Abstract
Leukotriene B4 (LTB4) is a potent lipid mediator of inflammation formed by the 5-lipoxygenase (5-LO)-catalyzed oxidation of arachidonic acid. We have previously shown that (i) LTB4 is generated during infection, (ii) its biosynthesis is essential for optimal antimicrobial host defense, (iii) LT deficiency is associated with clinical states of immunocompromise, and (iv) exogenous LTB4 augments antimicrobial functions in phagocytes. Here, we sought to determine whether the administration of LTB4 has therapeutic potential in a mouse model of pneumonia. Wild-type and 5-LO knockout mice were challenged with Streptococcus pneumoniae via the intranasal route, and bacterial burdens, leukocyte counts, and cytokine levels were determined. LTB4 was administered via the intraperitoneal, intravenous, and intranasal routes prior to pneumococcal infection and by aerosol 24 h following infection. Leukocytes recovered from mice given S. pneumoniae and treated with aerosolized LTB4 were evaluated for expression levels of the p47phox subunit of NADPH oxidase. Intrapulmonary but not systemic pretreatment with LTB4 significantly reduced the lung S. pneumoniae burden in wild-type mice. Aerosolized LTB4 was effective at improving lung bacterial clearance when administered postinoculation in animals with established infection and exhibited greater potency in 5-LO knockout animals, which also exhibited greater baseline susceptibility. Augmented bacterial clearance in response to LTB4 was associated with enhanced monocyte recruitment and leukocyte expression of p47phox. The results of the current study in an animal model serve as a proof of concept for the potential utility of treatment with aerosolized LTB4 as an immunostimulatory strategy in patients with bacterial pneumonia.
Pneumonia is associated with more disability-adjusted life years lost around the world than any other category of disease (31), and it is the leading cause of infection-related death in industrialized nations, as well as the leading cause of hospitalization in the United States (8). This enormous and growing impact of pneumonia is a result of an aging population, increased immunosuppression and iatrogenesis, and the emergence of antibiotic-resistant and new microbes (12, 32). The realization that antibiotics alone cannot stem this tide of disease mandates an improvement in our understanding of innate antimicrobial defense mechanisms and their potential for therapeutic augmentation. An effective host response against pulmonary bacterial infection requires the elaboration of proinflammatory cytokines and lipid mediators that activate antimicrobial functions in resident epithelial cells and alveolar macrophages (AMs) and recruit circulating leukocytes to the alveolar milieu (30). Among the lipid mediators produced in response to bacterial infection are the leukotrienes (LTs), potent proinflammatory molecules that are rapidly synthesized by the 5-lipoxygenase (5-LO)-catalyzed oxidation of arachidonic acid: the two classes of LTs synthesized under these conditions include LTB4 and the cysteinyl LTs (cysLTs) LTC4, LTD4, and LTE4 (21).
LTB4 is best known for its role as a neutrophil (polymorphonuclear leukocyte [PMN]) chemoattractant, and cysLTs are best known for their ability to induce protracted bronchoconstriction in asthma (36). However, LTs are now recognized to fulfill Koch's postulates as important participants in the host response against infection (35). They are produced at sites of infection and by phagocytes incubated in vitro with microbes (19, 21, 29, 48). Mice rendered LT deficient by targeted deletion of 5-LO or by pharmacologic inhibition of LT biosynthesis exhibited increased mortality and reduced microbial clearance following challenge with a variety of bacteria (3, 41), mycobacteria (34), fungi (28), and parasites (44). Likewise, LT-deficient AMs exhibited impaired phagocytosis and killing of bacteria in vitro, and these defects could be overcome in vitro by the addition of exogenous LTB4 or cysLTs (21, 42). Interestingly, endogenous LT deficiency is also observed in a number of clinical conditions (HIV infection, malnutrition, cigarette smoking, vitamin D deficiency, and following bone marrow transplantation) that are associated with impaired host defense against infection (4-6, 9, 10, 16).
While we have demonstrated that the exogenous administration of 5-LO products is an effective means to augment AM antibacterial function in vitro (21, 36, 42), the utility of this strategy in augmenting antibacterial pulmonary host defense in vivo has not been demonstrated. However, the exogenous provision of LTB4 intraperitoneally (i.p.) has been shown to reduce bacterial counts in a murine model of peritonitis (11). LTB4 may be expected to have greater immunostimulatory potential than cysLTs as an adjunctive treatment for pneumonia because of (i) its greater potency in enhancing microbial killing by AMs (42), (ii) its unique capacity not only to recruit but to augment antimicrobial functions of PMNs (22), in addition to those of AMs, and (iii) its inability to elicit bronchospasm. Indeed, LTB4 has previously been administered via aerosol or bronchoscope to the lungs of humans, where it resulted in increased PMN recruitment without eliciting symptoms or adverse effects on vital signs or lung function, even in asthmatics (24, 40). Since clinical application of novel therapeutic strategies requires initial proof-of-concept testing in animals, we evaluated the effects of LTB4 administration on the in vivo innate immune response in a murine model of pneumococcal pneumonia.
MATERIALS AND METHODS
Animals.
5-LO knockout (KO) (129-Alox5tm1Fun) (7) and strain-matched wild-type (WT) 129SvJ mice were bred in the University of Michigan Unit for Laboratory Animal Medicine from breeders obtained from Jackson Laboratories (Bar Harbor, ME) and maintained under specific-pathogen-free conditions. These studies were approved by the University Committee on Use and Care of Animals.
Streptococcus pneumoniae culture and inoculation of mice.
S. pneumoniae, serotype 3, ATCC 6303, was obtained from the American Type Culture Collection (Manassas, VA) and grown in Todd-Hewitt broth containing 0.5% yeast extract (THB) (Difco, Detroit, MI) to mid-logarithmic phase at 37°C (5% CO2). Mice were anesthetized and infected intranasally with 106 CFU of S. pneumoniae cells as previously described (2, 20).
LTB4 administration.
LTB4 (Cascade Biochem, Cork, Ireland) was prepared for intraperitoneal (i.p.), intravenous (i.v.), and intranasal (i.n.) administration by dilution in an aqueous solution containing 0.45% NaCl, 0.25% dextrose, and 0.01% bovine serum albumin (BSA) (vehicle) and was filter sterilized prior to use. In these experiments, LTB4 was administered 2 h (i.v. and i.p.) or 30 min (i.n.) prior to i.n. inoculation of S. pneumoniae. The number of PMNs recruited to the lungs and recovered from the bronchoalveolar lavage fluid (BALF) was used as a means to verify that an effective dose of LTB4 was delivered via the i.n. route. Macrophage inflammatory protein 2 (MIP-2) (R&D Systems, Minneapolis, MN) was used as a positive control for some experiments (45).
Administration of aerosolized LTB4.
Twenty-four hours following S. pneumoniae infection, mice were placed in a whole-body exposure chamber (Buxco Research Systems, Wilmington, NC) and exposed for 30 min to an aerosol containing the vehicle or LTB4 suspended in vehicle generated from an Aerogen nebulizer (Galway, Ireland). Pilot experiments were performed to determine the most effective doses of LTB4 in reducing pulmonary S. pneumoniae burdens in WT and 5-LO KO mice.
Bronchoalveolar lavage fluid cell differential count and cAMP measurements.
In a separate group of mice, total and differential leukocyte counts were performed as previously described (6) on BALF obtained at 30 min, 4 h, and 24 h following the administration of vehicle or LTB4 to mice infected with 106 CFU of S. pneumoniae 24 h previously. Cyclic AMP (cAMP) was assessed in leukocytes obtained from BALF 1 h following the administration of vehicle or LTB4 to mice infected with S. pneumoniae, using a commercially available assay kit (Assay Designs, Ann Arbor, MI).
Lung cytokine determinations.
Enzyme-linked immunosorbent assays (Duoset; R&D systems) were performed by the University of Michigan Cancer Center Cellular Immunology Core to determine the levels of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), IL-10, IL-12, and monocyte chemoattractant protein 1 (MCP-1) in lung homogenates obtained 8 and 24 h after vehicle or LTB4 treatment in mice challenged with 106 CFU of S. pneumoniae on the previous day. Cytokines were extracted from lung homogenates as previously described (19). Briefly, lungs were homogenized in phosphate-buffered saline (PBS) containing 0.05% Triton X-100 (Sigma), incubated for 30 min on ice, and centrifuged at 13,000 rpm for 3 min, and supernatants were collected and stored at −80°C until cytokine analyses.
Immunocytochemical quantification of p47phox in leukocytes obtained from BALF.
Leukocytes were obtained from the BALF of mice infected with S. pneumoniae and subsequently treated with aerosolized vehicle or LTB4 24 h postinfection. Cells were cytocentrifuged onto glass slides and prepared for immunocytochemical analysis by fixation with 4% phosphonoformate (PFA) for 30 min and permeabilization with 0.1% Triton X-100 in PBS for 3 min, followed by blocking with 1% BSA-PBS for 60 min. Detection of the NADPH oxidase (NADPHox) p47phox subunit was assessed by incubation with rabbit anti-mouse antibody (1:200; Santa Cruz Biotechnology, Santa Cruz, CA) for 60 min. Mounts were washed three times with 1% BSA-PBS, and fluorescein isothiocyanate FITC-conjugated goat anti-rabbit secondary antibody (1:200) was added for 1 h at 37°C. After being washed three times, preparations were mounted using Vectashield mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories). Fluorescence was visualized with a Nikon Labophot 2 microscope equipped for epifluorescence. Fluorescence was quantified using Image J (NIH) image analysis software on at least 50 cells/group.
Immunoblot analysis.
Cells were recovered from BALF of WT or 5-LO KO mice infected with S. pneumoniae and subsequently treated with aerosolized vehicle or LTB4 at 24 h postinfection. Cells were then lysed with ice-cold lysis buffer (radioimmunoprecipitation assay [RIPA] buffer; Sigma) and disrupted with sonication (10 bursts at 20% duty cycle). Twenty micrograms of protein, as determined using a Micro BCA protein assay kit (Pierce Chemical, Rockford, IL), were separated by SDS-PAGE under reducing conditions and transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were probed with rabbit polyclonal antibodies against p47phox (1:1,000) (Cell Signaling Technology, Danvers, MA), rabbit polyclonal antibody against p67phox (1:1,000) (Santa Cruz Biotechnology), goat polyclonal antibody against gp91phox (1:1,000), or GAPDH (Cell Signaling Technology). Primary antibodies were detected using alkaline phosphatase-conjugated goat anti-rabbit secondary antibody (titer of 1:5,000) and visualized with an ECF detection system (Amersham Pharmacia Biotech, Piscataway, NJ). The densities of the luminescent bands in appropriately exposed PVDF membranes were quantitated using Image Reader.
Statistical analysis.
Where appropriate, mean values were compared using a paired t test, a one-way analysis of variance, or a Kruskal-Wallis test on ranks for nonparametric data. The Bonferroni or Dunnett's test was used for mean separation. All experiments were performed on at least three separate occasions unless otherwise specified. In all cases, a P value of <0.05 was considered significant.
RESULTS
Impaired pulmonary bacterial clearance and increased bacteremia in 5-LO KO mice after S. pneumoniae challenge.
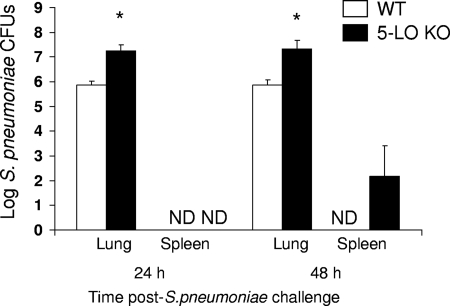
In order to confirm a protective role for endogenously generated LTs in pneumococcal pneumonia, we assessed the pulmonary and spleen bacterial loads following an i.n. challenge with 106 CFU of S. pneumoniae cells in WT and 5-LO KO mice. Compared with WT mice, we found 1 to 1.5 log higher S. pneumoniae CFU counts in the lung homogenates obtained from 5-LO KO mice 24 and 48 h after S. pneumoniae challenge (Fig. 1). In addition, we were able to culture S. pneumoniae from the spleen in 4 of 8 5-LO KO mice but in none of the WT mice 48 h after bacterial challenge. This finding suggests that endogenously produced LTs contribute to pulmonary bacterial clearance and limit bacterial dissemination to the peripheral circulation during pneumococcal pneumonia.
FIG. 1.
Increased lung and spleen bacterial burdens in 5-LO KO mice following S. pneumoniae challenge. WT and 5-LO KO mice were infected with 106 CFU of S. pneumoniae cells via the intranasal (i.n.) route, and lung and spleen homogenates were assessed for bacterial burdens 24 and 48 h postinfection. Bars represent the means ± standard errors of the means for 8 to 10 mice per group from two separate experiments. *, P < 0.05 versus results for WT mice, using the Student t test. ND, not detected.
Intranasal LTB4 administration prior to infection with S. pneumoniae improves pulmonary bacterial clearance in WT mice.
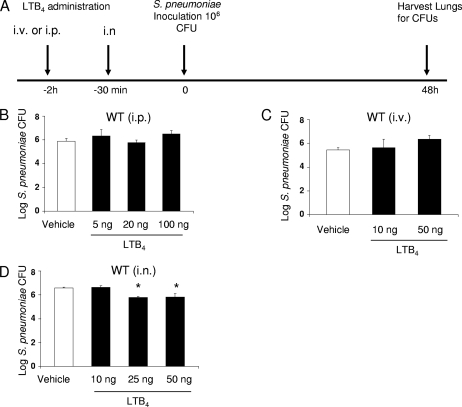
We first sought to confirm the bioactivity of intratracheally administered LTB4 in uninfected mice by assessing its classic ability to elicit PMN recruitment; the chemokine MIP-2 served as positive control (45). While we did not find any PMNs in BALF of mice given the vehicle, the percentages of PMNs in BALF of mice treated with 1 and 10 ng of LTB4 and 10 ng of MIP-2 were approximately 35%, 40%, and 50%, respectively (data not shown). Having confirmed a direct immunostimulatory effect of LTB4 in the lungs of uninfected mice, we next determined if exogenous LTB4 administered by various routes could enhance pulmonary host defense against pneumococcal pneumonia. In these experiments, we assessed the ability of i.p., i.v, or i.n. pretreatment with LTB4 to reduce pulmonary bacterial burdens in mice subsequently challenged with S. pneumoniae. Lung bacterial burdens were evaluated 48 h following an i.n. challenge with S. pneumoniae delivered 30 min (for i.n. delivery) or 2 h (for i.v. or i.p. delivery) after LTB4 administration (see protocol depicted in Fig. 2A). While we did not observe an improvement in pulmonary bacterial clearance in WT mice pretreated with LTB4 via the i.p. or i.v. route (Fig. 2B and C), i.n. LTB4 yielded significant improvement at doses of >25 ng (Fig. 2D). These data indicate that pretreatment with LTB4 can enhance pulmonary bacterial clearance when administered locally but not when administered systemically.
FIG. 2.
Effects on lung bacterial clearance of LTB4 pretreatment via various routes of administration following i.n. challenge with S. pneumoniae. (A) The protocol, with timing and routes of administration, is depicted. (B to D) WT mice were given LTB4 in the doses indicated via the i.p. (n = 6 mice) (B), i.v. (n = 6 mice) (C), or i.n. (n = 10 mice) (D) route 2 h or 30 min prior to an i.n. challenge with 106 CFU of S. pneumoniae cells. After 48 h, lungs were harvested to determine bacterial burdens. Bars represent the mean results ± standard errors of the means. *, P < 0.05 versus result for vehicle-treated control.
Aerosolized LTB4 reduces pulmonary bacterial burden in WT mice with established pneumococcal pneumonia.
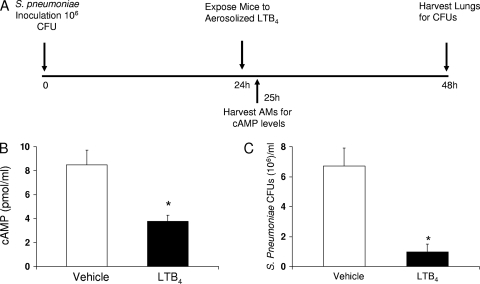
A more clinically relevant model of LTB4 administration for the treatment of bacterial pneumonia is its topical application to the lungs of mice with preexisting pneumonia. This was accomplished by exposing mice that had been infected with S. pneumoniae 24 h earlier to an aerosol of LTB4 in a whole-body exposure chamber. At this time point, no deaths had occurred but the mice typically exhibited sickness behavior, including a staggering gate, sunken eyes, hunched appearance, ruffled fur, and piloerection. This treatment regimen is summarized in Fig. 3A. To determine if aerosolized LTB4 was having a direct effect on lung leukocytes in vivo, we measured cAMP. Elevated levels of this cyclic nucleotide have been shown to suppress macrophage phagocytosis and killing of bacteria (2), while reduced levels are necessary for LTB4 enhancement of antimicrobial functions (33). We observed that cAMP levels were reduced in leukocytes recovered from infected mice 1 h after LTB4 treatment (Fig. 3B), confirming that in vivo administration of LTB4 directly activates lung leukocytes in mice with established pneumococcal pneumonia, just as it does when administered to leukocytes in vitro. In pilot experiments with aerosolized LTB4, doses of ≥100 ng loaded into the nebulizer (100, 500, and 1,000 ng) significantly and dramatically reduced lung bacterial burdens, while the 5- or 25-ng doses did not (see Fig. S1 in the supplemental material). Based on these data, we evaluated the 100-ng dose in subsequent experiments. We observed that LTB4 (100 ng) reduced the pulmonary pneumococcal burden in WT mice by approximately 85% (Fig. 3C). These data indicate that the intrapulmonary administration of LTB4 via aerosol is a very effective means of enhancing the innate immune response in the lungs of mice with established pneumonia.
FIG. 3.
Aerosolized LTB4 administered after S. pneumoniae challenge reduces cAMP levels in leukocytes recovered from BALF and improves pulmonary bacterial clearance in WT mice. WT mice were infected with 106 S. pneumoniae via the i.n. route and treated with aerosolized vehicle (PBS with 0.5% BSA) or indicated doses of LTB4 in vehicle 24 h later. (A) The protocol, with timing and routes of administration, is depicted. (B) One hour after LTB4 (100 ng) administration, leukocytes recovered from BALF of WT mice were assessed for cAMP levels. (C) In another group of mice, lungs from WT mice were assessed for bacterial CFU counts 24 h after LTB4 (100 ng) treatment (48 h postinfection). Bars represent the means ± standard errors of the means for 5 mice per group for cAMP assessment and 15 mice per group for CFU determinations. *, P < 0.05 versus result for vehicle, using the Student t test.
Aerosolized LTB4 increases pulmonary macrophage accumulation but not cytokine production in WT mice 24 h after S. pneumoniae challenge.
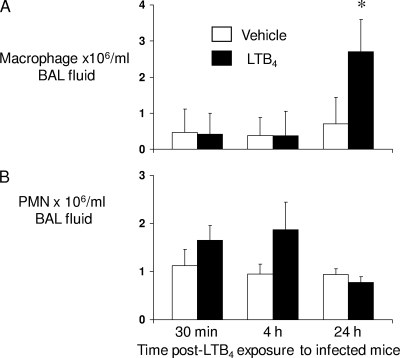
In order to explore the mechanism(s) by which LTB4 enhanced pulmonary bacterial clearance in mice with pneumococcal pneumonia, we assessed the total and differential cell counts in BALF and the cytokines in lung homogenates of WT mice challenged with S. pneumoniae and exposed to aerosolized LTB4 24 h later. While we did not see any differences in cytokines IL-6, IL-12, MCP-1, MIP-2, or TNF-α in lung homogenates (see Fig. S2 in the supplemental material), we did observe a statistically significant 3-fold increase in macrophage numbers in BALF of WT mice 24 h after exposure to aerosolized LTB4 (Fig. 4) . In addition, although a trend toward increased PMNs in BALF was noted at early time points (30 min and 4 h) after LTB4 administration, this increment was not statistically significant. These data indicate that the immunostimulatory action of LTB4 administration in WT mice is associated with increased recruitment and/or survival of monocytes/macrophages to the lungs.
FIG. 4.
Increased macrophage counts in BALF of WT mice treated with aerosolized LTB4 following S. pneumoniae challenge. WT mice were infected with 106 CFU of S. pneumoniae cells via the i.n. route and treated with aerosolized vehicle or LTB4 (100 ng) 24 h later. Lungs were lavaged 30 min, 4 h, and 24 h after LTB4 administration. Macrophage (A) and neutrophil (PMN) (B) counts were determined. Bars represent the means ± standard errors of the means for 6 mice per group. *, P < 0.05 versus results for WT mice, using the Student t test.
Aerosolized LTB4 improves pulmonary bacterial clearance in 5-LO KO mice.
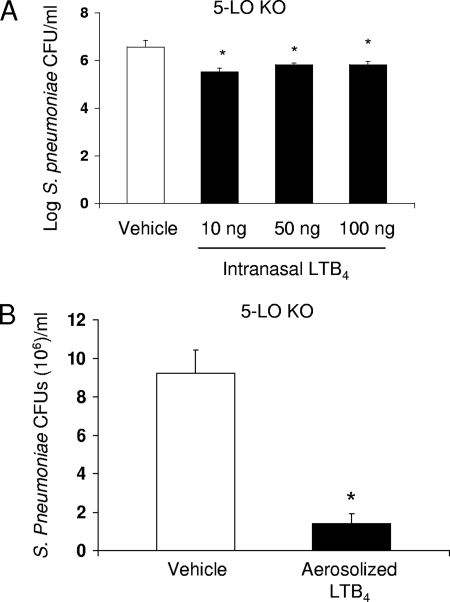
LT deficiency is frequently observed in immunocompromised patients (5, 6, 9, 10, 16), and we next sought to model this scenario by assessing the ability of exogenously administered LTB4 to improve pulmonary clearance of S. pneumoniae in 5-LO KO mice. As shown in Fig. 5, LT-deficient mice appeared to be more sensitive to the immunostimulatory effects of LTB4 than were WT mice. In particular, pretreatment with a dose of 10 ng i.n., which had no effect in WT mice (Fig. 2D), was maximally effective at improving lung bacterial clearance in 5-LO KO mice (Fig. 5A). Likewise, 25 ng of aerosolized LTB4 administered to 5-LO KO mice infected 24 h previously resulted in an 85% reduction in the pulmonary S. pneumoniae CFU count, a degree of enhanced clearance which required 100 ng of LTB4 in WT mice (Fig. 3C). Despite the increased sensitivity of KO mice, we did not observe differences in PMN or macrophage accumulation in 5-LO KO mice treated with LTB4 compared to the accumulation in mice treated with vehicle alone (data not shown). These data demonstrate the efficacy of intrapulmonary administration of LTB4 in LT-deficient animals, suggesting that this strategy would be an effective means of improving pulmonary host defense in the immunocompromised host.
FIG. 5.
Intrapulmonary LTB4 improves lung bacterial clearance in 5-LO KO mice. (A) 5-LO KO mice were given LTB4 via the i.n. route 30 min prior to an i.n. challenge with 106 CFU of S. pneumoniae. (B) 5-LO KO mice were given S. pneumoniae via the i.n. route and were exposed to an aerosol of LTB4 (25 ng) 24 h later. At 48 h after S. pneumoniae challenge, lungs were harvested to determine bacterial burdens. *, P < 0.05 versus results for 5-LO KO mice treated with vehicle, using the Student t test (5 to 15 mice per group).
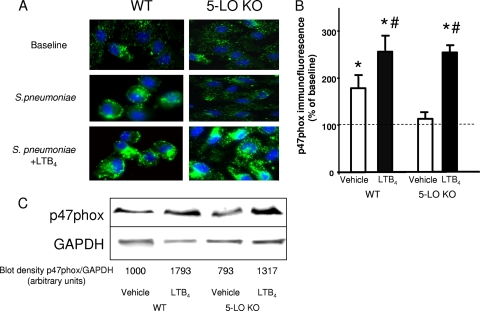
LTB4 increases p47phox expression in pulmonary macrophages in WT and 5-LO KO mice 24 h after S. pneumoniae challenge.
Our laboratory has previously reported that the ability of LTB4 to rapidly enhance bacterial killing and H2O2 production involves its ability to promote the assembly of the NADPH oxidase complex by stimulating phosphorylation and membrane translocation of its p47phox subunit in both macrophages (42) and PMNs (43). Here, we utilized p47phox immunostaining to assess the expression of this protein in leukocytes from infected lungs 24 h after treatment with vehicle or LTB4. Lung leukocytes obtained from the BALF of uninfected WT and 5-LO KO mice showed little p47phox staining at baseline. Infection elicited a striking increase in p47phox immunostaining in cells from WT mice, but this was not observed in 5-LO KO mice (Fig. 6A and B). However, aerosolized LTB4 administration resulted in a striking increase in p47phox immunostaining in leukocytes from infected 5-LO KO mice; a more modest increase above the level seen with infection alone was observed with LTB4 administration to WT mice. As judged by morphological criteria, the increased immunostaining for p47phox observed both with infection and in response to aerosolized LTB4 was limited to macrophages. This ability of LTB4 to enhance the expression of p47phox but not gp91phox or p67phox was confirmed by immunoblot analysis (Fig. 6C). Enhanced macrophage expression of p47phox, an essential component of the leukocyte NADPHox complex that produces reactive oxygen species necessary to kill S. pneumoniae (37), therefore represents a novel mechanism by which LTB4 may enhance bacterial clearance in vivo.
FIG. 6.
Increased p47phox expression in leukocytes recovered from WT and 5-LO KO mice treated with aerosolized LTB4 following S. pneumoniae challenge. WT and 5-LO KO mice were infected with 106 CFU of S. pneumoniae cells via the i.n. route and treated with aerosolized vehicle or LTB4 (100 ng) 24 h later. (A) Confocal images of cells recovered by lavage 24 h after LTB4 (48 h postinfection) and immunostained for p47phox are shown. (B) Fluorescence was quantitated in a minimum of 50 cells per condition as described in Materials and Methods. *, P < 0.05 versus results for cells from uninfected WT or 5-LO KO mice, and #, P < 0.05 versus results for cells from infected WT or 5-LO KO mice treated with vehicle, using the Student t test. Data are expressed as the percentages of the uninfected and untreated value, and bars represent the mean results ± standard errors of the means for fluorescence determined from confocal images of cells of 3 to 5 animals per group. (C) The immunoblot of p47phox in BALF cell lysates is from a single experiment that is representative of a total of 3 independent experiments. The numbers under the blots represent the arbitrary densitometric units for each band.
DISCUSSION
LT-deficient mice exhibit impaired clearance of lung infections caused by different types of microorganisms, including Klebsiella pneumoniae, Mycobacterium tuberculosis, and Histoplasma capsulatum (3, 27, 28, 34). Here, we report a similar phenomenon in the context of infection with S. pneumoniae, the most common cause of bacterial pneumonia (23), as greater pulmonary bacterial burdens and a greater degree of bacteremia were noted in 5-LO KO mice than in WT animals. Our prior studies further established that exogenously added LTB4 restored the defective in vitro phagocytosis and killing of bacteria observed in LT-deficient phagocytes and augmented those same functions even in LT-sufficient cells. In this report, we evaluated the in vivo responses of both WT and 5-LO KO mice following an i.n. challenge with S. pneumoniae. This approach permitted us to evaluate the potential of exogenously administered LTB4 to augment in vivo pulmonary bacterial clearance in WT mice and to restore defective clearance in 5-LO KO mice. Importantly, we were able to demonstrate that a single intrapulmonary dose of LTB4 administered via aerosol 24 h after S. pneumoniae challenge substantially enhanced pulmonary pneumococcal clearance in 5-LO KO and WT mice. Improved bacterial clearance was associated with an increase in pulmonary macrophage recruitment in WT mice and of macrophage p47phox expression in both genotypes. These results provide new insights into the mechanisms by which LTB4 promotes antibacterial defense and support the potential of exogenously administered LTB4 as an immunostimulatory agent for the treatment of patients with established pneumonia.
Our experiments utilizing both WT and 5-LO KO mice serve as relevant models of patients with bacterial pneumonia. For example, the WT animals with normal LT-synthetic capacity are representative of immunocompetent hosts, while 5-LO KO mice are representative of patients with a variety of immunocompromised conditions who have been demonstrated to lack the ability to produce normal levels of LTs in response to pulmonary infections (5, 6, 9, 10, 16). Data from each of these genotypes could predict that both of the parallel categories of patients would demonstrate an improved ability to clear pulmonary bacterial pathogens after the exogenous administration of LTB4.
LTB4 is well known for its role as a PMN chemoattractant factor (14), and bronchoscopic instillation of LTB4 (38) elicited PMN recruitment to the human lung. For this reason, we had anticipated a substantial increase in PMNs following LTB4 administration in both WT and 5-LO KO mice. Indeed, we verified that i.n. LTB4 elicited PMN recruitment to the lungs of uninfected mice, verifying its bioactivity. However, ours is the first study to examine LTB4 administration in the setting of infection, and we observed no increase in PMN accumulation in this context. This finding is reminiscent of our previous observation that 5-LO KO mice with K. pneumoniae pneumonia exhibited no reduction in lung PMNs (3). It was also surprising that we did not observe significant changes in cytokines following LTB4 treatment, since this 5-LO product has been shown to enhance the ability of leukocytes to synthesize IL-6, IL-8, and TNF-α in vitro (26, 38, 39). We speculate that both PMN accumulation and cytokine production in the infected lung were already maximized by virtue of the ongoing elaboration of LTB4 and chemokines and the activation of transcription factors driven by microbial products.
In contrast, intrapulmonary administration of LTB4 elicited a significant increase in BALF macrophage numbers in infected WT animals. LTB4 also has in vitro chemotactic activity for monocytes (17), has been shown to contribute to monocyte recruitment to the infected peritoneum (25), and has been implicated in monocyte recruitment to the atherosclerotic vessel wall (1). Recruited monocytes, which replace the majority of the resident AMs in the alveolar space 24 h after S. pneumoniae infection, play an essential role in host defense against pneumococcal pneumonia (47). This conclusion is based on the observations that reduced monocyte recruitment impairs pulmonary bacterial clearance and survival while enhanced recruitment of these cells improves these responses following pulmonary S. pneumoniae challenge in mice (49, 50). Within the alveolar space, newly recruited macrophages mediate pulmonary clearance in vivo by killing ingested pathogens, a process that likewise can be enhanced with LTB4.
It has been demonstrated that LTs enhance microbicidal activities of AMs and other leukocytes by upregulating nitric oxide production (18, 46), increasing the elaboration of antimicrobial peptides (13), and activating NADPHox to generate reactive oxygen intermediates (42). NADPHox is a multicomponent enzyme consisting of two membrane-bound phox proteins (gp91 and p22) that form a flavocytochrome with cytosolic p20, p40, p47, and p67 phox proteins, as well as Rac, a small G protein (15). We have previously demonstrated that LTB4 elicits the protein kinase C δ (PKC δ)-dependent phosphorylation and translocation of p47phox to the cell membrane to activate NADPHox-mediated production of H2O2 in AMs in vitro (42). In the current study, we observed that aerosolized LTB4 administration to mice with preexisting pneumonia increased the expression of p47phox in leukocytes recovered from BALF. Presumably, these two effects of LTB4, increasing both the amount of p47phox and its level of activation, would act synergistically to enhance the generation of reactive oxygen intermediates and the destruction of ingested S. pneumoniae cells by AMs. This is the first report, to our knowledge, that LTB4 can enhance the expression of p47phox in leukocytes, and the mechanism underlying this response is a subject for future investigation. The fact that aerosolized LTB4 was able to enhance macrophage p47phox expression in 5-LO KO and WT mice, whereas it only increased macrophage numbers in WT animals, suggests that the enhanced bacterial clearance in LT-deficient mice may be related to the former and not the latter action.
The intrapulmonary route of LTB4 administration was the most effective means to improve pulmonary bacterial clearance in our studies. The inability of systemically administered LTB4 to improve bacterial clearance from the lung suggests the importance of delivering this substance directly to the lungs, where it can stimulate the antimicrobial functions of resident and recruited leukocytes, and perhaps epithelia, locally at the site of infection. This approach could readily be applied in intubated patients with severe pneumonia, could be used in nonintubated patients with pneumonia, and could be used as a prophylactic measure in those with LT deficiency or other forms of immunosuppression. Although our studies demonstrated no efficacy of i.v. administration, we cannot at this time rule out the possibility that this route could be useful in patients with bacteremic pneumococcal disease, as i.v. administration of LTB4 to primates was shown to upregulate the expression of several PMN-derived antimicrobial peptides (13). Although our data provide proof of concept for the potential of intrapulmonary LTB4 in the treatment of pneumonia, we also acknowledge that a number of additional and as-yet-unexplored issues must be addressed before this approach can be applied to patients. These include the timing of LTB4 administration, the duration of therapy, the use of LTB4 as an adjuvant with antibiotics, and the use of clinically relevant endpoints, such as survival and the potential for lung injury.
In summary, we have demonstrated for the first time that the administration of aerosolized LTB4 to mice with preexisting pneumococcal pneumonia increases mononuclear phagocyte/macrophage accumulation in the lungs, p47phox expression in pulmonary macrophages, and pulmonary bacterial clearance. The efficacy of this approach in both WT and 5-LO KO mice suggests that aerosolized LTB4 may be a very effective adjunctive therapeutic agent for the treatment of bacterial pneumonia in both normal hosts and LT-deficient or otherwise immunocompromised patients. In contrast to immunostimulatory approaches employing recombinant proteins, such as the cytokines gamma interferon (IFN-γ) and granulocyte colony-stimulating factor (G-CSF), LTB4 can be administered topically, and this lipid has the advantage of being less immunogenic, shorter-lived, and less expensive than recombinant proteins. Moreover, if overexuberant inflammation were to result, specific antagonists to the high-affinity BLT1 receptor for LTB4 could be administered to limit any unwanted damage.
Supplementary Material
Acknowledgments
We thank Pierre Borgeat (Laval University, Quebec, Canada) for generously providing the LTB4 used in these studies.
This work was supported by a grant from LTB4 Sweden AB and grant no. HL077417 from the NIH to P.M.
P. Mancuso, C. H. Serezani, C, Lewis, and D. Goel have no financial disclosures to report. Peters-Golden has received consulting fees in the last 3 years from Pfizer, Merck, Nycomed, Boehringer Ingelheim, Eli Lilly, Actelion, and Ono. He has also received lecture honoraria from Merck and is a member of an advisory board for Merck. He shares a patent with the University of Michigan for the intrapulmonary administration of leukotriene B4 as a local immunostimulant for respiratory infections, which is licensed to LTB4 Sweden AB.
Editor: A. Camilli
Footnotes
Published ahead of print on 15 March 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Aiello, R. J., P.-A. Bourassa, S. Lindsey, W. Weng, A. Freeman, and H. J. Showell. 2002. Leukotriene B4 receptor antagonism reduces monocytic foam cells in mice. Arterioscler. Thromb. Vasc. Biol. 22:443-449. [DOI] [PubMed] [Google Scholar]
- 2.Aronoff, D. M., C. Lewis, C. H. Serezani, K. A. Eaton, D. Goel, J. C. Phipps, M. Peters-Golden, and P. Mancuso. 2009. E-prostanoid 3 receptor deletion improves pulmonary host defense and protects mice from death in severe Streptococcus pneumoniae infection. J. Immunol. 183:2642-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailie, M. B., T. J. Standiford, L. L. Laichalk, M. J. Coffey, R. M. Strieter, and M. Peters-Golden. 1996. Leukotriene-deficient mice manifest enhanced lethality from Klebsiella pneumonia in association with decreased alveolar macrophage phagocytic and bactericidal activities. J. Immunol. 157:5221-5224. [PubMed] [Google Scholar]
- 4.Ballinger, M. N., L. L. Hubbard, T. R. McMillan, G. B. Toews, M. Peters-Golden, R. Paine III, and B. B. Moore. 2008. Paradoxical role of alveolar macrophage-derived granulocyte-macrophage colony-stimulating factor in pulmonary host defense post-bone marrow transplantation. Am. J. Physiol. Lung Cell. Mol. Physiol. 295:L114-L122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balter, M., G. Toews, and M. Peters-Golden. 1989. Different patterns of arachidonate metabolism in autologous human blood monocytes and alveolar macrophages. J. Immunol. 142:602-608. [PubMed] [Google Scholar]
- 6.Cederholm, T., J. Å. Lindgren, and J. Palmblad. 2000. Impaired leukotriene C4 generation in granulocytes from protein-energy malnourished chronically ill elderly. J. Intern. Med. 247:715-722. [DOI] [PubMed] [Google Scholar]
- 7.Chen, X., J. Sheller, E. Johnson, and C. Funk. 1994. Role of leukotrienes revealed by targeted disruption of the 5-lipoxygenase gene. Nature 372:179-182. [DOI] [PubMed] [Google Scholar]
- 8.Christensen, K. L. Y., R. C. Holman, C. A. Steiner, J. J. Sejvar, B. J. Stoll, and L. B. Schonberger. 2009. Infectious disease hospitalizations in the United States. Clin. Infect. Dis. 49:1025-1035. [DOI] [PubMed] [Google Scholar]
- 9.Coffey, M. J., C. S. Wheeler, K. B. Gross, W. L. Eschenbacher, P. H. Sporn, and M. Peters-Golden. 1996. Increased 5-lipoxygenase metabolism in the lungs of human subjects exposed to ozone. Toxicology 114:187-197. [DOI] [PubMed] [Google Scholar]
- 10.Coffey, M. J., S. E. Wilcoxen, S. M. Phare, R. U. Simpson, M. R. Gyetko, and M. Peters-Golden. 1994. Reduced 5-lipoxygenase metabolism of arachidonic acid in macrophages from 1,25-dihydroxyvitamin D3-deficient rats. Prostaglandins 48:313-329. [DOI] [PubMed] [Google Scholar]
- 11.Demitsu, T., H. Katayama, T. Saito-Taki, H. Yaoita, and M. Nakano. 1989. Phagocytosis and bactericidal action of mouse peritoneal macrophages treated with leukotriene B4. Int. J. Immunopharmacol. 11:801-808. [DOI] [PubMed] [Google Scholar]
- 12.Donowitz, G. R., and H. L. Cox. 2007. Bacterial community-acquired pneumonia in older patients. Clin. Geriatr. Med. 23:515-534. [DOI] [PubMed] [Google Scholar]
- 13.Flamand, L., M. J. Tremblay, and P. Borgeat. 2007. Leukotriene B4 triggers the in vitro and in vivo release of potent antimicrobial agents. J. Immunol. 178:8036-8045. [DOI] [PubMed] [Google Scholar]
- 14.Ford-Hutchinson, A. W., M. A. Bray, and M. V. Doig. 1980. Leukotriene B4, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature 286:264-265. [DOI] [PubMed] [Google Scholar]
- 15.Groemping, Y., and K. Rittinger. 2005. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem. J. 386:401-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jubiz, W., R. E. Draper, J. Gale, and G. Nolan. 1984. Decreased leukotriene B4 synthesis by polymorphonuclear leukocytes from male patients with diabetes mellitus. Prostaglandins Leukot. Med. 14:305-311. [DOI] [PubMed] [Google Scholar]
- 17.Koyama, S., A. Takamizawa, E. Sato, T. Masubuchi, S. Nagai, and T. Izumi. 2001. Cyclophosphamide stimulates lung fibroblasts to release neutrophil and monocyte chemoattractants. Am. J. Physiol. Lung Cell. Mol. Physiol. 280:L1203-L1211. [DOI] [PubMed] [Google Scholar]
- 18.Larfars, G. L. F., M. A. Devynck, J. Palmblad, and H. Gyllenhammer. 1999. Activation of nitric oxide release and oxidative metabolism by leukotrienes B4, C4, and D4 in human polymorphonuclear leukocytes. Blood 93:1399-1405. [PubMed] [Google Scholar]
- 19.Mancuso, P., A. Gottschalk, S. M. Phare, M. Peters-Golden, N. W. Lukacs, and G. B. Huffnagle. 2002. Leptin-deficient mice exhibit impaired host defense in gram-negative pneumonia. J. Immunol. 168:4018-4024. [DOI] [PubMed] [Google Scholar]
- 20.Mancuso, P., G. B. Huffnagle, M. A. Olszewski, J. Phipps, and M. Peters-Golden. 2006. Leptin corrects host defense defects following acute starvation in murine pneumococcal pneumonia. Am. J. Respir. and Crit. Care Med. 173:212-218. [DOI] [PubMed] [Google Scholar]
- 21.Mancuso, P., T. Marshall, T. Standiford, and M. Peters-Golden. 1998. 5-Lipoxygenase reaction products modulate alveolar macrophage phagocytosis of Klebsiella pneumoniae. Infect. Immun. 66:5140-5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mancuso, P., P. Nana-Sinkam, and M. Peters-Golden. 2001. Leukotriene B4 augments neutrophil phagocytosis of Klebsiella pneumoniae. Infect. Immun. 69:2011-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandell, L. A., R. G. Wunderink, A. Anzueto, J. G. Bartlett, G. D. Campbell, N. C. Dean, S. F. Dowell, T. M. File, Jr., D. M. Musher, M. S. Niederman, A. Torres, and C. G. Whitney. 2007. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 44(Suppl. 2):S27-S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin, T., B. Pistorese, E. Chi, R. Goodman, and M. Matthay. 1989. Effect of leukotriene B4 in the human lung: recruitment of neutrophils into the alveolar spaces without a change in protein permeability. J. Clin. Invest. 89:1009-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsukawa, A., C. M. Hogaboam, N. W. Lukacs, P. M. Lincoln, R. M. Strieter, and S. L. Kunkel. 1999. Endogenous monocyte chemoattractant protein-1 (MCP-1) protects mice in a model of acute septic peritonitis: cross-talk between MCP-1 and leukotriene B4. J. Immunol. 163:6148-6154. [PubMed] [Google Scholar]
- 26.McCain, R., E. Holden, T. Blackwell, and J. Christman. 1994. Leukotriene B4 stimulates human polymorphonuclear leukocytes to synthesize and release interleukin-8 in vitro. Am. J. Respir. Cell Mol. Biol. 10:651-657. [DOI] [PubMed] [Google Scholar]
- 27.Medeiros, A. I., A. Sa-Nunes, E. G. Soares, C. M. Peres, C. L. Silva, and L. H. Faccioli. 2004. Blockade of endogenous leukotrienes exacerbates pulmonary histoplasmosis. Infect. Immun. 72:1637-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medeiros, A. I., A. Sa-Nunes, W. M. Turato, A. Secatto, F. G. Frantz, C. A. Sorgi, C. H. Serezani, G. S. Deepe, Jr., and L. H. Faccioli. 2008. Leukotrienes are potent adjuvant during fungal infection: effects on memory T cells. J. Immunol. 181:8544-8551. [DOI] [PubMed] [Google Scholar]
- 29.Medeiros, A. I., C. L. Silva, A. Malheiro, C. M. L. Maffei, and L. H. Faccioli. 1999. Leukotrienes are involved in leukocyte recruitment induced by live Histoplasma capsulatum or by the {beta}-glucan present in their cell wall. Br. J. Pharmacol. 128:1529-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizgerd, J. P. 2008. Acute lower respiratory tract infection. N. Engl. J. Med. 358:716-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizgerd, J. P. 2006. Lung infection—a public health priority. PLoS Med. 3:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mueller, N. J. 2008. New immunosuppressive strategies and the risk of infection. Transpl. Infect. Dis. 10:379-384. [DOI] [PubMed] [Google Scholar]
- 33.Peres, C. M., D. M. Aronoff, C. H. Serezani, N. Flamand, L. H. Faccioli, and M. Peters-Golden. 2007. Specific leukotriene receptors couple to distinct G proteins to effect stimulation of alveolar macrophage host defense functions. J. Immunol. 179:5454-5461. [DOI] [PubMed] [Google Scholar]
- 34.Peres, C. M., L. de Paula, A. I. Medeiros, C. A. Sorgi, E. G. Soares, D. Carlos, M. Peters-Golden, C. L. Silva, and L. H. Faccioli. 2007. Inhibition of leukotriene biosynthesis abrogates the host control of Mycobacterium tuberculosis. Microbes Infect. 9:483-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters-Golden, M., C. Canetti, P. Mancuso, and M. J. Coffey. 2005. Leukotrienes: underappreciated mediators of innate immune responses. J. Immunol. 174:589-594. [DOI] [PubMed] [Google Scholar]
- 36.Peters-Golden, M., and W. R. Henderson, Jr. 2007. Leukotrienes. N. Engl. J. Med. 357:1841-1854. [DOI] [PubMed] [Google Scholar]
- 37.Pitt, J., and H. Bernheimer. 1974. Role of peroxide in phagocytic killing of pneumococci. Infect. Immun. 9:48-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poubelle, P. E., J. Stankova, J. Grassi, and M. Rola-Pleszczynski. 1991. Leukotriene B4 up-regulates IL-6 rather than IL-1 synthesis in human monocytes. Agents Actions 34:42-45. [DOI] [PubMed] [Google Scholar]
- 39.Rola-Pleszczynski, M., and J. Stankova. 1992. Leukotriene B4 enhances interleukin-6 (IL-6) production and IL-6 messenger RNA accumulation in human monocytes in vitro: transcriptional and posttranscriptional mechanisms. Blood 80:1004-1011. [PubMed] [Google Scholar]
- 40.Sampson, S., J. Costello, and A. Sampson. 1997. The effect of inhaled leukotriene B4 in normal and in asthmatic subjects. Am. J. Respir. Crit. Care Med. 155:1789-1792. [DOI] [PubMed] [Google Scholar]
- 41.Schultz, M. J., J. Wijnholds, M. P. Peppelenbosch, M. Vervoordeldonk, P. Speelman, S. van Deventer, P. Borst, and T. van der Poll. 2001. Mice lacking the multidrug resistance protein 1 are resistant to Streptococcus pneumoniae-induced pneumonia. J. Immunol. 166:4059-4064. [DOI] [PubMed] [Google Scholar]
- 42.Serezani, C., D. Aronoff, S. Jancar, P. Mancuso, and M. Peters-Golden. 2005. Leukotrienes enhance the bactericidal activity of alveolar macrophages against Klebsiella pneumoniae through the activation of NADPH oxidase. Blood 106:1067-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serezani, C. H., D. M. Aronoff, S. Jancar, and M. Peters-Golden. 2005. Leukotriene B4 mediates p47phox phosphorylation and membrane translocation in polyunsaturated fatty acid-stimulated neutrophils. J. Leukoc. Biol. 78:976-984. [DOI] [PubMed] [Google Scholar]
- 44.Serezani, C. H., J. H. Perrela, M. Russo, M. Peters-Golden, and S. Jancar. 2006. Leukotrienes are essential for the control of Leishmania amazonensis infection and contribute to strain variation in susceptibility. J. Immunol. 177:3201-3208. [DOI] [PubMed] [Google Scholar]
- 45.Standiford, T. J., S. L. Kunkel, M. J. Greenberger, L. L. Laichalk, and R. M. Strieter. 1996. Expression and regulation of chemokines in bacterial pneumonia. J. Leukoc. Biol. 59:24-28. [DOI] [PubMed] [Google Scholar]
- 46.Talvani, A., F. Machado, G. Santana, A. Klein, L. Barcelos, J. Silva, and M. Teixeira. 2002. Leukotriene B4 induces nitric oxide synthesis in Trypanosoma cruzi-infected murine macrophages and mediates resistance to infection. Infect. Immun. 70:4247-4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taut, K., C. Winter, D. E. Briles, J. C. Paton, J. W. Christman, R. Maus, R. Baumann, T. Welte, and U. A. Maus. 2008. Macrophage turnover kinetics in the lungs of mice infected with Streptococcus pneumoniae. Am. J. Respir. Cell Mol. Biol. 38:105-113. [DOI] [PubMed] [Google Scholar]
- 48.Volovitz, B., R. C. Welliver, G. De Castro, D. A. Krystofik, and P. L. Ogra. 1988. The release of leukotrienes in the respiratory tract during infection with respiratory syncytial virus: role in obstructive airway disease. Pediatr. Res. 24:504-507. [DOI] [PubMed] [Google Scholar]
- 49.Winter, C., W. Herbold, R. Maus, F. Langer, D. E. Briles, J. C. Paton, T. Welte, and U. A. Maus. 2009. Important role for CC chemokine ligand 2-dependent lung mononuclear phagocyte recruitment to inhibit sepsis in mice infected with Streptococcus pneumoniae. J. Immunol. 182:4931-4937. [DOI] [PubMed] [Google Scholar]
- 50.Winter, C., K. Taut, M. Srivastava, F. Langer, M. Mack, D. Briles, J. Paton, R. Maus, T. Welte, M. Gunn, and U. Maus. 2007. Lung-specific overexpression of CC chemokine ligand (CCL) 2 enhances the host defense to Streptococcus pneumoniae infection in mice: role of the CCL2-CCR2 axis. J. Immunol. 178:5828-5838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.