilized to compare the galactose-PTS activities in the wild-type and mutant strains. As presented in Table 3, significantly lower galactose-PTS activities were seen in the manL and lacE mutants growing on galactose versus the wild-type strain. When the manL lacE double mutant was grown in BHI broth supplemented with 0.5% of galactose, since the mutant could not grow on galactose alone, galactose PTS activities were at the lower limit of detection for this assay. These results strongly support that EIIMan and EIILac are the primary routes for PTS-dependent transport of galactose by S. mutans.
TABLE 3.
PTS activity in wild-type and selected mutant strains
| Strain | Avg galactose-PTS activity (nmol of NADH oxidized mg of protein−1 min−1) ± SDa |
|---|---|
| UA159 | 184.3 ± 11 |
| manL mutant | 134.0 ± 16 |
| lacE mutant | 84.7 ± 11 |
| manL lacE mutant | 6.4 ± 3 |
PEP-dependent galactose-PTS activity was measured in cells that were growing exponentially in TV with 0.5% galactose (UA159 and manL and lacE mutants) or BHI supplemented with 0.5% galactose (manL lacE double mutant). Values are presented as the average results from three independent cultures.
Apparent toxicity of intermediates of galactose and lactose metabolism.
As noted above, disruption of the lacA, -B, -D, or -G genes severely impaired the ability of S. mutans to grow on lactose or galactose. Also, our laboratory previously demonstrated that inactivation of the gene for galactokinase (galK) of the Leloir pathway in S. mutans resulted in a near complete loss of growth on galactose (3). Since galactose should be catabolizable through both the Leloir and the tagatose pathways (Fig. 1) and because free glucose released by LacG from internalized lactose should support the growth of the lacA, lacB, and lacD mutants, these findings point to a potential growth inhibitory role of intermediates of galactose metabolism. Thus, we tested the hypothesis that some of these mutants lost their ability to grow on galactose or lactose because accumulation of pathway intermediates was inhibitory to growth.
Various mutant strains were grown to mid-exponential phase in TV medium containing 0.5% sorbitol, the cultures were then diluted 1:200 into TV supplemented with 0.5% sorbitol, with or without the addition of galactose or lactose (0.5%), and incubated for 24 h (Table 2). Sorbitol was selected for this test because it is not effective at eliciting CCR (45), and it is transported by a sorbitol-specific PTS permease (10). Whereas all strains could grow with sorbitol as the sole carbohydrate, the lacA, lacB, and lacAB mutants were unable to grow on sorbitol when galactose or lactose was included in the medium. Complete inhibition of growth occurred when as little as 1 mM galactose was used, and concentration-dependent inhibition was observed with galactose at levels as low as 30 μM in the lacA mutant (data not shown). Notably, in a MIC test, an ∼10-fold lower concentration of lactose was required to inhibit the growth of the lacA mutant compared to galactose (data not shown), probably because lactose is more efficiently internalized by S. mutans than galactose. Consistent with this idea, introduction of a lacF mutation, which blocks lactose uptake, into the lacA mutant yielded a strain with very low sensitivity to lactose (Table 2). Collectively, these results indicate that the accumulation of Gal-6-P in mutants lacking Gal-6-P isomerase leads to growth inhibition by lactose or galactose.
Growth of the lacD mutant, lacking the tagatose-1,6-bP aldolase, on sorbitol was also sensitive to galactose, though less sensitive than strains deficient in LacA or LacB (Table 2 and see Fig. S1 in the supplemental material). Poor, albeit measurable, growth by the lacD mutant was obtained in TV containing 0.5% of sorbitol and galactose, but little growth inhibition was seen in the presence of lactose, compared to the wild-type strain. Thus, it seems that tagatose-1,6-bP, which should accumulate in the absence of LacD, may also be inhibitory to growth of S. mutans, albeit less so than Gal-6-P. Another possibility is that tagatose-1,6-bP is not inhibitory to growth, but loss of the aldolase causes accumulation of Gal-6-P as the system backs up.
To add further support to the idea that Gal-6-P is required for the inhibitory effects of galactose or lactose, we cultured the cells on the α-galactoside melibiose. Melibiose is internalized in an unphosphorylated form by the multiple sugar metabolism (msm) pathway (41), which is a typical ABC transporter that transports a variety of galactosides, and is cleaved by a cytoplasmic α-galactosidase to release unphosphorylated glucose and galactose (5). The cells can then use the galactose via the Leloir pathway and the glucose could enter the Embden-Meyerhoff-Parnas pathway after phosphorylation by glucokinase. Both the lacAB and lacG mutants were able to grow on melibiose alone, likely because no Gal-6-P is generated. Growth inhibition of the lacA, -B, or -G mutants was also observed in medium containing both melibiose and galactose (data not shown), further supporting a growth inhibitory role of Gal-6-P. Notably, when a galK nonpolar mutant (JAM2) (3) was tested for its ability to grow in the presence of sorbitol and galactose or lactose, no growth inhibition was seen by galactose or lactose. However, the JAM2 strain could grow, albeit poorly, on galactose (Fig. 2), proving that the tagatose-6-P pathway is sufficient to allow for some growth on galactose.
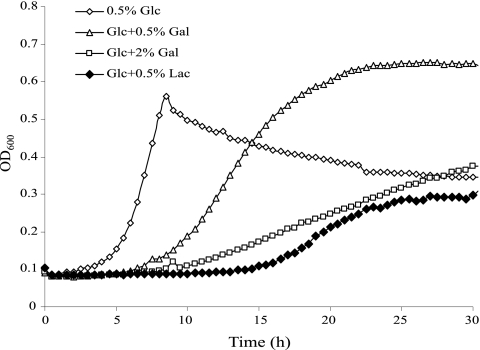
The observation of toxicity of galactose or lactose in the lacA and lacB mutants prompted us to investigate the possibility that galactose might inhibit the growth of the wild-type strain when other rapidly metabolized sugars are present. When glucose was used instead of sorbitol in the endpoint growth tests described above, the addition of galactose or lactose failed to significantly inhibit growth of the lacA or lacB mutant (data not shown). To better detect inhibitory effects of galactose or lactose, growth of strain UA159 and the lacA mutant in the presence of 0.5% glucose and 0.5 or 2% galactose or lactose was monitored in a Bioscreen C. The results (Fig. 3) revealed that the addition of 0.5% galactose to TV-glucose medium was able to significantly reduce the growth rate of the lacA mutant, and growth inhibition was more severe when 2% galactose was used. Remarkably, 0.5% lactose was able to completely inhibit growth on glucose of the lacA mutant during the first 12 h of incubation, which is consistent with the results from MIC testing that indicated a lower tolerance of the lacA mutant for lactose than galactose. In contrast, the wild-type strain UA159 showed no change in the growth rate or final yield of cells when galactose or lactose (up to 0.5%) was added to TV-glucose medium (data not shown). The reduced sensitivity of the lacA mutant toward lactose/galactose when growing on glucose, relative to the same strain growing on sorbitol, could be due to the fact that S. mutans catabolizes glucose at a much greater rate than it does sorbitol, possibly providing cells with more energy to overcome the inhibitory effect of Gal-6-P. It is also noteworthy that the repressive effects of glucose on lac gene expression (see below) would affect internalization of galactose by EIILac. Also, glucose is transported by EIIMan at much higher affinity than galactose, so glucose should prevent or diminish internalization of galactose, whereas sorbitol should not have these effects. Lastly, the apparent decrease in cell density starting at the ninth hour of the growth on glucose, is likely due to post-exponential-phase autolysis that is enhanced by low-pH conditions resulting from the rapid catabolism of glucose (S. J. Ahn and R. A. Burne, unpublished data).
FIG. 3.
Growth curves of the lacA:em mutant generated using TV medium containing 0.5% of glucose (Glc), or the combination of 0.5% glucose and 0.5% galactose (Gal), 2% galactose, or 0.5% lactose (Lac).
Examination of the basis for the effects of the lacG mutation.
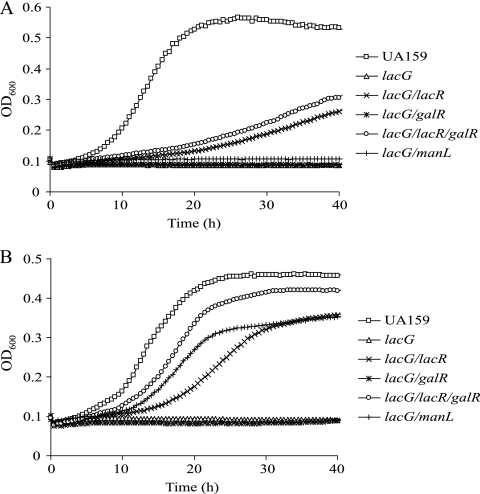
Whereas the failure of the lacG mutant to grow on lactose is predictable, it is not immediately clear why loss of the phospho-β-galactosidase should impact growth on galactose. Moreover, the lacG mutant lost the ability to grow on sorbitol if 0.5% galactose was present in the medium but was able to grow on sorbitol and lactose (Table 2). The simplest explanation for these results is that the expression of the lac operon is substantially reduced in the lacG mutant (Table 1), likely resulting in decreased lactose transport via LacEF. Likewise, the inhibitory effects of galactose on the lacG mutant may be due to lower expression of the lac operon (Table 1), which leads to accumulation of Gal-6-P that is generated by transport of galactose through EIIMan. Consistent with this idea, a lacG/manL double mutant grew as well as the parental strain in the combination of sorbitol and galactose (Table 2 and Fig. 4). Still, the lacG/manL double mutant was incapable of growing on galactose alone, likely as a result of poor expression of the lac operon.
FIG. 4.
Growth curves of UA159, lacG and various lacG derived mutants on (A) 0.5% galactose or (B) the combination of 0.5% sorbitol and 0.5% of galactose.
To probe the basis of the phenotype of the LacG-deficient strain in more detail, a mutant strain designated S. mutans LacGE375Q, containing a point mutation that replaced glutamic acid residue 375 with glutamine to create a catalytically inactive enzyme (50), was engineered by using a PCR-based approach. Consistent with the phenotype of a similar lacGE375Q mutant in Staphylococcus aureus (50), strain LacGE375Q showed no growth on lactose. However, this mutant also failed to grow on galactose, with or without addition of sorbitol, and the expression levels from PlacA-cat in strain LacGE375Q growing on various sugars closely resembled that of the lacG deletion mutant (data not shown). To further confirm that the phenotypes associated with the lacG mutant were attributable only to loss of LacG activity, a wild-type copy of the S. mutans lacG gene was integrated into the gtfA locus in the lacG (em) mutant using the integration vector pBGE (52). In this case, the gtfA promoter was used to drive lacG transcription. Complementation with the wild-type lacG restored growth in both TV-lactose and TV-galactose media. Moreover, CAT activities measured in the complemented strain carrying the PlacA-cat fusion were similar to those in the wild-type background (data not shown). Collectively, these data provide evidence that a catalytically active phospho-β-galactosidase enzyme is required for optimal expression of the lac operon.
Since reduced lac gene expression appears to be the main defect in the lacG mutants, we attempted to rescue growth of these mutants on galactose by enhancing expression of the lac and galKTE operons. To accomplish this, the genes encoding the negative regulators of these operons, lacR and galR, were inactivated as previously described (3, 8, 9), individually or together, in a lacG mutant. The resultant strains were tested for their ability to grow on galactose or galactose and sorbitol. Partial rescue of growth on galactose was noted in the lacG lacR double mutant and lacG lacR galR triple mutant (Fig. 4 and Table 2). However, a lacG galR double mutant remained incapable of growing in TV-galactose or in TV supplemented with both galactose and sorbitol. When quantitative real-time RT-PCR was applied to the lacG lacR galR triple-mutant strain grown in glucose, the levels of lacC mRNA were comparable to that in the wild-type strain grown in galactose (Table 4) . However, in the same cells, the levels of galK transcript were ∼10-fold lower than in S. mutans UA159 grown in galactose and only modestly higher (4-fold) than those in UA159 grown in glucose (Table 4). Similar levels of galK mRNA were found in the galR mutant growing on glucose (Table 4). Although it is not completely clear why the galKTE operon is not fully activated in the absence of GalR (7, 8), these results provide strong evidence that both the tagatose and the Leloir pathways need to be as least partially induced for S. mutans to grow on galactose or lactose (3).
TABLE 4.
Quantitative real-time RT-PCR in wild-type and various mutant strainsa
| Gene | Strain | Avg copies (μg of total RNA−1)b (SD) in TV supplemented with: |
||
|---|---|---|---|---|
| Glucose | Galactose | Lactose | ||
| lacC | UA159 | 2.42 × 105 (2.2 × 104) | 3.15 × 108 (5.0 × 107) | 3.24 × 108 (9.4 × 107) |
| lacG lacR galR mutant | 2.08 × 108 (6.4 × 107) | ND | ND | |
| lacD | UA159 | 1.95 × 105 (4.3 × 104) | ND | ND |
| lacF | UA159 | 2.10 × 105 (1.5 × 104) | ND | ND |
| lacE | UA159 | 8.16 × 105 (6.4 × 105) | ND | ND |
| lacG | UA159 | 6.25 × 106 (9.1 × 105) | 2.09 × 109 (2.5 × 108) | 2.03 × 109 (4.1 × 108) |
| galK | UA159 | 1.14 × 106 (2.7 × 105) | 6.92 × 107 (2.3 × 107) | 6.96 × 106 (2.6 × 106) |
| lacG lacR galR mutant | 4.96 × 106 (6.8 × 105) | ND | ND | |
| galR mutant | 6.99 × 106 (1.0 × 106) | 9.30 × 106 (7.0 × 105) | ND | |
RNA was isolated from cells growing exponentially in TV supplemented with 0.5% glucose, galactose, or lactose.
The data are presented as averages of results from three independent cultures. The standard deviations are indicated in parentheses. ND, not determined.
ManL is required for growth inhibition by galactose/lactose in the lacAB, lacD, and lacG mutants.
Our results provide evidence that the majority of galactose that is internalized by S. mutans comes through EIIMan. Thus, deletion of manL in the lacAB, lacD, and lacG mutant strains should alleviate the inhibitory effects of galactose on these strains. Indeed, introduction of a manL mutation into the lacAB double mutant strain restored the ability to grow in TV containing 0.5% of sorbitol and galactose (Table 2). Interestingly, the lacAB manL mutant strain also regained the ability to grow on TV containing 0.5% of sorbitol and lactose, but no growth was detected on TV containing 0.5% lactose alone. To further assess the role of ManL in galactose/lactose-mediated inhibition of growth, a manL deletion was also introduced into the lacD and lacG mutant strains. Compared to the lacD mutant, the lacD manL double mutant strain grew better in medium containing sorbitol and galactose, or lactose alone (see Fig. S1 in the supplemental material and Table 2). Also, the lacG manL double mutant grew better than the lacG mutant on 0.5% of sorbitol and galactose (Table 2 and Fig. 4). Since there does not appear to be any direct role for ManL in the uptake of lactose, ManL may play additional roles beyond CCR (see below) in the regulation of uptake or catabolism of galactose and lactose, e.g., modulation of the activity of the lactose PTS or allosteric control of catabolic enzymes. Notably, the better growth on sorbitol of the strains lacking ManL may be partly attributable to enhanced expression of the genes for sorbitol utilization, since these genes have been shown to be more highly expressed in strain JAM1 (1, 10).
To gain a more comprehensive picture of the regulation of the lac operon by preferred carbohydrates, transcriptional activity of the lacA promoter was measured using the PlacA-cat reporter fusion in both UA159 and various EII mutants. As presented in Table 1, lacA expression was inducible by both galactose and lactose, compared to the levels of expression in glucose or fructose. If cells were growing in combinations of lactose plus fructose or glucose, CCR by the preferred carbohydrates was evident. Introduction of the manL mutation resulted in alleviation of lacA expression in TV containing lactose and glucose. In contrast, the expression level from the lacA promoter in the manL mutant growing in the combination of galactose and glucose was very low. Since ManL contributes to galactose transport, these results probably arise, at least in part, from diminished uptake of galactose that leads to a failure to induce the lac operon. To confirm the results obtained with the gene fusion strategy, we also measured the transcript levels of lacC and lacG in the wild-type strain using a quantitative real-time RT-PCR. The results showed nearly a 3-log increase in expression of both genes in S. mutans UA159 growing in TV-galactose or TV-lactose medium, compared to cells growing in TV-glucose (Table 4). However, lacG mRNA levels were nearly 10-fold higher than those of lacC in the same cells growing in galactose or lactose. In cells growing in TV-glucose, lacG mRNA levels were ∼25-fold higher than that of lacC mRNA. The higher basal level of expression of lacG compared to the genes for the EIILac components on glucose was noted elsewhere (33). Interestingly, when the mRNA levels of lacD, lacF, and lacE in strain UA159 growing in TV-glucose were also measured by using real-time RT-PCR, the results (Table 4) indicated that the expression of lacD and lacF was comparable to that of lacC. At the same time, the level of the lacE transcript was modestly higher (∼4-fold) than that of lacF, but still nearly one log lower than lacG. To probe the possibility that a cryptic promoter may drive the expression of lacG, three DNA fragments carrying various lengths of lacE coding sequence were each fused with the promoterless cat gene. The resultant fusion constructs failed to produce any detectable CAT activities in all conditions tested (data not shown). Collectively, these observations suggest the higher levels of lacG expression compared to other lac genes is probably caused by differential degradation of mRNAs.
The expression level of the lac operon was also tested in mutant strains harboring defects in the primary fructose PTS permeases, fruI, fruCD, and levD (Table 1). Fructose could effectively repress lac gene expression, but CAT assays showed no alleviation of CCR due to fructose in either the levD mutant (data not shown) or the fruI fruCD levD triple mutant background, regardless of whether galactose or lactose was used to induce expression. Interestingly, a similar level of alleviation of CCR as that seen in the manL mutant was noted in the fruI fruCD levD triple mutant growing on a combination of glucose and lactose, likely due to the ability of these porters to also transport glucose, albeit not as effectively as ManL (47, 53). Consistent with the diminished role of CcpA in CCR in S. mutans, a ccpA mutant showed no relief of CCR under any of the conditions tested.
EIIMan and EIILac are both required for galactose-mediated growth inhibition in a galT mutant.
In the Leloir pathway (Fig. 1B), galactose is phosphorylated by GalK at the 1 position and then converted to UDP-galactose by GalT and isomerized to glucose-1-P by GalE. Deletion of galTE in S. mutans has been reported to result in sensitivity to galactose, probably due to accumulation of Gal-1-P (34). When we disrupted galT with a nonpolar Km marker, the mutant failed to grow in TV-sorbitol supplemented with galactose or the α-galactoside melibiose, indicating that galactose that is internalized through PTS or non-PTS routes can enter the Leloir pathway. Mutations in manL, lacE, and msmE of the msm pathway (41) were introduced into the galT mutant strain to test whether any of these transporters were responsible for supplying galactose to the Leloir pathway. The galT msmE, galT lacE, and galT manL double mutants showed no growth on galactose, with or without the addition of sorbitol. However, a manL lacE galT triple mutant, while retaining sensitivity to melibiose, was able to grow in medium containing both sorbitol and galactose, likely arising from an inability of this mutant to internalize galactose. On the other hand, the galT msmE double mutant was no longer sensitive to the presence of melibiose, a finding consistent with the fact that melibiose is internalized only by the Msm transporter (41). In accordance with our earlier conclusions, we believe that EIIMan and EIILac are responsible for the uptake of galactose and can supply both the Leloir and tagatose-6-P pathways, but it is unlikely that another galactose permease exists. Of note, the galT mutant exhibited growth on lactose that was similar to the wild-type strain.
DISCUSSION
An important observation in this study is that lacA, lacB, and lacG mutants of the lac operon were unable to grow on lactose or galactose and presented with a galactose/lactose-sensitive phenotype. Since the enzymes affected in these mutants are involved in the catabolism of both lactose and galactose, we propose that the accumulation of intermediates generated in this process, in particular Gal-6-P, is responsible for the growth-inhibitory effects observed. This model is consistent with the effects of loss of the galactose-6-P isomerase, constituted by LacA and LacB, since this enzyme is required for converting Gal-6-P into tagatose-6-P (Fig. 1B). However, the basis for why LacG-deficient strains display a similar phenotype to the lacAB mutants is more complex.
LacG, the phospho-β-galactosidase, is responsible for hydrolyzing lactose-6-P and releasing glucose and Gal-6-P, the first step in the catabolism of lactose after its internalization by the PTS (Fig. 1B). Although the inability to utilize lactose by the lacG mutant is readily explained by the failure to cleave lactose-6-P, the inability to utilize galactose and the sensitivity of the lacG mutant to this hexose are intriguing, especially in light of the facts that the Leloir pathway is able to catabolize galactose and the tagatose pathway should not require LacG for utilization of galactose. We believe there is a logical explanation for these results. First, for reasons that are not currently understood, disruption of lacG led to loss or diminution of the induction of the lac operon in the presence of galactose. Evidence to date supports that production of Gal-6-P is required for removal of repression mediated by LacR. This theory is consistent with previous studies of similar systems in S. aureus and L. lactis (38, 46) and is supported by the failure of lactose to induce the lac gene in the lacG mutant and the enhanced lac expression noted in the lacA and lacB mutants, which should accumulate Gal-6-P. Second, EIIMan plays a significant role in galactose uptake via the PTS. ManL-dependent uptake of galactose does not appear to be adversely affected in the lacG mutant, so it is likely that Gal-6-P is entering the cell. Third, Gal-6-P has been suggested to cause growth inhibition when accumulated by bacteria (29). Therefore, whereas Gal-6-P is continuously generated by EIIMan-dependent transport of galactose, insufficient expression of the genes for Gal-6-P isomerase of the lac operon in the lacG mutant leads to accumulation of growth-inhibiting intermediates and thus the galactose-sensitive phenotype of the lacG mutant.
The use of a complete deletion of lacG, as well as a point mutant that produces a nonfunctional phospho-β-galactosidase enzyme caused downregulation of the lac operon. Although further research is needed to fully appreciate why a catalytically active LacG enzyme is needed for optimal lac gene expression, one possibility is that LacG has an as-yet-unidentified activity that converts Gal-6-P into a different product, one which serves as the actual inducing molecule that releases LacR from binding to the lac promoter region. In a parallel situation, the transgalactosylation activity of the LacZ β-galactosidase enzyme of E. coli creates the inducing signal for the lac operon by converting lactose into allolactose (galactose-β-1,6-glucose), which is bound by LacI to allow derepression of the operon (13). Alternatively, an interaction between a catalytically active LacG and the LacR protein or components of the lactose PTS permease may be required for derepression of the lac genes. Experiments are under way to begin to explore these and other possibilities for how LacG exerts its influence on gene expression in S. mutans.
Prior to the present study, a number of publications dissecting the function and regulation of the lactose/galactose utilization mechanisms in S. mutans were unable to conclusively identify the primary routes for galactose-transport in this bacterium, and instead, a non-PTS permease was proposed to perform this task (3, 7-9). Surprisingly, when we knocked out both the primary glucose- and lactose-PTS EII enzymes by disrupting manL and lacFE, respectively, the double mutants displayed virtually no growth on galactose. This result is supported by PEP-dependent sugar PTS assays performed on these mutants. Furthermore, a mutant lacking EI of the PTS that is incapable of catalyzing PTS-dependent sugar uptake (16) also failed to grow on galactose (Fig. 2). Although we cannot exclude that other very-low-affinity uptake systems may be able to internalize galactose, the collective evidence presented here identifies EIIMan and EIILac as the biologically relevant uptake pathways for galactose in S. mutans; however, certain galactose-containing sugars, such as melibiose, can enter the cell through the Msm pathway (41). Importantly, however, galactose-transport by the PTS appears to have an affinity at least 100-fold lower than for typical PTS sugars. If commensal organisms in oral biofilms are more effective at obtaining galactose, which can be liberated from salivary glycoproteins and the diet in the oral cavity, galactose, or its derivatives could prove to be useful for modifying the composition and/or pathogenic potential of the oral microbiome.
The demonstration of the critical role of the PTS and lac operons in galactose metabolism detailed here is not entirely consistent with previous reports suggesting a more dominant role for the Leloir pathway of S. mutans in galactose utilization. As shown previously, and as confirmed here, a galK mutant showed slow growth on galactose, and it did not appear that the reduced growth was due to toxicity caused by galactose. The logical explanation for these findings is that galactokinase (GalK) is required for the majority of the galactose catabolic activity and that the tagatose-6-P pathway plays only a minor role when galactose is the sole carbohydrate. However, loss of LacG led to loss of growth on, and induction of, the lac operon by galactose, and enhancing the expression of the Leloir pathway in a lacG mutant via deletion of the galR gene failed to rescue growth on galactose (Tables 2 and 4). Since overexpression of the Leloir pathway is not sufficient to bypass poor expression of the tagatose pathway, there must be a critically important role for the tagatose pathway in galactose utilization by S. mutans. Furthermore, since transport by the PTS appears to be the sole route for galactose to enter both the Leloir and tagatose pathways, the cells must have the ability to dephosphorylate Gal-6-P internalized by the PTS if they are to direct galactose into Leloir pathway (Fig. 1). We propose that the primary route for galactose utilization in the wild-type strain is through the tagatose pathway and that a small amount of galactose is siphoned off to the Leloir pathway to provide intermediates for anabolic processes (8, 24).
Besides catabolizing galactose, another function of the Leloir pathway is to generate UDP-galactose required for bacterial cell-wall biogenesis. UDP-galactose is generated by GalT from Gal-1-P, and UDP-galactose 4-epimerase (GalE) catalyzes the interconversion between UDP-glucose and UDP-galactose, the latter being needed for glycosylation and cell wall synthesis in Escherichia coli (37). In L. lactis, a galE mutant showed a defect in cell separation and formed longer chains than the wild-type strain when cultured on glucose (24). Despite the lack of direct evidence suggesting a similar role for UDP-galactose in cell wall maintenance in S. mutans, galactose has been reported to be a significant component of the cellular structure (49). Notably, a galE mutant created by us via allelic exchange using a km marker showed impaired growth on both galactose and lactose (see Fig. S2 in the supplemental material), whereas the galT mutant grows normally on lactose. The additional defect seen in the galE mutant agrees with the proposed role of UDP-galactose in cell envelope maintenance and suggests a critical role for the Leloir pathway in the ability of the bacterium to metabolize lactose, as well as galactose. Consistent with this notion, gal operon transcript levels were elevated ∼6-fold in cells growing in the presence of lactose (Table 4). We postulate that in the presence of galactose, the Leloir pathway is required for production of UDP-galactose, and this function is not fulfilled by components encoded in the lac operon. If this is the case, then the growth defect on galactose displayed by the galK mutant may have been due to the inability of the mutant to provide sufficient UDP-galactose for structural integrity of the cell.
It was previously reported that expression of the lactose-utilizing enzymes of S. mutans was repressible by the presence of glucose, but not fructose, although both sugars are preferentially internalized over lactose by S. mutans (33). Recent developments in understanding CCR in S. mutans have indicated essential roles for a number of glucose-, fructose-, or mannose-specific PTS permeases in the transcriptional regulation of carbohydrate catabolic genes, including the fructan hydrolase (fruAB) operon and fructose/mannose permease (levDEFGX) genes (51). In an ongoing project, we have learned that both the Ser46-phosphorylated form of HPr and the glucose porter ManL are intimately involved in CcpA-independent CCR of the fruA and lev operons. An engineered mutant bearing a point mutation in the HPr kinase/phosphatase (hprKV265F) (35) produced elevated levels of HPr(Ser-P) and showed reduced growth on lactose (see Fig. S3 in the supplemental material). However, in the same strain, the transcription level of the lac operon, as measured by monitoring lacG, was not significantly altered, suggesting that HPr-mediated effects on the lac operon probably occur at the level of lactose-PTS activity (54), i.e., inducer exclusion. In this case, we propose that there is sufficient lactose PTS activity to allow induction of the operon, but not to support growth of the cells.
In the present study, the results obtained from promoter-reporter gene fusion confirmed that the primary glucose permease EIIMan (ManL) is involved in CCR of the lac operon in the presence of glucose and lactose (Table 1). On the other hand, disruption of three fructose permeases had little effect on CCR of the lac genes when fructose was present to induce CCR of the lac operon. Interestingly, compared to the wild-type strain, there appears to be alleviated lac expression in the fruI fruCD levD triple fructose-PTS mutant, similar to the level of alleviation observed in the manL mutant, when cells were growing on glucose and lactose. This phenotype could be attributed to the fact that all three PTS permeases appear to contribute to transport of glucose (47, 53). In addition, we did observe significant repression by fructose on the induction of lacA expression by galactose or lactose (Table 1), although the effects were not as pronounced as when glucose was the preferred carbohydrate. As mentioned above, when contrasted against the fruA/levD system (51, 53), HPr-Ser-P-mediated effects on the transport of lactose appear to be the dominant mechanism by which glucose or fructose effect CCR of the lac operon. Also of note, when it was compared to other CCR-sensitive catabolic pathways in S. mutans, e.g., the fruA and levD operons, and the cellobiose-utilizing pathway (52), the lac operon appears to be less susceptible to CCR, suggesting that lactose is relatively preferred by the bacterium over certain other secondary carbohydrate sources.
Unlike glucose, sucrose, and fructose, which are abundant in the diet, free galactose probably exists in low quantities in the oral cavity, arising from cleavage of galactose moieties from host-derived glycoproteins or dietary substrates. In contrast to S. mutans, a number of other streptococci, including Streptococcus pneumoniae, group A streptococcus, group B streptococcus, and Streptococcus gordonii, appear to possess PTS permeases dedicated to galactose uptake (SP_0645 to SP_0647, genome sequence of Streptococcus pneumoniae TIGR4; SPy_1709 to SPy_1711 of Streptococcus pyogenes M1 GAS; SAG1933 to SAG1935 of Streptococcus agalactiae 2603V/R; and SGO_1520 to SGO_1522 of S. gordonii Challis substrain CH1 [http://www.oralgen.lanl.gov]). In many cases, these organisms also encode at least one extracellular β-galactosidase, presumably involved in the release of galactose from host glycoproteins (11, 28, 30, 44). Our preliminary studies have indicated that the abundant oral commensal S. gordonii can grow well on galactose at nearly 10-fold lower concentrations than S. mutans (data not shown). Thus, either S. mutans failed to acquire, or has lost, the genetic material for high-affinity galactose transport during evolution in oral cavity. Interestingly, it may be possible to exploit these apparent differences in transport and regulation of galactose in the pathogen S. mutans and in oral commensals to modify the oral microbiome in a way that is beneficial to the host and prevents dental diseases.
Supplementary Material
Acknowledgments
This study was supported by grant DE12236 from the National Institute of Dental and Craniofacial Research.
We thank Bryan Korithoski for helpful discussions.
Footnotes
Published ahead of print on 26 February 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Abranches, J., M. M. Candella, Z. T. Wen, H. V. Baker, and R. A. Burne. 2006. Different roles of EIIABMan and EIIGlc in regulation of energy metabolism, biofilm development, and competence in Streptococcus mutans. J. Bacteriol. 188:3748-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abranches, J., Y. Y. Chen, and R. A. Burne. 2003. Characterization of Streptococcus mutans strains deficient in EIIABMan of the sugar phosphotransferase system. Appl. Environ. Microbiol. 69:4760-4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abranches, J., Y. Y. Chen, and R. A. Burne. 2004. Galactose metabolism by Streptococcus mutans. Appl. Environ. Microbiol. 70:6047-6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abranches, J., M. M. Nascimento, L. Zeng, C. M. Browngardt, Z. T. Wen, M. F. Rivera, and R. A. Burne. 2008. CcpA regulates central metabolism and virulence gene expression in Streptococcus mutans. J. Bacteriol. 190:2340-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aduse-Opoku, J., L. Tao, J. J. Ferretti, and R. R. Russell. 1991. Biochemical and genetic analysis of Streptococcus mutans α-galactosidase. J. Gen. Microbiol. 137:757-764. [DOI] [PubMed] [Google Scholar]
- 6.Ahn, S. J., J. A. Lemos, and R. A. Burne. 2005. Role of HtrA in growth and competence of Streptococcus mutans UA159. J. Bacteriol. 187:3028-3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ajdic, D., and J. J. Ferretti. 1997. Regulation of the galactose operon of Streptococcus mutans. Adv. Exp. Med. Biol. 418:1015-1018. [DOI] [PubMed] [Google Scholar]
- 8.Ajdic, D., and J. J. Ferretti. 1998. Transcriptional regulation of the Streptococcus mutans gal operon by the GalR repressor. J. Bacteriol. 180:5727-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ajdic, D., I. C. Sutcliffe, R. R. Russell, and J. J. Ferretti. 1996. Organization and nucleotide sequence of the Streptococcus mutans galactose operon. Gene 180:137-144. [DOI] [PubMed] [Google Scholar]
- 10.Boyd, D. A., T. Thevenot, M. Gumbmann, A. L. Honeyman, and I. R. Hamilton. 2000. Identification of the operon for the sorbitol (glucitol) phosphoenolpyruvate:sugar phosphotransferase system in Streptococcus mutans. Infect. Immun. 68:925-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burnaugh, A. M., L. J. Frantz, and S. J. King. 2008. Growth of Streptococcus pneumoniae on human glycoconjugates is dependent upon the sequential activity of bacterial exoglycosidases. J. Bacteriol. 190:221-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burne, R. A., Z. T. Wen, Y. Y. Chen, and J. E. Penders. 1999. Regulation of expression of the fructan hydrolase gene of Streptococcus mutans GS-5 by induction and carbon catabolite repression. J. Bacteriol. 181:2863-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burstein, C., M. Cohn, A. Kepes, and J. Monod. 1965. Role of lactose and its metabolic products in the induction of the lactose operon in Escherichia coli. Biochim. Biophys. Acta 95:634-639. [PubMed] [Google Scholar]
- 14.Calmes, R. 1978. Involvement of phosphoenolpyruvate in the catabolism of caries-conducive disaccharides by Streptococcus mutans: lactose transport. Infect. Immun. 19:934-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cha, R. S., H. Zarbl, P. Keohavong, and W. G. Thilly. 1992. Mismatch amplification mutation assay (MAMA): application to the c-H-ras gene. PCR Methods Appl. 2:14-20. [DOI] [PubMed] [Google Scholar]
- 16.Cvitkovitch, D. G., D. A. Boyd, T. Thevenot, and I. R. Hamilton. 1995. Glucose transport by a mutant of Streptococcus mutans unable to accumulate sugars via the phosphoenolpyruvate phosphotransferase system. J. Bacteriol. 177:2251-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deutscher, J. 2008. The mechanisms of carbon catabolite repression in bacteria. Curr. Opin. Microbiol. 11:87-93. [DOI] [PubMed] [Google Scholar]
- 18.de Vos, W. M., and E. E. Vaughan. 1994. Genetics of lactose utilization in lactic acid bacteria. FEMS Microbiol. Rev. 15:217-237. [DOI] [PubMed] [Google Scholar]
- 19.Frey, P. A. 1996. The Leloir pathway: a mechanistic imperative for three enzymes to change the stereochemical configuration of a single carbon in galactose. FASEB J. 10:461-470. [PubMed] [Google Scholar]
- 20.Fridovich-Keil, J. L. 2006. Galactosemia: the good, the bad, and the unknown. J. Cell Physiol. 209:701-705. [DOI] [PubMed] [Google Scholar]
- 21.Frostell, G., P. H. Keyes, and R. H. Larson. 1967. Effect of various sugars and sugar substitutes on dental caries in hamsters and rats. J. Nutr. 93:65-76. [DOI] [PubMed] [Google Scholar]
- 22.Gorke, B., and J. Stulke. 2008. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat. Rev. Microbiol. 6:613-624. [DOI] [PubMed] [Google Scholar]
- 23.Griswold, A. R., Y. Y. Chen, and R. A. Burne. 2004. Analysis of an agmatine deiminase gene cluster in Streptococcus mutans UA159. J. Bacteriol. 186:1902-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grossiord, B. P., E. J. Luesink, E. E. Vaughan, A. Arnaud, and W. M. de Vos. 2003. Characterization, expression, and mutation of the Lactococcus lactis galPMKTE genes, involved in galactose utilization via the Leloir pathway. J. Bacteriol. 185:870-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamilton, I. R., and H. Lebtag. 1979. Lactose metabolism by Streptococcus mutans: evidence for induction of the tagatose 6-phosphate pathway. J. Bacteriol. 140:1102-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton, I. R., and G. C. Lo. 1978. Co-induction of β-galactosidase and the lactose-P-enolpyruvate phosphotransferase system in Streptococcus salivarius and Streptococcus mutans. J. Bacteriol. 136:900-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jagusztyn-Krynicka, E. K., J. B. Hansen, V. L. Crow, T. D. Thomas, A. L. Honeyman, and R. Curtiss III. 1992. Streptococcus mutans serotype c tagatose 6-phosphate pathway gene cluster. J. Bacteriol. 174:6152-6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeong, J. K., O. Kwon, Y. M. Lee, D. B. Oh, J. M. Lee, S. Kim, E. H. Kim, T. N. Le, D. K. Rhee, and H. A. Kang. 2009. Characterization of the Streptococcus pneumoniae BgaC protein as a novel surface β-galactosidase with specific hydrolysis activity for the Galβ1-3GlcNAc moiety of oligosaccharides. J. Bacteriol. 191:3011-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kadner, R. J., G. P. Murphy, and C. M. Stephens. 1992. Two mechanisms for growth inhibition by elevated transport of sugar phosphates in Escherichia coli. J. Gen. Microbiol. 138:2007-2014. [DOI] [PubMed] [Google Scholar]
- 30.King, S. J., K. R. Hippe, and J. N. Weiser. 2006. Deglycosylation of human glycoconjugates by the sequential activities of exoglycosidases expressed by Streptococcus pneumoniae. Mol. Microbiol. 59:961-974. [DOI] [PubMed] [Google Scholar]
- 31.Lau, P. C., C. K. Sung, J. H. Lee, D. A. Morrison, and D. G. Cvitkovitch. 2002. PCR ligation mutagenesis in transformable streptococci: application and efficiency. J. Microbiol. Methods 49:193-205. [DOI] [PubMed] [Google Scholar]
- 32.LeBlanc, D. J., V. L. Crow, L. N. Lee, and C. F. Garon. 1979. Influence of the lactose plasmid on the metabolism of galactose by Streptococcus lactis. J. Bacteriol. 137:878-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liberman, E. S., and A. S. Bleiweis. 1984. Role of the phosphoenolpyruvate-dependent glucose phosphotransferase system of Streptococcus mutans GS5 in the regulation of lactose uptake. Infect. Immun. 43:536-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merritt, J., P. Tsang, L. Zheng, W. Shi, and F. Qi. 2007. Construction of a counterselection-based in-frame deletion system for genetic studies of Streptococcus mutans. Oral Microbiol. Immunol. 22:95-102. [DOI] [PubMed] [Google Scholar]
- 35.Monedero, V., S. Poncet, I. Mijakovic, S. Fieulaine, V. Dossonnet, I. Martin-Verstraete, S. Nessler, and J. Deutscher. 2001. Mutations lowering the phosphatase activity of HPr kinase/phosphatase switch off carbon metabolism. EMBO J. 20:3928-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morse, M. L., K. L. Hill, J. B. Egan, and W. Hengstenberg. 1968. Metabolism of lactose by Staphylococcus aureus and its genetic basis. J. Bacteriol. 95:2270-2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neidhardt, F. C., and R. Curtiss. 1996. Escherichia coli and Salmonella typhimurium: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 38.Oskouian, B., and G. C. Stewart. 1990. Repression and catabolite repression of the lactose operon of Staphylococcus aureus. J. Bacteriol. 172:3804-3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1993. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 57:543-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosey, E. L., and G. C. Stewart. 1992. Nucleotide and deduced amino acid sequences of the lacR, lacABCD, and lacFE genes encoding the repressor, tagatose 6-phosphate gene cluster, and sugar-specific phosphotransferase system components of the lactose operon of Streptococcus mutans. J. Bacteriol. 174:6159-6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russell, R. R., J. Aduse-Opoku, I. C. Sutcliffe, L. Tao, and J. J. Ferretti. 1992. A binding protein-dependent transport system in Streptococcus mutans responsible for multiple sugar metabolism. J. Biol. Chem. 267:4631-4637. [PubMed] [Google Scholar]
- 42.Simpson, C. L., and R. R. Russell. 1998. Identification of a homolog of CcpA catabolite repressor protein in Streptococcus mutans. Infect. Immun. 66:2085-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smorawinska, M., J. C. Hsu, J. B. Hansen, E. K. Jagusztyn-Krynicka, Y. Abiko, and R. Curtiss III. 1983. Clustered genes for galactose metabolism from Streptococcus mutans cloned in Escherichia coli. J. Bacteriol. 153:1095-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terra, V. S., K. A. Homer, S. G. Rao, P. W. Andrew, and H. Yesilkaya. 2009. Characterization of a novel β-galactosidase activity contributing to glycoprotein degradation and virulence in Streptococcus pneumoniae. Infect. Immun. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 45.Vadeboncoeur, C., and M. Pelletier. 1997. The phosphoenolpyruvate:sugar phosphotransferase system of oral streptococci and its role in the control of sugar metabolism. FEMS Microbiol. Rev. 19:187-207. [DOI] [PubMed] [Google Scholar]
- 46.van Rooijen, R. J., K. J. Dechering, C. Niek, J. Wilmink, and W. M. de Vos. 1993. Lysines 72, 80 and 213 and aspartic acid 210 of the Lactococcus lactis LacR repressor are involved in the response to the inducer tagatose-6-phosphate leading to induction of lac operon expression. Protein Eng. 6:201-206. [DOI] [PubMed] [Google Scholar]
- 47.Wen, Z. T., C. Browngardt, and R. A. Burne. 2001. Characterization of two operons that encode components of fructose-specific enzyme II of the sugar:phosphotransferase system of Streptococcus mutans. FEMS Microbiol. Lett. 205:337-342. [DOI] [PubMed] [Google Scholar]
- 48.Wen, Z. T., and R. A. Burne. 2002. Analysis of cis- and trans-acting factors involved in regulation of the Streptococcus mutans fructanase gene (fruA). J. Bacteriol. 184:126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wetherell, J. F., Jr., and A. S. Bleiweis. 1978. Antigens of Streptococcus mutans: isolation of a serotype-specific and a cross-reactive antigen from walls of strain V-100 (serotype e). Infect. Immun. 19:160-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Witt, E., R. Frank, and W. Hengstenberg. 1993. 6-Phospho-β-galactosidases of Gram-positive and 6-phospho-β-glucosidase B of Gram-negative bacteria: comparison of structure and function by kinetic and immunological methods and mutagenesis of the lacG gene of Staphylococcus aureus. Protein Eng. 6:913-920. [DOI] [PubMed] [Google Scholar]
- 51.Zeng, L., and R. A. Burne. 2008. Multiple sugar: phosphotransferase system permeases participate in catabolite modification of gene expression in Streptococcus mutans. Mol. Microbiol. 70:197-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeng, L., and R. A. Burne. 2009. Transcriptional regulation of the cellobiose operon of Streptococcus mutans. J. Bacteriol. 191:2153-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeng, L., Z. T. Wen, and R. A. Burne. 2006. A novel signal transduction system and feedback loop regulate fructan hydrolase gene expression in Streptococcus mutans. Mol. Microbiol. 62:187-200. [DOI] [PubMed] [Google Scholar]
- 54.Zeng, L., and R. A. Burne. 2010. Seryl-phosphorylated HPr regulates CcpA-independent carbon catabolite repression in conjunction with PTS permeases in Streptococcus mutans. Mol. Microbiol. 75:1145-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.