Abstract
Fc gamma receptor IIIA (CD16/FcγRIIIA) on monocytes/macrophages may play an important role in the pathogenesis of severe malarial anemia (SMA) by promoting phagocytosis of IgG-coated uninfected red cells and by allowing the production of tumor necrosis factor alpha (TNF-α) upon cross-linking by immune complexes (ICs). However, not much is known about the differential expression of this receptor on monocytes of children with severe malaria and uncomplicated malaria. Therefore, we investigated the expression of CD16/FcγRIIIA on monocytes of children with SMA, cerebral malaria (CM), and their age-matched uncomplicated malaria controls by flow cytometry. Since CD14low (CD14+) monocytes are considered more mature and macrophage-like than CD14high (CD14++) monocytes, we also compared the level of expression of CD16/FcγRIIIA according to the CD14 level and studied the relationship between CD16/FcγRIIIA expression and intracellular TNF-α production upon stimulation by ICs. CD16/FcγRIIIA expression was the highest overall on CD14+ CD16+ monocytes of children with SMA at enrollment. At convalescence, SMA children were the only ones to show a significant decline in the same parameter. In contrast, there were no significant differences among groups in the expression of CD16/FcγRIIIA on CD14++ CD16+ monocytes. A greater percentage of CD14+ CD16+ monocytes produced TNF-α upon stimulation than any other monocyte subset, and the amount of intracellular TNF-α correlated positively with CD16/FcγRIIIA expression. Furthermore, there was an inverse correlation between hemoglobin levels and CD16/FcγRIIIA expression in children with SMA and their controls. These data suggest that monocytes of children with SMA respond differently to Plasmodium falciparum infection by overexpressing CD16/FcγRIIIA as they mature, which could enhance erythrophagocytosis and TNF-α production.
Severe malarial anemia (SMA) and cerebral malaria (CM) are two of the most serious manifestations of Plasmodium falciparum malaria and are important causes of childhood mortality and morbidity in sub-Saharan Africa. However, the pathogenesis of these complications remains unclear. A better understanding of the factors involved in the pathogenesis of severe malaria is essential for the identification of at-risk populations and the development of effective prophylactic and therapeutic measures.
There is abundant evidence to suggest that destruction of uninfected red cells plays an important role in the development of SMA. Uninfected red cells of humans and mice infected with P. falciparum or rodent malaria have a reduced life span (26, 27, 48), and the life span is more reduced in patients with splenomegaly (26), a common finding in children with SMA. Uninfected erythrocytes can continue to be removed from circulation even following malaria treatment, suggesting that this phenomenon is not due to their continued invasion and rupture by parasites (27). A mathematical model suggested that for each lysed infected erythrocyte, a further 8.5 uninfected erythrocytes are destroyed (19). Erythrophagocytosis by macrophages in the spleen and liver is the most likely mechanism responsible for excessive destruction of uninfected red cells, since it is a common finding in these organs in patients who die from malaria (28, 56).
Fc-gamma receptors (FcγRs) on macrophages may play an important role in the pathogenesis of severe malaria by mediating phagocytosis of red cells and triggering the production of proinflammatory cytokines. Three different classes of FcγRs have been found on human leukocytes, differing in their cellular distribution, function, and affinity for IgG subclasses (44, 47). FcγRIII exists in two isoforms, FcγRIIIA and FcγRIIIB. The latter is expressed only on polymorphonuclear leukocytes (47). FcγRIIIA has little affinity for monomeric IgG but binds polymeric IgG and immune complexes (ICs) efficiently (44, 47). The interaction of ICs with FcγRIIIA expressed on monocytes/macrophages leads to their activation and triggers various effector functions, such as phagocytosis and secretion of proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) (44), which has been linked to the pathogenesis of severe malaria (8). Children with severe P. falciparum malaria have relatively high levels of ICs (29, 30, 52), and red blood cells of children with SMA have increased surface IgG (52). The antigens in these ICs are released by the parasites into the culture media or into the circulation (4, 5, 43). Some of these antigens, such as erythrocyte binding antigen 175 (EBA-175), merozoite surface protein 1 (MSP-1), or ring surface protein 2 (RSP2), can bind to the surface of red cells either passively or during aborted invasions (5, 24, 35). In the case of RSP2, it was shown that antibodies against it can promote phagocytosis of uninfected red cells and erythroid precursors (24). Therefore, children with severe malaria have the immunological environment that could trigger FcγR-mediated effector mechanisms resulting in increased erythrophagocytosis and production of proinflammatory cytokines.
Debets et al. (1988) (11) demonstrated that TNF-α secretion by monocytes after FcγR cross-linking varied considerably from one individual to another. Furthermore, clearance half-times of IgG-sensitized red cells were dissimilar in patients with acute P. falciparum malaria and directly correlated with hematocrit values (18, 25), indicating that FcγR-mediated clearance of uninfected erythrocytes may be important in the development of severe anemia in P. falciparum malaria. The heterogeneity of responses to stimulatory signals among monocytes from different individuals suggests the possibility that those individuals with responses leading to enhanced erythrophagocytic activity and/or production of high levels of TNF-α may be more susceptible to the development of severe malaria. This heterogeneity may arise from factors such as the presence of polymorphic variants of FcγR that differ in the efficiencies of their signal transduction cascades, affinities for ICs, or levels of expression. A number of polymorphisms of FcγRIIA and FcγRIIIA have been identified that influence the binding affinity for IgG and their subclass specificity (13, 21, 22, 59, 60).
Another possible explanation for the diversity of response to FcγR engagement is the composition and maturation profile of the monocyte population. Different monocyte subpopulations with distinct phenotypic and functional properties have been found in the human peripheral blood based on the level of expression of the lipopolysaccharide (LPS) receptor CD14 (CD14low [CD14+] or CD14high [CD14++]) and the presence or absence of the CD16/FcγRIIIA receptor (53). In vitro studies have shown that cells with the CD14+ CD16+ phenotype are derived from the more common CD14++ CD16− blood monocytes by maturation that includes upregulation of CD16 and downregulation of CD14 (1). There is also an intermediate monocyte phenotype that expresses CD16 and is strongly positive for CD14 (CD14++ CD16+) (61). The fourth monocyte subset, CD14+ CD16−, is known to be a precursor subpopulation for dendritic cells (61). The CD14+ CD16+ monocyte subpopulation has been shown to be more proinflammatory, as evidenced by higher production of TNF-α than that of CD14++ CD16− monocytes (2). Therefore, the balance between the different subpopulations of monocytes may be an important determinant of the response to stimulatory signals and the outcome of any infection.
Despite the importance of FcγRIIIA in determining the immunological response to IC-mediated stimulation, very little is known about the contribution of this receptor to the pathogenesis of severe malaria. Therefore, the purpose of this study was to determine if there are differences in the expression patterns of CD16/FcγRIIIA between the different monocyte subpopulations, defined by their CD14 levels among children with SMA, CM, or uncomplicated malaria, that could explain differential susceptibility to malaria complications.
MATERIALS AND METHODS
Patients and study design.
Participants were recruited under a human use protocol approved by the Human Use Research Committee of the Walter Reed Army Institute of Research, Silver Spring, MD, and the National Ethics Review Committee of the Kenya Medical Research Institute, Nairobi, Kenya. Informed consent was obtained from all parents or guardians. The study had a matched case-control design. SMA cases, defined as children with asexual P. falciparum parasitemia as determined by Giemsa-stained thick and thin blood smears and a hemoglobin (Hb) level of ≤6 g/dl, were recruited from the pediatric ward of the Nyanza Provincial General Hospital (NPGH), Kisumu, Kenya, where malaria is holoendemic. Because CM is uncommon in this area, CM cases were recruited from the pediatric ward of the Kisii District Hospital (KDH), as well as from the NPGH. KDH is located in the highlands of western Kenya, where transmission is seasonal, and consequently receives many more CM cases than the NPGH (16). CM was defined as asexual P. falciparum parasitemia by Giemsa-stained blood smear and a Blantyre coma score of ≤2 (31), lasting at least 30 min if there was a history of convulsions. Uncomplicated malaria controls matched by age ±2 months were assigned to each case at a case-control ratio of 1:1 for SMA and 1:1 to 1:2 for CM and were identified from the outpatient clinic of the same hospital where the corresponding case was recruited. Uncomplicated malaria controls were defined as children with a normal mental status, an Hb level of >6 g/dl, a Giemsa-stained blood smear positive for asexual P. falciparum, and an axillary temperature of ≥37.5°C. In the absence of fever, we required two of the following signs or symptoms: nausea/vomiting, irritability, poor feeding, myalgias, or headache. General exclusion criteria also included evidence of concomitant serious infections (i.e., meningitis excluded by lumbar puncture when indicated, pneumonia, and sepsis), chronic illness, or a history of blood transfusion in the 3 months preceding enrollment to avoid the influence of donor red cells in our measurements.
All study participants were evaluated in a standardized fashion at enrollment (visit 1) and at follow-up 2 months later (visit 2). During follow-up, a blood sample was obtained once it was confirmed that the child was asymptomatic and free of parasitemia. If malaria persisted at the first follow-up visit, the child was re-treated and reevaluated 2 weeks later. Inpatient treatment for malaria consisted of intravenous quinine, and outpatient therapy was with artemether/lumefantrine (23).
Blood collection and processing.
Giemsa-stained thick and thin blood smears were prepared from capillary blood obtained by finger prick. A 2.5-ml sample of venous blood was obtained at enrollment and 5.0 ml at follow-up. The blood was aliquoted into collection tubes containing sodium heparin or EDTA (Becton-Dickinson, San Diego, CA). EDTA-anticoagulated blood was used for determination of complete blood count using a hematology analyzer (Coulter, Hialeah, FL). Samples were kept at 4°C until arrival in the laboratory, where a single investigator blinded to the clinical status of the participants processed all the specimens. Heparinized blood was used to obtain plasma for storage at −80°C and for in vitro stimulation and flow cytometry.
Preparation of ICs.
Bovine serum albumin (BSA) (Sigma-Aldrich, St. Louis, MO) and rabbit anti-BSA IgG antibody (Sigma-Aldrich) were made endotoxin free by passage through endotoxin-removing gel (Thermo Fisher Scientific, Rockford, IL). ICs were prepared as previously described (40), by mixing the endotoxin-free BSA and rabbit anti-BSA in an antigen/antibody ratio of 1:163 (wt/wt) in order to obtain precipitating complexes at the point of antigen-antibody equivalence. The reaction mixture was incubated at 37°C for 1 h and thereafter at 4°C overnight. The formed ICs were centrifuged at 10,000 rpm for 10 min at 4°C, and the supernatant was discarded. The ICs were washed three times by resuspending them in sterile phosphate-buffered saline (PBS). A 10-μl aliquot of the ICs was dissolved in 90 μl of 0.1 N NaOH prior to determination of the protein concentration by UV spectrophotometry against a known protein standard. The IC was aliquoted in volumes of 30 μl and stored at −70°C.
Detection of CD16/FcγRIIIA on monocytes from whole blood.
All staining procedures were carried out in 96-well U-bottom plates (Nunclon, Denmark), and all incubations were at 4°C for 30 min. Fifty microliters of heparinized whole blood was added to each of five wells, and the cells were then washed twice with cold wash buffer (PBS [pH 7.2] containing 1% BSA). For direct fluorescence staining, a panel of directly conjugated antibodies (Becton-Dickinson, Belgium) consisting of a combination of peridinin chlorophyll protein (PerCP)-conjugated anti-CD14 (clone MφP9) and fluorescein isothiocyanate (FITC)-conjugated anti-CD16 (clone 3G8) or FITC-conjugated IgG1 isotype control (clone MOPC-21) was used at 1:10 dilutions in PBS. The erythrocytes were lysed in 200 μl of lysis solution (NH4Cl [1.5 mM], NaHCO3 ′100 mM], and Na2EDTA [10 mM]) for 3 min on ice three times. Following lysis of the erythrocytes, the leukocytes were washed and then fixed in 150 μl of 1% paraformaldehyde and stored in the dark at 4°C until acquisition within 24 h of staining.
In vitro whole blood stimulation and intracellular TNF-α staining.
Fifty-microliter aliquots of each heparinized blood sample was added to 100 μl of serum-free RPMI 1640 (Sigma-Aldrich) and incubated in a 96-well flat-bottom tissue culture plate (Costar, Cambridge, MA) with 50 μg/ml of endotoxin-free BSA-anti-BSA immune complex in the presence of brefeldin A (BA) (Sigma-Aldrich) or with BA and no IC (unstimulated control) for 4 h at 37°C in an atmosphere containing 5% CO2. Other control stimuli included 1 μg/ml of Escherichia coli 055:B5 LPS (Sigma-Aldrich) and IgG anti-BSA at 50 μg/ml. The final volume in each well was 150 μl, and the concentration of BA in the culture was 10 μg/ml. At the end of the incubation period, the cells were directly stained with a combination of PerCP-conjugated anti-CD14 (clone MφP9) and FITC-conjugated anti-CD16 (clone 3G8) as described above. After surface labeling, the cells were subsequently incubated with 100 μl of 1% saponin in wash buffer (PBS [pH 7.2] containing 1% BSA, 0.1% sodium azide) containing a 1:10 dilution of one of the following: phycoerythrin (PE)-conjugated anti-TNF-α (Clone Mab11), PE-conjugated IgG1 isotype control (Clone MOPC21), or no antibody for 30 min at 4°C. The stained cells were washed once using cold wash buffer, fixed in 150 μl of 1% paraformaldehyde, and stored in the dark at 4°C until acquisition within 24 h of staining.
Flow cytometric analysis.
For each sample, a total of 40,000 events were acquired and analyzed with the Win FCM and EXPO version 2 software packages (Applied Cytometry Systems, Sheffield, United Kingdom). Monocytes were identified and gated on a plot of side scatter (SSC) versus anti-CD14 FITC (Fig. 1A) and additionally by their staining pattern with anti-CD16 in combination with anti-CD14 (Fig. 1B). The regions associated with CD16+ monocytes were analyzed for the level of expression of this marker. In addition, all subpopulations were analyzed for intracellular TNF-α production in response to IC stimulation. Expression data are presented as the median fluorescence intensity (MFI) of the population or as the percent positive cells from the total gated monocytes.
FIG. 1.
Identification and analysis of monocyte subpopulations. (A) Monocytes were identified and gated based on CD14 staining and side-scatter characteristics. (B) Gated monocytes were subdivided into monocyte subpopulations on the basis of CD14 and CD16 staining characteristics. Subpopulations are defined as CD14+ CD16+ (quadrant 1), CD14++ CD16+ (quadrant 2), CD14++ CD16− (quadrant 3), and CD14+ CD16− (quadrant 4).
Statistical analysis.
Statistical analysis was performed using SPSS for Windows, version 11.5, software (SPSS Inc., Chicago, IL). Statistical differences of continuous numerical data between enrollment and follow-up values or between matched cases and controls were determined using the general linear model (GLM), a one-way analysis of variance procedure, with matching. Statistical differences between SMA cases, CM cases, and CM controls were explored using GLM with Dunnett's test for multiple comparisons using SMA cases as a reference. Parasite densities were log10 (parasite density + 1) transformed to normalize the data prior to statistical analysis. The Mann-Whitney U test or the independent-samples t test was used to identify statistical differences between any two unrelated groups. Pearson's correlation was used to examine the relationship between any two variables. All tests were two-tailed with α values of ≤0.05.
RESULTS
Patient population.
Sixteen CM cases and 41 SMA cases were enrolled and matched to 25 and 40 uncomplicated malaria controls, respectively. The demographics and hemoglobin levels of these children are summarized in Table 1. In keeping with previous reports (51, 57), children with cerebral malaria were older than children with severe malarial anemia. There was no significant difference in parasite density between cases and controls or between SMA and CM cases.
TABLE 1.
Demographic data and hemoglobin levels of study participants
| Parameter | Value for groupa |
|||
|---|---|---|---|---|
| Cerebral malaria |
Severe anemia |
|||
| CM | CMC | SMA | SMAC | |
| No. enrolled | 16 | 25 | 41 | 40 |
| Mean age in mo (SD) | 31.9 (14.1) | 29.7 (13.8) | 18.0 (11.8) | 18.1 (11.7) |
| No. (%) female | 6 (37.5) | 9 (36.0) | 16 (39.0) | 15 (37.5) |
| Mean no. of parasites × 10−3/μl (SD) | 61.0 (59.4) | 118.9 (151.0) | 99.2 (89.6) | 92.3 (135.9) |
| Mean hemoglobin (g/dl) (SD) | 8.9 (2.1) | 10.0 (1.6) | 5.0 (0.8) | 9.1 (1.6) |
CM, cerebral malaria cases; CMC, cerebral malaria controls; SMA, severe anemia cases; SMAC, severe anemia controls.
Monocyte subsets and expression of CD16/FcγRIIIA.
Children with SMA had higher concentrations of leukocytes, monocytes, and monocyte subpopulations at enrollment than other groups (Table 2), but these differences disappeared when expressed as percentages. SMA cases, SMA controls, and CM cases all showed a significant increase in the proportion of CD16+ monocytes at enrollment compared to convalescent-phase levels, the majority of which were of the CD14++ phenotype. Only SMA cases and SMA controls showed significant elevation in the concentration of CD14+ CD16+ monocytes at enrollment compared to that at convalescence.
TABLE 2.
Concentrations and proportions of leukocytes and monocyte subsets in the different clinical groups at visit 1 and visit 2
| Parameter | Value for group and visit (na)b |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SMA |
SMAC |
CM |
CMC |
|||||||||
| Visit 1 (41) | Visit 2 (35) | P valuec | Visit 1 (40) | Visit 2 (33) | P valuec | Visit 1 (16) | Visit 2 (14) | P valuec | Visit 1 (25) | Visit 2 (22) | P valuec | |
| Total leukocytes (×10−3)/μl | 15.2 (7.5) | 10.2 (3.0) | <0.001 | 11.4 (3.9)d | 10.7 (4.2) | 0.394 | 10.5 (3.1)d | 9.1 (2.0) | 0.417 | 8.5 (3.0)d | 9.8 (4.0) | 0.093 |
| % Monocytes | 11.3 (5.5) | 5.7 (2.3) | <0.001 | 9.2 (3.9) | 5.9 (2.8) | <0.001 | 7.6 (4.4)e | 5.9 (1.9) | 0.145 | 6.8 (3.6)d | 4.9 (1.3) | 0.033 |
| Total monocytes/μl | 1797 (1567) | 574 (270) | <0.001 | 1036 (562)d | 570 (225) | <0.001 | 803 (501)d | 541 (237) | 0.089 | 587 (404)d | 489 (298) | 0.474 |
| Monocyte subsets | ||||||||||||
| % CD16+ | 45.6 (17.0) | 33.3 (16.9) | <0.001 | 47.7 (17.2) | 29.4 (13.9) | <0.001 | 51.1 (19.8) | 36.9 (18.4) | 0.023 | 43.7 (14.6) | 39.3 (17.5) | 0.366 |
| No. of CD16+ monocytes/μl | 895 (960) | 210 (182) | <0.001 | 498 (319)e | 159 (98) | <0.001 | 429 (374)e | 179 (86) | 0.018 | 253 (206)d | 213 (244) | 0.680 |
| % CD14+ CD16+ | 15.3 (13.2) | 14.4 (5.2) | 0.269 | 18.8 (13.8) | 14.0 (7.3) | 0.061 | 14.1 (9.3) | 16.8 (6.9) | 0.333 | 16.8 (11.2) | 16.9 (6.2) | 0.980 |
| No. of CD14+ CD16+ monocytes/μl | 247 (273) | 86 (57) | <0.001 | 184 (152) | 73 (46) | <0.001 | 98 (69)e | 81 (33) | 0.485 | 108 (138)e | 76 (35) | 0.370 |
| % CD14++ CD16+ | 30.6 (13.6) | 19.1 (16.1) | <0.001 | 29.1 (15.3) | 15.8 (11.3) | <0.001 | 37.2 (18.4) | 20.3 (15.7) | 0.001 | 27.2 (12.4) | 22.6 (17.1) | 0.249 |
| No. of CD14++ CD16+ monocytes/μl | 653 (859) | 124 (143) | <0.001 | 313 (260)e | 86 (76) | <0.001 | 333 (341) | 98 (73) | 0.008 | 148 (103)d | 137 (218) | 0.960 |
| % CD14+ CD16− | 9.2 (13.5) | 11.1 (5.3) | <0.001f | 8.7 (4.7) | 9.4 (4.0) | 0.584 | 8.1 (3.6) | 14.8 (13.3) | 0.107 | 10.0 (4.3) | 12.3 (6.4) | 0.167 |
| No. of CD14+ CD16− monocytes/μl | 124 (105) | 58 (27) | <0.001 | 80 (41) | 51 (25) | 0.002 | 55 (27) | 71 (44) | 0.290 | 57 (41) | 52 (21) | 0.781 |
| % CD14++ CD16− | 45.3 (15.6) | 55.5 (14.1) | <0.001 | 43.7 (15.0) | 61.3 (12.8) | <0.001 | 40.9 (18.1) | 48.4 (19.5) | 0.058 | 46.4 (14.4) | 48.5 (16.3) | 0.643 |
| No. of CD14++ CD16 monocytes/μl | 782 (675) | 308 (155) | <0.001 | 459 (309)d | 350 (186) | 0.091 | 320 (248)d | 284 (232) | 0.502 | 277 (207)d | 220 (93) | 0.354 |
n, no. of patients.
CM, cerebral malaria cases; CMC, cerebral malaria controls; SMA, severe anemia cases; SMAC, severe anemia controls. Values are means(SDs).
P value for results for visit 1 versus those for visit 2 using the general linear model(GLM) with matching.
P < 0.01 for the comparison with SMA cases using GLM with Dunnett's multiple-comparison test.
P < 0.05 for the comparison with SMA cases using GLM with Dunnett's multiple comparison test.
P = 0.44 by independent-samples t test.
Due to a concern that endogenous ICs could block the anti-CD16 antibody, we carried out in vitro experiments to confirm that the range of IC concentration found in children with severe malaria (29, 30) did not result in significant inhibition of this antibody (data not shown). The expression of CD16/FcγRIIIA on the total CD16+ monocytes at enrollment was higher in children with SMA than in other groups, but this difference did not reach statistical significance compared to results for SMA controls (Fig. 2A). There were no differences among groups in CD16/FcγRIIIA expression at follow-up. When CD16+ monocytes were analyzed according to the level of CD14 expression, we observed that CD14+ CD16+ monocytes of SMA cases had the highest expression of CD16/FcγRIIIA at enrollment (Fig. 2B). Although the difference in CD16/FcγRIIIA expression between SMA cases and controls was of borderline significance (P = 0.05), exclusion of a single outlier from the SMA control group resulted in much greater significance (P = 0.02). Worth noting also is the observation that the only group that showed a significant decline in the expression of CD16/FcγRIIIA among CD14+ CD16+ monocytes at follow-up was the SMA group, which is consistent with the finding that the enrollment value represented a significant elevation above baseline that is unique to this group. With respect to the CD14++ CD16+ subset, we found a significant difference in CD16/FcγRIIIA expression only between SMA and CM controls at enrollment (Fig. 2C). Unlike the situation with the CD14+ CD16+ subset, we found significant or near-significant declines in the expression of CD16/FcγRIIIA on CD14++ CD16+ monocytes at follow-up in all groups.
FIG. 2.
The expression of CD16/FcγRIIIA on total CD16+ monocytes (A) or the CD14+ CD16+ (B) or CD14++ CD16+ (C) monocyte subpopulation from children within the SMA and CM cohorts at enrollment (visit 1) and follow-up visits (visit 2).
Intracellular TNF-α expression by monocyte subpopulations in response to IC stimulation.
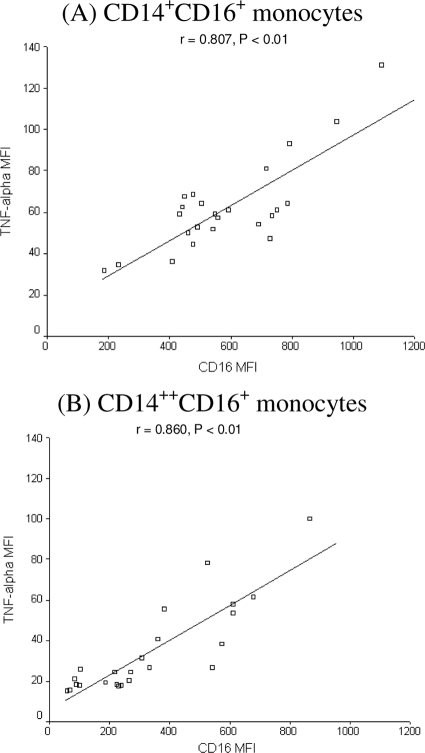
In order to assess the functional significance of the higher expression of CD16/FcγRIIIA, we measured the intracellular expression of TNF-α in response to stimulation with ICs. Whole-blood samples from 24 children at convalescence (for the SMA group, n = 9; for SMA controls, n = 10; for CM controls, n = 5), were stimulated with IC, and the intracellular expression of TNF-α was measured by flow cytometry. CD14+ CD16+ monocytes had the highest percentage of TNF-α positive cells, followed by CD14++ CD16+ cells (Fig. 3). On the other hand, very few CD16− monocytes produced TNF-α after stimulation. In addition, the level of expression of CD16/FcγRIIIA on CD14+ CD16+ (Fig. 4A) and CD14++ CD16+ (Fig. 4B) monocytes correlated positively with their levels of intracellular TNF-α production in response to IC stimulation. These data suggest that the increases in CD16/FcγRIIIA expression on monocytes, in particular CD14+ CD16+ monocytes, during P. falciparum infection can enhance their capacity to produce TNF-α in response to ICs.
FIG. 3.
Intracellular TNF-α production by different monocyte subpopulations stimulated with IC compared to that of unstimulated, IgG and LPS-treated controls. Results are expressed as mean values ± standard deviations of percentages of monocytes from 24 children (SMA group [n = 9], SMA controls [n = 10], and CM controls [n = 5]) that produced TNF-α. CD14+ CD16+ monocytes had the highest percentage of TNF-α-positive cells, followed by the CD14++ CD16+ subset. TNF-α expression by CD14+ CD16− and CD14++ CD16− monocytes in response to IC stimulation was negligible.
FIG. 4.
Correlation between CD16/FcγRIIIA expression levels on CD14+ CD16+ (A) and CD14++ CD16+ (B) monocyte subpopulations with TNF-α production levels in response to IC stimulation.
Relationship between CD16/FcγRIIIA expression on monocytes and hemoglobin levels of children with SMA and their controls.
Children with SMA are known to have circulating red cells coated with IgG (58). Because IgG-coated red cells formed during P. falciparum infection could engage CD16/FcγRIIIA on monocytes and macrophages, leading to their accelerated destruction by erythrophagocytosis, we explored the relationship between hemoglobin and the CD16/FcγRIIIA expression level in children with SMA and their controls at enrollment. We observed a significant inverse correlation between CD16/FcγRIIIA expression on CD16+ cells and hemoglobin levels (Fig. 5A). This relationship was stronger for CD14+ CD16+ monocytes (Fig. 5B) than for CD14++ CD16+ monocytes (Fig. 5C), suggesting that the former make a greater contribution to the decline in hemoglobin. These relationships were not observed in children with CM or in CM controls (data not shown).
FIG. 5.
Correlation between hemoglobin and CD16/FcγRIIIA expression levels on total CD16+ monocytes (A) or the CD14+ CD16+ (B) or CD14++ CD16+ (C) monocyte subpopulation from SMA cases and controls.
DISCUSSION
Monocytes/macrophages are thought to play an important role in the pathogenesis of SMA. Macrophage effector mechanisms that can contribute to this complication include phagocytosis and production of proinflammatory cytokines. These effector mechanisms can be mediated via engagement of FcγRIIIA receptors by IgG-coated red cells, leading to erythrophagocytosis, and/or by ICs, leading to production of proinflammatory cytokines. We hypothesized that children with SMA have increased levels of FcγRIIIA on their monocytes, which may predispose them to this complication. Therefore, we set out to measure the expression of FcγRIIIA on monocytes of children with SMA, CM, and uncomplicated malaria. Because of the important functional differences between CD14+ and CD14++ monocytes, we examined these two subpopulations separately.
The pattern of CD16/FcγRIIIA expression on monocytes of children with SMA was very distinctive compared to findings for the other forms of clinical malaria. At enrollment, CD16+ monocytes of children with SMA had high expression of CD16/FcγRIIIA (Fig. 2A), which was mostly due to the contribution of CD14+ monocytes (Fig. 2B). Children with SMA had the highest expression of CD16/FcγRIIIA on CD14+ CD16+ monocytes at enrollment and were the only group in which this expression was higher at enrollment than at follow-up. Assuming that the follow-up sample is representative of the baseline state, this suggests that CD14+ CD16+ monocytes of children at risk for development of SMA overexpress CD16/FcγRIIIA in response to parasite infection whereas those of children who develop other forms of clinical malaria do not. In contrast, the expression of CD16/FcγRIIIA on CD14++ CD16+ monocytes did not differ significantly between children with SMA and most other groups at enrollment and seemed to be lower at follow-up than at enrollment for all the groups.
Data from various studies suggest that CD14+ CD16+ monocytes are more-mature cells with properties of tissue macrophages (61, 62). Moreover, CD16/FcγRIIIA expression increases with activation and maturation of monocytes to macrophages (1, 9, 61). Therefore, the finding of higher CD16/FcγRIIIA expression on CD14+ CD16+ monocytes of children with SMA than in other children with complicated or uncomplicated malaria together with the observed increases in the number of CD16+ monocytes may reflect distinct phenotypic and functional differences in the way monocytes of these different groups of children mature in response to P. falciparum infection. In mice, Ly6Clow monocytes are analogous to CD14+ CD16+ monocytes in humans whereas Ly6Chi monocytes are analogous to CD14++ CD16− monocytes in humans (54). Adaptive transfer experiments have shown that CD14+ CD16+ monocytes preferentially populate the liver and spleen (14). On the other hand, another study showed that Ly6Chi and Ly6Clow in spleens reflect peripheral levels (55). Therefore, it is likely that peripheral changes in these monocyte populations reflect changes in the spleen and liver, where active erythrophagocytosis of infected and uninfected red cells takes place.
Few other studies have explored the makeup and phenotypic distribution of monocytes in patients with malaria, but none in patients with severe malaria. A recent study revealed expansion of the CD14+ CD16+ subset in pregnant women with malaria compared with pregnant women without malaria, but this was only statistically significant for HIV+ women (20). Monocytic intervillous infiltrates are seen commonly in placentas from pregnant women with malaria and are associated with low birth weight (46). However, the phenotype of these placental monocytes has not been described. Nonetheless, it is interesting to speculate on whether peripheral and placental CD14+ CD16+ monocytes may play a role in severe malarial anemia and fetal complications with malaria during pregnancy. Last, expansion of the CD14+ CD16+ monocyte population in peripheral blood was also recently documented in adult patients with uncomplicated malaria, supporting our results (7).
Our findings demonstrate that there are important functional differences between CD14+ CD16+ and CD14++ CD16+ monocytes. Although both CD14+ CD16+ and CD14++ CD16+ monocytes showed a strong correlation between CD16/FcγRIIIA expression and TNF-α production upon IC stimulation (Fig. 4), a greater percentage of the former were able to produce this cytokine in response to stimulation (Fig. 3). Monocytes with a high level of CD16/FcγRIIIA expression may demonstrate more-pronounced effector mechanisms, such as phagocytosis and the production of proinflammatory cytokines (3, 36), such as TNF-α, which has been implicated in the pathogenesis of SMA by decreasing erythropoiesis (8). However, although TNF-α levels were elevated at enrollment for all groups, they did not differ between groups (data not shown). It is possible that bone marrow and spleen cytokine levels may be more relevant, as has been found for placental malaria, where placental TNF-α is higher than peripheral levels (45). The level of expression of CD16/FcγRIIIA on monocytes has also been shown to correlate with the in vivo clearance of autologous IgG-coated erythrocytes by tissue macrophages in patients with systemic lupus erythematosus (SLE) (49). Since red cells of children with SMA are known to have increased surface IgG (58), the higher expression of CD16/FcγRIIIA on their CD14+ CD16+ cells may result in increased erythrophagocytosis and more-severe anemia. Our finding of an inverse correlation between the level of expression of CD16/FcγRIIIA in CD14+ CD16+ monocytes and the hemoglobin level in children with SMA and their controls is consistent with this proposition.
The molecular basis of the overexpression of FcγRIIIA is not clear. Increased expression of FcγRIIIA could result from the engagement of toll-like receptors by parasite ligands, such as glycosylphosphatidyl inositol and/or parasite DNA/hemozoin complexes (34, 42). A number of studies have shown that patients with rheumatoid arthritis, an IC-mediated disease (12, 41), overexpress FcγRIIIA on peripheral as well as synovial macrophages (3, 17). Individuals with the FcγRIIIA 158V allele have increased transcription and expression of this receptor on NK cells, but the same has not been reported for macrophages (15). Furthermore, individuals with the same allele have also been shown to be at increased risk for rheumatoid arthritis (6, 32). These findings suggest that increased expression of FcγRIIIA may be in part driven by more-efficient transcription of polymorphic variants and may affect susceptibility to diseases in which ICs make an important contribution to pathogenesis.
Very little is known about the relationship between FcγRIIIA polymorphisms and malaria. In the only study done to date, Omi et al. (37) did not observe any association between the presence of cerebral malaria or other types of severe malaria and the presence of FcγRIIIA 158-F/V polymorphisms. However, in this study there was not sufficient power to analyze the relationship between these polymorphisms and SMA. Other studies have analyzed more extensively the relationship between the FcγRIIA H/R-131 polymorphism and severe malaria. FcγRIIA is another low-affinity IgG receptor that is found on monocytes and granulocytes. The results of these studies have been inconsistent. While one study found that the H/R genotype is more common in children at risk for severe malaria (50), other studies have shown an association between the H/H genotype or the H allele and severe malaria (10, 38). Yet others have found no association (39) or that the H/H genotype is protective (33). The discrepancy in results likely comes from differences in study design, ethnic groups studied, and the pattern of malaria transmission at each study site.
To the best of our knowledge, our study is the first to provide a link between a distinct monocyte/macrophage phenotype and the development of SMA by demonstrating that a subpopulation of mature monocytes from these children responds differently to P. falciparum infection by overexpressing CD16/FcγRIIIA. Further studies of the molecular mechanisms involved in the upregulation of CD16/FcγRIIIA during malaria infection and of the identification of genetic polymorphisms that may explain the distinct phenotypic characteristic of monocytes from children with SMA should be pursued.
Acknowledgments
This work was supported by NIH grant HL71502 to José A. Stoute.
We thank the children and their parents for their participation and cooperation.
Editor: J. H. Adams
Footnotes
Published ahead of print on 15 March 2010.
REFERENCES
- 1.Andreesen, R., W. Brugger, C. Scheibenbogen, M. Kreutz, H. G. Leser, A. Rehm, and G. W. Lohr. 1990. Surface phenotype analysis of human monocyte to macrophage maturation. J. Leukoc. Biol. 47:490-497. [DOI] [PubMed] [Google Scholar]
- 2.Belge, K. U., F. Dayyani, A. Horelt, M. Siedlar, M. Frankenberger, B. Frankenberger, T. Espevik, and L. Ziegler-Heitbrock. 2002. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J. Immunol. 168:3536-3542. [DOI] [PubMed] [Google Scholar]
- 3.Blom, A. B., T. R. Radstake, A. E. Holthuysen, A. W. Sloetjes, G. J. Pesman, F. G. Sweep, F. A. van de Loo, L. A. Joosten, P. Barrera, P. L. van Lent, and W. B. van den Berg. 2003. Increased expression of Fcgamma receptors II and III on macrophages of rheumatoid arthritis patients results in higher production of tumor necrosis factor alpha and matrix metalloproteinase. Arthritis Rheum. 48:1002-1014. [DOI] [PubMed] [Google Scholar]
- 4.Bygbjerg, I. C., S. Jepsen, T. G. Theander, and N. Odum. 1985. Specific proliferative response of human lymphocytes to purified soluble antigens from Plasmodium falciparum in vitro cultures and to antigens from malaria patients' sera. Clin. Exp. Immunol. 59:421-426. [PMC free article] [PubMed] [Google Scholar]
- 5.Camus, D., and T. J. Hadley. 1985. A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science 230:553-556. [DOI] [PubMed] [Google Scholar]
- 6.Chen, J. Y., C. M. Wang, J. M. Wu, H. H. Ho, and S. F. Luo. 2006. Association of rheumatoid factor production with FcgammaRIIIa polymorphism in Taiwanese rheumatoid arthritis. Clin. Exp. Immunol. 144:10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chimma, P., C. Roussilhon, P. Sratongno, R. Ruangveerayuth, K. Pattanapanyasat, J. L. Perignon, D. J. Roberts, and P. Druilhe. 2009. A distinct peripheral blood monocyte phenotype is associated with parasite inhibitory activity in acute uncomplicated Plasmodium falciparum malaria. PLoS Pathog. 5:e1000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark, I. A., and G. Chaudhri. 1988. Tumour necrosis factor may contribute to the anaemia of malaria by causing dyserythropoiesis and erythrophagocytosis. Br. J. Haematol. 70:99-103. [DOI] [PubMed] [Google Scholar]
- 9.Clarkson, S. B., and P. A. Ory. 1988. CD16. Developmentally regulated IgG Fc receptors on cultured human monocytes. J. Exp. Med. 167:408-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooke, G. S., C. Aucan, A. J. Walley, S. Segal, B. M. Greenwood, D. P. Kwiatkowski, and A. V. Hill. 2003. Association of Fcgamma receptor IIa (CD32) polymorphism with severe malaria in West Africa. Am. J. Trop. Med. Hyg. 69:565-568. [PubMed] [Google Scholar]
- 11.Debets, J. M., C. J. van der Linden, I. E. Dieteren, J. F. Leeuwenberg, and W. A. Buurman. 1988. Fc-receptor cross-linking induces rapid secretion of tumor necrosis factor (cachectin) by human peripheral blood monocytes. J. Immunol. 141:1197-1201. [PubMed] [Google Scholar]
- 12.De Clerck, L. S. 1995. B lymphocytes and humoral immune responses in rheumatoid arthritis. Clin. Rheumatol. 14:14-18. [DOI] [PubMed] [Google Scholar]
- 13.de Haas, M., H. R. Koene, M. Kleijer, E. de Vries, S. Simsek, M. J. van Tol, D. Roos, and A. E. von dem Borne. 1996. A triallelic Fc gamma receptor type IIIA polymorphism influences the binding of human IgG by NK cell Fc gamma RIIIa. J. Immunol. 156:2948-2955. [PubMed] [Google Scholar]
- 14.Geissmann, F., S. Jung, and D. R. Littman. 2003. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19:71-82. [DOI] [PubMed] [Google Scholar]
- 15.Hatjiharissi, E., L. Xu, D. D. Santos, Z. R. Hunter, B. T. Ciccarelli, S. Verselis, M. Modica, Y. Cao, R. J. Manning, X. Leleu, E. A. Dimmock, A. Kortsaris, C. Mitsiades, K. C. Anderson, E. A. Fox, and S. P. Treon. 2007. Increased natural killer cell expression of CD16, augmented binding and ADCC activity to rituximab among individuals expressing the Fc(gamma)RIIIa-158 V/V and V/F polymorphism. Blood 110:2561-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hay, S. I., A. M. Noor, M. Simba, M. Busolo, H. L. Guyatt, S. A. Ochola, and R. W. Snow. 2002. Clinical epidemiology of malaria in the highlands of western Kenya. Emerg. Infect. Dis. 8:543-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hepburn, A. L., J. C. Mason, and K. A. Davies. 2004. Expression of Fcgamma and complement receptors on peripheral blood monocytes in systemic lupus erythematosus and rheumatoid arthritis. Rheumatology (Oxford) 43:547-554. [DOI] [PubMed] [Google Scholar]
- 18.Ho, M., N. J. White, S. Looareesuwan, Y. Wattanagoon, S. H. Lee, M. J. Walport, D. Bunnag, and T. Harinasuta. 1990. Splenic Fc receptor function in host defense and anemia in acute Plasmodium falciparum malaria. J. Infect. Dis. 161:555-561. [DOI] [PubMed] [Google Scholar]
- 19.Jakeman, G. N., A. Saul, W. L. Hogarth, and W. E. Collins. 1999. Anaemia of acute malaria infections in non-immune patients primarily results from destruction of uninfected erythrocytes. Parasitology 119:127-133. [DOI] [PubMed] [Google Scholar]
- 20.Jaworowski, A., D. D. Kamwendo, P. Ellery, S. Sonza, V. Mwapasa, E. Tadesse, M. E. Molyneux, S. J. Rogerson, S. R. Meshnick, and S. M. Crowe. 2007. CD16+ monocyte subset preferentially harbors HIV-1 and is expanded in pregnant Malawian women with Plasmodium falciparum malaria and HIV-1 infection. J. Infect. Dis. 196:38-42. [DOI] [PubMed] [Google Scholar]
- 21.Kim, T. D., S. E. Cho, C. H. Yang, and J. Kim. 2001. Analysis of Fc gammaRIII and IgG Fc polymorphism reveals functional and evolutionary implications of protein-protein interaction. J. Mol. Evol. 53:1-9. [DOI] [PubMed] [Google Scholar]
- 22.Koene, H. R., M. Kleijer, J. Algra, D. Roos, A. E. von dem Borne, and M. de Haas. 1997. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood 90:1109-1114. [PubMed] [Google Scholar]
- 23.Kokwaro, G., L. Mwai, and A. Nzila. 2007. Artemether/lumefantrine in the treatment of uncomplicated falciparum malaria. Expert Opin. Pharmacother. 8:75-94. [DOI] [PubMed] [Google Scholar]
- 24.Layez, C., P. Nogueira, V. Combes, F. T. Costa, I. Juhan-Vague, L. H. da Silva, and J. Gysin. 2005. Plasmodium falciparum rhoptry protein RSP2 triggers destruction of the erythroid lineage. Blood 106:3632-3638. [DOI] [PubMed] [Google Scholar]
- 25.Lee, S. H., S. Looareesuwan, Y. Wattanagoon, M. Ho, V. Wuthiekanun, N. Vilaiwanna, D. J. Weatherall, and N. J. White. 1989. Antibody-dependent red cell removal during P. falciparum malaria: the clearance of red cells sensitized with an IgG anti-D. Br. J. Haematol. 73:396-402. [DOI] [PubMed] [Google Scholar]
- 26.Looareesuwan, S., M. Ho, Y. Wattanagoon, N. J. White, D. A. Warrell, D. Bunnag, T. Harinasuta, and D. J. Wyler. 1987. Dynamic alteration in splenic function during acute falciparum malaria. N. Engl. J. Med. 317:675-679. [DOI] [PubMed] [Google Scholar]
- 27.Looareesuwan, S., A. H. Merry, R. E. Phillips, R. Pleehachinda, Y. Wattanagoon, M. Ho, P. Charoenlarp, D. A. Warrell, and D. J. Weatherall. 1987. Reduced erythrocyte survival following clearance of malarial parasitaemia in Thai patients. Br. J. Haematol. 67:473-478. [DOI] [PubMed] [Google Scholar]
- 28.Maegraith, B. 1948. Pathological processes in malaria and blackwater fever. Blackwell Scientific, Oxford, United Kingdom.
- 29.Mibei, E. K., A. S. Orago, and J. A. Stoute. 2005. Immune complex levels in children with severe Plasmodium falciparum malaria. Am. J. Trop. Med. Hyg. 72:593-599. [PubMed] [Google Scholar]
- 30.Mibei, E. K., W. O. Otieno, A. S. Orago, and J. A. Stoute. 2008. Distinct pattern of class and subclass antibodies in immune complexes of children with cerebral malaria and severe malarial anaemia. Parasite Immunol. 31:61-63. [DOI] [PubMed] [Google Scholar]
- 31.Molyneux, M. E., T. E. Taylor, J. J. Wirima, and A. Borgstein. 1989. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q. J. Med. 71:441-459. [PubMed] [Google Scholar]
- 32.Morgan, A. W., B. Griffiths, F. Ponchel, B. M. Montague, M. Ali, P. P. Gardner, H. C. Gooi, R. D. Situnayake, A. F. Markham, P. Emery, and J. D. Isaacs. 2000. Fcgamma receptor type IIIA is associated with rheumatoid arthritis in two distinct ethnic groups. Arthritis Rheum. 43:2328-2334. [DOI] [PubMed] [Google Scholar]
- 33.Nasr, A., N. C. Iriemenam, M. Troye-Blomberg, H. A. Giha, H. A. Balogun, O. F. Osman, S. M. Montgomery, G. ElGhazali, and K. Berzins. 2007. Fc gamma receptor IIa (CD32) polymorphism and antibody responses to asexual blood-stage antigens of Plasmodium falciparum malaria in Sudanese patients. Scand. J. Immunol. 66:87-96. [DOI] [PubMed] [Google Scholar]
- 34.Nebl, T., M. J. De Veer, and L. Schofield. 2005. Stimulation of innate immune responses by malarial glycosylphosphatidylinositol via pattern recognition receptors. Parasitology 130:S45-S62. [DOI] [PubMed] [Google Scholar]
- 35.Nikodem, D., and E. Davidson. 2000. Identification of a novel antigenic domain of Plasmodium falciparum merozoite surface protein-1 that specifically binds to human erythrocytes and inhibits parasite invasion, in vitro. Mol. Biochem. Parasitol. 108:79-91. [DOI] [PubMed] [Google Scholar]
- 36.Nockher, W. A., and J. E. Scherberich. 1998. Expanded CD14+ CD16+ monocyte subpopulation in patients with acute and chronic infections undergoing hemodialysis. Infect. Immun. 66:2782-2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Omi, K., J. Ohashi, J. Patarapotikul, H. Hananantachai, I. Naka, S. Looareesuwan, and K. Tokunaga. 2002. Absence of association between the Fc gamma receptor IIIA-176F/V polymorphism and the severity of malaria in Thai. Jpn. J. Infect. Dis. 55:167-169. [PubMed] [Google Scholar]
- 38.Omi, K., J. Ohashi, J. Patarapotikul, H. Hananantachai, I. Naka, S. Looareesuwan, and K. Tokunaga. 2002. Fcgamma receptor IIA and IIIB polymorphisms are associated with susceptibility to cerebral malaria. Parasitol. Int. 51:361-366. [DOI] [PubMed] [Google Scholar]
- 39.Ouma, C., C. C. Keller, D. A. Opondo, T. Were, R. O. Otieno, M. F. Otieno, A. S. Orago, J. M. Ong'Echa, J. M. Vulule, R. E. Ferrell, and D. J. Perkins. 2006. Association of FCgamma receptor IIA (CD32) polymorphism with malarial anemia and high-density parasitemia in infants and young children. Am. J. Trop. Med. Hyg. 74:573-577. [PubMed] [Google Scholar]
- 40.Owuor, B. O., C. O. Odhiambo, W. O. Otieno, C. Adhiambo, D. W. Makawiti, and J. A. Stoute. 2007. Reduced immune complex binding capacity and increased complement susceptibility of red cells from children with severe malaria-associated anemia. Mol. Med. 14:89-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panayi, G. S. 2005. B cells: a fundamental role in the pathogenesis of rheumatoid arthritis? Rheumatology (Oxford) 44:ii3-ii7. [DOI] [PubMed] [Google Scholar]
- 42.Parroche, P., F. N. Lauw, N. Goutagny, E. Latz, B. G. Monks, A. Visintin, K. A. Halmen, M. Lamphier, M. Olivier, D. C. Bartholomeu, R. T. Gazzinelli, and D. T. Golenbock. 2007. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc. Natl. Acad. Sci. U. S. A. 104:1919-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perlmann, H., K. Berzins, M. Wahlgren, J. Carlsson, A. Bjorkman, M. E. Patarroyo, and P. Perlmann. 1984. Antibodies in malarial sera to parasite antigens in the membrane of erythrocytes infected with early asexual stages of Plasmodium falciparum. J. Exp. Med. 159:1686-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ravetch, J. V., and S. Bolland. 2001. IgG Fc receptors. Annu. Rev. Immunol. 19:275-290. [DOI] [PubMed] [Google Scholar]
- 45.Rogerson, S. J., H. C. Brown, E. Pollina, E. T. Abrams, E. Tadesse, V. M. Lema, and M. E. Molyneux. 2003. Placental tumor necrosis factor alpha but not gamma interferon is associated with placental malaria and low birth weight in Malawian women. Infect. Immun. 71:267-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogerson, S. J., E. Pollina, A. Getachew, E. Tadesse, V. M. Lema, and M. E. Molyneux. 2003. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am. J. Trop. Med. Hyg. 68:115-119. [PubMed] [Google Scholar]
- 47.Salmon, J. E., and L. Pricop. 2001. Human receptors for immunoglobulin G: key elements in the pathogenesis of rheumatic disease. Arthritis Rheum. 44:739-750. [DOI] [PubMed] [Google Scholar]
- 48.Salmon, M. G., J. B. de Souza, G. A. Butcher, and J. H. Playfair. 1997. Premature removal of uninfected erythrocytes during malarial infection of normal and immunodeficient mice. Clin. Exp. Immunol. 108:471-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seres, T., I. Csipo, E. Kiss, G. Szegedi, and M. Kavai. 1998. Correlation of Fc gamma receptor expression of monocytes with clearance function by macrophages in systemic lupus erythematosus. Scand. J. Immunol. 48:307-311. [DOI] [PubMed] [Google Scholar]
- 50.Shi, Y. P., B. L. Nahlen, S. Kariuki, K. B. Urdahl, P. D. McElroy, J. M. Roberts, and A. A. Lal. 2001. Fcgamma receptor IIa (CD32) polymorphism is associated with protection of infants against high-density Plasmodium falciparum infection. VII. Asembo Bay Cohort Project. J. Infect. Dis. 184:107-111. [DOI] [PubMed] [Google Scholar]
- 51.Snow, R. W., I. Bastos de Azevedo, B. S. Lowe, E. W. Kabiru, C. G. Nevill, S. Mwankusye, G. Kassiga, K. Marsh, and T. Teuscher. 1994. Severe childhood malaria in two areas of markedly different falciparum transmission in east Africa. Acta Trop. 57:289-300. [DOI] [PubMed] [Google Scholar]
- 52.Stoute, J. A., A. O. Odindo, B. O. Owuor, E. K. Mibei, M. O. Opollo, and J. N. Waitumbi. 2003. Loss of red blood cell-complement regulatory proteins and increased levels of circulating immune complexes are associated with severe malarial anemia. J. Infect. Dis. 187:522-525. [DOI] [PubMed] [Google Scholar]
- 53.Strauss-Ayali, D., S. M. Conrad, and D. M. Mosser. 2007. Monocyte subpopulations and their differentiation patterns during infection. J. Leukoc. Biol. 82:244-252. [DOI] [PubMed] [Google Scholar]
- 54.Sunderkotter, C., T. Nikolic, M. J. Dillon, N. Van Rooijen, M. Stehling, D. A. Drevets, and P. J. Leenen. 2004. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 172:4410-4417. [DOI] [PubMed] [Google Scholar]
- 55.Swirski, F. K., M. Nahrendorf, M. Etzrodt, M. Wildgruber, V. Cortez-Retamozo, P. Panizzi, J. L. Figueiredo, R. H. Kohler, A. Chudnovskiy, P. Waterman, E. Aikawa, T. R. Mempel, P. Libby, R. Weissleder, and M. J. Pittet. 2009. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325:612-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taliaferro, W. H., and H. W. Mulliga. 1936. The histopathology of malaria with special reference to the function and origin of the macrophages in defence. Thacker, Spink & Co., Ltd., Calcutta, India.
- 57.Waitumbi, J. N., B. Donvito, A. Kisserli, J. H. Cohen, and J. A. Stoute. 2004. Age-related changes in red blood cell complement regulatory proteins and susceptibility to severe malaria. J. Infect. Dis. 190:1183-1191. [DOI] [PubMed] [Google Scholar]
- 58.Waitumbi, J. N., M. O. Opollo, R. O. Muga, A. O. Misore, and J. A. Stoute. 2000. Red cell surface changes and erythrophagocytosis in children with severe Plasmodium falciparum anemia. Blood 95:1481-1486. [PubMed] [Google Scholar]
- 59.Warmerdam, P. A., P. W. Parren, A. Vlug, L. A. Aarden, J. G. van de Winkel, and P. J. Capel. 1992. Polymorphism of the human Fc gamma receptor II (CD32): molecular basis and functional aspects. Immunobiology 185:175-182. [DOI] [PubMed] [Google Scholar]
- 60.Warmerdam, P. A., J. G. van de Winkel, E. J. Gosselin, and P. J. Capel. 1990. Molecular basis for a polymorphism of human Fc gamma receptor II (CD32). J. Exp. Med. 172:19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ziegler-Heitbrock, H. W. 1996. Heterogeneity of human blood monocytes: the CD14+ CD16+ subpopulation. Immunol. Today 17:424-428. [DOI] [PubMed] [Google Scholar]
- 62.Ziegler-Heitbrock, H. W., G. Fingerle, M. Strobel, W. Schraut, F. Stelter, C. Schutt, B. Passlick, and A. Pforte. 1993. The novel subset of CD14+/CD16+ blood monocytes exhibits features of tissue macrophages. Eur. J. Immunol. 23:2053-2058. [DOI] [PubMed] [Google Scholar]