Abstract
The Bordetella species are Gram-negative bacterial pathogens that are characterized by long-term colonization of the mammalian respiratory tract and are causative agents of respiratory diseases in humans and animals. Despite widespread and efficient vaccination, there has been a world-wide resurgence of pertussis, which remains the leading cause of vaccine-preventable death in developed countries. It has been proposed that current acellular vaccines (Pa) composed of only a few bacterial proteins may be less efficacious because of vaccine-induced antigenic shifts and adaptations. To gain insight into the development of a newer generation of vaccines, we constructed a Bordetella bronchiseptica strain (LPaV) that does not express the antigenic homologs included in any of the Pa vaccines currently in use. This strain also lacks adenylate cyclase toxin, an essential virulence factor, and BipA, a surface protein. While LPaV colonized the mouse nose as efficiently as the wild-type strain, it was highly deficient in colonization of the lower respiratory tract and was attenuated in induction of inflammation and injury to the lungs. Strikingly, to our surprise, we found that in an intranasal murine challenge model, LPaV elicited cross-species protection against both B. bronchiseptica and Bordetella pertussis. Our data suggest the presence of immunogenic protective components other than those included in the pertussis vaccine. Combined with the whole-genome sequences of many Bordetella spp. that are available, the results of this study should serve as a platform for strategic development of the next generation of acellular pertussis vaccines.
Bacteria belonging to the genus Bordetella are readily transmitted either by direct contact or through aerosol transmission via respiratory secretions or fomites (6, 30). Bordetella bronchiseptica has a broad host range and infects a wide variety of animals. It either is the etiological agent or is associated with a number of veterinary syndromes, such as kennel cough in dogs, atrophic rhinitis and pneumonia in pigs, and bronchopneumonia in guinea pigs, rabbits, horses, rats, mice, cats, and nonhuman primates (19). Bordetella parapertussis strains can be divided into two genetically distinct types, strains which infect humans and cause a pertussis-like illness and strains which cause respiratory infections in sheep (15, 16, 42). In contrast, Bordetella pertussis is pathogen of only humans and causes the acute respiratory disease known as pertussis or whooping cough (6).
Several vaccines against pertussis are currently available. The whole-cell vaccines (Pw) consisting of killed B. pertussis whole cells were the first generation of vaccines developed and are still being used in several countries (30). In many industrialized countries, the so-called acellular pertussis vaccines (Pa), which may include up to five antigens (filamentous hemagglutinin [FHA], pertactin [Prn], pertussis toxin [PT], and two fimbrial proteins [Fim]) have replaced the Pw vaccines (30, 36). These Pa vaccines differ greatly in antigen composition and the amounts of antigens in a dose (27, 30). Despite the availability of these vaccines, pertussis continues to be a significant cause of morbidity and mortality in infants and young children throughout the world (11). Although vaccination has decreased mortality considerably, B. pertussis continues to circulate and persist even in populations that have traditionally achieved high vaccination coverage. It is estimated that 20 to 30% of adolescents and adults who have a chronic cough lasting for more than 1 week are infected with B. pertussis (18, 57). Adults and adolescents carrying B. pertussis may act as reservoirs for infection of young children, in whom the disease can be severe and sometimes lethal (9). In most reported instances of such human-to-human transmission, infants and children were generally exposed to adults who did not have disease symptoms typical of pertussis but rather had only a simple prolonged cough (55, 56). Immunity following vaccination or infection is incomplete and wanes in a short time (26).
Several explanations and hypotheses have been suggested for the reemergence of this disease. These include waning immunity following vaccination in the absence of natural and vaccinal boosters, lower efficacy of the current vaccines, and changes in the circulating strains because of vaccine-induced adaptation (7, 11). It has been suggested that vaccination may select for vaccine escape mutants that have a different genotype and/or different antigenic expression pattern than the parent vaccine strains (4). Such concerns are heightened by the recent discovery of circulating strains that are deficient in two of the antigens included in the Pa vaccines, PT and Prn (3). Strains having alterations in the ptx promoter that result in increased production of PT have also been isolated (38). Thus, recent reports raise the extreme possibility that a strain that has lost all of the antigens included in the Pa vaccines could emerge, rendering Pa vaccines ineffective.
In this study, we constructed a strain of B. bronchiseptica (LPaV [lacks components of the acellular pertussis vaccine]) that has deletions in homologs of the genes encoding all of the antigens included in Pa vaccines. The LPaV strain also has deletions in the cyaA gene, which encodes adenylate cyclase toxin (ACT), and bipA, which encodes the surface protein Bordetella intermediate phase protein A (BipA). While ACT is not included in any of the Pa vaccines, we deleted cyaA because ACT is a critical virulence factor (23, 54). The bipA gene was deleted based on previous reports that BipA is highly immunogenic in both naturally and experimentally infected animals (17, 53). In a murine model of intranasal infection, LPaV colonized and persisted in the noses of mice. Because this strain did not colonize the tracheas and lungs of mice and induced less respiratory inflammation than the wild-type strain, we tested its efficacy for protection against two Bordetella species, B. bronchiseptica and B. pertussis. Surprisingly, we found that this mutant strain elicited protection against both species, as defined by reduced colonization in the respiratory tract. These results suggest that there are immunogenic cross-protective antigens and accentuate the need to develop a new generation of pertussis vaccines by identifying novel protective antigens.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
B. pertussis wild-type strain Bp536 and B. bronchiseptica strain RB50 used in this study have been described previously (37, 51). The LPaV strain has in-frame deletions in five B. bronchiseptica open reading frames, fhaB, prn, cyaA, fim, and bipA. The parental strain, RB71, an isogenic derivative of RB50, was a gift from Jeff F. Miller. To construct the LPaV strain, we deleted the bipA gene from RB71 as described previously (49). The genotype of this strain was confirmed by PCR.
All of the strains were maintained on Bordet-Gengou (BG) agar supplemented with 7.5% defibrinated sheep blood and containing 50 μg/ml of streptomycin. For animal inoculation and other assays, the B. bronchiseptica strains were grown at 37°C in Stainer-Scholte (SS) (45) broth on a roller drum. For Bp536, the SS medium was supplemented with heptakis (2,6-di-O-methyl-β-cyclodextrin).
Animal experiments.
Four- to six-week-old female C57/BL6 mice obtained from Jackson Laboratories were lightly anesthetized with isoflurane and intranasally inoculated with 5 × 105 CFU of RB50 or the LPaV strain in 25 μl of phosphate-buffered saline (PBS) by deposition onto the nares. The number of CFU administered was verified by plating an aliquot of the inoculum on BG agar plates containing streptomycin. For time course colonization experiments, groups of 4 or 5 mice were sacrificed 1, 3, 14, and 30 days postinoculation, the entire nasal septum, 1 cm of the trachea, and the left lung of each mouse were homogenized in PBS, and serial dilutions of the suspensions were plated on BG agar containing streptomycin to determine the numbers of viable CFU. To obtain anti-Bordetella antibodies, sera were collected from infected mice 30 days postinoculation. All experimental procedures complied with institutional regulations.
Immunizations.
For immunization studies, groups of 4 or 5 C57BL/6 mice were intranasally immunized on day 0 with 5 × 105 CFU of the LPaV strain in 25 μl of PBS. The control group of mice received 25 μl of sterile PBS. Four weeks after the primary infection with the LPaV strain or inoculation of PBS, mice were challenged with 5 × 105 CFU of either RB50 or Bp536. Mice were sacrificed 7 days after challenge with RB50 and 3 and 7 days after challenge with Bp536. The bacterial burdens in the lungs, trachea, and nasal septum were determined as described above.
The colonies of the RB50 and LPaV strains recovered from the mouse respiratory tract were distinguished based on hemolytic activity and colony morphology. Due to deletion of the cyaA gene, the LPaV strain is nonhemolytic. To distinguish between the Bp536 and LPaV strains, bacteria were plated on BG agar plates containing streptomycin and nalidixic acid. The LPaV strain is susceptible to nalidixic acid.
For passive immunization, groups of 4 or 5 mice were injected intraperitoneally with either 200 μl of pooled anti-LPaV convalescent-phase serum or sterile PBS. Three to four hours later, mice were challenged intranasally with 5 × 105 CFU of RB50 in 25 μl of sterile PBS. Mice were sacrificed 7 days postchallenge, and colonization levels were determined as described above.
Histopathology.
Immediately following sacrifice, the right lungs were harvested, fixed using neutral buffered formalin for 24 h, trimmed, and processed for histology as described previously (50). Hematoxylin and eosin (H&E)-stained sections were examined by light microscopy for evidence of injury in a blind manner by an American College of Veterinary Pathologists board-certified veterinary pathologist (N.D.K.). The sections were evaluated for overall cellularity and consolidation, alveolar wall thickness, amount of edema fluid present, and infiltrating neutrophils and macrophages in the airways (alveoli and bronchioles) and interstitium. Each parameter was scored subjectively using scores ranging from 0 to 5; 0 was considered normal or unaffected, and 5 indicated that there was a marked change. The averages for the parameters were added to obtain the final inflammatory score for each group.
Enzyme-linked immunosorbent assay (ELISA).
Serum antibody responses to RB50 or LPaV were quantified by coating 96-well flat-bottom plates with one of the strains. Overnight cultures of the strains were diluted to obtain an optical density at 600 nm (OD600) of 0.05 in 100 μl of 0.1 M sodium carbonate-sodium bicarbonate buffer, and the plates were incubated at 4°C overnight. Washing and blocking were carried out as described previously (50). Serum from RB50- or LPaV-infected mice was serially diluted and added to the plates as the primary antibody. The total IgG antibodies were detected using horseradish peroxidase (HRP)-conjugated goat anti-mouse antibody (1:2,000). Absorbance at 450 nm was determined using a Labsystems Multiskan Plus plate reader. The OD450 were plotted against the corresponding dilutions, and endpoint titers were determined as previously described (50).
Opsonophagocytosis assays.
Opsonophagocytosis assays were carried out using murine macrophage cell line J774A.1, as described previously (50). Briefly, PBS or different dilutions of sera from naïve LPaV-infected mice were heat inactivated at 55°C and incubated with 2 × 106 CFU of RB50. Subsequently, the bacterium-serum suspensions were added to the macrophage cells at a multiplicity of infection (MOI) of 1:10. The plates were incubated at 37°C for 1 h. Following gentamicin treatment, the number of bacteria that were phagocytosed was determined by lysing the macrophage cells and plating different dilutions.
Statistical analysis.
An unpaired two-tailed Student t test was used to determine the statistical significance of differences between treatment groups. P values of ≤0.05 were considered significant.
RESULTS
B. bronchiseptica strain LPaV lacking genes encoding five of the known adhesins and toxins colonizes the mouse nose.
B. bronchiseptica establishes a life-long infection in the nasopharyngeal cavity of laboratory animals (22, 40). In experimental animal models of intranasal infection, B. bronchiseptica initially colonizes both the upper and lower respiratory tracts. While this species is eventually cleared from the lower respiratory tract, high numbers of bacteria are recovered from the nasal cavity up to 270 days postinoculation (22, 40). Previous results have shown that loss of individual virulence factors (FHA, Fim, Prn, ACT, or BipA) has little, if any, effect on the ability of B. bronchiseptica to colonize the nasal cavity (12, 28, 31, 39, 49). Since Bordetella species express multiple adhesins and toxins, we hypothesized that while the lack of individual factors may not have an effect, simultaneous absence of five factors may result in a defect in nasal colonization. In order to test this hypothesis, we constructed a penta-mutant (LPaV) with in-frame deletions in prn, cyaA, fhaB, fimBCD, and bipA. Immunoblotting of the outer membrane fractions of the LPaV strain using convalescent-phase sera from an RB50-infected mouse confirmed that the FHA, Fim, Prn, ACT, and BipA proteins were not present (data not shown). Moreover, B. bronchiseptica does not express PT because of inactivating mutations in the ptx promoter (21). Thus, LPaV does not produce any of the protein homologs included in the current Pa vaccines.
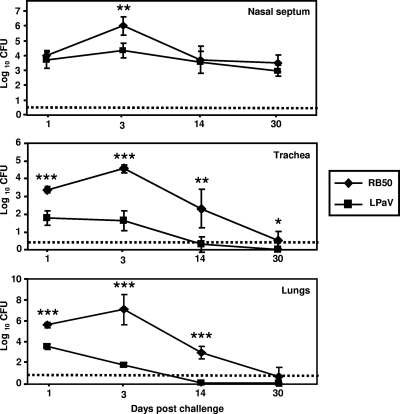
The kinetics of colonization of the mouse respiratory tract by the LPaV strain were compared with those of wild-type strain RB50. Consistent with previous results, high numbers of RB50 bacteria were recovered from the nasal septa, tracheas, and lungs of all of the animals at 1, 3, and 14 days postinoculation (Fig. 1) (22). At 30 days postinfection, while RB50 was cleared from the tracheas and lungs, more than 1,000 CFU was recovered from the nasal septa. In contrast, the LPaV mutant displayed a drastic defect in colonization of the trachea and the lungs at all time points examined (Fig. 1). As early as 1 day postchallenge, significantly lower numbers of bacteria were recovered from the lungs and tracheas of the LPaV-infected mice than from these lungs and tracheas of the wild-type strain-infected mice (Fig. 1). By 14 days postinfection, the LPaV strain was not recovered from the lungs. Three of five mice completely cleared the LPaV infection from the trachea, and in two mice the bacterial load was at the limit of detection. However, the ability of this mutant strain to colonize the nasal septum at 14 and 30 days postinfection was indistinguishable from that of the wild-type strain (Fig. 1). These data suggest that concomitant deletion of fhaB, cyaA, fim, prn, and bipA does not significantly affect the ability to persist in the upper respiratory tract.
FIG. 1.
Kinetics of colonization of the respiratory tract of C57BL/6 mice by the RB50 and LPaV strains. Mice were intranasally inoculated with 5 × 105 CFU of either the RB50 or LPaV strain in 25-μl droplets. At the indicated time points postinoculation, mice were sacrificed, and the bacterial burdens in the nasal septum, trachea, and lungs were enumerated. Each symbol indicates the results for a single mouse. The dashed line indicates the limit of detection. The bars indicate standard deviations. A statistical analysis was carried out using an unpaired two-tailed Student t test to compare the numbers of CFU obtained from the groups of RB50-infected mice to the numbers of CFU obtained from mice inoculated with the LPaV strain. The asterisks indicate P values (one asterisk, P ≤ 0.05; two asterisks, P ≤ 0.005; three asterisks, P ≤ 0.0005).
The LPaV mutant induces less pathology in the infected mouse lung.
In order to determine if decreased colonization of the lower respiratory tract by the mutant strain correlated with reduced pulmonary injury, lungs were collected at 1 and 14 days postchallenge, and sections were fixed, processed for H&E staining, and examined microscopically. At 1 day postinfection, lungs of RB50-infected mice had extensive alveolar wall thickening, mostly due to infiltration of neutrophils, and the mean pathology score was 4.0 (Fig. 2). At 14 days postinfection, the lungs of this group of infected mice showed enhanced infiltration by macrophages but contained fewer neutrophils (average pathology score, 3.5). In contrast, lungs from mice infected with LPaV exhibited modest changes, and the average pathology scores were 1.5 and 1.0 at 1 and 14 days, respectively (Fig. 2). The average pathology score for the lungs from PBS-inoculated mice was 1.0 at both 1 and 14 days postinfection, and there was no evidence of significant injury (Fig. 2). These observations suggest that LPaV causes less injury in the lungs, possibly due to decreased colonization of the lungs.
FIG. 2.
Infection with the LPaV strain results in reduced pathology in mouse lungs. Representative photomicrographs of lungs from mice inoculated with RB50, LPaV, or PBS at 1 and 14 days postchallenge are shown. H&E staining; magnification, ×100.
Antibody responses to LPaV infection.
B. bronchiseptica elicits a strong antibody response during experimental animal infections (25). Because many of the factors missing from the LPaV strain differentially contribute to generation of humoral responses against B. bronchiseptica (12, 31, 39, 49), we measured the anti-Bordetella titers in sera of mice infected with this mutant. ELISAs using RB50 cells as the antigen revealed that LPaV-infected mice had significantly lower anti-Bordetella titers than RB50-infected mice at 30 days postinoculation (Fig. 3). However, the serum antibody responses were similar in LPaV-infected mice and RB50-infected mice when LPaV cells were used as the antigen (Fig. 3), indicating that despite the absence of five antigens, infection with LPaV induces anti-Bordetella antibodies. These data further suggest that there are other known or yet-to-be-identified antigens in the LPaV strain.
FIG. 3.
Serum antibody responses in mice following intranasal inoculation with the RB50 or LPaV strain. Total Bordetella-specific IgG titers were determined by ELISA using either RB50 or LPaV whole cells as the antigen. The bars indicate the mean titers for 4 to 7 mice, and the error bars indicate the standard deviations. A statistical analysis was carried out using an unpaired two-tailed Student t test to compare the antibody titers obtained for sera of RB50-infected mice to the antibody titers obtained for sera from LPaV-infected mice. The asterisks indicate that the P value is ≤0.0005.
LPaV-specific serum is sufficient to clear B. bronchiseptica infection from the lower respiratory tract.
Anti-Bordetella antibodies are critical for resolving Bordetella infections, as well as for vaccine-mediated immune responses (20, 25). Previous studies have demonstrated that passive immunization with sera from RB50-infected convalescent mice leads to rapid elimination of B. bronchiseptica from the lower respiratory tract (25, 50). Therefore, we investigated if adoptive transfer of sera from LPaV-infected animals provided protection from colonization with wild-type strain RB50 in the mouse respiratory tract. Pooled sera collected from LPaV-infected mice or sterile PBS was administered intraperitoneally, and this was followed by challenge with RB50. Seven days postchallenge the bacterial burdens in the noses, tracheas, and lungs were determined. While mock-treated mice harbored more than 105 CFU in their lungs, 4 of 5 mice treated with the LPaV serum completely cleared RB50 from the lungs (Fig. 4). Although not as drastic as that for the lungs, passive immunization with LPaV sera also resulted in a significant lowering of the bacterial load in the trachea, and 2 of 5 mice that received the LPaV serum had no detectable bacteria at this site (Fig. 4). Adoptive transfer of LPaV antibodies did not have a significant effect on nasal colonization by RB50 (Fig. 4). Previous studies (25, 50) demonstrated that adoptively transferred anti-Bordetella antibodies have no effect on colonization of the upper respiratory tract by Bordetella in mice. Our results suggest that in spite of lower titers, antibodies elicited during infection of mice with the LPaV strain efficiently provide protection against wild-type B. bronchiseptica infection in the lower respiratory tract.
FIG. 4.
Adoptively transferred anti-LPaV serum confers protection against challenge with RB50 in mice. Mice were intraperitoneally injected with LPaV-specific serum or sterile PBS 3 to 4 h prior to intranasal challenge with 5 × 105 CFU of RB50 in 25 μl. Seven days postchallenge, mice were sacrificed, and bacterial colonization in the nasal septum, trachea, and lungs was determined. The dashed line indicates the limit of detection. Each symbol indicates the results for a single mouse. The horizontal lines indicate the means for the groups. An unpaired two-tailed Student t test was used to determine statistical significance. The asterisks indicate P values (one asterisk, P ≤ 0.05; two asterisks, P ≤ 0.005).
Opsonization with anti-LPaV serum enhances phagocytosis of B. bronchiseptica.
Antibody-mediated bacterial uptake by phagocytes is often correlated with the protective efficacy of passively transferred immune sera (43, 50). We examined the efficiency of LPaV serum for promoting phagocytosis of RB50 by the murine macrophage cell line J774. PBS and sera collected from naïve mice were utilized as negative controls. There was a dose-dependent increase in the uptake of RB50 by J774 cells when they were opsonized with the LPaV serum compared to the results obtained for opsonization with either the serum from naïve mice or only PBS (Fig. 5). These results suggest that one of the mechanisms for the observed passive protection mediated by anti-LPaV serum is increased opsonization of B. bronchiseptica for phagocytosis.
FIG. 5.
Opsonization with anti-LPaV serum augments the phagocytosis of RB50 by J774 murine macrophages. Approximately 2 × 106 CFU of RB50 was incubated with either 1, 5, or 10% heat-inactivated anti-LPaV serum, 10% naïve mouse serum, or sterile PBS at 37°C for 30 min, and this was followed by incubation with 2 × 105 J774 cells for 1 h. Gentamicin (100 μg/ml) was added to kill the extracellular bacteria, and subsequently the wells were washed twice with sterile PBS to remove adherent bacteria. The cells were lysed with water, and the CFU of phagocytosed bacteria were enumerated as described in Materials and Methods. Results are representative of two independent experiments performed in four replicates. The error bars indicate standard deviations. A statistical analysis was carried out using an unpaired two-tailed Student t test. Two asterisks indicate that the P value was ≤0.005.
Immunization with the LPaV strain elicits cross-protective immunity to both B. bronchiseptica and B. pertussis.
Next we investigated the utility of LPaV as a potential live vaccine against B. bronchiseptica. Thirty days after inoculation with LPaV, mice were challenged with RB50. The bacterial burdens in the lungs, trachea, and nasal septum were then determined 7 days after the challenge with RB50. Although LPaV was cleared from the trachea and lungs at 30 days postinoculation, it was still present in the nose at this time point (Fig. 1). Thus, we utilized hemolysis to distinguish between the colonies of RB50 and the colonies of LPaV. RB50 produces colonies with a distinct hemolytic zone, whereas LPaV produces nonhemolytic colonies because of deletion of the cyaA gene. Compared to the mock-treated mice, LPaV-vaccinated mice completely cleared an RB50 infection from the lungs and trachea, and bacteria were not recovered from these organs (Fig. 6A). Similarly, there was close to a 3-log reduction in the number of RB50 colonies obtained from the noses of LPaV-vaccinated mice compared to the number of RB50 colonies obtained from the noses of mock-treated mice (Fig. 6A). For two of the five vaccinated mice the level of colonization was near the threshold of detection (Fig. 6A). Although there were no significant differences in growth between RB50 and the LPaV strain in broth cultures (data not shown) and the LPaV strain colonized the nose as efficiently as RB50 (Fig. 1), we cannot exclude the possibility that the observed reduction in the number of RB50 colonies was due to competition with the resident LPaV bacteria in the nasal cavity at the time of challenge.
FIG. 6.
Intranasal immunization with the LPaV strain protects mice against challenge with B. bronchiseptica and B. pertussis. (A) Groups of 5 mice were intranasally inoculated with 5 × 105 CFU of the LPaV strain or sterile PBS. On day 30 postimmunization mice were challenged with 5 × 105 CFU of RB50, and the numbers of bacteria were determined 7 days postinfection. (B) Mice were intranasally inoculated with 5 × 105 CFU of the LPaV strain or sterile PBS. Thirty days postimmunization mice were challenged with 5 × 105 CFU of Bp536, and the bacterial burdens in the respiratory tract were determined 3 and 7 days postinfection. Each symbol indicates the results for a single mouse. The dashed lines indicate the limits of CFU detection. The horizontal lines indicate the means of the groups. Statistical significance was determined by an unpaired two-tailed Student t test. The asterisks indicate P values (one asterisk, P ≤ 0.05; two asterisks, P ≤ 0.005; three asterisks, P ≤ 0.0005).
Based on the protection observed with RB50, we determined whether LPaV generated cross-protection against the human pathogen B. pertussis. Despite the fact that a B. bronchiseptica strain was utilized to examine protection against B. pertussis, the B. bronchiseptica-mouse model is a natural-host animal model and is also analogous to and representative of human B. pertussis infections (22). To precisely count the number of B. pertussis bacteria obtained from the nose, bacteria harvested from this site were plated on BG agar plates containing nalidixic acid and streptomycin. LPaV is susceptible to nalidixic acid. At 3 days postchallenge, the greatest decline in the bacterial load was observed in the trachea, while there was a small but significant decline in the lungs (Fig. 6B). While the colonization of the nose by B. pertussis at this time point was highly variable, for two of the LPaV-vaccinated mice the numbers of CFU obtained were at or close to the limit of detection. At 7 days postchallenge, all the immunized mice had cleared B. pertussis from the trachea. Although at 7 days we did not recover bacteria from the lungs of one of the control mice, in contrast to the results for the rest of the mice in this group, on average the LPaV-immunized mice harbored 50-fold fewer bacteria in the lungs (Fig. 6B). Compared to the burden at 3 days postinoculation, the burden of B. pertussis in the noses of the LPaV-vaccinated mice was further decreased at 7 days postinoculation, and in two of the mice there were no detectable bacteria at this site (Fig. 6B). These results suggest that intranasal immunization with LPaV provides substantial cross-immunity to both B. bronchiseptica and B. pertussis.
Vaccination with LPaV reduces lung pathology in mice challenged with B. bronchiseptica and B. pertussis.
We also examined the lungs of LPaV-vaccinated mice following challenge with RB50 or Bp536 to determine whether prior inoculation with this strain reduced pulmonary injury resulting from infection with wild-type B. bronchiseptica or B. pertussis. While the lungs of control mice infected with RB50 had alveolar wall thickening that was mostly due to infiltration of mononuclear phagocytes and had an average pathology score of 4.3, the lungs of LPaV-immunized mice exhibited less injury, and the mean pathology score was 1.5 (Fig. 7A). Similarly vaccinated mice that were challenged with Bp536 exhibited reduced lung injury with a mean pathology score of 1.6, while the lungs of control mice infected with Bp536 were edematous with an average pathology score of 3.0 (Fig. 7B). These results show that immunization with the LPaV strain decreases the lung pathology associated with B. bronchiseptica and B. pertussis infection (Fig. 7).
FIG. 7.
Immunization with the LPaV strain reduces lung pathology in B. bronchiseptica- and B. pertussis-infected mice. Mice were immunized with the LPaV strain or sterile PBS and challenged with RB50 (A) or B. pertussis (B) as described in the text. On day 7 postchallenge, lungs were harvested, fixed, and processed for H&E staining. The sections were examined, and pathology was scored in a blinded manner. Representative H&E-stained lung sections are shown along with the mean pathology score for each group. (A) The LPaV-RB50 image shows that there was prominent bronchiole-associated lymphoid tissue, but the airways were generally clear and the alveolar walls were fairly normal (pathology score, 1.5). In contrast, the lung from the mouse treated with PBS and challenged with RB50 had diffuse alveolar wall thickening and airway inflammation (pathology score, 4.3). Magnification, ×100. (B) The LPaV-Bp536 image shows that there was prominent bronchiole-associated lymphoid tissue, patchy alveolar edema, and minimal inflammation (pathology score, 1.6), while the lung from the mouse treated with PBS and challenged with Bp536 was markedly infiltrated by inflammatory cells and edema fluid and there was a reduction in the clear airway space (pathology score, 3.0). Magnification, ×100.
DISCUSSION
Pertussis is an important and sometimes the only vaccine-preventable infectious disease whose occurrence is increasing in countries with long-standing and very high vaccination coverage. B. pertussis continues to circulate by persisting in the human nasopharynx, which results in horizontal transmission (8, 10). Similarly, animals and birds continue to be carriers despite vaccination with the animal pathogens B. bronchiseptica and Bordetella avium, respectively (19, 44, 52). Animals and birds frequently shed bacteria, resulting in outbreaks in herds (14). Previous studies have not identified a significant role for the known surface proteins and toxins in nasal colonization (12, 28, 31, 39, 49). We hypothesized that efficient nasopharyngeal colonization by bordetellae involves coordinated participation of multiple virulence factors. We constructed mutant strain LPaV with the expectation that concurrent deletion of genes encoding five of the well-studied surface proteins and toxins would drastically reduce colonization of the upper respiratory tract. However, to our surprise, this mutant strain was as effective as the wild-type strain in colonization of the mouse nasal cavity (Fig. 1). In contrast, it was essentially cleared from the trachea and lungs, and an LPaV infection resulted in reduced pulmonary injury (Fig. 1 and 2).
Encouraged by the observed attenuation in the trachea and lungs and the resultant minimal damage to the host following infection with the LPaV strain, we determined the effectiveness of this mutant as a live vaccine for B. bronchiseptica. We found that prior infection of mice with this mutant conferred protection against a wild-type strain of B. bronchiseptica (Fig. 6A). Immunized animals had a significant, albeit weaker, humoral immune response compared to animals infected with the wild-type strain (Fig. 3). Adoptive transfer of sera from immunized animals rapidly eliminated B. bronchiseptica from the lungs and reduced the bacterial burden in the trachea, suggesting that the protection mediated by this strain in the lower respiratory tract is mediated in part by antibodies (Fig. 4). One potential use of this mutant strain is as a live attenuated vaccine against B. bronchiseptica in animals. The currently available vaccines against B. bronchiseptica contain live, attenuated, or heat-killed bacteria (2, 28, 32, 46, 48). One of the problems associated with these vaccines is that the genetic mutations that result in attenuation are not known. It is possible that under survival pressure in the host, including coinfections with other pathogens, such vaccine strains might revert to a virulent form (47, 48). Since the LPaV mutant strain carries genetically defined mutations, it may be a safer vaccine strain. However, because of its ability to persist in the noses of experimentally infected animals the potential for herd-to-herd transmission of such a vaccine strain exists.
The animal pathogen B. bronchiseptica is considered the evolutionary progenitor of the human pathogen B. pertussis (41). Despite this evolutionary relationship, there are distinct differences in the genome sizes and gene expression patterns between these two species. B. pertussis has a smaller genome and contains a large number of pseudogenes, many of which have been inactivated by insertion elements, in-frame stop codons, and frameshift mutations (13). Our finding that immunization with LPaV provided cross-species protection against B. pertussis (Fig. 6B) is important because this strain does not produce any protein homologs of the components included in the different acellular pertussis vaccines. Not only did we observe decreased colonization of the entire respiratory tract by B. pertussis following immunization with LPaV, but there was also a significant decrease in the overall lung pathology in immunized mice. It appears that the protection against B. pertussis afforded by LPaV is mediated by antigens that are shared by B. bronchiseptica and B. pertussis. It was recently demonstrated that a B. pertussis strain lacking three of the major toxins, PT, dermonecrotic toxin (Dnt), and tracheal cytotoxin (Tct), provided protection against B. pertussis colonization of the lungs (34). B. bronchiseptica expresses both Dnt and Tct. Although the results of this study and the results of our study cannot be directly compared, it is possible that Dnt and Tct do not play a role in protection mediated by the LPaV strain. We are currently engineering deletions of dnt and tct in the LPaV strain to examine whether these genes have any role in the protection mediated by this strain. Our laboratory has recently identified a new outer membrane protein, Bordetella colonization factor A (BcfA), in B. bronchiseptica (51) and has shown that immunization with this protein significantly lowered the bacterial burden in the respiratory tract (50), which makes it one of the likely candidate protective antigens in the mutant strain. Our preliminary results suggest that there is a BcfA homolog in B. pertussis, and we are currently attempting to identify this protein in B. pertussis. Recently, it has been shown that Bps22, a protein secreted by the Bordetella type III secretion system, protects mice against B. bronchiseptica infection (33). While Bsp22 or other type III components may be antigens that protect against B. bronchiseptica infection, a recent study showed that a B. bronchiseptica strain lacking cyaA and type III secretion provided cross-species protection (28). The fact that Bp536, the B. pertussis strain utilized in this study, does not express an active type III secretion complex or the secreted proteins makes it unlikely that Bsp22 mediates the protection against B. pertussis observed. Other possible candidate protective antigens include the iron-repressible outer membrane proteins (5), lipopolysaccharide (LPS) (24), and autotransporters like BrKA (29). Utilizing immunoproteomics, multiple novel antigens from B. pertussis extracts that are recognized by immune sera were identified recently (1). Taken together, the results of this study and other studies highlight the synergistic contribution of known and yet-to-be-identified proteins in maximizing protection against B. pertussis.
In the past 2 decades, there has been an increase in the number of pertussis cases reported in many developed countries, including the United States (30). It has been discovered that currently circulating isolates of B. pertussis have genotypic changes in some of the vaccine antigens, such as Prn and PT (3). Consequently, antigens that are included in the currently available acellular vaccines may eventually not provide efficient protection. Thus, it is critical that we remain open to alternatives to Pa vaccine-based approaches to pertussis immunization. Use of live attenuated B. bronchiseptica and B. pertussis strains as vaccines for pertussis has been advocated (35). Similarly, the LPaV strain has the potential to be a highly effective and safe complement to current pertussis vaccines because it induces immunity against antigens that are not part of the Pa vaccines. Realistically, however, because of safety issues associated with Pw vaccines, it may be difficult for the public health authorities in countries where the Pa vaccines are currently used to return to use of whole-cell vaccines. Thus, observations reported here highlight the need to develop a new generation of pertussis vaccines by identification and inclusion of additional protective antigens.
Acknowledgments
We thank Jeffery F. Miller for the generous gift of the RB71 strain. We thank Manish S. Bharadwaj and Haiping Lu for technical assistance. We also thank two anonymous reviewers for providing a number of discussion points in their critiques.
Research in the laboratory of R.D. was supported by funds from National Research Initiative grant 2006-35604-16874 from the USDA National Institute of Food and Agriculture and by funds from the Microbial Functional Genomics Program, from NIH (grant 1R01AI075081), and from the American Heart Association. M.S.C. was supported by NIH predoctoral training grant T32 AI07401.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 22 February 2010.
REFERENCES
- 1.Altindis, E., B. E. Tefon, V. Yildirim, E. Ozcengiz, D. Becher, M. Hecker, and G. Ozcengiz. 2009. Immunoproteomic analysis of Bordetella pertussis and identification of new immunogenic proteins. Vaccine 27:542-548. [DOI] [PubMed] [Google Scholar]
- 2.Bey, R. F., F. J. Shade, R. A. Goodnow, and R. C. Johnson. 1981. Intranasal vaccination of dogs with liver avirulent Bordetella bronchiseptica: correlation of serum agglutination titer and the formation of secretory IgA with protection against experimentally induced infectious tracheobronchitis. Am. J. Vet. Res. 42:1130-1132. [PubMed] [Google Scholar]
- 3.Bouchez, V., D. Brun, T. Cantinelli, G. Dore, E. Njamkepo, and N. Guiso. 2009. First report and detailed characterization of B. pertussis isolates not expressing pertussis toxin or pertactin. Vaccine 27:6034-6041. [DOI] [PubMed] [Google Scholar]
- 4.Bouchez, V., V. Caro, E. Levillain, G. Guigon, and N. Guiso. 2008. Genomic content of Bordetella pertussis clinical isolates circulating in areas of intensive children vaccination. PLoS One 3:e2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brickman, T. J., T. Hanawa, M. T. Anderson, R. J. Suhadolc, and S. K. Armstrong. 2008. Differential expression of Bordetella pertussis iron transport system genes during infection. Mol. Microbiol. 70:3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbonetti, N. H. 2007. Immunomodulation in the pathogenesis of Bordetella pertussis infection and disease. Curr. Opin. Pharmacol. 7:272-278. [DOI] [PubMed] [Google Scholar]
- 7.Cherry, J. D. 1997. Comparative efficacy of acellular pertussis vaccines: an analysis of recent trials. Pediatr. Infect. Dis. J. 16:S90-S96. [DOI] [PubMed] [Google Scholar]
- 8.Cherry, J. D. 1999. Epidemiological, clinical, and laboratory aspects of pertussis in adults. Clin. Infect. Dis. 28(Suppl. 2):S112-S117. [DOI] [PubMed] [Google Scholar]
- 9.Cherry, J. D. 2005. The epidemiology of pertussis: a comparison of the epidemiology of the disease pertussis with the epidemiology of Bordetella pertussis infection. Pediatrics 115:1422-1427. [DOI] [PubMed] [Google Scholar]
- 10.Cherry, J. D. 1998. Pertussis in adults. Ann. Intern. Med. 128:64-66. [DOI] [PubMed] [Google Scholar]
- 11.Cherry, J. D., and P. Olin. 1999. The science and fiction of pertussis vaccines. Pediatrics 104:1381-1383. [DOI] [PubMed] [Google Scholar]
- 12.Cotter, P. A., M. H. Yuk, S. Mattoo, B. J. Akerley, J. Boschwitz, D. A. Relman, and J. F. Miller. 1998. Filamentous hemagglutinin of Bordetella bronchiseptica is required for efficient establishment of tracheal colonization. Infect. Immun. 66:5921-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cummings, C. A., M. M. Brinig, P. W. Lepp, S. van de Pas, and D. A. Relman. 2004. Bordetella species are distinguished by patterns of substantial gene loss and host adaptation. J. Bacteriol. 186:1484-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edinboro, C. H., M. P. Ward, and L. T. Glickman. 2004. A placebo-controlled trial of two intranasal vaccines to prevent tracheobronchitis (kennel cough) in dogs entering a humane shelter. Prev. Vet. Med. 62:89-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eldering, G., and P. Kendrick. 1938. Bacillus para-pertussis: a species resembling both Bacillus pertussis and Bacillus bronchisepticus but identical with neither. J. Bacteriol. 35:561-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eldering, G., and P. L. Kendrick. 1952. Incidence of parapertussis in the Grand Rapids area as indicated by 16 years' experience with diagnostic cultures. Am. J. Public Health Nations Health 42:27-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchslocher, B., L. L. Millar, and P. A. Cotter. 2003. Comparison of bipA alleles within and across Bordetella species. Infect. Immun. 71:3043-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilberg, S., E. Njamkepo, I. P. Du Chatelet, H. Partouche, P. Gueirard, C. Ghasarossian, M. Schlumberger, and N. Guiso. 2002. Evidence of Bordetella pertussis infection in adults presenting with persistent cough in a French area with very high whole-cell vaccine coverage. J. Infect. Dis. 186:415-418. [DOI] [PubMed] [Google Scholar]
- 19.Goodnow, R. A. 1980. Biology of Bordetella bronchiseptica. Microbiol. Rev. 44:722-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gopinathan, L., G. S. Kirimanjeswara, D. N. Wolfe, M. L. Kelley, and E. T. Harvill. 2007. Different mechanisms of vaccine-induced and infection-induced immunity to Bordetella bronchiseptica. Microbes Infect. 9:442-448. [DOI] [PubMed] [Google Scholar]
- 21.Gross, R., and R. Rappuoli. 1988. Positive regulation of pertussis toxin expression. Proc. Natl. Acad. Sci. U. S. A. 85:3913-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harvill, E. T., P. A. Cotter, and J. F. Miller. 1999. Pregenomic comparative analysis between Bordetella bronchiseptica RB50 and Bordetella pertussis Tohama I in murine models of respiratory tract infection. Infect. Immun. 67:6109-6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hewlett, E. L., G. M. Donato, and M. C. Gray. 2006. Macrophage cytotoxicity produced by adenylate cyclase toxin from Bordetella pertussis: more than just making cyclic AMP! Mol. Microbiol. 59:447-459. [DOI] [PubMed] [Google Scholar]
- 24.Higgins, S. C., A. G. Jarnicki, E. C. Lavelle, and K. H. Mills. 2006. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J. Immunol. 177:7980-7989. [DOI] [PubMed] [Google Scholar]
- 25.Kirimanjeswara, G. S., P. B. Mann, and E. T. Harvill. 2003. Role of antibodies in immunity to Bordetella infections. Infect. Immun. 71:1719-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le, T., J. D. Cherry, S. J. Chang, M. D. Knoll, M. L. Lee, S. Barenkamp, D. Bernstein, R. Edelman, K. M. Edwards, D. Greenberg, W. Keitel, J. Treanor, and J. I. Ward. 2004. Immune responses and antibody decay after immunization of adolescents and adults with an acellular pertussis vaccine: the APERT study. J. Infect. Dis. 190:535-544. [DOI] [PubMed] [Google Scholar]
- 27.Locht, C. 2008. A common vaccination strategy to solve unsolved problems of tuberculosis and pertussis? Microbes Infect. 10:1051-1056. [DOI] [PubMed] [Google Scholar]
- 28.Mann, P., E. Goebel, J. Barbarich, M. Pilione, M. Kennett, and E. Harvill. 2007. Use of a genetically defined double mutant strain of Bordetella bronchiseptica lacking adenylate cyclase and type III secretion as a live vaccine. Infect. Immun. 75:3665-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marr, N., D. C. Oliver, V. Laurent, J. Poolman, P. Denoel, and R. C. Fernandez. 2008. Protective activity of the Bordetella pertussis BrkA autotransporter in the murine lung colonization model. Vaccine 26:4306-4311. [DOI] [PubMed] [Google Scholar]
- 30.Mattoo, S., and J. D. Cherry. 2005. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin. Microbiol. Rev. 18:326-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattoo, S., J. F. Miller, and P. A. Cotter. 2000. Role of Bordetella bronchiseptica fimbriae in tracheal colonization and development of a humoral immune response. Infect. Immun. 68:2024-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McArthur, J. D., N. P. West, J. N. Cole, H. Jungnitz, C. A. Guzman, J. Chin, P. R. Lehrbach, S. P. Djordjevic, and M. J. Walker. 2003. An aromatic amino acid auxotrophic mutant of Bordetella bronchiseptica is attenuated and immunogenic in a mouse model of infection. FEMS Microbiol. Lett. 221:7-16. [DOI] [PubMed] [Google Scholar]
- 33.Medhekar, B., R. Shrivastava, S. Mattoo, M. Gingery, and J. F. Miller. 2009. Bordetella Bsp22 forms a filamentous type III secretion system tip complex and is immunoprotective in vitro and in vivo. Mol. Microbiol. 71:492-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mielcarek, N., A. S. Debrie, D. Raze, J. Bertout, C. Rouanet, A. B. Younes, C. Creusy, J. Engle, W. E. Goldman, and C. Locht. 2006. Live attenuated B. pertussis as a single-dose nasal vaccine against whooping cough. PLoS Pathog. 2:e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mielcarek, N., A. S. Debrie, D. Raze, J. Quatannens, J. Engle, W. E. Goldman, and C. Locht. 2006. Attenuated Bordetella pertussis: new live vaccines for intranasal immunisation. Vaccine 24(Suppl. 2):S2-54-S2-55. [DOI] [PubMed] [Google Scholar]
- 36.Mills, K. H., M. Ryan, E. Ryan, and B. P. Mahon. 1998. A murine model in which protection correlates with pertussis vaccine efficacy in children reveals complementary roles for humoral and cell-mediated immunity in protection against Bordetella pertussis. Infect. Immun. 66:594-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mishra, M., G. Parise, K. D. Jackson, D. J. Wozniak, and R. Deora. 2005. The BvgAS signal transduction system regulates biofilm development in Bordetella. J. Bacteriol. 187:1474-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mooi, F. R., I. H. van Loo, M. van Gent, Q. He, M. J. Bart, K. J. Heuvelman, S. C. de Greeff, D. Diavatopoulos, P. Teunis, N. Nagelkerke, and J. Mertsola. 2009. Bordetella pertussis strains with increased toxin production associated with pertussis resurgence. Emerg. Infect. Dis. 15:1206-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicholson, T. L., S. L. Brockmeier, and C. L. Loving. 2009. Contribution of Bordetella bronchiseptica filamentous hemagglutinin and pertactin to respiratory disease in swine. Infect. Immun. 77:2136-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parise, G., M. Mishra, Y. Itoh, T. Romeo, and R. Deora. 2007. Role of a putative polysaccharide locus in Bordetella biofilm development. J. Bacteriol. 189:750-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parkhill, J., M. Sebaihia, A. Preston, L. D. Murphy, N. Thomson, D. E. Harris, M. T. Holden, C. M. Churcher, S. D. Bentley, K. L. Mungall, A. M. Cerdeno-Tarraga, L. Temple, K. James, B. Harris, M. A. Quail, M. Achtman, R. Atkin, S. Baker, D. Basham, N. Bason, I. Cherevach, T. Chillingworth, M. Collins, A. Cronin, P. Davis, J. Doggett, T. Feltwell, A. Goble, N. Hamlin, H. Hauser, S. Holroyd, K. Jagels, S. Leather, S. Moule, H. Norberczak, S. O'Neil, D. Ormond, C. Price, E. Rabbinowitsch, S. Rutter, M. Sanders, D. Saunders, K. Seeger, S. Sharp, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, L. Unwin, S. Whitehead, B. G. Barrell, and D. J. Maskell. 2003. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat. Genet. 35:32-40. [DOI] [PubMed] [Google Scholar]
- 42.Porter, J. F., K. Connor, and W. Donachie. 1994. Isolation and characterization of Bordetella parapertussis-like bacteria from ovine lungs. Microbiology 140:255-261. [DOI] [PubMed] [Google Scholar]
- 43.Schlageter, A. M., and T. R. Kozel. 1990. Opsonization of Cryptococcus neoformans by a family of isotype-switch variant antibodies specific for the capsular polysaccharide. Infect. Immun. 58:1914-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spears, P. A., L. M. Temple, D. M. Miyamoto, D. J. Maskell, and P. E. Orndorff. 2003. Unexpected similarities between Bordetella avium and other pathogenic bordetellae. Infect. Immun. 71:2591-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stainer, D. W., and M. J. Scholte. 1970. A simple chemically defined medium for the production of phase I Bordetella pertussis. J. Gen. Microbiol. 63:211-220. [DOI] [PubMed] [Google Scholar]
- 46.Stevenson, A., and M. Roberts. 2004. Intranasal immunisation against tetanus with an attenuated Bordetella bronchiseptica vector expressing FrgC: improved immunogenicity using a Bvg-regulated promoter to express FrgC. Vaccine 22:4300-4305. [DOI] [PubMed] [Google Scholar]
- 47.Stevenson, A., and M. Roberts. 2002. Use of a rationally attenuated Bordetella bronchiseptica as a live mucosal vaccine and vector for heterologous antigens. Vaccine 20:2325-2335. [DOI] [PubMed] [Google Scholar]
- 48.Stevenson, A., and M. Roberts. 2003. Use of Bordetella bronchiseptica and Bordetella pertussis as live vaccines and vectors for heterologous antigens. FEMS Immunol. Med. Microbiol. 37:121-128. [DOI] [PubMed] [Google Scholar]
- 49.Stockbauer, K. E., B. Fuchslocher, J. F. Miller, and P. A. Cotter. 2001. Identification and characterization of BipA, a Bordetella Bvg-intermediate phase protein. Mol. Microbiol. 39:65-78. [DOI] [PubMed] [Google Scholar]
- 50.Sukumar, N., C. F. Love, M. S. Conover, N. D. Kock, P. Dubey, and R. Deora. 2009. Active and passive immunizations with Bordetella colonization factor A protect mice against respiratory challenge with Bordetella bronchiseptica. Infect. Immun. 77:885-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sukumar, N., M. Mishra, G. P. Sloan, T. Ogi, and R. Deora. 2007. Differential Bvg phase-dependent regulation and combinatorial role in pathogenesis of two Bordetella paralogs, BipA and BcfA. J. Bacteriol. 189:3695-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Temple, L. M., A. A. Weiss, K. E. Walker, H. J. Barnes, V. L. Christensen, D. M. Miyamoto, C. B. Shelton, and P. E. Orndorff. 1998. Bordetella avium virulence measured in vivo and in vitro. Infect. Immun. 66:5244-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vergara-Irigaray, N., A. Chavarri-Martinez, J. Rodriguez-Cuesta, J. F. Miller, P. A. Cotter, and G. Martinez de Tejada. 2005. Evaluation of the role of the Bvg intermediate phase in Bordetella pertussis during experimental respiratory infection. Infect. Immun. 73:748-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vojtova, J., J. Kamanova, and P. Sebo. 2006. Bordetella adenylate cyclase toxin: a swift saboteur of host defense. Curr. Opin. Microbiol. 9:69-75. [DOI] [PubMed] [Google Scholar]
- 55.Wendelboe, A. M., M. G. Hudgens, C. Poole, and A. Van Rie. 2007. Estimating the role of casual contact from the community in transmission of Bordetella pertussis to young infants. Emerg. Themes Epidemiol. 4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wendelboe, A. M., E. Njamkepo, A. Bourillon, D. D. Floret, J. Gaudelus, M. Gerber, E. Grimprel, D. Greenberg, S. Halperin, J. Liese, F. Munoz-Rivas, R. Teyssou, N. Guiso, and A. Van Rie. 2007. Transmission of Bordetella pertussis to young infants. Pediatr. Infect. Dis. J. 26:293-299. [DOI] [PubMed] [Google Scholar]
- 57.Wilson, T. R. 2006. Update on adolescent immunization: review of pertussis and the efficacy, safety, and clinical use of vaccines that contain tetanus-diphtheria-acellular pertussis. J. Pediatr. Health Care 20:229-237. [DOI] [PubMed] [Google Scholar]