Abstract
Neisseria gonorrhoeae is the etiologic agent of gonorrhea, which has been among the most frequently reported communicable diseases in the United States since 1960. Women frequently do not exhibit symptoms, which can lead to chronic infection. N. gonorrhoeae readily forms biofilms over abiotic surfaces, over primary and transformed cervical epithelial cells, and over cervical tissues in vivo. Biofilms are often associated with chronic infection, which suggests a link between biofilm formation and asymptomatic gonorrhea in women. Proteins involved in anaerobic metabolism and oxidative-stress tolerance are critical for normal biofilm formation of N. gonorrhoeae. Therefore, we examined the spatial profiles of anaerobic respiration in N. gonorrhoeae, using an aniA′-′gfp transcriptional fusion. Nitric oxide (NO) can elicit biofilm dispersal when present at sublethal concentrations in the surrounding medium. Some reports indicate that NO may also encourage biofilm formation at higher, potentially lethal concentrations. NO is produced by polymorphonuclear lymphocytes (PMNs) and cervical endothelial and epithelial cells. Thus, we also examined the effect of NO on N. gonorrhoeae biofilms. We found that anaerobic respiration occurs predominantly in the substratum of gonococcal biofilms and that expression of aniA is induced over time in biofilms. Treatment with high concentrations of a rapid-release NO donor prevents biofilm formation when supplied early in biofilm development but can also enhance biofilm formation once anaerobic respiration is initiated. NO treatment partially restores biofilm formation in an aniA::kan insertion mutant, which suggests that N. gonorrhoeae in biofilms may use NO as a substrate for anaerobic growth but prefer nitrite.
Sixty-two million new cases of gonorrhea, which are caused by the bacterium Neisseria gonorrhoeae (31, 35, 37), are reported annually worldwide (26). Gonorrhea is one of the oldest known human illnesses (31, 37), and it remains prevalent, as gonorrhea is among the most frequently reported communicable diseases in the United States today (8, 9). Both men and women may become infected with N. gonorrhoeae, although the mechanism of infection differs between men and women (21). Men who become infected with N. gonorrhoeae typically develop acute anterior urethritis with urethral discharge and/or dysuria (31, 37), while up to 80% of infected women do not develop any noticeable symptoms (4, 31, 37, 47, 50). Asymptomatic infection may occur in men, but is rare, occurring in only 1% of those infected (30, 31). Undiagnosed infection in women can lead to prolonged or persistent infection (31, 36, 37). Women with persistent infection may develop pelvic inflammatory disease (PID), ectopic pregnancy, chronic pain, infertility, and/or disseminated gonococcal infection (DGI) (1, 31, 36, 37). In addition, gonorrhea increases the risk of infection with other sexually transmitted pathogens, including HIV (8, 10, 25).
It was recently acknowledged that biofilm formation frequently contributes to infection by pathogenic and opportunistic bacterial species (12-14, 19, 28, 29, 65). Biofilm formation can facilitate persistence within the human host, as the majority of biofilms are inherently resistant to antimicrobials and host immune defenses (2, 14, 18, 19, 28). N. gonorrhoeae is capable of forming biofilms over glass and over primary and transformed human cervical epithelial cells (THCEC) (27). Microscopic examination of biopsied human cervical tissue also indicates that N. gonorrhoeae forms biofilms during natural cervical infection (64). It has been demonstrated that the ability of N. gonorrhoeae to perform anaerobic respiration and tolerate oxidative stress is critical for normal biofilm formation (22, 44, 52, 53, 61). Mutations in a number of stress tolerance genes, including trxB, estD, gor, oxyR, prx, mntABC, ccp, and norB, impair the ability of the gonococcus to form biofilms over THCEC and/or glass surfaces (22, 44, 52, 53, 61).
The capacity to tolerate oxidative and nitrosative stress associated with the innate immune response of the human body can be an important virulence determinant, and mechanisms for oxidative-stress tolerance are abundant in human pathogens (32, 41, 60). N. gonorrhoeae can neutralize reactive oxygen and nitrogen species, such as hydrogen peroxide (H2O2) and nitric oxide (NO) (59,60). NO is toxic to many bacterial species (15, 23, 45, 72), and the production of NO may be a strategy used by the innate immune system to respond to bacterial infection (23), as it is produced by polymorphonuclear lymphocytes (PMNs) (7, 23, 45, 46) and cervical endothelial and epithelial cells (43, 66). N. gonorrhoeae is inherently resistant to NO-mediated killing and can rapidly reduce NO to achieve noninflammatory steady-state levels (6). N. gonorrhoeae uses NorB, a respiratory nitric oxide reductase to reduce NO (39). NorB is also responsible for the reduction of NO produced by the respiratory nitrite reductase AniA, and together NorB and AniA facilitate anaerobic growth of gonococcus (38). Although both norB and aniA insertion mutants are defective in some aspect of biofilm formation, a norB insertion mutant is more severely attenuated for biofilm formation (22). This appears to be due to the accumulation of NO in the norB mutant, as nanomolar concentrations of NO can impair biofilm formation and/or elicit biofilm dispersal in N. gonorrhoeae (22). This has been also observed in Pseudomonas aeruginosa (3) and staphylococcal (58) biofilms. However, higher concentrations of NO (μM to mM) can also enhance biofilm formation in P. aeruginosa (3, 71).
We elected to investigate the anaerobic respiratory profiles of N. gonorrhoeae biofilms, using a green fluorescent protein (GFP) transcriptional fusion to aniA, as mutations in aniA and norB impair but do not entirely prevent biofilm formation (22). We hypothesized that N. gonorrhoeae uses a combination of anaerobic/microaerobic and aerobic metabolism to support biofilm growth. The observations that biofilm formation is more severely attenuated in a norB mutant and that NO can affect P. aeruginosa biofilm in a concentration-dependent manner (3, 71) prompted us to investigate the influence of higher concentrations of NO on gonococcal biofilm formation. We found that anaerobic metabolism occurs primarily at the biofilm-surface interface or substrata of gonococcal biofilms and that higher NO concentrations can enhance biofilm formation, especially in the absence of nitrite. NO may also help to sustain anaerobic growth, as the addition of NO enhances biofilm formation in an aniA insertion mutant, which cannot utilize nitrite.
MATERIALS AND METHODS
Bacteria.
N. gonorrhoeae strain 1291, a piliated clinical isolate that expresses Opa proteins, was used in this study. This strain was reconstituted from frozen stock cultures and propagated at 37°C with 5% CO2 on GC agar (Becton Dickinson, Franklin Lakes, NJ) supplemented with 1% IsoVitaleX (Becton Dickinson) (Table 1).
TABLE 1.
Strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Genotype or sequence | Reference |
|---|---|---|
| Strains | ||
| N. gonorrhoeae 1291 | Wild type | |
| aniA::kan | aniA::kan ΔaniA pGFP | 22 |
| aniA′-′gfp | aniA′-′gfp | This study |
| Plasmids | ||
| pCTS32 | pGEM-T easy/Sptr/ΔproB | 64 |
| pGFP | pLES98/GFP/Chlr | 20 |
| paniA′-′gfp | pCTS32/aniA′-′gfp | This study |
| Primers | ||
| aniA promoter For | 5′-TACCCGGGAACTGCCTTTGCCTGCTCTG-3′ | |
| aniA promoter Rev | 5′-TCTTCTCTTTTACTCATAATGTTTCCTTTT-3′ | |
| GFP For | 5′-AAAAGGAAAACATTATGAGTAAAAGGAGAAGA-3′ | |
| GFP Rev | 5′-GTCTTAAGTTCTGCAGGAGGTCTGGACATT-3′ |
Strains, plasmids, and primers used during this study are listed above with a description of their relevant genotypes or sequences (primers only). The source of each strain or plasmid is also denoted by a citation. Strains and plasmids were constructed during the course of this study. Primers are named for the region they amplify.
Construction of an aniA′-′gfp transcriptional fusion.
The approximately 190-bp promoter region of the aniA gene was PCR amplified from N. gonorrhoeae strain 1291 genomic DNA, using primers that generated an XmaI site at the 5′ end. The coding sequence for GFP was amplified from the pGFP plasmid (20), using primers that generated an AflII site at the 3′ end. These fragments were then used as a template for PCR splicing by overlap extension (PCR-SOE) to fuse the GFP coding sequence (beginning at the ATG start codon) to the aniA promoter from N. gonorrhoeae 1291. The resulting fragment was cloned into pGEM-T easy (Promega, Madison, WI). This fragment was then subcloned into pCTS32 (64) for recombination into the proline B synthesis gene (proB) of N. gonorrhoeae. The restriction sites generated by PCR amplification were selected for ligation into the pCTS32 plasmid in the opposite orientation of the Sptr promoter to allow transcription to be initiated from the aniA promoter only. The resulting construct was sequenced and used to transform wild-type N. gonorrhoeae strain 1291. Transformants were selected on GC agar containing spectinomycin and supplemented with proline. Integration of these constructs was confirmed via PCR and Southern blotting (Table 1).
Biofilm growth of the aniA′-′gfp fusion over glass.
The N. gonorrhoeae 1291 aniA′-′gfp fusion was assayed for its ability to form biofilm. This strain was propagated from frozen stock culture on GC agar with 1% IsoVitaleX (Becton Dickinson, Franklin Lakes, NJ) and 0.2 g/liter proline and was incubated at 37°C and 5% CO2. An overnight plate culture was used to create a cell suspension for inoculation of biofilm flow chambers. N. gonorrhoeae was grown in continuous-flow chambers over glass as described previously (22). The media was supplemented with 0.2 g/liter of proline to facilitate growth of the aniA′-′gfp strain. After 43 h of biofilm formation, chambers were stained with the 2C3 antibody to gonococcal H.8. H.8 is present in the outer membrane of the gonococcus and was used to visualize all cells within the biofilm for overlay with cells expressing GFP from the aniA′-′gfp fusion. Staining was performed as follows. A 1:100 dilution of 2C3 (in biofilm media) was pumped through the chambers for 2 h, after which the media was replaced and pumped through the chambers for another 30 min to remove unbound 2C3. Finally, a 1:500 dilution of the secondary antibody Alexa Fluor 568 goat anti-mouse IgG (Molecular Probes, Invitrogen Corp., Carlsbad, CA) was pumped through the chambers for 2 h in the dark. Biofilm formation was then examined via confocal microscopy.
Confocal microscopy of continuous-flow chambers.
z-Series photomicrographs of flow chamber biofilms were taken with a Nikon PCM-2000 confocal microscope scanning system (Nikon, Melville, NY), using a modified stage for flow cell microscopy. GFP was excited at 450 to 490 nm, and Alexa Fluor 568 dye was excited at 540 to 580 nm for biofilm imaging. Three-dimensional images of the biofilms were created from each z-series, using Volocity high-performance three-dimensional imaging software (Improvision, Lexington, MA). The images were adjusted to incorporate the pixel sizes for the x, y, and z axes of each image stack.
Treatment of biofilms with the NO donors SNP and DETA/NO adduct.
Wild-type N. gonorrhoeae strain 1291 biofilms were treated with sodium nitroprusside (SNP), a rapid-release NO donor, at concentrations ranging from 1 mM to 20 μM at the start of biofilm formation. For most experiments, SNP was added to the biofilm medium prior to initiating flow but not to the medium that was used to create suspensions for inoculation of the biofilm. Nitrite was added to the biofilm media at a concentration of 100 μM. Biofilms were also treated with a gradual-release NO donor, diethylenetriamine/nitric oxide (DETA/NO) in the absence of nitrite. Wild-type N. gonorrhoeae strain 1291 biofilms were treated with 10 μM and 20 μM concentrations of DETA/NO and assessed for biofilm formation relative to that of biofilms grown in the absence of nitrite and NO. The impact of SNP treatment following growth as a biofilm in the presence of nitrite for 24 h was also investigated. Wild-type N. gonorrhoeae strain 1291 biofilms were grown for 24 h in the presence of nitrite and then transitioned to media with 20 μM SNP or media without nitrite and NO. Biofilm formation was evaluated after another 24 h of growth under these conditions. Biofilm assays were run in quadruplicate in a minimum of two experiments for each described condition. Biofilm formation was evaluated by confocal microscopy and analyzed by COMSTAT software (available at http://www.dtu.dk/centre/CSM/Instrument%20Center/Resources/COMSTAT%20Software.aspx).
Measurement of dissolved NO in NO donor stocks and biofilm reservoir media.
A Sievers NOA 280i nitric oxide analyzer (GE Analytical Instruments, Boulder, CO) was used to measure dissolved NO in the SNP and DETA/NO stock solutions, as well as in biofilm media containing 500 nM SNP, 20 μM SNP, and 20 μM DETA/NO. Concentrations were determined from a NO standard curve immediately prior to treating biofilm flow cells with these media.
COMSTAT analysis of confocal z-series.
Quantitative analysis of each z-series was performed using COMSTAT (34). COMSTAT is a mathematical script written for MATLAB 5.3 (The Mathworks, Inc., Natick, MA) that quantifies three-dimensional biofilm structures by evaluating confocal image stacks so that pixels may be converted to relevant measurements of biofilm, including total biomass and average thickness. To complete COMSTAT analysis, an information file was created for each z-series to adjust for the pixel sizes of the x, y, and z axes and the number of images in each z series. COMSTAT was then used to obtain threshold images to reduce the background. The biomass and the average and maximum thicknesses in each z-series were calculated by COMSTAT, using the threshold images.
Statistical analysis of COMSTAT results.
Statistical analysis was performed with Prism 4 software (GraphPad Software, Inc., La Jolla, CA). Student's t tests were used to compare the biomasses and average thicknesses of untreated biofilms to those treated with the NO donors SNP and DETA/NO. Values that met a P value cutoff of 0.05 were considered statistically different.
RESULTS
Microscopic examination of anaerobic respiration in biofilm.
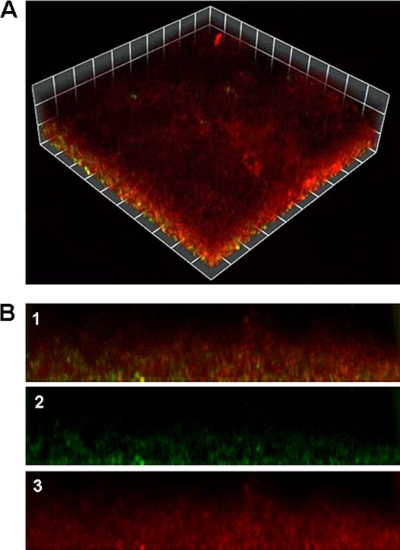
Confocal imaging of the aniA′-′gfp transcriptional fusion strain under typical biofilm growth conditions (22) indicated that transcription of aniA occurs in the majority of cells in the substratum of N. gonorrhoeae biofilms, while few cells express the fusion in other regions of the biofilm (Fig. 1). aniA is induced during anaerobic growth and is tightly repressed under aerobic growth conditions (38). Thus, it can be concluded that anaerobic respiration occurs most readily near the attachment surface in gonococcal biofilms. Since expression of aniA′-′gfp does not occur in all regions of the biofilm, this indicates that the partial oxygen pressure is sufficient to repress anaerobic gene expression. Although the majority of GFP-positive cells can be found in the substratum, they are also present in other areas of the biofilm. The intensity of the GFP reporter was also greater in areas of the biofilm where water channels were less abundant. Water channels can facilitate the diffusion of oxygen into the biofilm (12, 13, 19), and this may lead to inhibition of anaerobic gene expression. Almost no GFP expression could be detected at the surface-liquid interface of the biofilm, where oxygen would be most abundant in the bulk fluid (Fig. 1).
FIG. 1.
Biofilm formation by the aniA′-′gfp fusion strain. (A) Biomass of N. gonorrhoeae strain 1291 aniA′-′gfp after 2 days of growth. This image is a three-dimensional reconstruction of a representative stacked z series taken at a magnification of ×200 and rendered by Volocity. Cells expressing GFP appear green or yellow (colocalization of GFP and 2C3), while cells stained with 2C3 that are not expressing GFP appear red. (B) Series of side views of the biofilm depicted in panel A. These images are oriented so that the bottom of each image is the substratum of the biofilm, or the attachment surface. The top of each image is the portion of the biofilm that is exposed to the fluid flow. The highest point of biofilm formation in these images is approximately 130 μM. Image 1 in panel B is a merged image, in which the red and green channels are overlaid. Image 2 is the green channel alone (aniA-gfp), while image 3 is the red channel alone (2C3).
Effect of high concentrations of SNP on biofilm formation.
A previous report determined that low concentrations (500 nM) of the NO donor SNP inhibit biofilm formation when administered at the start or after 24 h of biofilm formation (22). However, studies of P. aeruginosa indicate that SNP can also enhance biofilm formation when administered at higher doses (25 to 100 μM) (3, 71). This observation prompted us to investigate the effect of NO concentration on gonococcal biofilm formation. We first treated N. gonorrhoeae biofilms with 1 mM SNP at the start of biofilm formation and examined biofilms after 48 h of growth, using confocal microscopy. We found that 1 mM SNP completely prevented biofilm formation in N. gonorrhoeae, as no cells were associated with the glass surface in continuous-flow chambers. This was an unexpected result, because N. gonorrhoeae is thought to be inherently resistant to NO, at least more so than P. aeruginosa (59, 60). To eliminate the possibility that the presence of SNP was interfering with the initial adherence of biofilm cells, we used media without SNP to inoculate flow chambers. SNP was administered to these biofilms when flow was initiated, as SNP was added directly to the medium reservoir. However, this did not significantly improve biofilm formation. We then tested lower concentrations of SNP (500, 250, 50, and 20 μM), yet all concentrations completely prevented biofilm formation. SNP is a rapid-release NO donor. Therefore, high concentrations of SNP administered at the start of biofilm formation may overwhelm the NO defenses of the gonococcus. Expression of norB is induced under anaerobic conditions, and the norB transcript is virtually undetectable under aerobic growth conditions (39). This expression pattern is similar to that of aniA (38).
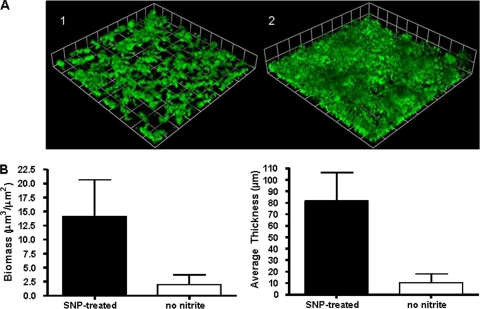
To assess the induction of anaerobic respiration in gonococcal biofilms, we used confocal microscopy to examine GFP expression in N. gonorrhoeae strain 1291 aniA′-′gfp in overnight plate cultures and the biofilm inoculum and after 24 h of biofilm growth. GFP expression could not be detected in cells from overnight plate cultures or the biofilm inoculum after these cells were spotted on glass slides. However, GFP could be detected in biofilms grown for 24 h in the presence of nitrite (data not shown). Therefore, we elected to examine the effect of SNP on biofilms that were first grown for 24 h in the presence of nitrite. Biofilms that were transitioned to media containing 20 μM SNP and no source of nitrite formed biofilms with significantly increased biomasses and average thicknesses compared to those of biofilms that were transitioned to media without SNP or nitrite (Fig. 2). Biofilms grown in the presence of SNP for the final 24 h of growth formed biofilms similar to those grown for 48 h in the presence of nitrite, as described in an earlier publication (22). This finding suggests that NO may be able to enhance growth in biofilms undergoing anaerobic respiration. The same is true for nitrite, which enhances but is not required for biofilm growth (22).
FIG. 2.
NO enhances biofilm formation in biofilms that are undergoing anaerobic respiration. (A) Biofilm mass of wild-type N. gonorrhoeae strain 1291 in the absence of nitrite (1) and in the presence of 20 μM SNP (2) after 2 days of growth. Biofilms were grown for 24 h in the presence of nitrite and then transitioned to media without nitrite that either did or did not contain SNP. Experiments were performed a minimum of two times, and a representative result is depicted in panel A. N. gonorrhoeae was visualized by GFP expression, and these images are three-dimensional reconstructions of stacked z series taken at a magnification of ×200 and rendered by Volocity. (B) Graphs showing COMSTAT analyses of biomass and average thickness. There was a significant difference between biofilms with nitrite and those without nitrite, as determined by Student's t test (P < 0.001).
Measurements of dissolved NO in the SNP stock solution and biofilm media indicated that the concentration of NO was substantially lower than the prepared SNP concentration. We found that SNP degrades rapidly in the biofilm media and that the concentration of NO is greatest in the headspace above the media. Authentic NO is present under these conditions, which results in the observed biofilm phenotype. However, the concentration of NO in the biofilm media is not likely to exceed 20 nM, which would be within the normal physiological range in vivo.
Effect of DETA/NO on biofilm formation.
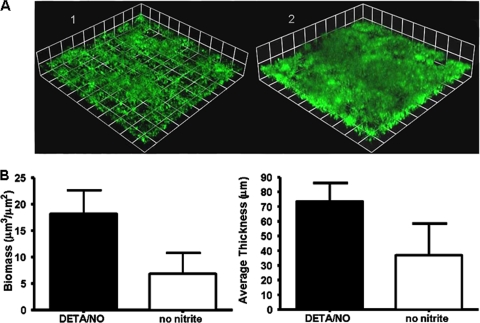
SNP treatment enhanced biofilm formation after nitrite was removed from the media. Therefore, we decided to examine the effect of NO treatment on biofilms that were not previously treated with nitrite to determine if NO could be substituted for nitrite. This line of study was of interest because anaerobic respiration plays an important role in biofilm formation and nitrite and NO are both consumed during anaerobic respiration (22). Therefore, we treated biofilms with DETA/NO, a more-stable, slow-release NO donor. SNP proved unsuitable for these experiments, as treatment with concentrations of 20 μM or greater at the start blocked biofilm formation. Biofilm formation was enhanced when wild-type N. gonorrhoeae strain 1291 biofilms were grown in the presence of 20 μM DETA/NO for 48 h. Biofilms treated with 20 μM DETA/NO at the start of and throughout biofilm growth formed thicker biofilms with significantly more biomass than biofilms grown in the absence of nitrite and NO (Fig. 3). Biofilms grown with DETA/NO also formed biofilms with biomasses and average thicknesses that were similar to those of wild-type biofilms grown in the presence of nitrite (22).
FIG. 3.
DETA/NO enhances biofilm formation in biofilms without nitrite. (A) Biofilm mass of wild-type N. gonorrhoeae strain 1291 in the absence of nitrite (1) and in the presence of 20 μM DETA/NO (2) after 2 days of growth. Experiments were performed a minimum of three times, and a representative result is depicted in panel A. N. gonorrhoeae was visualized by GFP expression, and these images are three-dimensional reconstructions of stacked z series taken at a magnification of ×200 and rendered by Volocity. (B) Graphs showing COMSTAT analyses of biomass and average and maximum thickness. Statistical differences between biofilms with and without nitrite were determined via Student's t test (P < 0.001).
Measurements of dissolved NO in the DETA/NO stock and biofilm media indicated that DETA/NO is indeed more stable than SNP. DETA/NO releases NO slowly and steadily, providing a continuous low concentration of NO to the biofilm. The average concentration of NO in the media was between 15 and 22 nM.
Effect of DETA/NO on aniA::kan insertion mutant (22) biofilms.
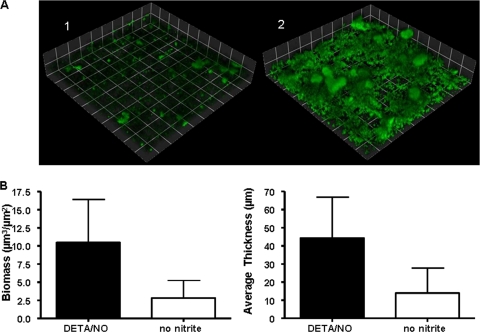
In order to evaluate the ability of N. gonorrhoeae to use NO as a substrate for anaerobic respiration during growth in a biofilm, we elected to examine the effect of DETA/NO treatment on an aniA::kan insertion mutant. An aniA::kan mutant cannot reduce nitrite, but it can reduce NO via NorB (22). Biofilm formation was enhanced in N. gonorrhoeae 1291 aniA::kan after treatment with 20 μM DETA/NO in the absence of nitrite. Biofilms grown in the presence of DETA/NO had significantly increased biomasses and average thicknesses compared to those of aniA::kan biofilms grown in the absence of NO or nitrite (Fig. 4). However, treatment with DETA/NO did not restore biofilm formation to levels similar to that of the wild type grown in the presence of nitrite. Complementation of the aniA::kan mutation fully restores biofilm formation (data not shown). Therefore, it would appear that NO can enhance biofilm formation when nitrite is unavailable or cannot be consumed. However, NO cannot entirely compensate for the inability to utilize nitrite, indicating that NO may partially sustain anaerobic respiration in biofilm, although nitrite is the preferred substrate. It is also possible that the level of NO supplied is not optimal to fully support anaerobic growth in these biofilms. Overall, these data indicate that N. gonorrhoeae biofilms are capable of utilizing both nitrite and NO to enhance biofilm formation in a concentration-dependent manner.
FIG. 4.
Biofilm formation by the aniA::kan mutant is enhanced in the presence of DETA/NO. (A) Biofilm mass of N. gonorrhoeae strain 1291 aniA::kan in the absence of nitrite (1) and in the presence of 20 μM DETA/NO (2) after 2 days of growth. Experiments were performed a minimum of three times, and a representative result is depicted in panel A. N. gonorrhoeae was visualized by GFP expression, and these images are three-dimensional reconstructions of stacked z series taken at a magnification of ×200 and rendered by Volocity. (B) Graphs showing COMSTAT analyses of biomass and average and maximum thickness. Statistical differences between biofilms with and without nitrite were determined via Student's t test (P < 0.001).
DISCUSSION
N. gonorrhoeae biofilms can use a combination of anaerobic and aerobic metabolism to support growth. Genes that are anaerobically induced and/or encode proteins involved in anaerobic respiration are required for normal biofilm formation, while the transcripts of some genes involved in aerobic respiration are less abundant in biofilms (22). We found that the addition of NO can improve biofilm formation in the absence of nitrite and help to restore biofilm formation in an aniA::kan mutant that is incapable of reducing nitrite. However, NO does not fully restore biofilm formation in this mutant, which may indicate that nitrite is the preferred substrate for anaerobic respiration. Biofilm formation may also be inhibited by NO when it is present at a sublethal concentration within the biofilm, even when nitrite is available, which suggests that the gonococcus is capable of sensing and responding to the concentration of NO in the surrounding media, as well as to the availability of nitrite.
The gonococcus was initially considered to be incapable of anaerobic growth (40), although N. gonorrhoeae was often isolated in the presence of obligate anaerobes (62). It was subsequently determined that nitrite is required for the anaerobic growth of N. gonorrhoeae on plates (42). AniA (nitrite reductase) reduces nitrite to NO (48), which is then reduced to nitrous oxide (N2O) by NorB (NO reductase) (39). N. gonorrhoeae does not evolve nitrogen gas, as there is a frameshift mutation in the nos genes that would normally encode proteins that reduce N2O (49). Therefore, both nitrite and NO, both of which are present at the site of infection in women (43, 66, 67), could be reduced during anaerobic growth. Previous studies demonstrated that an aniA insertion mutant cannot respire anaerobically but can survive incubation under anaerobic conditions (38). However, NO was not present under these growth conditions. We found that exogenously supplied NO can partially rescue biofilm formation in an aniA::kan mutant, which suggests that strains with an impaired aniA function may be capable of anaerobic respiration in the presence of NO if it is present at concentrations that enhance biofilm formation. Naturally, this may be difficult to achieve outside of a biofilm or other chemostatic system.
NorB establishes a NO steady state that rapidly reduces NO from proinflammatory (1 μM) to anti-inflammatory levels (100 nM) (6). This indicates that NorB is efficient at reducing endogenously and exogenously produced NO, which is produced by PMNs (7, 23, 45, 46) and cervical endothelial and epithelial cells in the host (43, 66). This poses a potentially serious threat to bacterial cells that cannot reduce NO (59, 60). NorB not only reduces nitrosative stress, but it also participates in anaerobic respiration. We previously demonstrated that normal biofilm formation in N. gonorrhoeae is dependent on the ability to grow anaerobically (22). In this study, we determined that NO can partially restore biofilm formation in an aniA::kan mutant that is incapable of anaerobic growth using nitrite. Therefore, our results suggest that NorB may be sufficient to support anaerobic growth when NO is present at concentrations that enhance biofilm formation. Thus, reduction of endogenous NO may contribute to anaerobic respiration, especially if nitrite is unavailable or the function of AniA is impaired. It has been demonstrated that Neisseria meningitidis can respire anaerobically (16, 55-57). However, AniA is functional in only some strains of N. meningitidis, and it is not required for pathogenesis (63). If an aniA::kan insertion mutant can anaerobically respire through the NorB-mediated reduction of NO, this may also help to explain why biofilm formation is more severely attenuated in the norB::kan mutant (22).
NorB is the simplest form of a respiratory nitric oxide reductase. It uses ubiquinol as an electron donor and reduces NO at the outer face of the cytoplasmic membrane (17). As a consequence, this enzyme does not conserve energy. In some cases, its sole functional role appears to be to detoxify NO, as in the cyanobacterium Synechocystis (5). However, provided that NO respiration was coupled to the activity of proton-translocating NADH dehydrogenase, the respiratory pathway to NO would generate a proton motive force. The AniA nitrite reductase is located in the outer membrane of N. gonorrhoeae (11). Although the respiratory proteins that shuttle electrons across the periplasm to AniA have not been definitively identified, the sensitivity of nitrite respiration to the inhibitor myxothiazol is a clear indication that the cytochrome bc1 complex is involved (16). This means that electron transfer from NADH to nitrite involves two energy-conserving steps. This may explain why nitrite is a superior electron acceptor for respiration compared to NO. Neisseria species possess a single electrogenic cytochrome oxidase (cytochrome cbb3). Thus, respiration from NADH to oxygen involves three energy-conserving steps. It is notable that cytochrome cbb3 has a very high affinity for oxygen, with a Km in the nM range (51). This means that oxygen consumption and energy conservation via aerobic respiration probably take place under very low concentrations of oxygen, which would induce expression of aniA and norB. Under these conditions, NorB may have an additional role in detoxification. It has been established that NADH dehydrogenase is susceptible to inhibition by NO; therefore, NorB may play a key role in preventing inhibition of this key energy-conserving enzyme.
We determined that N. gonorrhoeae uses a combination of aerobic and anaerobic/microaerobic metabolism to support its growth as a biofilm. This agrees with our previous findings that biofilm formation is impaired, but not prevented entirely, in the aniA::kan and norB::kan insertion mutants (22). Pseudomonas aeruginosa, the paradigm organism for the study of biofilm formation, also undergoes a combination of anaerobic and aerobic respiration during growth as a biofilm. Anaerobic respiration is believed to be the primary mode of respiration during cystic fibrosis infection (24, 33, 68, 70). Respiration profiles appear to be similar for N. gonorrhoeae, as a large proportion of the biofilm expresses aniA. Normal biofilm formation is dependent on the expression of anaerobic respiratory genes in P. aeruginosa (70), and N. gonorrhoeae is similar in this respect (22). We found that anaerobic respiration occurs most prevalently in the substratum of N. gonorrhoeae biofilms. Our images also demonstrate that anaerobic respiration does not occur in the upper portions of the biofilm, which are directly exposed to the fluid flow. This agrees with the finding that oxygen can penetrate approximately the first 50 μM P. aeruginosa biofilms (54, 69).
N. gonorrhoeae biofilms do not immediately catalyze anaerobic respiration. Overnight plate cultures and biofilm inocula do not produce detectable levels of GFP in the N. gonorrhoeae 1291 aniA′-′gfp transcriptional fusion strain. However, GFP can be detected after 24 h of growth as a biofilm, which indicates that N. gonorrhoeae biofilms do become anaerobic/microaerobic over time. This likely explains why high doses of SNP prevent biofilm formation when administered at the start of biofilm growth but can enhance growth in biofilms that are primed for anaerobic respiration after growth for 24 h in the presence of nitrite. Biofilms undergoing anaerobic respiration can effectively reduce NO, which contributes to nitrosative-stress tolerance and anaerobic metabolism in the gonococcus. However, nitrite appears to be the preferred substrate for anaerobic respiration in the gonococcus. DETA/NO-treated biofilms are indistinguishable from nitrite-treated biofilms, while those grown in the absence of nitrite and NO are severely attenuated. DETA/NO partially restores biofilm formation in the aniA::kan mutant, but these biofilms do not achieve the same level of biofilm production as wild-type biofilms grown in the presence of nitrite. Thus, wild-type biofilms that can utilize both NO and nitrite would have a distinct advantage over aniA::kan biofilms that can use only NO. Respiration is heterogeneous in N. gonorrhoeae biofilms, which employ a combination of aerobic and anaerobic or microaerobic metabolism. Aerobic respiration likely plays its most important role during initial biofilm formation, which allows time for these biofilms to induce the transcription of genes in the anaerobic respiratory chain.
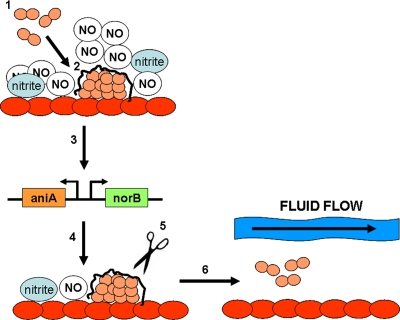
N. gonorrhoeae biofilms use a combination of aerobic and anaerobic respiration. Although aerobic respiration may largely support the initial growth of biofilms, anaerobic respiration uniquely confers protection against the oxidative stresses that are present in the natural cervical environment. The ability to sense and respond to NO also appears to be critical for biofilm formation by N. gonorrhoeae, as NO may contribute to anaerobic metabolism as well as influence the mechanisms that govern biofilm detachment and formation. The ability to sense the concentration of NO in the surrounding medium appears to influence biofilm formation, which in turn may regulate the gonococcal lifestyle, governing the switch between attached and planktonic modes of growth. See Fig. 5 for our proposed model, which illustrates the potential role of NO in biofilm formation and detachment. We acknowledge that further investigation is necessary to support or refute this model.
FIG. 5.
One possible model for the role of nitric oxide in biofilm formation. During step 1, N. gonorrhoeae cells (depicted in pink) recognize high levels of NO and bind to the surfaces of the cervical cells (depicted in red). During step 2, these cells differentiate into a biofilm, producing a biofilm matrix. In step 3, biofilm formation turns on the transcription of aniA and norB, which reduces the concentration of NO in the surrounding media. In step 4, low levels of NO signal dispersal of the biofilm, which likely occurs through degradation of the biofilm matrix (step 5). In step 6, cells that are released from the biofilm may be swept away by secretion, possibly allowing these cells to colonize new areas of cervical tissue.
Acknowledgments
The M.A.A. laboratory is supported by NIH program grant R-01AI045728. A.G.M. and M.P.J. thank the NH and MRC (Australia) for support.
There is no conflict of interest.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 15 March 2010.
REFERENCES
- 1.Anonymous. 1995. Ectopic pregnancy—United States, 1990-1992. MMWR Morb. Mortal. Wkly. Rep. 44:46-48. [PubMed] [Google Scholar]
- 2.Aparna, M. S., and S. Yadav. 2008. Biofilms: microbes and disease. Braz. J. Infect. Dis. 12:526-530. [DOI] [PubMed] [Google Scholar]
- 3.Barraud, N., D. J. Hassett, S. H. Hwang, S. A. Rice, S. Kjelleberg, and J. S. Webb. 2006. Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J. Bacteriol. 188:7344-7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bozicevic, I., K. A. Fenton, I. M. Martin, E. A. Rudd, C. A. Ison, K. Nanchahal, and K. Wellings. 2006. Epidemiological correlates of asymptomatic gonorrhea. Sex. Transm. Dis. 33:289-295. [DOI] [PubMed] [Google Scholar]
- 5.Büsch, A., B. Friedrich, and R. Cramm. 2002. Characterization of the norB gene, encoding nitric oxide reductase, in the nondenitrifying cyanobacterium Synechocystis sp. strain PCC6803. Appl. Environ. Microbiol. 68:668-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardinale, J. A., and V. L. Clark. 2005. Determinants of nitric oxide steady-state levels during anaerobic respiration by Neisseria gonorrhoeae. Mol. Microbiol. 58:177-188. [DOI] [PubMed] [Google Scholar]
- 7.Carreras, M. C., G. A. Pargament, S. D. Catz, J. J. Poderoso, and A. Boveris. 1994. Kinetics of nitric oxide and hydrogen peroxide production and formation of peroxynitrite during the respiratory burst of human neutrophils. FEBS Lett. 341:65-68. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2007. Sexually transmitted disease surveillance, 2006. U.S. Department of Health and Human Services, Atlanta, GA.
- 9.Centers for Disease Control and Prevention. 2008. Sexually transmitted disease surveillance, 2007. U.S. Department of Health and Human Services, Atlanta, GA.
- 10.Chen, A., I. C. Boulton, J. Pongoski, A. Cochrane, and S. D. Gray-Owen. 2003. Induction of HIV-1 long terminal repeat-mediated transcription by Neisseria gonorrhoeae. AIDS 17:625-628. [DOI] [PubMed] [Google Scholar]
- 11.Clark, V. L., L. A. Campbell, D. A. Palermo, T. M. Evans, and K. W. Klimpel. 1987. Induction and repression of outer membrane proteins by anaerobic growth of Neisseria gonorrhoeae. Infect. Immun. 55:1359-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costerton, J. W. 1999. Introduction to biofilm. Int. J. Antimicrob. Agents 11:217-221. [DOI] [PubMed] [Google Scholar]
- 13.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 14.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 15.Davis, K. L., E. Martin, I. V. Turko, and F. Murad. 2001. Novel effects of nitric oxide. Annu. Rev. Pharmacol. Toxicol. 41:203-236. [DOI] [PubMed] [Google Scholar]
- 16.Deeudom, M., J. Rock, and J. Moir. 2006. Organization of the respiratory chain of Neisseria meningitidis. Biochem. Soc. Trans. 34:139-142. [DOI] [PubMed] [Google Scholar]
- 17.de Vries, S., and I. Schroder. 2002. Comparison between the nitric oxide reductase family and its aerobic relatives, the cytochrome oxidases. Biochem. Soc. Trans. 30:662-667. [DOI] [PubMed] [Google Scholar]
- 18.Donlan, R. M. 2001. Biofilm formation: a clinically relevant microbiological process. Clin. Infect. Dis. 33:1387-1392. [DOI] [PubMed] [Google Scholar]
- 19.Dunne, W. M., Jr. 2002. Bacterial adhesion: seen any good biofilms lately? Clin. Microbiol. Rev. 15:155-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards, J. L., and M. A. Apicella. 2005. I-domain-containing integrins serve as pilus receptors for Neisseria gonorrhoeae adherence to human epithelial cells. Cell. Microbiol. 7:1197-1211. [DOI] [PubMed] [Google Scholar]
- 21.Edwards, J. L., and M. A. Apicella. 2004. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin. Microbiol. Rev. 17:965-981, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falsetta, M. L., T. B. Bair, S. C. Ku, R. N. Vanden Hoven, C. T. Steichen, A. G. McEwan, M. P. Jennings, and M. A. Apicella. 2009. Transcriptional profiling identifies the metabolic phenotype of gonococcal biofilms. Infect. Immun. 77:3522-3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang, F. C. 1997. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J. Clin. Invest. 99:2818-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filiatrault, M. J., K. F. Picardo, H. Ngai, L. Passador, and B. H. Iglewski. 2006. Identification of Pseudomonas aeruginosa genes involved in virulence and anaerobic growth. Infect. Immun. 74:4237-4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleming, D. T., and J. N. Wasserheit. 1999. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex. Transm. Infect. 75:3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerbase, A. C., J. T. Rowley, D. H. Heymann, S. F. Berkley, and P. Piot. 1998. Global prevalence and incidence estimates of selected curable STDs. Sex. Transm. Infect. 74(Suppl. 1):S12-S16. [PubMed] [Google Scholar]
- 27.Greiner, L. L., J. L. Edwards, J. Shao, C. Rabinak, D. Entz, and M. A. Apicella. 2005. Biofilm formation by Neisseria gonorrhoeae. Infect. Immun. 73:1964-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall-Stoodley, L., J. W. Costerton, and P. Stoodley. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2:95-108. [DOI] [PubMed] [Google Scholar]
- 29.Hall-Stoodley, L., and P. Stoodley. 2009. Evolving concepts in biofilm infections. Cell. Microbiol. 11:1034-1043. [DOI] [PubMed] [Google Scholar]
- 30.Handsfield, H. H., T. O. Lipman, J. P. Harnisch, E. Tronca, and K. K. Holmes. 1974. Asymptomatic gonorrhea in men. Diagnosis, natural course, prevalence and significance. N. Engl. J. Med. 290:117-123. [DOI] [PubMed] [Google Scholar]
- 31.Hansfield, H. H., and P. F. Sparling. 2005. Neisseria gonorrhoeae, p. 2514-2529. In G. L. Mandell et al. (ed.), Principles and practice of infectious diseases, 4th ed. Churchill Livingstone Inc., New York, NY.
- 32.Hassett, D. J., and M. S. Cohen. 1989. Bacterial adaptation to oxidative stress: implications for pathogenesis and interaction with phagocytic cells. FASEB J. 3:2574-2582. [DOI] [PubMed] [Google Scholar]
- 33.Hassett, D. J., J. Cuppoletti, B. Trapnell, S. V. Lymar, J. J. Rowe, S. S. Yoon, G. M. Hilliard, K. Parvatiyar, M. C. Kamani, D. J. Wozniak, S. H. Hwang, T. R. McDermott, and U. A. Ochsner. 2002. Anaerobic metabolism and quorum sensing by Pseudomonas aeruginosa biofilms in chronically infected cystic fibrosis airways: rethinking antibiotic treatment strategies and drug targets. Adv. Drug Deliv. Rev. 54:1425-1443. [DOI] [PubMed] [Google Scholar]
- 34.Heydorn, A., A. T. Nielsen, M. Hentzer, C. Sternberg, M. Givskov, B. K. Ersboll, and S. Molin. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146:2395-2407. [DOI] [PubMed] [Google Scholar]
- 35.Hook, E. W., III, and H. H. Handsfield. 1999. Biology of Neisseria gonorrhoeae, p. 433-449. In K. K. Holmes, P. A. Mardh, P. F. Sparling, S. M. Lemon, W. E. Stamm, P. Piot, and J. N. Wasserjeot (ed.), Sexually transmitted diseases, 3rd ed. McGraw HIll, New York, NY.
- 36.Hook, E. W., III, and H. H. Handsfield. 1999. Gender perspectives and STDs, p. 117-127. In K. K. Holmes, P. A. Mardh, P. F. Sparling, S. M. Lemon, W. E. Stamm, P. Piot, and J. N. Wasserjeot (ed.), Sexually transmitted diseases, 3rd ed. McGraw Hill, New York, NY.
- 37.Hook, E. W., III, and H. H. Handsfield. 1999. Gonococcal infections in the adult, p. 451-466. In K. K. Holmes, P. A. Mardh, P. F. Sparling, S. M. Lemon, W. E. Stamm, P. Piot, and J. N. Wasserjeot (ed.), Sexually transmitted diseases, 3rd ed. McGraw Hill, New York, NY.
- 38.Householder, T. C., W. A. Belli, S. Lissenden, J. A. Cole, and V. L. Clark. 1999. cis- and trans-acting elements involved in regulation of aniA, the gene encoding the major anaerobically induced outer membrane protein in Neisseria gonorrhoeae. J. Bacteriol. 181:541-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Householder, T. C., E. M. Fozo, J. A. Cardinale, and V. L. Clark. 2000. Gonococcal nitric oxide reductase is encoded by a single gene, norB, which is required for anaerobic growth and is induced by nitric oxide. Infect. Immun. 68:5241-5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.James-Holmquest, A. N., R. D. Wende, R. L. Mudd, and R. P. Williams. 1973. Comparison of atmospheric conditions for culture of clinical specimens of Neisseria gonorrhoeae. Appl. Microbiol. 26:466-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janssen, R., T. van der Straaten, A. van Diepen, and J. T. van Dissel. 2003. Responses to reactive oxygen intermediates and virulence of Salmonella typhimurium. Microbes Infect. 5:527-534. [DOI] [PubMed] [Google Scholar]
- 42.Knapp, J. S., and V. L. Clark. 1984. Anaerobic growth of Neisseria gonorrhoeae coupled to nitrite reduction. Infect. Immun. 46:176-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ledingham, M. A., A. J. Thomson, A. Young, L. M. Macara, I. A. Greer, and J. E. Norman. 2000. Changes in the expression of nitric oxide synthase in the human uterine cervix during pregnancy and parturition. Mol. Hum. Reprod. 6:1041-1048. [DOI] [PubMed] [Google Scholar]
- 44.Lim, K. H., C. E. Jones, R. N. vanden Hoven, J. L. Edwards, M. L. Falsetta, M. A. Apicella, M. P. Jennings, and A. G. McEwan. 2008. Metal binding specificity of the MntABC permease of Neisseria gonorrhoeae and its influence on bacterial growth and interaction with cervical epithelial cells. Infect. Immun. 76:3569-3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacMicking, J., Q. W. Xie, and C. Nathan. 1997. Nitric oxide and macrophage function. Annu. Rev. Immunol. 15:323-350. [DOI] [PubMed] [Google Scholar]
- 46.McCall, T. B., N. K. Boughton-Smith, R. M. Palmer, B. J. Whittle, and S. Moncada. 1989. Synthesis of nitric oxide from l-arginine by neutrophils. Release and interaction with superoxide anion. Biochem. J. 261:293-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCormack, W. M., R. J. Stumacher, K. Johnson, and A. Donner. 1977. Clinical spectrum of gonococcal infection in women. Lancet i:1182-1185. [DOI] [PubMed] [Google Scholar]
- 48.Mellies, J., J. Jose, and T. F. Meyer. 1997. The Neisseria gonorrhoeae gene aniA encodes an inducible nitrite reductase. Mol. Gen. Genet. 256:525-532. [DOI] [PubMed] [Google Scholar]
- 49.Overton, T. W., R. Whitehead, Y. Li, L. A. Snyder, N. J. Saunders, H. Smith, and J. A. Cole. 2006. Coordinated regulation of the Neisseria gonorrhoeae-truncated denitrification pathway by the nitric oxide-sensitive repressor, NsrR, and nitrite-insensitive NarQ-NarP. J. Biol. Chem. 281:33115-33126. [DOI] [PubMed] [Google Scholar]
- 50.Pedersen, A. H., and P. Bonin. 1971. Screening females for asymptomatic gonorrhea infection. Northwest Med. 70:255-261. [PubMed] [Google Scholar]
- 51.Pitcher, R. S., and N. J. Watmough. 2004. The bacterial cytochrome cbb3 oxidases. Biochim. Biophys. Acta 1655:388-399. [DOI] [PubMed] [Google Scholar]
- 52.Potter, A. J., S. P. Kidd, J. L. Edwards, M. L. Falsetta, M. A. Apicella, M. P. Jennings, and A. G. McEwan. 2009. Esterase D is essential for protection of Neisseria gonorrhoeae against nitrosative stress and for bacterial growth during interaction with cervical epithelial cells. J. Infect. Dis. 200:273-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Potter, A. J., S. P. Kidd, J. L. Edwards, M. L. Falsetta, M. A. Apicella, M. P. Jennings, and A. G. McEwan. 2009. Thioredoxin reductase is essential for protection of Neisseria gonorrhoeae against killing by nitric oxide and for bacterial growth during interaction with cervical epithelial cells. J. Infect. Dis. 199:227-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rani, S. A., B. Pitts, H. Beyenal, R. A. Veluchamy, Z. Lewandowski, W. M. Davison, K. Buckingham-Meyer, and P. S. Stewart. 2007. Spatial patterns of DNA replication, protein synthesis, and oxygen concentration within bacterial biofilms reveal diverse physiological states. J. Bacteriol. 189:4223-4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rock, J. D., M. R. Mahnane, M. F. Anjum, J. G. Shaw, R. C. Read, and J. W. Moir. 2005. The pathogen Neisseria meningitidis requires oxygen, but supplements growth by denitrification. Nitrite, nitric oxide and oxygen control respiratory flux at genetic and metabolic levels. Mol. Microbiol. 58:800-809. [DOI] [PubMed] [Google Scholar]
- 56.Rock, J. D., and J. W. Moir. 2005. Microaerobic denitrification in Neisseria meningitidis. Biochem. Soc. Trans. 33:134-136. [DOI] [PubMed] [Google Scholar]
- 57.Rock, J. D., M. J. Thomson, R. C. Read, and J. W. Moir. 2007. Regulation of denitrification genes in Neisseria meningitidis by nitric oxide and the repressor NsrR. J. Bacteriol. 189:1138-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schlag, S., C. Nerz, T. A. Birkenstock, F. Altenberend, and F. Gotz. 2007. Inhibition of staphylococcal biofilm formation by nitrite. J. Bacteriol. 189:7911-7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seib, K. L., H. J. Tseng, A. G. McEwan, M. A. Apicella, and M. P. Jennings. 2004. Defenses against oxidative stress in Neisseria gonorrhoeae and Neisseria meningitidis: distinctive systems for different lifestyles. J. Infect. Dis. 190:136-147. [DOI] [PubMed] [Google Scholar]
- 60.Seib, K. L., H. J. Wu, S. P. Kidd, M. A. Apicella, M. P. Jennings, and A. G. McEwan. 2006. Defenses against oxidative stress in Neisseria gonorrhoeae: a system tailored for a challenging environment. Microbiol. Mol. Biol. Rev. 70:344-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seib, K. L., H. J. Wu, Y. N. Srikhanta, J. L. Edwards, M. L. Falsetta, A. J. Hamilton, T. L. Maguire, S. M. Grimmond, M. A. Apicella, A. G. McEwan, and M. P. Jennings. 2007. Characterization of the OxyR regulon of Neisseria gonorrhoeae. Mol. Microbiol. 63:54-68. [DOI] [PubMed] [Google Scholar]
- 62.Smith, L. D. 1975. The pathogenic anaerobic bacteria. Charles C. Thomas Publisher, Springfield, IL.
- 63.Stefanelli, P., G. Colotti, A. Neri, M. L. Salucci, R. Miccoli, L. Di Leandro, and R. Ippoliti. 2008. Molecular characterization of nitrite reductase gene (aniA) and gene product in Neisseria meningitidis isolates: is aniA essential for meningococcal survival? IUBMB Life 60:629-636. [DOI] [PubMed] [Google Scholar]
- 64.Steichen, C. T., J. Q. Shao, M. R. Ketterer, and M. A. Apicella. 2008. Gonococcal cervicitis: a role for biofilm in pathogenesis. J. Infect. Dis. 198:1856-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stoodley, P., K. Sauer, D. G. Davies, and J. W. Costerton. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56:187-209. [DOI] [PubMed] [Google Scholar]
- 66.Tschugguel, W., C. Schneeberger, H. Lass, F. Stonek, M. B. Zaghlula, K. Czerwenka, C. Schatten, A. Kaider, P. Husslein, and J. C. Huber. 1999. Human cervical ripening is associated with an increase in cervical inducible nitric oxide synthase expression. Biol. Reprod. 60:1367-1372. [DOI] [PubMed] [Google Scholar]
- 67.Väisänen-Tommiska, M., M. Nuutila, K. Aittomaki, V. Hiilesmaa, and O. Ylikorkala. 2003. Nitric oxide metabolites in cervical fluid during pregnancy: further evidence for the role of cervical nitric oxide in cervical ripening. Am. J. Obstet. Gynecol. 188:779-785. [DOI] [PubMed] [Google Scholar]
- 68.Van Alst, N. E., K. F. Picardo, B. H. Iglewski, and C. G. Haidaris. 2007. Nitrate sensing and metabolism modulate motility, biofilm formation, and virulence in Pseudomonas aeruginosa. Infect. Immun. 75:3780-3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Werner, E., F. Roe, A. Bugnicourt, M. J. Franklin, A. Heydorn, S. Molin, B. Pitts, and P. S. Stewart. 2004. Stratified growth in Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 70:6188-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoon, S. S., R. F. Hennigan, G. M. Hilliard, U. A. Ochsner, K. Parvatiyar, M. C. Kamani, H. L. Allen, T. R. DeKievit, P. R. Gardner, U. Schwab, J. J. Rowe, B. H. Iglewski, T. R. McDermott, R. P. Mason, D. J. Wozniak, R. E. Hancock, M. R. Parsek, T. L. Noah, R. C. Boucher, and D. J. Hassett. 2002. Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev. Cell 3:593-603. [DOI] [PubMed] [Google Scholar]
- 71.Zaitseva, J., V. Granik, A. Belik, O. Koksharova, and I. Khmel. 2009. Effect of nitrofurans and NO generators on biofilm formation by Pseudomonas aeruginosa PAO1 and Burkholderia cenocepacia 370. Res. Microbiol. 160:353-357. [DOI] [PubMed] [Google Scholar]
- 72.Zumft, W. G. 1997. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61:533-616. [DOI] [PMC free article] [PubMed] [Google Scholar]