Abstract
In North America, tick-borne relapsing fever is caused by the species Borrelia hermsii, B. parkeri, and B. turicatae, which are transmitted to humans through the bite of the respective infected tick vectors. Here we describe the identification and functional characterization of a surface lipoprotein of B. parkeri, designated BpcA, that binds the human complement regulators factor H and factor H-related protein 1 and, simultaneously, the host protease plasminogen. In contrast, the homologous B. turicatae protein failed to bind human factor H and factor H-related protein 1 but retained its plasminogen binding capacity. Factor H bound to BpcA maintains its regulatory capacity to control C3b deposition and C3 convertase activity. Ectopic expression of BpcA in a serum-sensitive B. burgdorferi strain protects transformed cells from complement-mediated killing. Furthermore, bound plasminogen/plasmin endows B. parkeri and B. turicatae with the potential to degrade extracellular matrix components. These findings expand our understanding of the putative recent evolutionary separation of Borrelia parkeri and Borrelia turicatae, provide evidence that B. parkeri differs from B. turicatae in its ability to resist complement attack, and may help in understanding the pathological processes underlying tick-borne relapsing fever.
In North America, tick-borne relapsing fever (TBRF) is caused by the spirochete species Borrelia hermsii, B. parkeri, and B. turicatae. TBRF is transmitted to humans through the bite of infected Ornithodoros hermsi, O. parkeri, and O. turicata ticks. Their enzootic hosts and their distribution in North America are currently under investigation (8, 9). Rodents are the natural vertebrate hosts for B. hermsii, whereas B. parkeri and B. turicatae are assumed to be pathogenic in horses and dogs, respectively (41, 47). The relapsing fever ticks are primarily nocturnal feeders and can complete the intake of a blood meal in minutes. Previously, it was generally agreed that patients suffering from relapsing fever were infected with those Borrelia species that are associated with the geographical area and the occurrence of certain spirochete-infected ticks (7, 9, 41). Due to the availability of genetic tools and genome sequence data, discrimination between closely related species causing relapsing fever infections among humans is now feasible. Although B. hermsii has been isolated frequently from humans, direct evidence for B. parkeri and B. turicatae being pathogenic to humans is still missing (4, 44, 45).
In order to survive in human tissues, relapsing fever spirochetes have to escape innate and adaptive immune responses. Complement acts as an important part of host innate immunity, which is essential for recognition and elimination of microbes (52). However, pathogenic organisms can evade complement attack by various routes, in particular the acquisition of host regulators at the surface of the pathogen (53). Recruitment of fluid-phase complement regulators of the alternative complement pathway, such as factor H (CFH), to the spirochetal surface represents one sophisticated strategy to resist the host's innate immunity. The CFH protein family consists of seven structurally related proteins, including factor H-like protein 1 (CFHL-1) and factor H-related proteins (CFHRs), and all are composed of short consensus repeats (SCRs) (51).
For Borrelia burgdorferi, the etiological agent of Lyme disease, a strong correlation between serum resistance of a given isolate and its expression profile of CFH binding outer surface lipoproteins, termed CRASPs, was reported (1, 23-29, 50). Recent analysis of the tick-borne relapsing fever spirochete B. hermsii has demonstrated that certain isolates are able to bind the complement regulatory proteins CFH and CFHR-1 via outer surface lipoproteins, thereby conferring resistance to complement attack (19-21, 43). Surface-bound CFH controls complement activation by accelerating the decay of the C3 convertase of the alternative pathway and by inactivating newly formed C3b (31, 38). The ability of B. hermsii to bind host plasminogen (PLG) via BhCRASP-1, which is subsequently activated via a urokinase-type PLG activator, has been assessed previously (6). Plasmin exhibits broad substrate specificity and is able to dissolve blood clots, to degrade constituents of the extracellular matrices and basement membranes, and to activate latent collagenase (5, 10, 12). Thus, it could be assumed that for B. parkeri and B. turicatae, analogous PLG-binding proteins are involved in their dissemination processes.
CFH-binding proteins of B. parkeri have been proposed (19, 20, 34), but so far these proteins have not been identified. Here we provide evidence for a novel 17-kDa outer surface lipoprotein, termed BpcA, that displays binding specificities for the complement regulators CFH/CFHR-1 and in addition for PLG/plasmin. Thus, expression of BpcA on the surface of Borrelia most likely confers resistance to complement attack and may aid in the spirochete's dissemination.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
B. parkeri strain RML, B. turicatae strain 91E135, B. hermsii strains YOR and Frogner (provided by Tom G. Schwan, Rocky Mountain Laboratories), B. hermsii strain HS1, and B. burgdorferi B313 Lyme disease spirochetes were cultivated in BSK-H complete medium (Bio&Sell, Feucht/Nürnberg, Germany) supplemented with 5% rabbit serum (Cell Concept, Freiburg, Germany) at 30°C. B313 spirochetes harbor the plasmids cp32-1, cp32-2, cp32-3, cp32-4, cp26, and lp7 exclusively and therefore lack expression of several major outer surface proteins, e.g., OspA, DbpA, and DbpB, as well as several complement regulator binding proteins, BbCRASP-1 to -4 (15, 54, 55). Bacteria were harvested by centrifugation and washed with phosphate-buffered saline. The density of spirochetes was determined using dark-field microscopy and a Kova counting chamber (Hycor Biomedical, Garden Grove, CA). Escherichia coli JM109 was grown at 37°C in LB medium.
Preparation of whole borrelial cell lysate and immunoprecipitation of BpcA.
Whole-cell lysates of spirochetes were prepared as described elsewhere with minor modifications (43). Briefly, borrelial cultures were grown at 33°C in modified Barbour-Stoenner-Kelly (BSK) medium to late log phase and harvested by centrifugation at 6,000 × g for 10 min at 4°C. The resulting pellets were washed gently once with phosphate-buffered saline (PBS) and resuspended in ice-cold 50 mM Tris-HCl (pH 7.5), 25 mM KCl, 5 mM MgCl2, 1 mM EGTA, and 0.5% NP-40. Cell debris were removed by centrifugation, and for preclearing, lysates were incubated with protein G Sepharose (GE Healthcare, Munich, Germany) at 4°C for 1 h. For immunoprecipitation, precleared B. parkeri lysates were incubated with polyclonal anti-CFH antibody and purified human CFH coupled to protein G Sepharose for 12 h at 4°C. After washing in 50 mM NaH2PO4, 300 mM NaCl, and 10 mM imidazole (pH 8), bound proteins were eluted with 2× SDS sample buffer (Serva, Heidelberg, Germany) and subjected to 13% Tris-Tricine SDS-PAGE under reducing conditions. The selected protein band of approximately 18 kDa was cored from gels and subjected to mass spectrometry as previously described (15).
Cloning of the bpcA gene, construction of expression plasmids, and production of recombinant proteins.
The gene encoding BpcA was amplified by PCR using genomic B. parkeri DNA in combination with the primers F0 and FR (Table 1), and the amplified DNA fragment was cloned into the vector pGEM-T Easy (Promega, Mannheim, Germany). The resulting plasmid was used as a template for construction of expression plasmids by PCR amplification. For the recombinant full-length BpcA protein, the primers BpBam and BpSal were used. For construction of N- and C-terminal deletion mutants, these primers were applied in combination with the primer Δ48Bam, Δ78Bam, or Δ122Sal (Table 1), resulting in the recombinant proteins BpcAΔ48-183, BpcAΔ78-182, and BpcAΔ18-122, respectively. After digestion with the restriction enzymes BamHI and SalI, the amplified DNA fragments were ligated in frame into the His6-tag-encoding sequence of vector pQE-30Xa (Qiagen, Hilden, Germany). For expression of the His-tagged BpcA fusion proteins, the plasmids were transformed in E. coli JM109 and recombinant proteins were purified as recommended by the manufacturer (Qiagen).
TABLE 1.
Oligonucleotides used in this study
| Primer | Sequence (5′ to 3′) | Purpose |
|---|---|---|
| F0 | CAAAGATAGGCTACATTTATAGTC | Amplification of bpcA |
| FR | TCAATGAATTATGCAAATAAATTCG | Amplification of bpcA |
| BpBam | GTCTTGTTGGATCCGGTTTATTAAG | Generation of expression plasmid |
| BpSal | TATGTCGACCGAACTTAAATTTCTAAAACGG | Generation of expression plasmid |
| Δ48Bam | TTTGGATCCTTACTTCAAGATAAAAATC | Construction of deletion mutant |
| Δ78Bam | GAAAATGGATCCCTGCAAGATAAGC | Construction of deletion mutant |
| Δ122Sal | GTAAGGTCGACTTAGTTAATAGACTTTAAC | Construction of deletion mutant |
| BpTBam | CAAAGATAGGATCCATTTATAGTC | Construction of pBP |
| BpTSph | TCAATGAAGCATGCAAATAAATTCG | Construction of pBP |
Southern blot analysis, SDS-PAGE, and ligand affinity blot.
Southern blotting of total genomic DNA was done as described previously (48). Randomly primed gene probes were prepared by using the Random Primer labeling system (Gibco-BRL, Germany), and hybridization was conducted as described previously (49). Borrelial whole-cell lysates (15 μg) or purified recombinant proteins (500 ng) were subjected to 10% Tris-Tricine SDS-PAGE under reducing conditions and transferred to nitrocellulose as previously described (27).
In situ protease treatment of spirochetes.
Whole cells of B. parkeri were treated with proteases by modification of a method described previously (2). Briefly, intact borrelial cells were incubated with either proteinase K or trypsin, and whole-cell protein preparations (10 μl) were separated using Tris-Tricine SDS-PAGE via 4% stacking and 10% separating gels as described previously (27).
Expression of recombinant proteins CFH, CFHL-1, and CFHR-1.
Deletion constructs of CFH (containing only SCR1 to SCR7 [CFHSCR1-7] or only SCR8 to SCR20 [CFHSCR8-20]) and CFHR-1 were expressed in Spodoptera frugiperda Sf9 insect cells infected with a recombinant baculovirus. Cloning, expression, and purification have been described previously (31, 32). The CFH deletion mutant CFHSCR19-20 was amplified by PCR and ligated in frame into the pQE-30Xa vector. Expression and purification were done as recommended by the manufacturer (Qiagen).
Functional assay for cofactor activity of CFH.
Cofactor activity of CFH bound to immobilized recombinant BpcA or intact B. parkeri cells was analyzed by measuring factor I-mediated conversion of C3b to iC3b. Briefly, recombinant BpcA (20 μg/ml) immobilized on a microtiter plate was incubated with an excess of purified CFH (2 μg/ml). After washing, purified C3b (16 μg/ml) (Calbiochem, Bad Soden, Germany) and purified factor I (8 μg/ml) (Sigma, Deisenhofen, Germany) were added, and the mixture was incubated for 30, 60, and 240 min at 37°C. C3b degradation was quantified by Western blot analysis using biotinylated rabbit anti-C3c IgG (Dako, Hamburg, Germany), followed by peroxidase-conjugated streptavidin (GE Healthcare).
ELISA.
For ELISA using nondenatured recombinant proteins, the wells of microtiter plates (Nunc, Wiesbaden, Germany) were coated for 2 h at room temperature with BpcA or the deletion mutants thereof (100 μl; 2 μg/ml). The plates were washed three times with PBS-0.05% Tween and blocked by incubation with PBS-5% bovine serum albumin (BSA). CFH (1 μg/ml), PLG (10 μg/ml), or 50% normal human serum (NHS) was added to the wells, and after 1 h of incubation and three washes with PBS, goat anti-CFH (Calbiochem, Bad Soden, Germany), goat anti-PLG (Acris, Hiddeshausen, Germany), or CFHR-1-specific monoclonal antibody (MAb) JHD8 antibodies were added, respectively. For detection of specific antibodies, peroxidase-labeled rabbit anti-goat IgG (Dianova, Hamburg, Germany) or sheep antimouse antibodies (GE Healthcare) were used as conjugates. The substrate reaction was carried out with o-phenyldiamine dihydrochloride (Sigma) at room temperature.
For competition assays, microtiter plates were coated with BpcA (1 μg/ml). To analyze the ability of PLG to inhibit the binding of CFH to immobilized BpcA (1 μg/ml), plates were incubated simultaneously with constant amounts of CFH (0.2 μg/ml) and increasing amounts of PLG (0.001 to 100 μg/ml). The ability of CFH to inhibit binding of PLG to BpcA was determined by mixing increasing amounts of CFH (0.0001 to 10 μg/ml) with PLG at 20 μg/ml. CFH and PLG bound to immobilized BpcA were analyzed as described above.
Plasmin-dependent degradation of fibrinogen.
Microtiter plates were coated with BpcA (2 μg/ml) (Nunc). After blocking, 10 μg PLG (Chromogenix, Essen, Germany) was added and incubated for 30 min at 37°C. Following three washes, 10 ng urokinase-type plasminogen activator (uPA) (Chemicon, Hampshire, United Kingdom) and 500 ng fibrinogen (Calbiochem) were added. After 0.5, 2, and 6 h at 37°C, the reaction was terminated and probes were subjected to 13% Tris-Tricine SDS-PAGE under reducing conditions and transferred to nitrocellulose. Membranes were blocked and incubated with rabbit antifibrinogen IgG (Calbiochem) followed by a peroxidase-conjugated goat anti-rabbit IgG (Dianova, Hamburg, Germany).
Production of monoclonal antibodies.
A monoclonal antibody (BP3) directed against BpcA was generated by immunization of BALB/c mice with the respective purified recombinant protein according to a method described elsewhere (30).
Construction of a shuttle vector for transformation with BpcA.
The bpcA gene, including its native promoter region, was amplified by PCR using the BpTBam and BpTSph primers, containing the respective restriction sites. The resulting amplicon was cloned into pKFSS1, yielding the shuttle vector pBP. Transformation of B. burgdorferi B313 and characterization of transformants were described previously (15). Expression of BpcA of posttransformation B. burgdorferi B313 was determined by Western blotting using MAb BP3.
Serum susceptibility testing for Borrelia strains.
The serum susceptibilities of B. parkeri, B. turicatae, B. burgdorferi B313, and transformed B313/pBP were assessed using a survival assay. Cells grown to mid-logarithmic phase were harvested and washed, and 2 × 107 spirochetes were suspended in BSK-H medium (PAN Biotech, Aidenbach, Germany) supplemented with either 25% or 50% NHS or heat-inactivated serum (hiNHS), as appropriate. Cells were incubated in Eppendorf tubes at 30°C for 3 days. At days 0, 1, 2, and 3, cells were washed in 0.85% NaCl, transferred to microtiter plates, and incubated with the SYTO 9 green fluorescent nucleic acid stain (MoBiTec, Goettingen, Germany) as recommended by the manufacturer. Subsequently, relative growth of spirochetes compared to day 0 levels was determined by measuring the green fluorescence emission at 485 nm/530 nm using a microtiter plate reader (Victor2 plate reader; Perkin Elmer, Waltham, MA).
Statistical analysis.
Statistics were analyzed with the unpaired Student t test; P values less than 0.01 were considered significant.
Nucleotide sequence accession numbers.
The bpcA and btcA gene sequences reported in this article have been deposited in the EMBL/GenBank databases under accession numbers FN659904 and FN659905, respectively.
RESULTS
Serum resistance and interaction of B. parkeri and B. turicatae with human complement regulators.
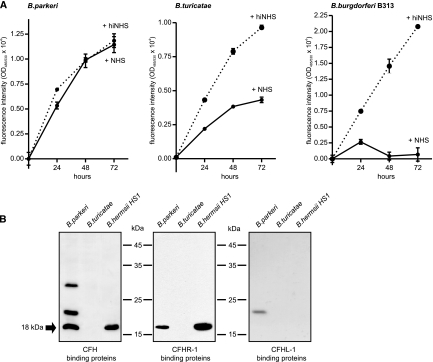
To assess the serum sensitivities of B. parkeri and B. turicatae, spirochetes were incubated in 50% NHS or 50% hiNHS for 3 days. Using a survival assay, different levels of serum susceptibility were observed. B. parkeri was resistant to complement-mediated lysis, whereas B. turicatae was classified as marginally serum resistant. In contrast, growth of the serum-sensitive B. burgdorferi isolate B313, which was used as a control, was strongly inhibited under the same conditions (Fig. 1A). Earlier observations indicating that B. parkeri relapsing fever spirochetes express CFH-binding surface proteins prompted us to identify the potential receptor(s) for CFH. A ligand affinity blot was employed using spirochetal whole-cell lysates in combination with human serum and anti-human CFH antibodies to determine if B. parkeri and B. turicatae can bind human complement regulators, CFH, CFHL-1, and CFHR-1. The CFH-specific antibody detected 18-, 21-, and 30-kDa proteins that were adsorbed by B. parkeri (Fig. 1B). When CFHR-1-specific antibodies were used in the ligand affinity assay, an approximately 18-kDa protein was adsorbed by both B. parkeri and B. hermsii (positive control). This finding is reminiscent of our previous observation that B. hermsii strain HS1 expresses a CFH and CFHR-1 binding protein, designated BhCRASP-1 (43). Screening with the anti-CFHL-1 antiserum revealed that an unidentified protein of approximately 21 kDa was adsorbed by B. parkeri, showing only low-level binding capacity. However, it remains to be determined whether the 21-kDa protein of B. parkeri can bind both CFH and CFHL-1. B. turicatae lysates did not adsorb either CFH, CFHL-1, or CFHR-1, indicating that the respective human complement regulator binding proteins are not expressed by in vitro-cultured B. turicatae spirochetes (Fig. 1B).
FIG. 1.
Serum susceptibility of spirochetes and binding of complement regulators CFH, CFHL-1, and CFHR-1. (A) To investigate susceptibility to human serum, B. parkeri, B. turicatae, and B. burgdorferi B313 cells were incubated with 50% normal human serum (NHS) (solid line) or heat-inactivated serum (hiNHS) (dashed line) at 30°C for 72 h. Cells were stained with the SYTO 9 green fluorescent nucleic acid stain, and the relative growth compared to day 0 was determined by measurement of the green fluorescence emission. Values represent the means ± standard errors of the means (SEM) of a single experiment performed in triplicate that is representative of three independent experiments. (B) Whole-cell lysates of Borrelia parkeri, B. turicatae, and B. hermsii were separated by 10% Tris-Tricine SDS-PAGE, transferred to nitrocellulose membranes, and incubated with NHS. CFH binding was analyzed employing specific MAb JHD7, and binding of CFHR-1 was analyzed using specific MAb JHD8. CFHL-1 binding capability was detected by using a rabbit immune serum against SCRs 1 to 4. For a control, cell lysates of B. hermsii HS1 were included. The arrow indicates an 18-kDa CFH binding protein.
Cloning and characterization of BpcA.
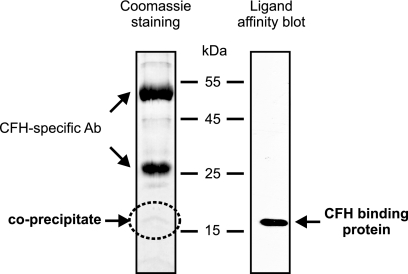
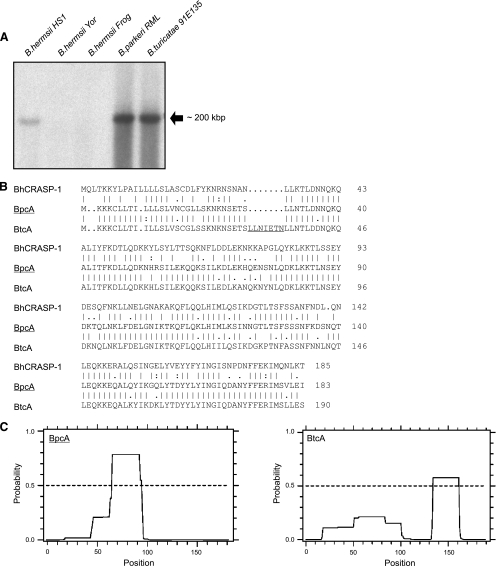
To test the assumption that the 18-kDa CFH and CFHR-1 binding proteins of B. parkeri and B. hermsii exhibit extensive sequence similarities, we used primers derived from the BhCRASP-1-encoding cspA gene sequence of B. hermsii. However, our attempts failed to PCR amplify a homologous gene sequence encoding the 18-kDa protein of B. parkeri. For this reason, immunoprecipitation in combination with mass spectrometry was used to identify the 18-kDa CFH binding protein of B. parkeri. Analysis of whole-cell lysates derived from B. parkeri spirochetes demonstrated that CFH bound to an 18-kDa protein. The respective protein was cored from a Coomassie-stained gel, and its identity was determined by mass spectrometry (Fig. 2). One individual peptide (LFDELGNIK) was identified as belonging to an open reading frame (ORF) by searching the complete B. parkeri genome sequence database (unpublished) and was selected for further analysis. The encoding gene was designated bpcA. Based upon the presence of a spirochetal lipobox at its N terminus, the protein represents a putative surface lipoprotein (3). A BLAST search detected a protein with significant homology in B. turicatae (accession no. ABQ85661). The bpcA gene is located on a B. parkeri linear plasmid of approximately 200 kb and encodes a full-length protein of 21.3 kDa, which after processing is predicted to be 19.5 kDa in size (Fig. 3A). The N terminus of the protein shows significant homology to the signal peptides of other bacterial lipoproteins (Fig. 3B). This motif includes three lysine residues near the N terminus, a hydrophobic region, and a sequence with significant similarity to the consensus signal peptidase II cleavage sequence, Leu(Ala, Ser)-4-Leu(Val, Phe, Ile)-3-Ile(Val, Gly)-2-Ala(Ser, Gly)-1-Cys+1 (14). Using LipoP for prediction of lipoproteins of Gram-negative bacteria (22), a unique cleavage site for signal peptidase II was found between amino acid residues 16 and 17, suggesting lipidation at cysteine residue 17 of BpcA. Amino acid alignment with the homologous protein of B. turicatae (here named BtcA) and BhCRASP-1 of B. hermsii detected a high degree of similarities, ranging from 67 to 84%, indicating that similar proteins can be found in other tick-borne relapsing fever spirochetes (Fig. 3B). The BpcA and BtcA amino acid sequences exhibited 84.2% identity, and most of the sequence differences were located in the central domain, with the exception of the 7-amino-acid (aa) insertion LLNIETN (aa 30 to 36) in the N-terminal domain of BtcA (Fig. 3B). To clarify whether this particular sequence is critically involved in the interaction with human CFH and CFHR-1, the N-terminal peptide LLNIETN was deleted from the BtcA protein and vice versa inserted at the corresponding amino acid position 29 of BpcA using a PCR-based mutagenesis approach. Wild-type and mutant recombinant proteins were assayed for CFH and CFHR-1 binding using ELISA. However, both mutations did not affect binding of complement regulators, making it unlikely that those additional amino acids exhibit a significant impact on the interaction of BpcA and BtcA with CFH/CFHR-1 (data not shown).
FIG. 2.
Identification and isolation of a CFH binding protein of B. parkeri. Protein G Sepharose beads were incubated with polyclonal anti-CFH antibodies (Ab) and purified human CFH. After several washings, whole-cell lysates of B. parkeri were added and immunoprecipitates were separated by SDS-PAGE. Proteins were detected by Coomassie staining, and the CFH-reactive protein of 18 kDa was identified by ligand affinity blotting. The CFH-reactive protein was excised and submitted to mass spectrometry.
FIG. 3.
Genomic localization of bpcA. (A) Total genomic DNA of B. hermsii HS1, B. hermsii Yor, B. hermsii Frog, B. parkeri RML, and B. turicatae 91E135 strains was separated by pulsed-field gel electrophoresis (PFGE) and subjected to Southern blot analysis using a full-length 32P-labeled bpcA gene probe. (B) Alignment of deduced amino acid sequences of proteins orthologous to BpcA. BpcA exhibits 84% amino acid sequence similarity to BtcA from B. turicatae and only 67% similarity to BhCRASP-1 from B. hermsii. (C) Output of coiled-coil analyses performed using the COILS program (33). The predicted probability of coiled-coil formation was assessed using a 28-amino-acid window. The predicted probability is indicated on the y axis, and the amino acid residues are indicated on the x axis.
In an earlier study, it was also demonstrated that coiled-coil elements are involved in the presentation of the CFH binding site of the FhbA protein of B. hermsii (19, 21). A computer-assisted structural analysis indicated that there was a high probability of coiled-coil formation for one domain in each of the two proteins BpcA and BtcA (33). However, the coiled-coil domain spanning residues 67 to 96 was predicted with high probability in the BpcA sequence but only with low probability in BtcA (Fig. 3C). In contrast, the C-terminal coiled-coil domain spanning residues 135 to 162 in BtcA could not be detected in the BpcA sequence, supporting the notion that the higher-order structures most likely abrogate binding of BtcA to CFH and CFHR-1.
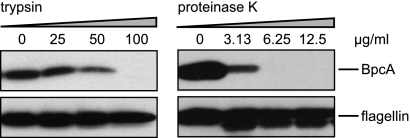
Surface exposure and protease sensitivity of BpcA.
To define surface localization of BpcA, B. parkeri spirochetes were pretreated with either proteinase K or trypsin, lysed, separated by SDS-PAGE, and assayed by Western blotting. As shown in Fig. 4, a significant reduction was observed for BpcA after incubation with proteinase K at concentrations of ≥6.25 μg/ml, whereas for treatment with the site-specific protease trypsin, only larger amounts (≥100 μg/ml) yielded complete degradation of BpcA. Signal intensity observed for flagellin remained constant, indicating that periplasmic flagella were not affected by proteolytic digestion. These data strongly suggest that BpcA is exposed on the outer surface of B. parkeri.
FIG. 4.
Surface exposition of BpcA. Proteinase K and trypsin treatment affects surface expression of native BpcA. B. parkeri cells were incubated with the indicated concentrations of proteinase K and trypsin, lysed, immunoblotted, and probed either with anti-BpcA MAb BP3 (upper panel) or with anti-flagellin MAb LA21 (lower panel).
Localization of CFH/CFHR-1 and PLG-binding domains of BpcA.
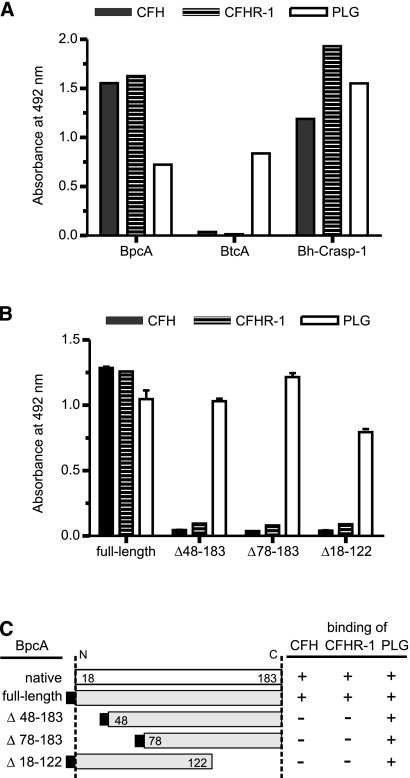
Previously, we reported that BhCRASP-1 of B. hermsii binds CFH and CFHR-1 and in addition interacts with PLG (43). To ascertain the binding capacities of BpcA of B. parkeri and BtcA of B. turicatae for CFH, CFHR-1, and PLG, multiwell plates were coated with the respective borrelial proteins, blocked, and then incubated with the indicated human serum proteins. While BpcA bound the human complement regulators CFH and CFHR-1, as well as PLG, BtcA interacted only with PLG (Fig. 5A). In order to localize the CFH and CFH-1 binding site on BpcA, deletion mutants with N- and C-terminal truncations were constructed as depicted in Fig. 5C and analyzed by ligand affinity blotting. Exclusively, the full-length BpcA protein bound to CFH (Fig. 5B and C). No binding to CFH and CFHR-1 was detected with any of the C- and N-terminal deletion mutants of BpcA. These data suggest that long-range intramolecular interactions are involved in the formation and presentation of the CFH binding site of BpcA. In contrast, full-length BpcA and all truncated versions of BpcA retained their PLG binding activity (Fig. 5B and C), indicating that the central domain of BpcA is indispensable for PLG-BpcA interactions.
FIG. 5.
Binding of CFH, CFHR-1, and PLG to BpcA. (A) Microtiter plates were coated with BpcA, BtcA, or BhCRASP-1 and incubated with either CFH, normal human serum (as a source of CFHR-1), or PLG. Binding was detected using goat anti-CFH, the CFHR-1-specific MAb JHD8, or goat anti-PLG immune serum, followed by the respective horseradish peroxidase (HRP)-conjugated IgGs. (B) Microtiter plates were coated with purified recombinant BpcA protein and various deletion mutants thereof and were then incubated with CFH, normal human serum (as a source of CFHR-1), or PLG. Binding was detected using goat anti-CFH, the CFHR-1-specific MAb JHD8, or goat anti-PLG immune serum, followed by the respective HRP-conjugated IgGs. (C) Diagrammatic representation of native and expressed recombinant BpcA proteins and their binding characteristics for the serum proteins CFH, CFH-1, and PLG as determined by ligand affinity blot analysis and ELISA. Numbers refer to amino acid residues.
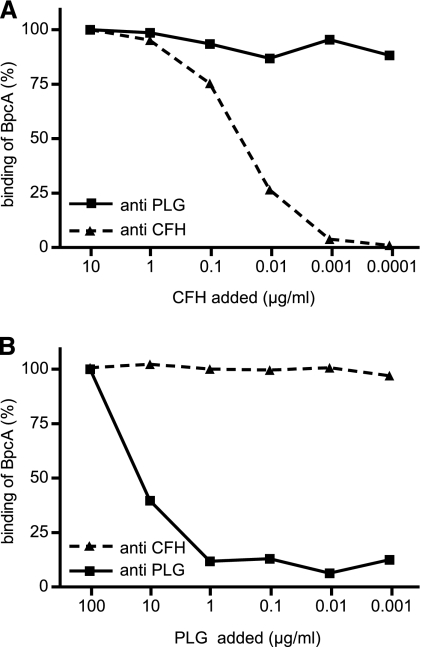
To verify the assumption that CFH and PLG bind to distinct, nonoverlapping domains of the BpcA molecule, competition assays were performed using immobilized BpcA and increasing amounts of PLG or CFH (up to 100 μg/ml) in combination with constant amounts of CFH (0.2 μg/ml) or PLG (20 μg/ml), respectively. As shown in Fig. 6A, CFH did not compete with PLG for binding to BpcA even at high concentrations (10 μg/ml) and vice versa: using up to 100 μg/ml of PLG, binding of CFH to BpcA was not inhibited (Fig. 6B).
FIG. 6.
Concurrent binding of CFH and PLG to BpcA. A competition inhibition assay was performed, adding different amounts of PLG or CFH to inhibit the binding of 20 μg/ml PLG (upper panel) or 0.2 μg/ml CFH (lower panel) to immobilized BpcA. Bound CFH and PLG were detected using goat anti-CFH (dashed line) and goat anti-PLG (solid line), respectively, followed by peroxidase-conjugated secondary antibody.
Mapping of the CFH binding site for BpcA.
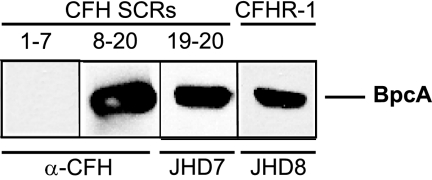
Members of the factor H family are composed exclusively of repetitive protein domains, termed short consensus repeats, and each domain contains about 60 amino acid residues (53). To determine the binding domain of CFH that interacts with BpcA, recombinant deletion constructs CFHSCR1-7, CFHSCR8-20, and CFHSCR19-20 and CFHR-1 were used. BpcA strongly bound to CFHR-1 (Fig. 7, lane 4) and in addition to CFHSCR8-20 (lane 2) and CFHSCR19-20 (lane 3) but not CFHSCR1-7 (lane 1), indicating that SCRs 19 and 20 of CFH are critical for interaction with BpcA. SCR 20 of CFH displays 97% sequence similarity to the most C-terminal domain (SCR 5) of CFHR-1, supporting the notion that the C termini of both molecules are involved in the interaction with BpcA.
FIG. 7.
Mapping of the CFH domain interacting with BpcA. Purified recombinant BpcA protein was separated by 10% Tris-Tricine SDS-PAGE and transferred to nitrocellulose. Membrane strips were incubated with either recombinant CFHL-1 (CFHSCR1-7), two deletion constructs of CFH (CFHSCR8-20 and CFHSCR19-20), or human serum. Bound proteins were visualized using either polyclonal anti-CFH immune serum (α-CFH) or MAb specific for SCRs 19 and 20 (JHD7) or CFHR-1 (JHD8), respectively.
CFH retains cofactor activity when bound to BpcA.
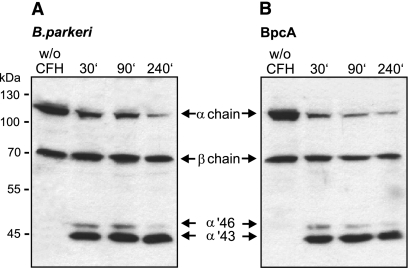
To assess whether BpcA-bound CFH maintains its complement regulatory function, cofactor activity of the attached CFH was analyzed by measuring factor I-mediated conversion of C3b to iC3b. Spirochetes were pretreated with CFH and incubated with C3b and factor I. The supernatant of this reaction was separated by SDS-PAGE, and C3b cleavage products were detected by Western blotting. Surface-bound CFH retained cofactor activity, as indicated by the presence of representative C3b cleavage products, the 46- and 43-kDa α′-chains (Fig. 8A). B. parkeri alone did not promote cleavage of C3b, demonstrating that B. parkeri organisms lack an endogenous C3b cleaving activity. Next, CFH attached to immobilized BpcA, followed by incubation with C3b and factor I, was tested. CFH efficiently mediated C3b conversion, as indicated by the appearance of the respective inactivation products (Fig. 8B). Incubation of immobilized BpcA in the absence of CFH had no effect on C3b conversion under similar conditions.
FIG. 8.
Cofactor activity of CFH bound to either BpcA or intact B. parkeri spirochetes. Functional activity of CFH was analyzed by measuring factor I-mediated conversion of C3b to iC3b. CFH bound to the surface of intact B. parkeri spirochetes (A) or BpcA-coated microtiter plates (B) was incubated with C3b and factor I. Reaction mixtures were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes. C3b degradation products were evaluated by specific detection of α′-chain cleavage fragments of 46 and 43 kDa using biotinylated rabbit anti-C3c IgG followed by peroxidase-conjugated streptavidin. w/o, without.
Activation of bound PLG by host-derived PLG activators.
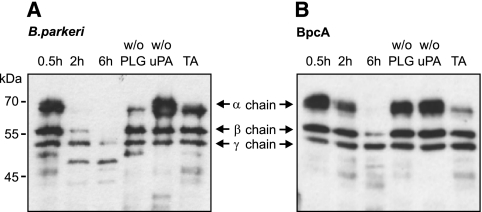
PLG bound to the surface of B. hermsii can be converted to plasmin (43). To determine whether BpcA-bound PLG can similarly be activated, multiwell plates were coated with BpcA, blocked, and incubated with PLG. uPA was added, and the proteolytic activity was quantified using a plasmin-specific chromogenic substrate. BpcA-bound PLG was converted to enzymatically active plasmin in the presence of exogenous uPA and led to degradation of the chromogenic substrate (data not shown). The proteolytic activity of BpcA-bound plasmin was further analyzed by assessing its ability to cleave the physiological substrate fibrinogen. When incubated with BpcA-bound plasmin, fibrinogen was degraded to low-molecular-mass fragments as assessed by Western blotting (Fig. 9). No degradation of fibrinogen was observed in the absence of either PLG or uPA. Furthermore, the addition of the lysine analog tranexamic acid to the reaction mixture abrogated degradation of fibrinogen (Fig. 9).
FIG. 9.
Activation and proteolytic activity of BpcA- and B. parkeri-bound plasmin(ogen). Intact B. parkeri organisms (A) or recombinant BpcA-coated microtiter plates (B) were incubated with PLG. Bound PLG was converted to plasmin by uPA addition, and the proteolytic activity was measured by degradation of fibrinogen. uPA-mediated PLG activation was inhibited by tranexamic acid (TA). The reaction mixtures were separated by 10% SDS-PAGE, transferred to nitrocellulose, and probed with rabbit antifibrinogen followed by peroxidase-conjugated IgG for detection of α, β, and γ chains (67, 57, and 47 kDa, respectively) and the small-size degradation products of fibrinogen.
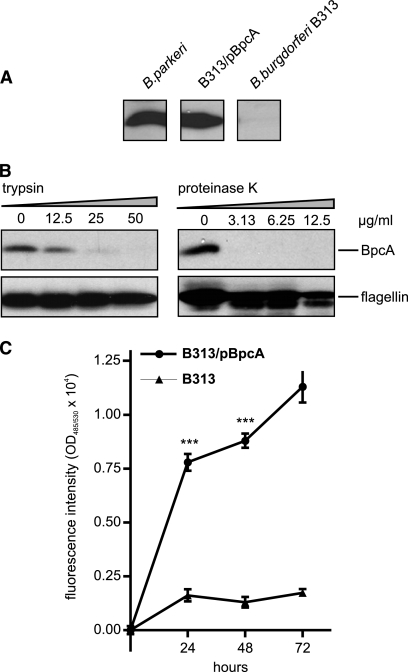
BpcA confers resistance to complement-mediated killing.
To further verify the significance of BpcA for complement resistance of borreliae and removal of C3b from the bacterial surface, the serum-sensitive B. burgdorferi B313 strain, lacking the CFH and CFHR-1 binding proteins, was transformed with the shuttle vector pBpcA containing the complete bpcA gene (B. burgdorferi B313/pBP). Expression of BpcA was determined by Western blot analysis using the BpcA-specific MAb (PB3). B. parkeri and the transformed B313/pBP isolate but not the CFH and CFHR-1-deficient B. burgdorferi B313 mutant showed ectopic expression of BpcA (Fig. 10A). In addition, expression and surface localization of BpcA was confirmed by proteolytic degradation using trypsin (Fig. 10B). To compare the susceptibilities of B313 and B313/pBP to complement-mediated killing, a growth assay was employed. Accordingly, B313 and B313/pBP were incubated with 25% NHS or 25% heat-inactivated serum for up to 3 days. As shown in Fig. 10C, B. burgdorferi B313/pBP spirochetes continuously grew within 72 h in the presence of NHS, demonstrating a pronounced resistance to human serum. Serum-sensitive B313 and B313 spirochetes containing the empty shuttle vector (not shown) did not grow under similar conditions. However, B. burgdorferi B313 and B313/pBP showed similar growth rates when cultured in heat-inactivated human serum, suggesting their susceptibility to complement-mediated lysis (not shown).
FIG. 10.
Ectopic expression of BpcA by B. burgdorferi B313 isolate. (A) Expression of BpcA by transformed B. burgdorferi B313 was assessed using immunoblot analysis. Whole-cell lysates were separated by 10% SDS-PAGE, transferred to nitrocellulose, and analyzed for CFH binding by incubation with NHS and a CFH-specific MAb (JHD-7). (B) Surface localization of BpcA by transformed B. burgdorferi B313 cells was assessed using trypsin and proteinase K treatment. B313/pBP cells were incubated with the indicated concentrations of trypsin, lysed, immunoblotted, and probed with either a BpcA-specific MAb, BP3, or antiflagellin MAb LA21 (lower panel). (C) To investigate susceptibility to human serum, transformed B313/pBP or B313 cells were incubated with 25% NHS at 30°C for 72 h. Cells were stained with a nucleic acid dye, and the relative growth compared to day 0 levels was determined by measurement of green fluorescence emission. Values represent the means ± SEM of results from a single experiment performed in triplicate that is representative of three independent experiments. ***, P < 0.0002.
DISCUSSION
B. parkeri and B. turicatae represent closely related spirochetes in the United States that are suspected to cause tick-borne relapsing fever in humans (9). Although epidemiological evidence is strong for B. turicatae as a cause of human infections, isolation of B. turicatae and B. parkeri from human patients has not yet been documented (45). In this study, we have identified a receptor from B. parkeri for CFH and CFHR-1, two key soluble-phase inhibitory molecules of the alternative complement pathway, and we showed that binding of CFH to BpcA permits resistance to complement-mediated killing by NHS. In contrast, the homologous protein of B. turicatae, BtcA, failed to bind human CFH and CFHR-1. These data provide a possible explanation for the observed serum resistance of B. parkeri compared to serum-sensitive B. turicatae strains.
Many spirochetes have evolved strategies to survive in both arthropod vectors and a variety of vertebrate hosts, including humans. These include their ability to escape innate and adaptive immune defense processes. Complement acts as an important part of the host innate immunity, which is essential for recognition and elimination of microbes. In fact, B. hermsii, the louse-borne relapsing fever spirochete Borrelia recurrentis, and many Lyme disease organisms of the B. burgdorferi sensu lato complex can evade human complement attack by acquiring host regulators at their surface (13, 19, 20, 26, 36, 43). Here we have identified and characterized the surface protein BpcA of B. parkeri, which binds to the human complement regulators CFH and CFHR-1, composed of five SCR domains. Recently it was demonstrated that CFHR-1 is an inhibitor of the alternative complement pathway that binds to C3b, inhibits C5 convertase activity, and interferes with C5b surface deposition and membrane attack complex formation (16). Bacterium-bound CFH maintained its ability to downregulate complement activation. Adsorption experiments with human plasma and intact B. parkeri organisms demonstrated that binding of CFH to BpcA is associated with its C-terminal domains SCRs 19 and 20. These data suggest that selective binding of CFH to BpcA is expected to occur in vivo and thus might be of relevance for bacterial virulence. This assumption is supported by the ability of bacterium-bound CFH to act as a cofactor to factor I in the inactivation of C3b and degradation of the formed C3 convertase. Binding of CFH has been demonstrated for strains of Streptococcus pyogenes (group A streptococcus) (18), Neisseria gonorrhoeae (39), Streptococcus pneumoniae (37), Borrelia duttoni (36), and B. parkeri (34). CFH also has the potential to function in adherence, since it binds to surface glycosaminoglycans and host cell receptors. It is noteworthy that the C-terminal part of CFH has previously been implicated in binding to other bacterial surface structures, e.g., the sialylated lipooligosaccharide of N. gonorrhoeae and the BbCRASP-3 to -5 lipoproteins of B. burgdorferi (17, 24, 25, 40). Acquisition of CFH molecules by surface-exposed BpcA seems to result in enhanced complement-regulatory activity, by which relapsing fever spirochetes can evade clearance by the immune system of the mammalian host. Although the biological significance of BpcA expression by B. parkeri awaits further elucidation, the present finding that the B. burgdorferi B313 mutant, deficient in CFH receptors, resists complement-mediated killing upon transformation with bpcA strongly suggests its involvement in immune avoidance.
To localize the binding determinants of BpcA required for CFH binding, BpcA proteins with N- and C-terminal truncations were constructed. Proteins with either an N-terminal 48-residue (BpcA48-183) or 78-residue (BpcA78-183) truncation or a C-terminal 61-residue (BpcA18-122) truncation showed a complete loss of CFH binding, suggesting that the determinants required for CFH binding are conformationally defined rather than being simple contiguous linear elements. This finding is reminiscent of the CFH binding potential as previously reported for B. hermsii HS1 (43). Moreover, the observed difference in the coiled-coil elements that were predicted in the BpcA and BtcA sequences in combination with the loss of the CFH/CFHR-1 binding site in BtcA suggests an important role of these elements for interaction with human complement regulators. The importance of coiled-coil elements in the presentation of the CFH-binding site has also been demonstrated for the B. burgdorferi complement regulator binding protein, OspE (35).
Here we also demonstrated that BpcA serves as a multifunctional ligand on the borrelial surface as it interacts with PLG. Binding of PLG to BpcA was not affected by any of the indicated truncations of the BpcA protein, indicating that PLG and CFH interact with distinct BpcA domains and suggesting that both host proteins can bind simultaneously to BpcA. This assumption is supported by our findings that PLG and CFH bind independently and coordinately to immobilized BpcA and do not compete with each other. Due to the efficient concurrent binding of CFH and PLG to immobilized BpcA, it is to be expected that both host proteins bind to the outer surface of B. parkeri. Binding of PLG to the surface of bacteria is often important for virulence, both by providing a means of initial anchoring to endothelium via PLG receptors and by adding a protein protease activity to the bacterial surface. Activation of plasmin on the surface of pathogens can thereby facilitate extracellular matrix degradation, allowing the pathogen to disseminate throughout the host (11). PLG has previously been shown to be important for B. burgdorferi dissemination in both vector ticks and mammalian hosts (5). In this study, we also show that BpcA-associated plasmin degrades the physiological substrate fibrinogen, suggesting that plasmin-loaded spirochetes may traverse tissue barriers to colonize distant organs and to propagate pathophysiological processes within the affected tissues. Furthermore, the outer surface-attached proteolytic activity may also protect spirochetes against serum-derived nonspecific and specific antimicrobial compounds, such as complement opsonin C3b or specific IgG antibodies (42). Some PLG-binding proteins are also immunosuppressive, such as enolase of Streptococcus sobrinus, which increases interleukin-10 production and dampens immune responses in mice (46). Binding of multiple serum components may be an advantage for spirochetes because fewer surface lipoproteins need to be expressed in order to disseminate and evade host immune responses. Further studies are required to better understand the molecular mechanisms of binding of CFH and PLG to BpcA and its role in the virulence and pathogenesis of B. parkeri in humans.
In summary, the analyses described here are the first examination of the simultaneous and noncompetitive interaction between CFH, CFHR-1, and PLG and the newly characterized CFH/CFHR-1 binding protein of B. parkeri, BpcA. In contrast, the homologous protein of the closely related spirochete B. turicatae binds only PLG but not CFH and CFHR-1. Our data provide evidence that some relapsing fever spirochetes can circumvent human complement attack through specific binding of regulators CFH and CFHR-1 and thus may account for human infections. Future efforts should be directed toward elucidating the sophisticated escape mechanisms of B. parkeri and B. turicatae for dissemination and persistence in the mammalian host.
Acknowledgments
We thank Jüri Habicht for skillful and expert technical assistance. We also thank D. Scott Samuels for providing pKFSS1 and Tom G. Schwan for providing relapsing fever spirochetes.
We are indebted for the financial support of the Deutsche Forschungsgemeinschaft to R.W. (Wa 533/7-1).
Editor: R. P. Morrison
Footnotes
Published ahead of print on 15 March 2010.
REFERENCES
- 1.Brooks, C. S., S. R. Vuppala, A. M. Jett, A. Alitalo, S. Meri, and D. R. Akins. 2005. Complement regulator-acquiring surface protein 1 imparts resistance to human serum in Borrelia burgdorferi. J. Immunol. 175:3299-3308. [DOI] [PubMed] [Google Scholar]
- 2.Bunikis, J., and A. G. Barbour. 1999. Access of antibody or trypsin to an integral outer membrane protein (P66) of Borrelia burgdorferi is hindered by Osp lipoproteins. Infect. Immun. 67:2874-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 4.Ciceroni, L., A. Bartoloni, P. Guglielmetti, F. Paradisi, H. G. Barahona, M. Roselli, S. Ciarrocchi, and B. Cacciapuoti. 1994. Prevalence of antibodies to Borrelia burgdorferi, Borrelia parkeri and Borrelia turicatae in human settlements of the Cordillera Province, Bolivia. J. Trop. Med. Hyg. 97:13-17. [PubMed] [Google Scholar]
- 5.Coleman, J. L., J. A. Gebbia, J. Piesman, J. L. Degen, T. H. Bugge, and J. L. Benach. 1997. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell 89:1111-1119. [DOI] [PubMed] [Google Scholar]
- 6.Colombo, M. J., and K. R. Alugupalli. 2008. Complement factor H-binding protein, a putative virulence determinant of Borrelia hermsii, is an antigenic target for protective B1b lymphocytes. J. Immunol. 180:4858-4864. [DOI] [PubMed] [Google Scholar]
- 7.Davis, H., J. M. Vincent, and J. Lynch. 2002. Tick-borne relapsing fever caused by Borrelia turicatae. Pediatr. Infect. Dis. J. 21:703-705. [DOI] [PubMed] [Google Scholar]
- 8.Dworkin, M. S., D. E. Anderson, Jr., T. G. Schwan, P. C. Shoemaker, S. N. Banerjee, B. O. Kassen, and W. Burgdorfer. 1998. Tick-borne relapsing fever in the northwestern United States and southwestern Canada. Clin. Infect. Dis. 26:122-131. [DOI] [PubMed] [Google Scholar]
- 9.Dworkin, M. S., T. G. Schwan, and D. E. Anderson, Jr. 2002. Tick-borne relapsing fever in North America. Med. Clin. North Am. 86:417-433, viii-ix. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs, H., R. Wallich, M. M. Simon, and M. D. Kramer. 1994. The outer surface protein A of the spirochete Borrelia burgdorferi is a plasmin(ogen) receptor. Proc. Natl. Acad. Sci. U. S. A. 91:12594-12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gebbia, J. A., J. C. Monco, J. L. Degen, T. H. Bugge, and J. L. Benach. 1999. The plasminogen activation system enhances brain and heart invasion in murine relapsing fever borreliosis. J. Clin. Invest. 103:81-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grab, D. J., G. Perides, J. S. Dumler, K. J. Kim, J. Park, Y. V. Kim, O. Nikolskaia, K. S. Choi, M. F. Stins, and K. S. Kim. 2005. Borrelia burgdorferi, host-derived proteases, and the blood-brain barrier. Infect. Immun. 73:1014-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grosskinsky, S., M. Schott, C. Brenner, S. J. Cutler, P. Kraiczy, P. F. Zipfel, M. M. Simon, and R. Wallich. 2009. Borrelia recurrentis employs a novel multifunctional surface protein with anti-complement, anti-opsonic and invasive potential to escape innate immunity. PLoS One 4:e4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haake, D. A. 2000. Spirochaetal lipoproteins and pathogenesis. Microbiology 146(Pt. 7):1491-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartmann, K., C. Corvey, C. Skerka, M. Kirschfink, M. Karas, V. Brade, J. C. Miller, B. Stevenson, R. Wallich, P. F. Zipfel, and P. Kraiczy. 2006. Functional characterization of BbCRASP-2, a distinct outer membrane protein of Borrelia burgdorferi that binds host complement regulators factor H and FHL-1. Mol. Microbiol. 61:1220-1236. [DOI] [PubMed] [Google Scholar]
- 16.Heinen, S., A. Hartmann, N. Lauer, U. Wiehl, H. M. Dahse, S. Schirmer, K. Gropp, T. Enghardt, R. Wallich, S. Halbich, M. Mihlan, U. Schlotzer-Schrehardt, P. F. Zipfel, and C. Skerka. 2009. Factor H-related protein 1 (CFHR-1) inhibits complement C5 convertase activity and terminal complex formation. Blood 114:2439-2447. [DOI] [PubMed] [Google Scholar]
- 17.Hellwage, J., T. Meri, T. Heikkila, A. Alitalo, J. Panelius, P. Lahdenne, I. J. Seppala, and S. Meri. 2001. The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi. J. Biol. Chem. 276:8427-8435. [DOI] [PubMed] [Google Scholar]
- 18.Horstmann, R. D., H. J. Sievertsen, J. Knobloch, and V. A. Fischetti. 1988. Antiphagocytic activity of streptococcal M protein: selective binding of complement control protein factor H. Proc. Natl. Acad. Sci. U. S. A. 85:1657-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hovis, K. M., J. P. Jones, T. Sadlon, G. Raval, D. L. Gordon, and R. T. Marconi. 2006. Molecular analyses of the interaction of Borrelia hermsii FhbA with the complement regulatory proteins factor H and factor H-like protein 1. Infect. Immun. 74:2007-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hovis, K. M., J. V. McDowell, L. Griffin, and R. T. Marconi. 2004. Identification and characterization of a linear-plasmid-encoded factor H-binding protein (FhbA) of the relapsing fever spirochete Borrelia hermsii. J. Bacteriol. 186:2612-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hovis, K. M., M. E. Schriefer, S. Bahlani, and R. T. Marconi. 2006. Immunological and molecular analyses of the Borrelia hermsii factor H and factor H-like protein 1 binding protein, FhbA: demonstration of its utility as a diagnostic marker and epidemiological tool for tick-borne relapsing fever. Infect. Immun. 74:4519-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juncker, A. S., H. Willenbrock, G. Von Heijne, S. Brunak, H. Nielsen, and A. Krogh. 2003. Prediction of lipoprotein signal peptides in Gram-negative bacteria. Protein Sci. 12:1652-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraiczy, P., K. Hartmann, J. Hellwage, C. Skerka, M. Kirschfink, V. Brade, P. F. Zipfel, R. Wallich, and B. Stevenson. 2004. Immunological characterization of the complement regulator factor H-binding CRASP and Erp proteins of Borrelia burgdorferi. Int. J. Med. Microbiol. 293(Suppl. 37):152-157. [DOI] [PubMed] [Google Scholar]
- 24.Kraiczy, P., J. Hellwage, C. Skerka, H. Becker, M. Kirschfink, M. M. Simon, V. Brade, P. F. Zipfel, and R. Wallich. 2004. Complement resistance of Borrelia burgdorferi correlates with the expression of BbCRASP-1, a novel linear plasmid-encoded surface protein that interacts with human factor H and FHL-1 and is unrelated to Erp proteins. J. Biol. Chem. 279:2421-2429. [DOI] [PubMed] [Google Scholar]
- 25.Kraiczy, P., J. Hellwage, C. Skerka, M. Kirschfink, V. Brade, P. F. Zipfel, and R. Wallich. 2003. Immune evasion of Borrelia burgdorferi: mapping of a complement-inhibitor factor H-binding site of BbCRASP-3, a novel member of the Erp protein family. Eur. J. Immunol. 33:697-707. [DOI] [PubMed] [Google Scholar]
- 26.Kraiczy, P., C. Skerka, V. Brade, and P. F. Zipfel. 2001. Further characterization of complement regulator-acquiring surface proteins of Borrelia burgdorferi. Infect. Immun. 69:7800-7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraiczy, P., C. Skerka, M. Kirschfink, V. Brade, and P. F. Zipfel. 2001. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and Factor H. Eur. J. Immunol. 31:1674-1684. [DOI] [PubMed] [Google Scholar]
- 28.Kraiczy, P., C. Skerka, M. Kirschfink, P. F. Zipfel, and V. Brade. 2001. Mechanism of complement resistance of pathogenic Borrelia burgdorferi isolates. Int. Immunopharmacol. 1:393-401. [DOI] [PubMed] [Google Scholar]
- 29.Kraiczy, P., C. Skerka, P. F. Zipfel, and V. Brade. 2002. Complement regulator-acquiring surface proteins of Borrelia burgdorferi: a new protein family involved in complement resistance. Wien. Klin. Wochenschr. 114:568-573. [PubMed] [Google Scholar]
- 30.Kramer, M. D., U. E. Schaible, R. Wallich, S. E. Moter, D. Petzoldt, and M. M. Simon. 1990. Characterization of Borrelia burgdorferi associated antigens by monoclonal antibodies. Immunobiology 181:357-366. [DOI] [PubMed] [Google Scholar]
- 31.Kuhn, S., C. Skerka, and P. F. Zipfel. 1995. Mapping of the complement regulatory domains in the human factor H-like protein 1 and in factor H. J. Immunol. 155:5663-5670. [PubMed] [Google Scholar]
- 32.Kuhn, S., and P. F. Zipfel. 1995. The baculovirus expression vector pBSV-8His directs secretion of histidine-tagged proteins. Gene 162:225-229. [DOI] [PubMed] [Google Scholar]
- 33.Lupas, A., M. Van Dyke, and J. Stock. 1991. Predicting coiled coils from protein sequences. Science 252:1162-1164. [DOI] [PubMed] [Google Scholar]
- 34.McDowell, J. V., E. Tran, D. Hamilton, J. Wolfgang, K. Miller, and R. T. Marconi. 2003. Analysis of the ability of spirochete species associated with relapsing fever, avian borreliosis, and epizootic bovine abortion to bind factor H and cleave c3b. J. Clin. Microbiol. 41:3905-3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDowell, J. V., J. Wolfgang, L. Senty, C. M. Sundy, M. J. Noto, and R. T. Marconi. 2004. Demonstration of the involvement of outer surface protein e coiled coil structural domains and higher order structural elements in the binding of infection-induced antibody and the complement-regulatory protein, factor H1. J. Immunol. 173:7471-7480. [DOI] [PubMed] [Google Scholar]
- 36.Meri, T., S. J. Cutler, A. M. Blom, S. Meri, and T. S. Jokiranta. 2006. Relapsing fever spirochetes Borrelia recurrentis and B. duttonii acquire complement regulators C4b-binding protein and factor H. Infect. Immun. 74:4157-4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neeleman, C., S. P. Geelen, P. C. Aerts, M. R. Daha, T. E. Mollnes, J. J. Roord, G. Posthuma, H. van Dijk, and A. Fleer. 1999. Resistance to both complement activation and phagocytosis in type 3 pneumococci is mediated by the binding of complement regulatory protein factor H. Infect. Immun. 67:4517-4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pangburn, M. K., R. D. Schreiber, and H. J. Muller-Eberhard. 1977. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J. Exp. Med. 146:257-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ram, S., F. G. Mackinnon, S. Gulati, D. P. McQuillen, U. Vogel, M. Frosch, C. Elkins, H. K. Guttormsen, L. M. Wetzler, M. Oppermann, M. K. Pangburn, and P. A. Rice. 1999. The contrasting mechanisms of serum resistance of Neisseria gonorrhoeae and group B Neisseria meningitidis. Mol. Immunol. 36:915-928. [DOI] [PubMed] [Google Scholar]
- 40.Ram, S., D. P. McQuillen, S. Gulati, C. Elkins, M. K. Pangburn, and P. A. Rice. 1998. Binding of complement factor H to loop 5 of porin protein 1A: a molecular mechanism of serum resistance of nonsialylated Neisseria gonorrhoeae. J. Exp. Med. 188:671-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rawlings, J. A. 1995. An overview of tick-borne relapsing fever with emphasis on outbreaks in Texas. Tex. Med. 91:56-59. [PubMed] [Google Scholar]
- 42.Rooijakkers, S. H., W. J. van Wamel, M. Ruyken, K. P. van Kessel, and J. A. van Strijp. 2005. Anti-opsonic properties of staphylokinase. Microbes Infect. 7:476-484. [DOI] [PubMed] [Google Scholar]
- 43.Rossmann, E., P. Kraiczy, P. Herzberger, C. Skerka, M. Kirschfink, M. M. Simon, P. F. Zipfel, and R. Wallich. 2007. Dual binding specificity of a Borrelia hermsii-associated complement regulator-acquiring surface protein for factor H and plasminogen discloses a putative virulence factor of relapsing fever spirochetes. J. Immunol. 178:7292-7301. [DOI] [PubMed] [Google Scholar]
- 44.Schwan, T. G., P. F. Policastro, Z. Miller, R. L. Thompson, T. Damrow, and J. E. Keirans. 2003. Tick-borne relapsing fever caused by Borrelia hermsii, Montana. Emerg. Infect. Dis. 9:1151-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwan, T. G., S. J. Raffel, M. E. Schrumpf, P. F. Policastro, J. A. Rawlings, R. S. Lane, E. B. Breitschwerdt, and S. F. Porcella. 2005. Phylogenetic analysis of the spirochetes Borrelia parkeri and Borrelia turicatae and the potential for tick-borne relapsing fever in Florida. J. Clin. Microbiol. 43:3851-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veiga-Malta, I., M. Duarte, M. Dinis, D. Tavares, A. Videira, and P. Ferreira. 2004. Enolase from Streptococcus sobrinus is an immunosuppressive protein. Cell Microbiol. 6:79-88. [DOI] [PubMed] [Google Scholar]
- 47.Walker, R. L., D. H. Read, D. C. Hayes, and R. W. Nordhausen. 2002. Equine abortion associated with the Borrelia parkeri-B. turicatae tick-borne relapsing fever spirochete group. J. Clin. Microbiol. 40:1558-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wallich, R., C. Brenner, M. D. Kramer, and M. M. Simon. 1995. Molecular cloning and immunological characterization of a novel linear-plasmid-encoded gene, pG, of Borrelia burgdorferi expressed only in vivo. Infect. Immun. 63:3327-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wallich, R., C. Helmes, U. E. Schaible, Y. Lobet, S. E. Moter, M. D. Kramer, and M. M. Simon. 1992. Evaluation of genetic divergence among Borrelia burgdorferi isolates by use of OspA, fla, HSP60, and HSP70 gene probes. Infect. Immun. 60:4856-4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wallich, R., J. Pattathu, V. Kitiratschky, C. Brenner, P. F. Zipfel, V. Brade, M. M. Simon, and P. Kraiczy. 2005. Identification and functional characterization of complement regulator-acquiring surface protein 1 of the Lyme disease spirochetes Borrelia afzelii and Borrelia garinii. Infect. Immun. 73:2351-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zipfel, P. F., and C. Skerka. 1994. Complement factor H and related proteins: an expanding family of complement-regulatory proteins? Immunol. Today 15:121-126. [DOI] [PubMed] [Google Scholar]
- 52.Zipfel, P. F., and C. Skerka. 2009. Complement regulators and inhibitory proteins. Nat. Rev. Immunol. 9:729-740. [DOI] [PubMed] [Google Scholar]
- 53.Zipfel, P. F., C. Skerka, J. Hellwage, S. T. Jokiranta, S. Meri, V. Brade, P. Kraiczy, M. Noris, and G. Remuzzi. 2002. Factor H family proteins: on complement, microbes and human diseases. Biochem. Soc. Trans. 30:971-978. [DOI] [PubMed] [Google Scholar]
- 54.Zuckert, W. R., J. E. Lloyd, P. E. Stewart, P. A. Rosa, and A. G. Barbour. 2004. Cross-species surface display of functional spirochetal lipoproteins by recombinant Borrelia burgdorferi. Infect. Immun. 72:1463-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zuckert, W. R., J. Meyer, and A. G. Barbour. 1999. Comparative analysis and immunological characterization of the Borrelia Bdr protein family. Infect. Immun. 67:3257-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]