Abstract
Vibrio cholerae O1 and enterotoxigenic Escherichia coli (ETEC) are major bacterial pathogens that cause dehydrating disease requiring hospitalization of children and adults. The cholera toxin (CT) produced by V. cholerae O1 and the heat-labile toxin (LT) and/or heat-stable toxin (ST) of ETEC are responsible for secretory diarrhea. We have observed that about 13% of hospitalized diarrheal patients are concomitantly infected with V. cholerae O1 and ETEC. In order to understand the outcome of such dual infections on the clinical and immunological responses in cholera patients, we studied patients infected with V. cholerae O1 (group VC; n = 25), those infected with both V. cholerae O1 and ETEC (group VCET; n = 25), and those infected with ETEC only (group ET; n = 25). The VCET group showed more severe dehydration and had a higher intake of intravenous fluid and more vomiting than the ETEC group (P = 0.01 to 0.003). The VCET patients showed higher vibriocidal responses and increased antibody titers to cholera toxin and lipopolysaccharide (LPS) in plasma than did the V. cholerae O1 patients (P = 0.02 to <0.001). All responses in the V. cholerae O1 and in the VCET groups were more robust than those seen in the group infected with ETEC only (P = 0.01 to <0.001). We thus show that concomitant colonization with ETEC induces immune responses to V. cholerae antigens that are more robust than those seen with V. cholerae O1 infection alone. It is possible that LT or other factors expressed by ETEC may play a role as a mucosal adjuvant in enhancing the immune responses to V. cholerae O1.
Vibrio cholerae O1 and enterotoxigenic Escherichia coli (ETEC) are two major bacterial pathogens responsible for community-based infections and hospitalizations of both adults and children. Cholera and ETEC diarrhea together may account for nearly 50% of the million or so cases of diarrhea occurring annually in Bangladesh (11, 37). The pathogenesis of disease caused by V. cholerae O1 and that of disease caused by ETEC are similar. The cholera toxin (CT) produced by V. cholerae O1 and the heat-labile enterotoxin (LT) of ETEC share 80% identity at the nucleotide level (39). LT produced by ETEC is structurally, functionally, and immunologically very similar to CT produced by Vibrio cholerae. Each toxin consists of a toxin A subunit that shares 75% homology with each other (CTA and LTA, respectively), which is noncovalently associated with five identical B subunits (CTB and LTB, respectively) (43). LTB and CTB also show a high degree of homology to each other, with an 85% conservation of amino acids in the mature proteins. There is evidence from crystallographic studies that LTB and CTB pentamers are also structurally similar (25, 41, 42). There has been a high degree of immunological cross-reactivity between these proteins reported by many investigators, with both shared and unique epitopes (45); the majority of antibodies are directed against epitopes on the assembled pentamers. Both V. cholerae and ETEC share similar type II secretion systems for the secretion of these toxins (LT and CT) (9, 47). The small polypeptide heat-stable enterotoxin (ST) is an additional virulence factor of ETEC and may be present in strains with or without LT (52).
Differences in the immune responses stimulated by CT and LT have been observed in experimental studies with mouse models; CT induces more T-helper-2 (Th2)-associated cytokines (51), while LT triggers a more balanced T-cell response involving both Th1 and Th2 cells, cytokines, and antibody isotypes (46).
The two pathogens can both cause dehydrating disease requiring hospitalization and have similar modes of transmission. Both ETEC and V. cholerae O1 also have similar patterns of seasonality, with epidemic peaks biannually in Bangladesh (17, 37). The peak incidence of ETEC usually precedes that of V. cholerae O1 by a few weeks in the spring in Bangladesh (17). Studies in Bangladesh and other countries have shown that both ETEC and V. cholerae O1 can cause severe disease as well asymptomatic infections in household contacts and in the community, and the asymptomatic infection rates may vary from 9 to 42% for studies that have been carried out to assess this (19, 36, 37).
It has been shown by numerous studies carried out in developing countries (3, 10, 27) that more than one enteric pathogen can be detected in stool samples of diarrheal patients. Mixed pathogens are identifiable in 12% to 26% of individuals with diarrhea; ETEC and V. cholerae O1 can be isolated together in about 13% of samples from patients with acute watery diarrhea in Bangladesh (5, 12, 33, 40, 44). In addition, asymptomatic infection with ETEC is also common in children as well as adults (36, 37). However, an analysis of the clinical and immunological aspects of concomitant infection with V. cholerae O1 and ETEC has not previously been done. In the present study, we have enrolled patients hospitalized with V. cholerae O1 or ETEC infection or with infections with both pathogens simultaneously and have evaluated clinical and immunological responses to infection.
MATERIALS AND METHODS
Study design and subject enrollment.
The hospital at the Clinical Research and Service Centre of the International Centre for Diarrheal Disease Research (ICDDR,B) cares for ca. 110,000 patients each year. We included 75 patients in this study based on our case definition and monitored this cohort of patients prospectively for a period of 21 days after the onset of illness. From April 2003 to May 2006, we enrolled patients who received care for acute watery diarrhea at the ICDDR,B. Inclusion criteria included a positive stool culture for V. cholerae O1, ETEC, or both and an absence of significant comorbid conditions. These patients were categorized into three groups: those with V. cholerae O1 and ETEC detected in stool (VCET group; n = 25), those with only V. cholerae O1 detected in stool (VC group; n = 25), and those with only ETEC detected in stool (ET group; n = 25). We excluded patients that were positive for other pathogens. We first identified dually infected patients and then included individuals in the VC and ET groups, matching these individuals to patients in the VCET group by age, gender, and year of infection. We also matched the serotype of V. cholerae O1, Ogawa, or Inaba between the VCET and VC groups. Clinical examination and history forms were completed by the study physicians.
We assessed the degree of dehydration of patients according to WHO guidelines (49). We analyzed all stool samples for the presence of enteric pathogens, including V. cholerae O1, ETEC, Salmonella spp., Shigella spp., and Campylobacter jejuni. We also examined stool samples by direct microscopy to detect enteric parasites (50). Stool samples were evaluated immediately after arrival at the hospital, minimizing the possibility that mixed infections occurred due to nosocomial transmission in the hospital. Study day 1 was defined as the day of identification of the patient; patients were enrolled on day 2 if they had a positive stool culture for V. cholerae O1, ETEC, or both. Informed consent for participation in this research study was obtained from all patients or their guardians, and approval for this study was obtained from the Research and Ethical Review Committees of the ICDDR,B as well as from the Institutional Review Board of the Massachusetts General Hospital. Information regarding clinical features and demographics was collected from patients at enrollment following clinical stabilization.
Specimen collection and microbiological analyses of stool specimens.
We collected blood for blood grouping on day 2 and for immunological measurements on days 2, 7, and 21 following the onset of illness. Blood grouping was available for only a subset of patients with ETEC infection. All cases of cholera were confirmed by culturing of stool samples for V. cholerae O1 by use of standard procedures (32). To detect ETEC, we analyzed six lactose-fermenting E. coli colonies isolated from fresh stool samples cultured on MacConkey agar plates (34). These E. coli colonies were tested for the presence of heat-labile enterotoxin (LT) or heat-stable enterotoxin (ST) by using a multiplex PCR assay for the rapid detection of ETEC (4, 44, 45). PCR-positive colonies were confirmed by means of a ganglioside enzyme-linked immunosorbent assay (ELISA) (GM1-ELISA) procedure for the detection of LT and ST (44, 45). The E. coli colonies that tested positive for either toxin type were plated onto colonization factor (CF) antigen (CFA) agar plates, with and without bile salts, and tested for 13 colonization factors, CFA/I, CS1 to CS8, CS12, CS14, CS17, and CS21, by a dot blot procedure described previously (34).
Immunological analyses.
We compared vibriocidal, anti-CTB, and anti-V. cholerae O1 lipopolysaccharide (LPS) responses of patients in the VCET, VC, and ET groups. We performed vibriocidal antibody assays as previously described by using guinea pig complement and the homologous serotype of V. cholerae O1 as the target organism (35). Plasma antibodies specific to LPS, CTB, and LTB were measured by use of previously described ELISA methods (32). Briefly, 96-well microtiter plates were coated with either a purified homologous LPS serotype (El Tor, O1, Ogawa, or Inaba) (250 ng/well) (38) or sequentially with GM1 ganglioside (100 ng/well), followed by recombinant CTB or LTB (50 ng/well) (gifts from the University of Gothenburg). CTB and LTB are immunologically cross-reactive (22). ELISAs were initially carried out with plasma samples from five ETEC-infected patients in our collection (30) and tested in parallel by ELISA of both CTB and LTB; the results showed comparable ELISA results regardless of the antigen used (geometric mean titers [GMTs] for IgA responses to CTB on days 2 and 21 of 223 and 437, respectively, and GMTs for IgA responses to LTB on days 2 and 21 of 260 and 572, respectively) (the P value was nonsignificant [NS] for both day 2 and day 21 responses). These results showed that the antibody responses using either antigen were comparable for ETEC patients, and based on this finding, we used CTB as the antigen for assessments of the immune responses of all groups of patients thereafter in the study.
We incubated plates with diluted patient plasma (starting dilution, 1:200) for IgG and IgA responses, with serial 3-fold dilutions thereafter. Plates were washed, and secondary horseradish peroxidase-conjugated anti-human IgG or anti-human IgA antibodies were applied (Jackson Laboratories, Bar Harbor, ME). We developed plates by using 0.1% ortho-phenylene diamine (Sigma, St. Louis, MO) in 0.1 M sodium citrate buffer with 0.1% hydrogen peroxide, and optical densities were measured at 492 nm. The end-point titer was defined as the reciprocal interpolated dilution giving an absorbance of 0.4 above the background. A reference standard from pooled convalescent cholera patient samples (31) was also included in duplicate on each plate to ensure assay validity for each plate. The acceptable range for the CTB-specific IgA titer in the control was set between 300 and 500, and the IgG titer for the other readings from that plate was between 800 to 12,00 to be considered valid. Titer calculations were carried out by use of a computer-based program in the Multiskan Ascent ELISA reader.
Statistical analyses.
Data analyses were performed by use of SPSS for Windows (version 11.5; SPSS Inc., Chicago, IL) and Epi Info (version 6.0; USD, Stone Mountain, GA). A P value of less than 0.05 was considered to be statistically significant. The strength of association was determined by calculating odds ratios (ORs) and their 95% confidence intervals (CIs). For groups of patients, antibody titers are presented as GMTs and standard deviations (SD). Comparisons were carried out by use of Mann-Whitney U tests for non-normally-distributed data. The χ2 test was used for comparisons of categorical variables. All reported P values are two tailed.
RESULTS
Clinical history of patients.
The diarrheal patients enrolled in this study were mostly adults (<5 years of age, n = 3; >5 years of age, n = 72; median age, 25 to 30 years for the three groups). Roughly equal numbers of females were included in each group (Table 1). An evaluation of the clinical characteristics showed that severe dehydration was seen more often for the VCET group (96%) and the VC group (84%) than for patients in the ET group (52%) (P = 0.03 to 0.001). The mean intravenous fluid replacement required was also higher for the VCET and VC groups than for the ET-group (P = 0.003), while the oral rehydration solution (ORS) intake was higher for the ETEC patients (P = 0.004). Those in the VC group had a higher frequency of rice watery stool than the other two groups (P = 0.001 to 0.008). More VC and VCET patients (>96%) vomited than in the ET group (76%; P = 0.01 to 0.04). A history of taking an antibiotic prior to hospitalization was highest among the cholera patients in the VC group, followed by the VCET group (68% and 44%, respectively; P = NS), and was lower among those in the ET group (24%; P = 0.004 to 0.04). The frequency of patients with severe or moderate dehydration was not significantly different for any of the groups studied based on whether or not they had received an antibiotic prior to hospitalization. Information on the specific antibiotic taken was not generally available. The duration of diarrhea at home prior to hospitalization was higher for the VCET and ET groups than for the VC group (P = 0.006 to <0.001), showing a longer interval between the onset of disease and admission in the former two groups. All patients with severe dehydration were treated with antibiotics according to ICDDR,B protocols for the management of acute watery diarrhea. Doxycycline was generally given to adults, while erythromycin was generally given to children as well as to pregnant women. Of the patients studied, 65 of 75 received antibiotics during hospitalization: 86% were given doxycycline, 11% were given erythromycin, and 3% were given ciprofloxacin. Patients with severe disease, irrespective of the type of infection, were given a similar treatment.
TABLE 1.
Microbiological and clinical features of patients with acute watery diarrhea caused by V. cholerae O1, ETEC, or botha
| Characteristic | Value for group |
P value(s) | ||
|---|---|---|---|---|
| VCET (n = 25) | VC (n = 25) | ET (n = 25) | ||
| Median age (yr) | 25 | 25.5 | 30 | NS |
| No. (%) female patients | 12 (48) | 11 (44) | 11 (44) | NS |
| No. (%) of patients with clinical feature of: | ||||
| Rice watery stool | 11 (44) | 21 (84) | 9 (36) | 0.008,b 0.001c |
| Watery stool | 25 (100) | 24 (96) | 23 (92) | NS |
| Dehydration | ||||
| Moderate | 1 (4) | 4 (16) | 12 (48) | 0.03,c 0.001b |
| Severe | 24 (96) | 21 (84) | 13 (52) | 0.03,c 0.001d |
| Vomiting | 25 (100) | 24 (96) | 19 (76) | 0.01,d 0.04c |
| Fever of >37.5°C | 0 | 1 (4) | 1 (4) | NS |
| Mean duration of diarrhea at home (h) ± SD | 33.8 ± 29.8 | 14.4 ± 10.12 | 31.6 ± 18.7 | 0.006,b <0.001c |
| No. (%) of patients who used antibiotic at home | 11 (44) | 17 (68) | 6 (24) | 0.04,d 0.004c |
| No. of patients who used antibiotic in hospital | 23 (Doxy); 1 (Ery) | 18 (Doxy); 5 (Ery); 2 (Cipro) | 15 (Doxy); 1 (Ery) | NS |
| Mean amt of intravenous fluids given (liters) ± SD | 6.1 ± 3.8 | 6.3 ± 4.2 | 3.3 ± 2.6 | 0.003,d 0.003c |
| Mean amt of ORS given (liters) ± SD | 3.9 ± 1.8 | 4.6 ± 1.9 | 10.7 ± 26.7 | 0.004,d 0.004c |
| No. (%) of patients of blood group | ||||
| O | 10 (40) | 10 (40) | 4 (44) | NS |
| A | 7 (28) | 6 (24) | 4 (16) | NS |
| B | 5 (20) | 8 (32) | 0 | NS |
| AB | 3 (12) | 1 (4) | 1 (11) | NS |
Statistical analyses were performed by use of the χ2 test for categorical variables or the Mann-Whitney U test for continuous variables. Blood grouping was done for only a subset (n = 9) of the patients in the ET group. Doxy, doxycycline; Ery, erythromycin; Cipro, ciprofloxacin; NA, not applicable; NS, not significant.
Significant differences between the VCET group and the VC group.
Significant differences between the VC group and the ET group.
Significant differences between the VCET group and the ET group.
Phenotypes of ETEC strains and absence of other pathogens.
The ETEC strains in the VCET group were mainly LT-producing types (72% [60% LT and 12% LT/ST]), while 28% produced ST only. Only 4 of the 25 ETEC strains in the VCET group were positive for the 13 colonization factors that were assayed (these 4 strains expressed CS6, CFA/I, or CS2 and CS3), and these 4 strains were all of the ST phenotype. In the ET group, 80% of strains were LT and LT/ST producing, while 20% were ST producing. About 60% of the LT strains were positive for CFs (CS7, CS17, CFA/I, CS4 and CS6, CS2 and CS3, or CS14). More strains in the ET group than in the VCET group were CF positive (P = 0.003). Stool microscopy revealed the presence of Giardia lamblia in one patient each in the VCET and ET groups; otherwise, no other enteropathogen was detected in the stool samples of the patients in the different groups that participated in this study.
Immune responses of patients.
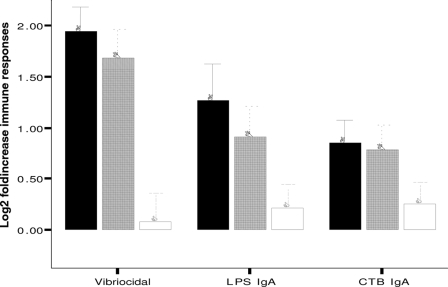
The magnitude of the vibriocidal antibody response was higher in the VCET group than in the VC group at convalescence on days 7 and 21 (P < 0.001) (Table 2). The increase in vibriocidal titer between day 2 and day 7 was also significantly higher for the VCET group than for the VC group (P = 0.02) (Fig. 1). As expected, the vibriocidal responses in the ET group did not increase significantly from day 2 to day 7 after the onset of disease (P = NS) (Table 3). The levels of CTB-specific IgA and IgG responses for the VCET group were also higher than those for the VC group on day 2, day 7, and day 21 after the onset of disease (P = <0.001 to 0.001) (Table 2). More patients in the VCET group than in the VC group had ≥2-fold increases in levels of CT-specific IgA (CT-IgA) responses at day 7 compared to day 2 after the onset of disease (P = 0.02) (Fig. 1). This was not seen in the analyses of CT-IgG responses. The VCET group of patients also had higher LPS-specific IgA and IgG responses than the VC group at day 7 or day 21 following illness (P = <0.001 to 0.02) (Table 2). The fold increases in levels of LPS-specific IgA responses were higher between day 2 and day 7 in the VCET group than in the VC group, but the fold increases in LPS-specific IgG responses were not significantly different for these groups of patients (Fig. 1).
TABLE 2.
Comparison of immunological responses of diarrheal patients with V. cholerae O1, those with ETEC infection, and those infected with both pathogensa
| Response and study day | Mean GMT (SE) for group |
P value(s) | ||
|---|---|---|---|---|
| VCET | VC | ET | ||
| Vibriocidal antibody | ||||
| 2 | 210 (49) | 118 (29) | 205 (68) | 0.29, 0.8 |
| 7 | 12,186 (2,694) | 4,013 (518) | 731 (262) | 0.002,b <0.001c |
| 21 | 8,070 (2,211) | 3,386 (468) | 188 (52) | 0.04,b <0.001c |
| CTB IgA antibody | ||||
| 2 | 492 (86) | 284 (60) | 115 (25) | 0.032,b <0.001c |
| 7 | 4,914 (1323) | 1,090 (199) | 275 (73) | 0.002,b <0.001c |
| 21 | 1,944 (457) | 800 (174) | 235 (57) | 0.007,b <0.001c |
| CTB IgG antibody | ||||
| 2 | 5,182 (905) | 964 (197) | 1,263 (195) | <0.001b,c |
| 7 | 17,342 (2,347) | 4,354 (1,016) | 2,643 (638) | <0.001b,c |
| 21 | 27,645 (3,630) | 5,680 (760) | 3,029 (755) | <0.001b,c |
| LPS IgA antibody | ||||
| 2 | 203 (63) | 164 (54) | 77 (19) | 0.97, 0.01c |
| 7 | 5,229 (1,119) | 1,271 (376) | 247 (95) | 0.004,b <0.001c |
| 21 | 1,713 (330) | 1,042 (304) | 131 (50) | 0.08, <0.001c |
| LPS IgG antibody | ||||
| 2 | 2,563 (312) | 1,480 (340) | 1,200 (239) | 0.003,b <0.001c |
| 7 | 11,204 (2,549) | 3,127 (649) | 1,525 (300) | <0.001b,c |
| 21 | 12,313 (3,825) | 3,866 (921) | 1,972 (345) | 0.001b,c |
Magnitudes of immune responses to vibriocidal antibody titers and CTB IgA and IgG and LPS IgA and IgG are shown by kinetic ELISA (monoclonal antibody [MAb]/min). GMTs and standard errors are shown. Statistical analyses were carried out using the Mann-Whitney U test for continuous variables.
Differences in responses between the VCET group and the VC group (P ≤ 0.05).
Differences in responses between thee VCET group and the ET group (P ≤ 0.05).
FIG. 1.
Increases of vibriocidal, V. cholerae LPS IgA, and CTB IgA responses among the VCET (black bars), VC (gray bars), and ET (white bars) groups of patients. Values are mean log2-transformed titers, and error bars represent 95% CIs of the means. Responses shown are the ratios between convalescence at day 7 compared with the acute stage on day 2.
TABLE 3.
Comparison of immunological responses on day 2 and day 7 after onset of diarrhea in patients infected with enterotoxigenic E. colia
| Immunological parameter | Mean GMT (SE) on study day: |
P value | |
|---|---|---|---|
| 2 | 7 | ||
| Vibriocidal antibody titer | 205 (68) | 731 (262) | 0.76 |
| CTB IgA antibody response | 115 (25) | 275 (73) | 0.04* |
| CTB IgG antibody response | 1,263 (195) | 2,643 (638) | 0.006* |
| V. cholerae LPS IgA antibody response | 77 (19) | 247 (95) | 0.24 |
| V. cholerae LPS IgG antibody response | 1,200 (239) | 1,525 (300) | 0.31 |
An asterisk indicates significant differences (P ≤ 0.05) in immune responses between the study days among patients infected with enterotoxigenic E. coli only. The Mann-Whitney U test was used for statistical analyses.
We examined the increased immune responses of the VCET group compared to those of the VC group, stratifying them by the type of ETEC strain coinfecting the VCET group, to see if the differences persisted whether the individual in the VCET group had LT (LT or LT/ST)-expressing or ST-only-expressing ETEC coinfection. We observed that significantly higher vibriocidal, as well as CTB- and LPS-specific, antibody responses were seen with both LT- and ST-producing ETEC coinfections in the VCET group.
Patients with only ETEC infection had significant increases in antitoxin responses when day 7 responses were compared to day 2 responses (P = 0.04 to 0.006) (Table 3), supporting ETEC as the etiology of their diarrhea. We analyzed the responses of patients in the ET group on the basis of whether they were infected with LT-expressing ETEC strains (LT or LT/ST; n = 20) or with ST-only-expressing ETEC strains (n = 5) (Table 4). We observed that significant increases in the CTB IgA- and IgG-specific immune responses were seen only patients infected with LT-producing ETEC (LT-ETEC) or LT/ST-producing ETEC but not for patients infected with ST-producing ETEC (P = 0.00001 and 0.0002 for differences, respectively), consistent with the cross-reactivity of LT and CT. Of note, increases in the antitoxin responses of patients infected with ETEC only were less prominent than those seen for patients in the VC group. Those patients in the ET group also had lower response rates than patients in the VC group for CTB-specific IgA (44%; P = 0.001) (Fig. 1) and IgG (40%; P = 0.03) responses between days 2 and 7. As expected, the ET group did not respond to V. cholerae O1 LPS.
TABLE 4.
Toxin-specific immune responses of patients with ETEC infections in the VCET and ET groups analyzed by type of ETEC straina
| Toxin type for VCET group (no. of patients) | % of patients with response in VCET group |
Toxin type for ET group (no. of patients) | % of patients with response in ET group |
P value(s) | ||
|---|---|---|---|---|---|---|
| CT-IgA | CT-IgG | CT IgA | CT IgG | |||
| ETEC (25) | 98 | 72 | ETEC (25) | 40 | 40 | <0.001,b <0.001c |
| LT (15) | 56 | 36 | LT (9) | 16 | 12 | <0.001,b < 0.001c |
| LT/ST (3 [CF = 0]) | 12 | 12 | LT/ST (11) | 20 | 24 | NS, 0.04 |
| ST (7) | 30 | 24 | ST (5) | 4 | 4% | <0.001,b <0.001c |
Percentages of patients responding with 2-fold-or-greater increases in cholera toxin (CTB)-specific immune responses between the acute stage (day 2) and convalescence (day 7) are shown. The χ2 test was used for statistical analyses.
Significant differences between the VCET group and the ET group in IgA responses.
Significant differences between the VCET group and ET group in IgG responses.
In order to determine if the presence of severe illness contributed to the magnitudes of the immune responses in the different groups, we further analyzed the data based on the different degrees of dehydration. Those patients with moderate (n = 12) or severe (n = 13) dehydration in the ET group had similar magnitudes and frequencies of CTB-specific responses (P = 0.54). There was only one patient with moderate dehydration in the VCET group, and this individual had immune responses similar to those of patients with severe dehydration. In the VC group, 4 of the 25 patients who had moderate dehydration also had a magnitude of response similar to those of the cholera patients with severe dehydration.
Patients in the VCET, VC, and ET groups were all approximately 40% blood group O positive (Table 1). When we compared the immune responses between the O blood group and other blood group types in each of these groups, there were no significant differences between them with the sizes of our study groups.
DISCUSSION
Although mixed bacterial infections of the gastrointestinal tract are common, comparisons of the clinical and immunological responses of patients coinfected and singly infected with major bacterial pathogens have been reported infrequently. In this study, we have focused on infections of hospitalized patients coinfected with the two most common bacterial diarrheal pathogens in Bangladesh, ETEC and V. cholerae O1. We show that mixed infection with the two pathogens is associated with more severe dehydration and vomiting and more intravenous fluid intake than what is seen for patients with ETEC diarrhea alone.
Studies of experimental animals have shown that dual infections of rotavirus and ETEC can cause more fluid loss and more prolonged diarrhea than infection with either pathogen alone (23, 47). Children infected with rotavirus together with other bacterial enteropathogens have more severe diarrhea and dehydration than children infected with rotavirus alone (15). In the present study, although dual infection with ETEC and V. cholerae O1 was more severe than infection with ETEC alone, there was no statistically significant difference in the severities of illness between dually infected patients and those with V. cholerae O1 infection alone. Between 24 and 68% of our patients had taken antibiotics prior to hospitalization. The clinical severity of diarrhea upon presentation was not affected by whether or not they had taken antibiotics prior to hospitalization. Although information on the specific antibiotic taken was generally not available, our previous data indicated that the antibiotics taken by patients prior to hospitalization are usually not those recommended for either ETEC or V. cholerae infection (our unpublished data).
We also observed that patients with ETEC alone and those with mixed infection sought care at the hospital after a comparatively longer duration of diarrhea than the cholera group. It is possible that for patients with mixed infection, ETEC was the primary pathogen, causing less severe initial diarrhea, followed by coinfection with V. cholerae O1 at a later point. This sequence might explain the longer duration of diarrhea at home in the VCET group than in the VC group.
The main finding of the study is that dual infection with both V. cholerae O1 and ETEC produced significantly higher immune responses to cholera antigens than did infection with V. cholerae O1 only. There are a number of possible explanations for this. Both CT and LT are powerful mucosal adjuvants (7, 9, 48). Thus, the presence of the enterotoxins in dual infections may have produced an immunoadjuvant effect for responses to cholera antigens following infection. Clinical studies with oral cholera vaccines containing the cholera toxin B subunit have found these vaccines not only to give protection against cholera but also to augment protection against other enteric infections as well (13). In Bangladesh, oral cholera vaccine protected against both LT-ETEC and LT/ST-ETEC diarrhea (7). In travelers, the vaccine was found to have a protective effect against mixed infections with LT-ETEC and Salmonella and also reduced ST-ETEC diarrhea (28). Similarly, a CTB-based ETEC vaccine also showed protection against C. jejuni and Salmonella enterica serovar Typhi infections (14). Derivatives of CT and LT have been explored as adjuvants in the development of Helicobacter pylori vaccines (24). Previous studies have also shown that immune responses to a bivalent Salmonella vaccine expressing the ETEC colonization factor CFA/I were enhanced by the coadministration of a mutant LT that was used as a mucosal adjuvant (16).
In addition to acting as an immunoadjuvant (18), recent data suggested that LT provides an advantage in the ability of bacteria to colonize epithelial cells in the small intestine. In addition, LT was shown to increase colonization by ETEC in both mouse and pig experimental models (1, 2). Adherence may be enhanced by the ADP-ribosylating activity of LT and by the levels of cyclic AMP (cAMP) that can affect host cell physiology (4), leading to an enhanced adherence of V. cholerae to the gut (20, 29).
However, we also observed increased immune responses in patients dually infected with V. cholerae O1 and ST-expressing ETEC compared to responses in patients infected with V. cholerae O1 alone. The heat-stable toxin (ST) is less well characterized for its immunomodulating properties than are LT and CT. Dual infections of children in developing countries with ETEC and enteropathogenic E. coli (EPEC) have been observed (10, 26), and recent studies have shown that purified ST or crude extracts of ETEC can enhance the virulence of EPEC by use of laboratory-based in vitro assay systems (8). This suggested a molecular basis for the role of ST in causing an increased severity of disease in EPEC diarrhea. By analogy, ST might also have played a role in mediating the enhanced immunological responses in the VCET patients infected with ST-ETEC in the present study compared to those infected with V. cholerae O1 alone.
It is possible that both the adjuvant activity of LT and the effect of host-derived cAMP produced by either LT or ST to increase the adherence of V. cholerae may have contributed to the enhanced immune responses seen in the dually infected patients compared to those infected with V. cholerae alone in the present study. The increased CTB responses seen for dually infected patients may also reflect the antigenic cross-reactivity seen between LT and CT (6).
In this study, we were not able to determine if the converse phenomenon is also true, i.e., if Vibrio cholerae O1 coinfection leads to enhanced immune responses to ETEC antigens as well. Since ETEC strains belong to a large number of different O and H groups, it was not possible to study the immune responses to these various antigens in this study with sufficient power to compare dually infected patients to those with ETEC infection alone. In addition, only 4 of the 25 patients with concomitant ETEC and V. cholerae O1 infection were positive for any of the 13 CFs that were tested in this study, and therefore, the responses to CFs were not determined either.
For preventive strategies against the major bacterial pathogens causing diarrhea, protective antitoxin immunity is an important target for vaccine development. Generally, for toxin-mediated enteric infections, robust responses are needed to overcome the effect of the toxins, for achieving protective efficacy for these major pathogens, and to formulate a wide-range global vaccine (8). Since the presence of O-blood-group antigen was previously found to be associated with susceptibility to cholera (18) and to be associated with the magnitude of immune responses generated after vaccination (21), we analyzed the effect of this antigen on the immune responses seen for our three study groups. However, with the sizes of our study groups, we did not see a significant effect of the O blood group on the immune responses in the patients. Also, the proportions of patients of different blood groups were comparable across our study groups, suggesting the this was not the explanation for the observed increased immune responses in the VCET group compared to the immune responses of the VC group.
There was a significant difference in the proportions of ETEC strains that produced colonization factors between the patients in the VCET group and those in the ET group. We do not have an explanation for this difference at present, but we did not see a difference in immune responses between individuals in the VCET group infected with CF-positive strains and those infected with CF-negative ETEC strains.
Previous studies may suggest additional possible mechanisms for the increased immune responses seen in the dually infected group in the present study. Peltola et al. (28) studied protective efficacy following vaccination with an oral cholera vaccine, an inactivated whole cell cholera toxin B subunit vaccine. In that study, in addition to the protective efficacy of this vaccine against ETEC diarrhea, there was also an unexpected protection against the combination of ETEC infection and infection with another pathogen, particularly mixed infections with Salmonella. That study suggested that toxin may have a broader role in facilitating other enteric infections.
Glenn et al. (13) recently suggested that LT and CT render the human gut more susceptible to colonization by enteric pathogens by disrupting innate mucosal defenses. Two possible mechanisms were suggested for this: toxin-mediated fluid secretion, disrupting the protective mucus layer, or toxin-mediated interference with the synthesis of antimicrobial peptides or other innate mucosal defenses. Those investigators suggested that the preconditioning of the gut by LT or CT enterotoxins might increase the efficiency of subsequent colonization by ETEC or other enteric pathogens. Thus, there may be advantages for V. cholerae O1 and ETEC as coinfecting pathogens in terms of an enhanced capacity for colonization of the gut. This hypothesis is consistent with the observations here of an increased immune response to cholera antigens in dually infected patients compared to the responses of patients with single infection with V. cholerae O1.
Both ETEC and V. cholerae O1 cause disease that gives protection from further infection and are thus considered to be vaccine preventable. Since vaccines are needed to protect individuals from these pathogens, a better understanding of the mechanisms leading to increased immune responses to dual infection with ETEC and V. cholerae O1 would be helpful in formulating appropriate and protective vaccines. Further studies of dual infection with ETEC and V. cholerae O1 and other bacterial pathogens in countries where these infections are endemic will shed additional light on these issues.
Acknowledgments
This research was supported by the ICDDR,B; by grants U01 AI058935 (S.B.C.), RO3 AI063079 (F.Q.), and U01 AI077883 (E.T.R.); and by the Swedish Agency for Research and Economic Cooperation (F.Q. and A.-M.S.) (Sida-SAREC; grant INT-ICDDR,B-HN-01-AV). F.C. is a recipient of the Fogarty/Ellison Fellowship in Global Health awarded by the Fogarty International Center at the National Institutes of Health (grant D43 TW005572).
Editor: S. M. Payne
Footnotes
Published ahead of print on 22 February 2010.
REFERENCES
- 1.Allen, K. P., M. M. Randolph, and J. M. Fleckenstein. 2006. Importance of heat-labile enterotoxin in colonization of the adult mouse small intestine by human enterotoxigenic Escherichia coli strains. Infect. Immun. 74:869-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berberov, E. M., Y. Zhou, D. H. Francis, M. A. Scott, S. D. Kachman, and R. A. Moxley. 2004. Relative importance of heat-labile enterotoxin in the causation of severe diarrheal disease in the gnotobiotic piglet model by a strain of enterotoxigenic Escherichia coli that produces multiple enterotoxins. Infect. Immun. 72:3914-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black, R. E., M. H. Merson, A. S. Rahman, M. Yunus, A. R. Alim, I. Huq, R. H. Yolken, and G. T. Curlin. 1980. A two-year study of bacterial, viral, and parasitic agents associated with diarrhea in rural Bangladesh. J. Infect. Dis. 142:660-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolin, I., G. Wiklund, F. Qadri, O. Torres, A. L. Bourgeois, S. Savarino, and A. M. Svennerholm. 2006. Enterotoxigenic Escherichia coli with STh and STp genotypes is associated with diarrhea both in children in areas of endemicity and in travelers. J. Clin. Microbiol. 44:3872-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman, P. A., and D. L. Mitchelmore. 1988. A two-year survey of the incidence of heat-labile enterotoxin-producing Escherichia coli and other enteric pathogens in travellers returning to the Sheffield area. Epidemiol. Infect. 101:239-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clements, J. D., and R. A. Finkelstein. 1978. Immunological cross-reactivity between a heat-labile enterotoxin(s) of Escherichia coli and subunits of Vibrio cholerae enterotoxin. Infect. Immun. 21:1036-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clements, J. D., N. M. Hartzog, and F. L. Lyon. 1988. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine 6:269-277. [DOI] [PubMed] [Google Scholar]
- 8.Crane, J. K., S. S. Choudhari, T. M. Naeher, and M. E. Duffey. 2006. Mutual enhancement of virulence by enterotoxigenic and enteropathogenic Escherichia coli. Infect. Immun. 74:1505-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elson, C. O., and W. Ealding. 1984. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J. Immunol. 132:2736-2741. [PubMed] [Google Scholar]
- 10.Faruque, A. S., D. Mahalanabis, A. Islam, and S. S. Hoque. 1994. Severity of cholera during concurrent infections with other enteric pathogens. J. Diarrhoeal Dis. Res. 12:214-218. [PubMed] [Google Scholar]
- 11.Faruque, A. S., M. A. Salam, S. M. Faruque, and G. J. Fuchs. 1998. Aetiological, clinical and epidemiological characteristics of a seasonal peak of diarrhoea in Dhaka, Bangladesh. Scand. J. Infect. Dis. 30:393-396. [DOI] [PubMed] [Google Scholar]
- 12.Gascon, J., M. Vargas, L. Quinto, M. Corachan, M. T. Jimenez de Anta, and J. Vila. 1998. Enteroaggregative Escherichia coli strains as a cause of traveler's diarrhea: a case-control study. J. Infect. Dis. 177:1409-1412. [DOI] [PubMed] [Google Scholar]
- 13.Glenn, G. M., D. H. Francis, and E. M. Danielsen. 2009. Toxin-mediated effects on the innate mucosal defenses: implications for enteric vaccines. Infect. Immun. 77:5206-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glenn, G. M., C. P. Villar, D. C. Flyer, A. L. Bourgeois, R. McKenzie, R. M. Lavker, and S. A. Frech. 2007. Safety and immunogenicity of an enterotoxigenic Escherichia coli vaccine patch containing heat-labile toxin: use of skin pretreatment to disrupt the stratum corneum. Infect. Immun. 75:2163-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grimprel, E. M., R. Carlos, and U. Desselberger. 2008. Rotavirus disease: impact of coinfections. Pediatr. Infect. Dis. J. 27:S3-S10. [Google Scholar]
- 16.Guillobel, H. C., J. I. Carinhanha, L. Cardenas, J. D. Clements, D. F. de Almeida, and L. C. Ferreira. 2000. Adjuvant activity of a nontoxic mutant of Escherichia coli heat-labile enterotoxin on systemic and mucosal immune responses elicited against a heterologous antigen carried by a live Salmonella enterica serovar Typhimurium vaccine strain. Infect. Immun. 68:4349-4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris, A. M., F. Chowdhury, Y. A. Begum, A. I. Khan, A. S. Faruque, A. M. Svennerholm, J. B. Harris, E. T. Ryan, A. Cravioto, S. B. Calderwood, and F. Qadri. 2008. Shifting prevalence of major diarrheal pathogens in patients seeking hospital care during floods in 1998, 2004, and 2007 in Dhaka, Bangladesh. Am. J. Trop. Med. Hyg. 79:708-714. [PMC free article] [PubMed] [Google Scholar]
- 18.Harris, J. B., A. I. Khan, R. C. LaRocque, D. J. Dorer, F. Chowdhury, A. S. Faruque, D. A. Sack, E. T. Ryan, F. Qadri, and S. B. Calderwood. 2005. Blood group, immunity, and risk of infection with Vibrio cholerae in an area of endemicity. Infect. Immun. 73:7422-7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris, J. B., R. C. LaRocque, F. Chowdhury, A. I. Khan, T. Logvinenko, A. S. Faruque, E. T. Ryan, F. Qadri, and S. B. Calderwood. 2008. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl. Trop. Dis. 2:e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson, A. M., R. S. Kaushik, N. J. Rotella, and P. R. Hardwidge. 2009. Enterotoxigenic Escherichia coli modulates host intestinal cell membrane asymmetry and metabolic activity. Infect. Immun. 77:341-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagos, R., A. Avendano, V. Prado, I. Horwitz, S. Wasserman, G. Losonsky, S. Cryz, Jr., J. B. Kaper, and M. M. Levine. 1995. Attenuated live cholera vaccine strain CVD 103-HgR elicits significantly higher serum vibriocidal antibody titers in persons of blood group O. Infect. Immun. 63:707-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lebens, M., V. Shahabi, M. Backstrom, T. Houze, N. Lindblad, and J. Holmgren. 1996. Synthesis of hybrid molecules between heat-labile enterotoxin and cholera toxin B subunits: potential for use in a broad-spectrum vaccine. Infect. Immun. 64:2144-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lecce, J. G., R. K. Balsbaugh, D. A. Clare, and M. W. King. 1982. Rotavirus and hemolytic enteropathogenic Escherichia coli in weanling diarrhea of pigs. J. Clin. Microbiol. 16:715-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, A., and M. Chen. 1994. Successful immunization against gastric infection with Helicobacter species: use of a cholera toxin B-subunit-whole-cell vaccine. Infect. Immun. 62:3594-3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merritt, E. A., T. K. Sixma, K. H. Kalk, B. A. van Zanten, and W. G. Hol. 1994. Galactose-binding site in Escherichia coli heat-labile enterotoxin (LT) and cholera toxin (CT). Mol. Microbiol. 13:745-753. [DOI] [PubMed] [Google Scholar]
- 26.Moyenuddin, M., K. M. Rahman, and D. A. Sack. 1987. The aetiology of diarrhoea in children at an urban hospital in Bangladesh. Trans. R. Soc. Trop. Med. Hyg. 81:299-302. [DOI] [PubMed] [Google Scholar]
- 27.Paniagua, G. L., E. Monroy, O. Garcia-Gonzalez, J. Alonso, E. Negrete, and S. Vaca. 2007. Two or more enteropathogens are associated with diarrhoea in Mexican children. Ann. Clin. Microbiol. Antimicrob. 6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peltola, H., A. Siitonen, H. Kyronseppa, I. Simula, L. Mattila, P. Oksanen, M. J. Kataja, and M. Cadoz. 1991. Prevention of travellers' diarrhoea by oral B-subunit/whole-cell cholera vaccine. Lancet 338:1285-1289. [DOI] [PubMed] [Google Scholar]
- 29.Pierce, N. F., J. B. Kaper, J. J. Mekalanos, and W. C. Cray, Jr. 1985. Role of cholera toxin in enteric colonization by Vibrio cholerae O1 in rabbits. Infect. Immun. 50:813-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qadri, F., F. Ahmed, T. Ahmed, and A. M. Svennerholm. 2006. Homologous and cross-reactive immune responses to enterotoxigenic Escherichia coli colonization factors in Bangladeshi children. Infect. Immun. 74:4512-4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qadri, F., F. Ahmed, M. M. Karim, C. Wenneras, Y. A. Begum, M. A. Salam, M. J. Albert, and J. R. McGhee. 1999. Lipopolysaccharide- and cholera toxin-specific subclass distribution of B-cell responses in cholera. Clin. Diagn. Lab. Immunol. 6:812-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qadri, F., T. Azim, A. Chowdhury, J. Hossain, R. B. Sack, and M. J. Albert. 1994. Production, characterization, and application of monoclonal antibodies to Vibrio cholerae O139 synonym Bengal. Clin. Diagn. Lab. Immunol. 1:51-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qadri, F., S. K. Das, A. S. Faruque, G. J. Fuchs, M. J. Albert, R. B. Sack, and A. M. Svennerholm. 2000. Prevalence of toxin types and colonization factors in enterotoxigenic Escherichia coli isolated during a 2-year period from diarrheal patients in Bangladesh. J. Clin. Microbiol. 38:27-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qadri, F., J. A. Giron, A. Helander, Y. A. Begum, M. Asaduzzaman, J. Xicohtencatl-Cortes, E. Negrete, and M. J. Albert. 2000. Human antibody response to longus type IV pilus and study of its prevalence among enterotoxigenic Escherichia coli in Bangladesh by using monoclonal antibodies. J. Infect. Dis. 181:2071-2074. [DOI] [PubMed] [Google Scholar]
- 35.Qadri, F., G. Mohi, J. Hossain, T. Azim, A. M. Khan, M. A. Salam, R. B. Sack, M. J. Albert, and A. M. Svennerholm. 1995. Comparison of the vibriocidal antibody response in cholera due to Vibrio cholerae O139 Bengal with the response in cholera due to Vibrio cholerae O1. Clin. Diagn. Lab. Immunol. 2:685-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qadri, F., A. Saha, T. Ahmed, A. Al Tarique, Y. A. Begum, and A. M. Svennerholm. 2007. Disease burden due to enterotoxigenic Escherichia coli in the first 2 years of life in an urban community in Bangladesh. Infect. Immun. 75:3961-3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qadri, F., A. M. Svennerholm, A. S. Faruque, and R. B. Sack. 2005. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin. Microbiol. Rev. 18:465-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qadri, F., C. Wenneras, M. J. Albert, J. Hossain, K. Mannoor, Y. A. Begum, G. Mohi, M. A. Salam, R. B. Sack, and A. M. Svennerholm. 1997. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect. Immun. 65:3571-3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salmond, R. J., J. A. Luross, and N. A. Williams. 2002. Immune modulation by the cholera-like enterotoxins. Expert Rev. Mol. Med. 4:1-16. [DOI] [PubMed] [Google Scholar]
- 40.Schultsz, C., J. van den Ende, F. Cobelens, T. Vervoort, A. van Gompel, J. C. Wetsteyn, and J. Dankert. 2000. Diarrheagenic Escherichia coli and acute and persistent diarrhea in returned travelers. J. Clin. Microbiol. 38:3550-3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sixma, T. K., K. H. Kalk, B. A. van Zanten, Z. Dauter, J. Kingma, B. Witholt, and W. G. Hol. 1993. Refined structure of Escherichia coli heat-labile enterotoxin, a close relative of cholera toxin. J. Mol. Biol. 230:890-918. [DOI] [PubMed] [Google Scholar]
- 42.Sixma, T. K., S. E. Pronk, K. H. Kalk, E. S. Wartna, B. A. van Zanten, B. Witholt, and W. G. Hol. 1991. Crystal structure of a cholera toxin-related heat-labile enterotoxin from E. coli. Nature 351:371-377. [DOI] [PubMed] [Google Scholar]
- 43.Spangler, B. D. 1992. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol. Rev. 56:622-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Svennerholm, A. M., and J. Holmgren. 1978. Identification of Escherichia coli heat labile enterotoxin by means of ganglioside immunosorbent assay (GM1 ELISA) procedure. Curr. Microbiol. 1:9-23. [Google Scholar]
- 45.Svennerholm, A. M., M. Wikstrom, M. Lindblad, and J. Holmgren. 1986. Monoclonal antibodies against Escherichia coli heat-stable toxin (STa) and their use in a diagnostic ST ganglioside GM1-enzyme-linked immunosorbent assay. J. Clin. Microbiol. 24:585-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi, I., et al. 1996. Mechanisms for mucosal immunogenicity and adjuvancy of Escherichia coli labile enterotoxin. J. Infect. Dis. 173:627-635. [DOI] [PubMed] [Google Scholar]
- 47.Tzipori, S. R., T. J. Makin, M. L. Smith, and F. L. Krautil. 1981. Clinical manifestations of diarrhea in calves infected with rotavirus and enterotoxigenic Escherichia coli. J. Clin. Microbiol. 13:1011-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vajdy, M., and N. Y. Lycke. 1992. Cholera toxin adjuvant promotes long-term immunological memory in the gut mucosa to unrelated immunogens after oral immunization. Immunology 75:488-492. [PMC free article] [PubMed] [Google Scholar]
- 49.WHO. 1990. Treatment of diarrhoea: a manual for physicians anal other senior health workers. World Health Organization, Geneva, Switzerland.
- 50.WHO. 1987. Manual for laboratory investigations of acute enteric infections, p. 9-20. World Health Organization, Geneva, Switzerland.
- 51.Wilson, A. D. 2002. The in vitro production of cytokines by mucosal lymphocytes immunized by oral administration of keyhole limpet hemocyanin using cholera toxin as an adjuvant. Eur. J. Immunol. 21:2333-2339. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, W., M. Zhao, L. Ruesch, A. Omot, and D. Francis. 2007. Prevalence of virulence genes in Escherichia coli strains recently isolated from young pigs with diarrhea in the US. Vet. Microbiol. 123:145-152. [DOI] [PubMed] [Google Scholar]