Abstract
The chemokine receptor CCR7 is a well-established homing receptor for dendritic cells and T cells. Interactions with its ligands, CCL19 and CCL21, facilitate priming of immune responses in lymphoid tissue, yet CCR7-independent immune responses can be generated in the presence of sufficient antigen. In these studies, we investigated the role of CCR7 signaling in the generation of protective immune responses to the intracellular protozoan parasite Toxoplasma gondii. The results demonstrated a significant increase in the expression of CCL19, CCL21, and CCR7 in peripheral and central nervous system (CNS) tissues over the course of infection. Unexpectedly, despite the presence of abundant antigen, CCR7 was an absolute requirement for protective immunity to T. gondii, as CCR7−/− mice succumbed to the parasite early in the acute phase of infection. Although serum levels of interleukin 12 (IL-12), IL-6, tumor necrosis factor alpha (TNF-α), and IL-10 remained unchanged, there was a significant decrease in CCL2/monocyte chemoattractant protein 1 (MCP-1) and inflammatory monocyte recruitment to the site of infection. In addition, CCR7−/− mice failed to produce sufficient gamma interferon (IFN-γ), a critical Th1-associated effector cytokine required to control parasite replication. As a result, there was increased parasite dissemination and a significant increase in parasite burden in the lungs, livers, and brains of infected mice. Adoptive-transfer experiments revealed that expression of CCR7 on the T-cell compartment alone is sufficient to enable T-cell priming, increase IFN-γ production, and allow the survival of CCR7−/− mice. These data demonstrate an absolute requirement for T-cell expression of CCR7 for the generation of protective immune responses to Toxoplasma infection.
The chemokine receptor CCR7 and its ligands, CCL19 and CCL21, are known to be critical for a number of essential immunological processes throughout the development of an immune response. These include the generation of thymocytes (6, 26), central and peripheral tolerance, T-cell homeostasis (36), regulatory T-cell (Treg) function (23, 29, 40), and the activation and homing of CCR7-expressing dendritic cells to lymph nodes (12, 28, 32). This central role is in part due to the constitutive expression of CCL19 and CCL21 in primary and secondary lymphoid organs and their roles in guiding the migration of developing, naïve, and central memory T cells. CCL21 is expressed on the high endothelial vessels (HEVs) of the lymph node and by fibroblastic reticular cells (FRC) and follicular dendritic cells (FDC) forming the conduits that are the structural core of the lymph node (2, 33). These structures enable the migration and interaction of CCR7+ cells, namely, naïve or memory T cells and dendritic cells (DCs). Following CCR7-mediated entry into the lymphatics, DCs present antigen to naïve T cells within the lymph node paracortex. T cells enter the lymph node via HEVs and show a “haptokinetic” mode of migration on the FRC surface in response to immobilized CCL21 (4). These chemokine interactions, therefore, influence the velocity of T-cell migration and the duration and likelihood of their contact with DCs presenting cognate antigen. As such, in the absence of the receptor, (CCR7−/− mice) or its ligands (plt/plt mice), lymph nodes have poorly organized T- and B-cell zones and a drastic decrease in the numbers of T cells and DCs in their secondary lymphoid organs (11, 16). Despite the apparent requirement for CCR7 during the priming of adaptive immune responses in the lymph node, studies have demonstrated robust immune responses in the absence of CCR7-CCL21 signaling during lymphocytic choriomeningitis virus (21, 22) and Listeria (24) infections, models of contact hypersensitivity, autoimmune disease, and asthma (15, 30, 34, 37). Indeed, the consensus is that in the presence of a large antigen dose, CCR7 is dispensable for protective immune responses (11).
Toxoplasma gondii is an obligate intracellular parasite that induces a strong systemic immune response. During primary acute infections, quickly replicating tachyzoites can invade any nucleated cell and disseminate rapidly throughout the body. This stage is associated with systemic Th1 immune responses. Production of interleukin 12 (IL-12) by DCs, macrophages, and neutrophils triggers gamma interferon (IFN-γ) production from NK and T cells (8, 18, 25, 27, 35). IFN-γ production is the major mediator of antitoxoplasma effector mechanisms, which include nitric oxide production and expression of IFN-γ-inducible genes, such as those encoding IGTP and LRG-47, which are necessary for controlling parasite growth during the acute infection phase (5, 42). This control of parasite replication initiates conversion of tachyzoites to slowly replicating, cyst-forming bradyzoites, enabling T. gondii to exist chronically for the lifetime of the infected host.
Previous work has demonstrated profound changes in the expression of CCL21 in the lymph node during acute T. gondii infection (20) and in the brain during chronic infection (45). However, as yet, there has been no demonstration of the requirement for CCR7/CCL21 signaling during Toxoplasma infection. To rectify this, we analyzed the kinetics of CCR7, CCL21, and CCL19 expression in the brain and peripheral organs, and we infected mice deficient in CCR7 and monitored their ability to control infection. In contrast to other infection models (21, 24), CCR7−/− mice succumbed during acute infection, associated with a failure to control parasite replication. The transfer of wild-type (WT) T cells indicates that CCR7 expression in the T-cell compartment is sufficient to increase IFN-γ production and control infection.
MATERIALS AND METHODS
Mice and parasites.
T. gondii strain Prugniaud was maintained in vitro in human foreskin fibroblasts (HFF) grown in Dulbecco's modified Eagle's medium (DMEM) complete (90% DMEM, 10% fetal bovine serum, 1% penicillin/streptomycin). After infecting HFF, parasites were grown in D10 medium (70% DMEM, 20% medium 199, 10% fetal bovine serum, 5% penicillin/streptomycin, 5% gentamicin). The parasites were purified by passing them through a 22.5-gauge needle, followed by passage through a 5.0-μm nylon filter, and were centrifuged at 3,000 rpm for 10 min at 4°C. After the supernatant was removed, the parasites were resuspended in 1 ml sterile phosphate-buffered saline (PBS) and counted. Ten thousand parasites were intraperitoneally (i.p.) injected in 200 μl of PBS. C57BL/6 mice were obtained from the Jackson Laboratory (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), and CCR7−/− (C57BL/6 background) mice were bred and maintained in a pathogen-free environment under IACUC-established protocols at the University of California, Riverside, CA.
Blood cytokine measurement.
Blood was collected from the tail vein at day 7 postinfection and again from the heart at the time of sacrifice (day 14). Samples were centrifuged at 14,000 rpm for 10 min at 4°C, and the serum was collected and used to measure the cytokines IFN-γ, CCL2, IL-12p70, IL-10, IL-6, and tumor necrosis factor alpha (TNF-α) using the BD Cytometric Bead Array (according to the manufacturer's instructions) on a BD FACSCanto II flow cytometer with FlowJo analysis software v.8.7.3 (Ashland, OR).
Cytospins.
At the time of sacrifice, peritoneal exudate cells (PECs) from the site of infection were removed in 5 ml intraperitoneally injected PBS. Cytospins were prepared using 100 μl of the cell mixture from each mouse in a cytocentrifuge and stained with a HEMA 3 Staining Kit (Fisher Scientific Company, Pittsburgh, PA).
Flow cytometry.
A single-cell suspension was prepared from spleen and lymph nodes by passing cells through a nylon 40-μm cell strainer (BD Falcon). The suspensions were washed with RPMI complete (10% fetal calf serum [FCS], 1% penicillin/streptomycin, 1% glutamine, 1% sodium pyruvate, 1% nonessential amino acids, 0.1% β-mercaptoethanol) and then centrifuged for 5 min at 1,200 rpm at 4°C. Red blood cells were lysed using 0.86% ammonium chloride solution, centrifuged, and resuspended in RPMI complete. The cells were centrifuged at 1,200 rpm for 5 min at 4°C and resuspended in fluorescence-activated cell sorter (FACS) buffer (PBS containing 4% bovine serum albumin [BSA], 0.01% EDTA). After a further wash in FACS buffer, the cells were preincubated with a saturating solution of Fc block (eBioscience, San Diego, CA) for 5 min on ice and stained with various conjugated antibodies against CD4, CD8, CD44, CD62L, CD11b, CD11c, Ly6C, and Ly6G (all purchased from eBioscience) for 25 min on ice. The cells were washed with FACS buffer and analyzed using the BD FACSCanto II flow cytometer and FlowJo analysis software v.8.7.3.
Restimulation assay and enzyme-linked immunosorbent assay (ELISA).
Single-cell suspensions of splenic cells were counted and diluted with RPMI complete to a cell density of 5 × 106/ml in a final volume of 100 μl. Cells were added in triplicate to a round-bottom 96-well culture plate. The cultures were then left in medium alone or stimulated with soluble Toxoplasma antigen (sTAg) at a final concentration of 25 μg/ml and incubated for 48 h at 37°C and 5% CO2.
ELISA plates (Costar, Corning, NY) were prepared by coating them with primary monoclonal anti-mouse IFN-γ (eBioscience clone AN-18; 0.5 mg/ml) in sterile PBS and left to incubate overnight at 4°C. Following incubation, the plates were washed 3 times in wash buffer (PBS, 0.05% Tween 20; Sigma-Aldrich). Samples and standards (recombinant IFN-γ [rIFN-γ]; eBiosciences) were added in 50 μl and incubated for a further 2 h at 37°C. The plates were washed as before, and secondary biotinylated antibody was added at 0.5 mg/ml (eBiosciences) for 1 h at 37°C. Following a further wash, streptavidin peroxidase at 0.5 μg/ml (Jackson ImmunoResearch) was added in 100 μl/well and incubated for 30 min at 37°C. Following washing, 100 μl/well of the substrate ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] (SouthernBiotech, Birmingham, AL) was added, and absorbance was measured.
Quantification of the T. gondii burden by real-time PCR.
The parasite burden was measured by amplifying the T. gondii B1 gene by real-time PCR, using SYBR GreenER SuperMix for iCycler (Invitrogen, Carlsbad, CA) with an MgCl2 concentration adjusted to 3.5 μM in a 30-μl reaction volume, 2 μg of total template DNA from each organ, 1.50 μl of 0.5 μM primer (IDT) (forward primer, 5′-TCCCCTCTGCTGGCGAAAAGT-3′, and reverse primer, 5′-AGCGTTCGTGGTCAACTATCGATTG-3′), and water for a final volume of 30 μl. The gene was amplified in an iCycler reverse transcription (RT)-PCR machine (Bio-Rad Laboratories, Hercules, CA) using a 10-min initial denaturation at 95°C, followed by 50 cycles that consisted of 15 s of denaturation at 95°C, 30 s of annealing at 60°C, and 30 s of extension at 72°C. The melting curve was generated to check for primer dimers, and threshold values (CT) were acquired and analyzed using the Bio-Rad iQ5 2.0 standard-edition optical-system software v.2.0.148.060623.
Real-time PCR.
Total RNA from the liver and brain tissue samples was extracted with TRIzol reagent (Invitrogen). DNase I treatment and first-strand cDNA synthesis were performed using a cDNA synthesis kit (Fermentas Life Sciences) according to the manufacturer's instructions. CCR7-, CCL19-, and CCL21-specific primers for real-time PCR were purchased from IDT. Primer sequences were as follows: CCR7, forward, 5′-TCATTGCCGTGGTGGTAGTCTTCA-3′, and reverse, 5′-ATGTTGAGCTGCTTGCTGGTTTCG-3′; CCL19, forward, 5′-ATGTGAATCACTCTGGCCCAGGAA-3′, and reverse, 5′-AAGCGGCTTTATTGGAAGCTCTGC-3′; and CCL21, forward, 5′-TGAGCTATGTGCAAACCCTGAGGA-3′, and reverse, 5′-TGAGGGCTGTGTCTGTTCAGTTCT-3′. Real-time PCR was performed using the iQ5 real-time PCR detection system (Bio-Rad) in a total 25-μl reaction mixture with 12.5 μl SYBR green/Rox qPCR Master Mix (2×) and 300 nM primer. The reaction conditions were as follows: 10 min at 95°C, followed by 40 cycles of 15 s at 95°C and 60 s at 60°C. The HPRT (Hypoxine phosphoribosyl transferase) forward primer (5′-CCCTCTGGTAGATTGTCGCTTA-3′) and reverse primer (5′-AGATGCTGTTACTGATAGGAAATTGA-3′) were used as endogenous controls. The quantified results represent the fold induction of target gene expression on different days postinfection in comparison to the target gene expression in naïve cDNA samples. Analyses on 1.5% agarose gels were performed to exclude nonspecific amplification. A no-template control (reagent alone without a template) was included in each assay to detect any possible contamination of the PCR reagents.
Immunohistochemistry.
Immediately following organ excisions, brains, livers, and lungs were fixed in 10% formalin solution, neutral buffered (Sigma-Aldrich, St. Louis, MO), for 10 days. The organs were then put into a standard Tissue-Tek cryomold filled with optimal-cutting-temperature (OCT) solution (also from Tissue-Tek, manufactured for Sakura, Torrance, CA), put on dry ice, and subsequently stored at −80°C. Sections were sliced, stained with hematoxylin and eosin (H&E) (Harris modified hematoxylin from Fisher Scientific Company and eosin from Protocol, Kalamazoo, MI), and stored at −20°C. Series of 10-μm sections were prepared on a standard cryostat machine (Leica CM1850; Simi Valley, CA).
Adoptive transfer.
T cells were purified from naive WT and CCR7−/− splenic cells using T-cell enrichment columns (R&D Systems). The purity of the T cells was assessed by flow cytometry analysis of splenic cells (preisolation) and purified T cells using T-cell-specific marker (CD3), and the population was at least 88% CD3+. WT or CCR7−/− T cells were resuspended in PBS, and 5 × 106 cells (in 200 μl of PBS) were injected i.p. into mice.
RESULTS
Upregulation of CCR7 and ligands at sites of infection.
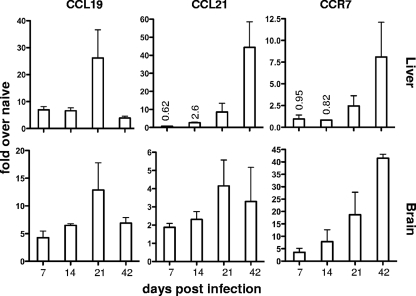
It was previously reported that CCL21 is upregulated in the brain following T. gondii infection (45). To establish the kinetics of expression of CCR7 and its ligands in nonlymphoid organs during infection, mice were infected with the Prugniaud strain of T. gondii, and real-time PCR was conducted on livers and whole brains at various time points following infection (Fig. 1; see Fig. S1 in the supplemental material). At 7 days postinfection, a time point considered to be the peak of the acute systemic immune response, there was a significant increase in the level of CCL19 in the livers of infected mice compared to those of naïve uninfected mice. By 21 days postinfection, transcript levels had reached a peak >20-fold above that of naïve mice, and by day 42, once chronic infection had been established, CCL19 had been downregulated in the liver to levels similar to those in early infection. In contrast, CCL21 transcript was delayed, with no significant increase detected until 14 days after infection. CCL21 continued to increase over the course of infection, reaching a >40-fold increase at 42 days postinfection. Transcript levels of CCR7 followed a pattern similar to that of CCL21, reaching a >8-fold increase in the liver at day 42 postinfection. At this later time point, the parasites had been controlled in the periphery and were predominantly found in the brain of the host. The kinetics of transcript levels for all three molecules were mimicked in the brain, although the fold increases and copy number ratio of CCR7 were significantly greater in the brain at all time points, reflecting the accumulation of parasites and inflammation in this area. At all time points in the brain, the copy number ratio of CCL19 was greater than that of CCL21 (see Fig. S1 in the supplemental material). These data demonstrate that following Toxoplasma infection there is significant upregulation of CCR7, CCL19, and CCL21 in peripheral tissues associated with sites of infection.
FIG. 1.
CCL19, CCL21, and CCR7 are upregulated during the course of T. gondii infection. Infected mice were sacrificed at days 7, 14, 21, and 42 postinfection, and total RNA was extracted from the livers and brains. Real-time RT-PCR was conducted to determine the absolute copy numbers of CCL19, CCL21, and CCR7 using a standard curve, and they were compared to those of the internal reference HPRT gene. The data are represented as mean fold changes over naïve mice, with the error bars representing standard errors of the mean (SEM) of at least 3 biological replicates.
CCR7−/− mice produce significantly less IFN-γ in response to infection.
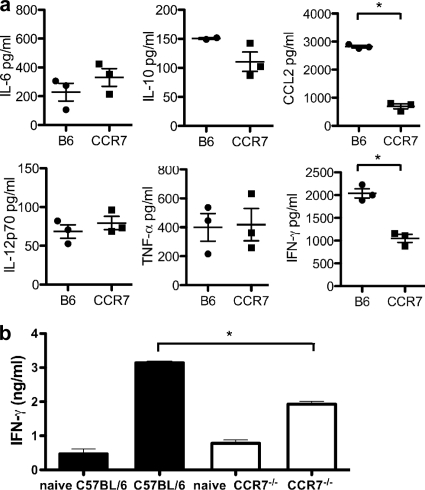
To determine if CCR7 interactions are required for the generation of protective immune responses to T. gondii, CCR7−/− mice were infected and serum levels of cytokines were compared to those of wild-type C57BL/6 mice. At day 7 postinfection, the peak of the systemic immune response, equivalent concentrations of IL-6, IL-12p70, and TNF-α were present in the sera of CCR7−/− mice and control mice (Fig. 2a). Measurement of IL-10, required to prevent infection-induced immunopathology (44, 46), demonstrated a trend toward decreased production, although it did not reach statistical significance. However, the concentrations of CCL2 and the proinflammatory cytokine IFN-γ were significantly decreased in mice deficient in CCR7. CCL2 is required for the recruitment of monocytes to the site of infection (38), while IFN-γ, produced by NK and Th1 cells during T. gondii infection, is required for the activation of monocytes, and ultimately effector mechanisms, against the parasite (3, 49). In addition, the defect in IFN-γ production remained following restimulation of splenic cells from naïve mice with sTAg. Thus, although both wild-type and CCR7−/− cultures increased the production of IFN-γ compared to cultures from uninfected naïve mice, cultures from infected CCR7−/− mice remained deficient in IFN-γ compared to cells from wild-type controls (Fig. 2b).
FIG. 2.
Deficiency in proinflammatory cytokine production during acute infection in CCR7−/− mice. (a) Serum samples were collected from C57BL/6 and CCR7−/− mice at day 7 postinfection, and the protein levels of IFN-γ, CCL2, IL-12p70, IL-10, IL-6, and TNF-α were measured. The levels (averages ± SEM) for individual mice (n = 3) are plotted. (b) Single-cell suspensions were generated from naïve and infected spleens and restimulated with sTAg. IFN-γ production was measured after 48 h using ELISA. Averages and SEM of at least 3 biological replicates are plotted and are representative of 3 independent experiments containing a minimum of 3 biological replicates. *, P < 0.001.
CCR7−/− mice are susceptible to Toxoplasma infection.
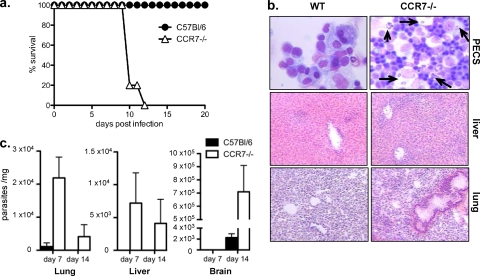
Survival and parasite burdens were monitored in CCR7−/− and wild-type mice. Consistent with the failure to produce IFN-γ, CCR7−/− mice succumbed to infection at around 10 days postinfection (Fig. 3a). Histological analysis of livers and lungs revealed increased inflammation and necrosis (Fig. 3b). Examination of the inflammatory infiltrate at the site of infection revealed increased numbers of cells in the PECs, many of which were infected, and replicating parasites were readily detectable compared to infected wild-type controls (Fig. 3b). To quantify this increase in the parasite burden, real-time RT-PCR was conducted for the parasite-specific B1 gene (46) in peripheral and central nervous system (CNS) tissues. At 7 days postinfection, parasites could be detected in the lungs of wild-type mice but remained undetectable in the liver and brain. By day 14, a time point at which we saw contraction of systemic infection and inflammation, parasites could be detected only in the brain (Fig. 3c). In contrast, parasites in CCR7−/− mice were detectable in the liver and were significantly increased in the lung compared to infected control mice. In addition, although parasites had not yet migrated to the brain by day 7, by day 14, there was a significant increase in the number of parasites in the brain compared to control mice (Fig. 3c). These data demonstrate that CCR7 is required to generate protective immune responses to T. gondii, as in its absence, there is increased parasite survival and dissemination and failure to survive infection.
FIG. 3.
CCR7−/− mice fail to survive acute Toxoplasma infection due to uncontrolled parasite replication. (a) C57BL/6 WT (n = 5) and CCR7−/− (n = 5) mice were infected with 104 T. gondii parasites, and survival was monitored daily. (b) PECs (the arrows indicate replicating parasites) and liver and lung histology at day 7 postinfection indicating increased parasite burden and inflammation in CCR7−/− compared to C57BL/6 mice. (c) Measurement of parasite DNA in lung, liver, and brain at days 7 and 14 postinfection using real-time RT-PCR as described in Materials and Methods. Averages and SEM of 3 biological replicates are plotted.
Defective recruitment of effector cells in CCR7−/− mice.
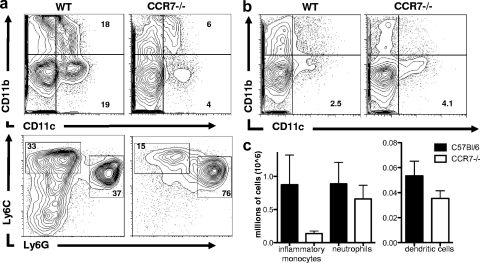
CCR7 and its ligands can play roles in multiple cell types. To determine which cells during Toxoplasma infection require CCR7 signaling, analysis of the cellular infiltrate was conducted using flow cytometry. Following T. gondii infection, neutrophils and inflammatory monocytes, differentiated by their expression of Ly6C and Ly6G, are among the first effector cells at the site of infection and are actively involved in killing the parasite (9, 10). Analysis of inflammatory monocytes (CD45+ CD11b+ Ly6G− Ly6Chi) and neutrophils (CD45+ CD11b+ Ly6Ghi Ly6Cmed) (14) at day 7 postinfection revealed significant populations of both cell types in the peritoneal cavity and increased numbers of these cells in the lymph node and spleen following infection (Fig. 4a and data not shown). (Hi and Med are high and medium levels of expression, respectively.) In contrast, CCR7−/− mice had a significant defect in the proportion of inflammatory monocytes at the site of infection, with on average a 50% reduction in the percentage of CD45+ CD11b+ Ly6G− Ly6Chi cells in the peritoneal cavity and decreased numbers in the lymph node (Fig. 4a and c). These data are consistent with the significant defect in serum levels of CCL2 (Fig. 2a), and this is a likely cause of the delay in the control of parasite replication (9, 31).
FIG. 4.
Analysis of cellular composition using flow cytometry at the site of infection (PECs) (a) and draining lymph nodes (b) from 7-day-infected C57BL/6 and CCR7−/− mice. The numbers represent proportions of dendritic cells (CD11c+) as percentages of live cells (top) and proportions of inflammatory monocytes (CD45+ CD11b+ Ly6Chi Ly6G−) and neutrophils (CD45+ CD11b+ Ly6Cmed Ly6Ghi) as a percentage of CD11b+ cells from PECs (a, bottom). (c) Absolute numbers of neutrophils and dendritic cells in the draining lymph nodes from 7-day-infected C57BL/6 and CCR7−/− mice. Averages and SEM are shown.
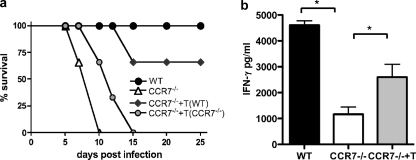
As previously reported, phenotypic analysis of cells in lymphoid compartments revealed very few T cells in the lymph nodes of naïve CCR7-deficient mice and increased numbers of CD4 and CD8 T cells in peripheral sites, including the peritoneal cavity, before and after infection (references 4, 7, and 12 and data not shown). Analysis of the proportion of recently activated T cells (CD44hi CD62Llo) that are present at these sites revealed little to no defect in their activation status (percentage [average ± SEM] of CD44hi CD62Llo: [24.9 ± 1.0 in WT and 20 ± 0.9 in CCR7−/−), suggesting that the small numbers of T cells that are in the lymph node are capable of being primed. CCR7 is also required for DC migration into the lymph node and migration within the T-cell zone. As a result, DC numbers in the lymph nodes of CCR7−/− mice are vastly reduced during inflammation (17, 19, 48). Analysis of the dendritic cell population at the site of Toxoplasma infection revealed a substantial decrease in the number of CD45+ CD11b− CD11c+ cells in the peritoneal exudate cells (Fig. 4a). However, analysis of lymph nodes following infection revealed an increase in the proportion of CD45+ CD11b− CD11c+ dendritic cells compared to wild-type control mice (Fig. 4b), although absolute cell numbers never reached the levels of wild-type controls (Fig. 4c). Unlike in some viral infections (39), dendritic cells play an intrinsic role in the generation of immunity to Toxoplasma via production of IL-12 and antigen presentation (1, 35). CCR7-independent recruitment of dendritic cells during infection has previously been demonstrated (31). To determine if the inflammation-induced dendritic cells present in the lymph nodes of CCR7−/− mice were sufficient to generate protective immune responses, adoptive transfer of wild-type CCR7-expressing T cells was conducted. One day following transfer of wild-type or CCR7-deficient T cells, CCR7−/− mice were infected as before and monitored for survival (Fig. 5). As previously shown, CCR7-deficient mice succumbed to infection within the first 2 weeks. CCR7−/− mice that received additional CCR7−/− T cells also succumbed to infection, with similar kinetics. However, mice that received CCR7-expressing T cells had significantly increased survival rates (Fig. 5a). In addition, measuring serum IFN-γ from wild-type mice, CCR7−/− mice, and CCR7−/− mice that received wild-type T cells revealed a significant increase in the concentration of IFN-γ following T-cell transfer (Fig. 5b). These data demonstrate that CCR7+ T cells can be primed in CCR7−/− mice, producing IFN-γ during T. gondii infection that is sufficient to rescue CCR7-deficient mice.
FIG. 5.
CCR7+ T cells are sufficient for protective immune responses in CCR7−/− mice. (a) T cells were isolated from spleens and lymph nodes of naive C57BL/6 or CCR7−/− mice, and 5 × 106 cells were adoptively transferred into CCR7−/− mice (n = 5). C57BL/6 (n = 5) and nontransferred CCR7−/− mice (n = 5) were sham injected with PBS. The survival curve is representative of four independent experiments with similar results. (b) Serum IFN-γ levels at day 7 postinfection. Averages and SEM are shown.
DISCUSSION
Previous reports have established CCR7 as a crucial chemokine receptor for promoting T-cell migration and coordinating antigen presentation by DCs in the lymph node (33, 47). Despite this central role, multiple studies have demonstrated that, although CCR7-deficient mice can show delayed kinetics, CCR7 signaling is not an absolute requirement for effective immune responses in experimental models of inflammation (15, 30, 40) or for protective immunity during infection (11, 21, 22, 24). The prevailing consensus is that during strong inflammatory responses with abundant antigen, the requirement for antigen presentation in the lymph node, and thus the need for CCR7, can be bypassed. The present study investigated the role of CCR7 in the generation of protective immune responses to the intracellular pathogen T. gondii. This infection is often described as the “atomic bomb” of Th1 immune responses, characterized by the invasion of any nucleated cell by quickly replicating parasites, resulting in systemic infection and tissue damage during the acute phase of infection. Despite this high antigen load, we found that CCR7−/− mice were incapable of mounting protective immune responses, with severe defects in inflammatory monocyte recruitment and in the production of the key effector cytokine IFN-γ.
The decrease in CCL2 and monocyte recruitment to the site of infection has a significant detrimental effect on the ability of CCR7−/− mice to innately control parasite replication. Inflammatory monocytes are required for protection during infection by many intracellular pathogens, including Toxoplasma (9, 10, 38). These cells are recruited to inflamed sites and can directly kill pathogens by the production of nitric oxide (38, 43). In addition, as they are capable of differentiating into dendritic cells, they may play an important role in shaping the adaptive immune response (10, 14). Inflammatory monocytes are not thought to express CCR7, and therefore, the decrease in recruitment of these cells at day 7 following infection may reflect an absence of sustained Th1 immune responses. In addition, there is a loss of cells that express low levels of both Ly6C and Ly6G in the absence of CCR7. The downregulation of Ly6C has been associated with recently activated inflammatory monocytes (41). To our knowledge, this defect in inflammatory monocyte recruitment in CCR7−/− mice has not previously been reported. The lack of inflammatory monocytes most likely leads to impaired parasite killing and a significant increase in parasite numbers and dissemination in CCR7-deficient mice.
In addition to this defect in the innate immune response, CCR7−/− mice, while producing equivalent levels of IL-12, failed to mount a significant type 1 adaptive immune response. A recent paper has characterized the dynamic early responses following Toxoplasma infection in the lymph node (20). These studies demonstrate significant recruitment of naïve CCR7hi CD8+ T cells and sustained contact with DCs in the subcapsular region alongside profound structural changes in lymphoid architecture. The studies presented here address whether an alternate priming site is enough for efficient T-cell-DC interaction, which is required for a robust Th1 immune response following T. gondii infection. Despite the significant numbers of T cells at the site of infection in CCR7−/− mice, the low levels of IFN-γ suggest that they are not sufficiently activated. This implies a dominant role for the lymph node in antigen presentation and T-cell priming during Toxoplasma infection. To support this, adoptive transfer of CCR7+ T cells, and not CCR7−/− T cells, led to a significant increase in IFN-γ and increased survival of CCR7−/− mice. Thus, CCR7 signaling on only T cells enabled sufficient T-cell priming to produce protective immunity in T. gondii-infected mice. This is likely aided by the presence of inflammation-induced DCs at the site of infection and their migration to the lymph node (31). Analysis of the cellular infiltrate demonstrated that although the numbers of dendritic cells were never equivalent to those of wild-type mice, there was an increase in these cells in the lymph node following infection. Toxoplasma is known to induce CCL2 (13, 38) and to stimulate the migration of blood-derived inflammatory dendritic cells to the lymph node in a CCR7-independent manner (31). This process would enable the priming of CCR7+ T cells in CCR7-deficient mice. Intriguingly, CCR7 and its ligands accumulate in tissue during chronic infection, suggesting that these molecules are not only required for priming of T cells, but may play a role in chronic immune responses at the site of infection (45).
In summary, these studies have established a requirement for CCR7 signaling for the generation of protective immune responses during Toxoplasma infection. T-cell expression of CCR7 is sufficient to facilitate the priming of a type 1 adaptive immune response and generation of IFN-γ.
Supplementary Material
Acknowledgments
We thank J. Crane and W. Carter for animal husbandry.
This work was supported by funding from the State of California (to E.H.W.).
Editor: J. H. Adams
Footnotes
Published ahead of print on 1 March 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Aliberti, J., D. Jankovic, and A. Sher. 2004. Turning it on and off: regulation of dendritic cell function in Toxoplasma gondii infection. Immunol. Rev. 201:26-34. [DOI] [PubMed] [Google Scholar]
- 2.Bajenoff, M., J. G. Egen, H. Qi, A. Y. Huang, F. Castellino, and R. N. Germain. 2007. Highways, byways and breadcrumbs: directing lymphocyte traffic in the lymph node. Trends Immunol. 28:346-352. [DOI] [PubMed] [Google Scholar]
- 3.Bliss, S. K., L. C. Gavrilescu, A. Alcaraz, and E. Y. Denkers. 2001. Neutrophil depletion during Toxoplasma gondii infection leads to impaired immunity and lethal systemic pathology. Infect. Immun. 69:4898-4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bromley, S. K., T. R. Mempel, and A. D. Luster. 2008. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat. Immunol. 9:970-980. [DOI] [PubMed] [Google Scholar]
- 5.Collazo, C. M., G. S. Yap, G. D. Sempowski, K. C. Lusby, L. Tessarollo, G. F. Woude, A. Sher, and G. A. Taylor. 2001. Inactivation of LRG-47 and IRG-47 reveals a family of interferon gamma-inducible genes with essential, pathogen-specific roles in resistance to infection. J. Exp. Med. 194:181-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davalos-Misslitz, A. C., J. Rieckenberg, S. Willenzon, T. Worbs, E. Kremmer, G. Bernhardt, and R. Forster. 2007. Generalized multi-organ autoimmunity in CCR7-deficient mice. Eur. J. Immunol. 37:613-622. [DOI] [PubMed] [Google Scholar]
- 7.Debes, G. F., C. N. Arnold, A. J. Young, S. Krautwald, M. Lipp, J. B. Hay, and E. C. Butcher. 2005. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat. Immunol. 6:889-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denkers, E. Y., and A. Sher. 1997. Role of natural killer and NK1+ T-cells in regulating cell-mediated immunity during Toxoplasma gondii infection. Biochem. Soc. Trans. 25:699-703. [DOI] [PubMed] [Google Scholar]
- 9.Dunay, I. R., R. A. Damatta, B. Fux, R. Presti, S. Greco, M. Colonna, and L. D. Sibley. 2008. Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity 29:306-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egan, C. E., W. Sukhumavasi, A. L. Bierly, and E. Y. Denkers. 2008. Understanding the multiple functions of Gr-1(+) cell subpopulations during microbial infection. Immunol. Res. 40:35-48. [DOI] [PubMed] [Google Scholar]
- 11.Forster, R., A. C. Davalos-Misslitz, and A. Rot. 2008. CCR7 and its ligands: balancing immunity and tolerance. Nat. Rev. Immunol. 8:362-371. [DOI] [PubMed] [Google Scholar]
- 12.Forster, R., A. Schubel, D. Breitfeld, E. Kremmer, I. Renner-Muller, E. Wolf, and M. Lipp. 1999. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99:23-33. [DOI] [PubMed] [Google Scholar]
- 13.Fouts, A. E., and J. C. Boothroyd. 2007. Infection with Toxoplasma gondii bradyzoites has a diminished impact on host transcript levels relative to tachyzoite infection. Infect. Immun. 75:634-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geissmann, F., S. Jung, and D. R. Littman. 2003. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19:71-82. [DOI] [PubMed] [Google Scholar]
- 15.Grinnan, D., S. S. Sung, J. A. Dougherty, A. R. Knowles, M. B. Allen, C. E. Rose III, H. Nakano, M. D. Gunn, S. M. Fu, and C. E. Rose, Jr. 2006. Enhanced allergen-induced airway inflammation in paucity of lymph node T cell (plt) mutant mice. J. Allergy Clin. Immunol. 118:1234-1241. [DOI] [PubMed] [Google Scholar]
- 16.Gunn, M. D., S. Kyuwa, C. Tam, T. Kakiuchi, A. Matsuzawa, L. T. Williams, and H. Nakano. 1999. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 189:451-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hintzen, G., L. Ohl, M. L. del Rio, J. I. Rodriguez-Barbosa, O. Pabst, J. R. Kocks, J. Krege, S. Hardtke, and R. Forster. 2006. Induction of tolerance to innocuous inhaled antigen relies on a CCR7-dependent dendritic cell-mediated antigen transport to the bronchial lymph node. J. Immunol. 177:7346-7354. [DOI] [PubMed] [Google Scholar]
- 18.Hunter, C. A., C. S. Subauste, and J. S. Remington. 1994. The role of cytokines in toxoplasmosis. Biotherapy 7:237-247. [DOI] [PubMed] [Google Scholar]
- 19.Jang, M. H., N. Sougawa, T. Tanaka, T. Hirata, T. Hiroi, K. Tohya, Z. Guo, E. Umemoto, Y. Ebisuno, B. G. Yang, J. Y. Seoh, M. Lipp, H. Kiyono, and M. Miyasaka. 2006. CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes. J. Immunol. 176:803-810. [DOI] [PubMed] [Google Scholar]
- 20.John, B., T. H. Harris, E. D. Tait, E. H. Wilson, B. Gregg, L. G. Ng, P. Mrass, D. S. Roos, F. Dzierszinski, W. Weninger, and C. A. Hunter. 2009. Dynamic imaging of CD8(+) T cells and dendritic cells during infection with Toxoplasma gondii. PLoS Pathog. 5:e1000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Junt, T., H. Nakano, T. Dumrese, T. Kakiuchi, B. Odermatt, R. M. Zinkernagel, H. Hengartner, and B. Ludewig. 2002. Antiviral immune responses in the absence of organized lymphoid T cell zones in plt/plt mice. J. Immunol. 168:6032-6040. [DOI] [PubMed] [Google Scholar]
- 22.Junt, T., E. Scandella, R. Forster, P. Krebs, S. Krautwald, M. Lipp, H. Hengartner, and B. Ludewig. 2004. Impact of CCR7 on priming and distribution of antiviral effector and memory CTL. J. Immunol. 173:6684-6693. [DOI] [PubMed] [Google Scholar]
- 23.Kocks, J. R., A. C. Davalos-Misslitz, G. Hintzen, L. Ohl, and R. Forster. 2007. Regulatory T cells interfere with the development of bronchus-associated lymphoid tissue. J. Exp. Med. 204:723-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kursar, M., U. E. Hopken, M. Koch, A. Kohler, M. Lipp, S. H. Kaufmann, and H. W. Mittrucker. 2005. Differential requirements for the chemokine receptor CCR7 in T cell activation during Listeria monocytogenes infection. J. Exp. Med. 201:1447-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieberman, L. A., and C. A. Hunter. 2002. The role of cytokines and their signaling pathways in the regulation of immunity to Toxoplasma gondii. Int. Rev. Immunol. 21:373-403. [DOI] [PubMed] [Google Scholar]
- 26.Liu, C., T. Ueno, S. Kuse, F. Saito, T. Nitta, L. Piali, H. Nakano, T. Kakiuchi, M. Lipp, G. A. Hollander, and Y. Takahama. 2005. The role of CCL21 in recruitment of T-precursor cells to fetal thymi. Blood 105:31-39. [DOI] [PubMed] [Google Scholar]
- 27.Liu, C. H., Y. T. Fan, A. Dias, L. Esper, R. A. Corn, A. Bafica, F. S. Machado, and J. Aliberti. 2006. Cutting edge: dendritic cells are essential for in vivo IL-12 production and development of resistance against Toxoplasma gondii infection in mice. J. Immunol. 177:31-35. [DOI] [PubMed] [Google Scholar]
- 28.Martin-Fontecha, A., S. Sebastiani, U. E. Hopken, M. Uguccioni, M. Lipp, A. Lanzavecchia, and F. Sallusto. 2003. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J. Exp. Med. 198:615-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menning, A., U. E. Hopken, K. Siegmund, M. Lipp, A. Hamann, and J. Huehn. 2007. Distinctive role of CCR7 in migration and functional activity of naive- and effector/memory-like Treg subsets. Eur. J. Immunol. 37:1575-1583. [DOI] [PubMed] [Google Scholar]
- 30.Mori, S., H. Nakano, K. Aritomi, C. R. Wang, M. D. Gunn, and T. Kakiuchi. 2001. Mice lacking expression of the chemokines CCL21-ser and CCL19 (plt mice) demonstrate delayed but enhanced T cell immune responses. J. Exp. Med. 193:207-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakano, H., K. L. Lin, M. Yanagita, C. Charbonneau, D. N. Cook, T. Kakiuchi, and M. D. Gunn. 2009. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat. Immunol. 10:394-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohl, L., M. Mohaupt, N. Czeloth, G. Hintzen, Z. Kiafard, J. Zwirner, T. Blankenstein, G. Henning, and R. Forster. 2004. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity 21:279-288. [DOI] [PubMed] [Google Scholar]
- 33.Okada, T., and J. G. Cyster. 2007. CC chemokine receptor 7 contributes to Gi-dependent T cell motility in the lymph node. J. Immunol. 178:2973-2978. [DOI] [PubMed] [Google Scholar]
- 34.Pahuja, A., R. A. Maki, P. A. Hevezi, A. Chen, G. M. Verge, S. M. Lechner, R. B. Roth, A. Zlotnik, and D. G. Alleva. 2006. Experimental autoimmune encephalomyelitis develops in CC chemokine receptor 7-deficient mice with altered T-cell responses. Scand. J. Immunol. 64:361-369. [DOI] [PubMed] [Google Scholar]
- 35.Pepper, M., F. Dzierszinski, E. Wilson, E. Tait, Q. Fang, F. Yarovinsky, T. M. Laufer, D. Roos, and C. A. Hunter. 2008. Plasmacytoid dendritic cells are activated by Toxoplasma gondii to present antigen and produce cytokines. J. Immunol. 180:6229-6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ploix, C., D. Lo, and M. J. Carson. 2001. A ligand for the chemokine receptor CCR7 can influence the homeostatic proliferation of CD4 T cells and progression of autoimmunity. J. Immunol. 167:6724-6730. [DOI] [PubMed] [Google Scholar]
- 37.Ploix, C., R. I. Zuberi, F. T. Liu, M. J. Carson, and D. D. Lo. 2009. Induction and effector phase of allergic lung inflammation is independent of CCL21/CCL19 and LT-beta. Int. J. Med. Sci. 6:85-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robben, P. M., M. LaRegina, W. A. Kuziel, and L. D. Sibley. 2005. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J. Exp. Med. 201:1761-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scandella, E., K. Fink, T. Junt, B. M. Senn, E. Lattmann, R. Forster, H. Hengartner, and B. Ludewig. 2007. Dendritic cell-independent B cell activation during acute virus infection: a role for early CCR7-driven B-T helper cell collaboration. J. Immunol. 178:1468-1476. [DOI] [PubMed] [Google Scholar]
- 40.Schneider, M. A., J. G. Meingassner, M. Lipp, H. D. Moore, and A. Rot. 2007. CCR7 is required for the in vivo function of CD4+ CD25+ regulatory T cells. J. Exp. Med. 204:735-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sunderkotter, C., T. Nikolic, M. J. Dillon, N. Van Rooijen, M. Stehling, D. A. Drevets, and P. J. Leenen. 2004. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 172:4410-4417. [DOI] [PubMed] [Google Scholar]
- 42.Taylor, G. A., C. M. Collazo, G. S. Yap, K. Nguyen, T. A. Gregorio, L. S. Taylor, B. Eagleson, L. Secrest, E. A. Southon, S. W. Reid, L. Tessarollo, M. Bray, D. W. McVicar, K. L. Komschlies, H. A. Young, C. A. Biron, A. Sher, and G. F. Vande Woude. 2000. Pathogen-specific loss of host resistance in mice lacking the IFN-gamma-inducible gene IGTP. Proc. Natl. Acad. Sci. U. S. A. 97:751-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Voisin, M. B., D. Buzoni-Gatel, D. Bout, and F. Velge-Roussel. 2004. Both expansion of regulatory GR1+ CD11b+ myeloid cells and anergy of T lymphocytes participate in hyporesponsiveness of the lung-associated immune system during acute toxoplasmosis. Infect. Immun. 72:5487-5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wille, U., E. N. Villegas, L. Craig, R. Peach, and C. A. Hunter. 2002. Contribution of interleukin-12 (IL-12) and the CD28/B7 and CD40/CD40 ligand pathways to the development of a pathological T-cell response in IL-10-deficient mice. Infect. Immun. 70:6940-6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson, E. H., T. H. Harris, P. Mrass, B. John, E. D. Tait, G. F. Wu, M. Pepper, E. J. Wherry, F. Dzierzinski, D. Roos, P. G. Haydon, T. M. Laufer, W. Weninger, and C. A. Hunter. 2009. Behavior of parasite-specific effector CD8+ T cells in the brain and visualization of a kinesis-associated system of reticular fibers. Immunity 30:300-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson, E. H., U. Wille-Reece, F. Dzierszinski, and C. A. Hunter. 2005. A critical role for IL-10 in limiting inflammation during toxoplasmic encephalitis. J. Neuroimmunol. 165:63-74. [DOI] [PubMed] [Google Scholar]
- 47.Worbs, T., and R. Forster. 2007. A key role for CCR7 in establishing central and peripheral tolerance. Trends Immunol. 28:274-280. [DOI] [PubMed] [Google Scholar]
- 48.Worbs, T., T. R. Mempel, J. Bolter, U. H. von Andrian, and R. Forster. 2007. CCR7 ligands stimulate the intranodal motility of T lymphocytes in vivo. J. Exp. Med. 204:489-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao, Y., D. Wilson, S. Matthews, and G. S. Yap. 2007. Rapid elimination of Toxoplasma gondii by gamma interferon-primed mouse macrophages is independent of CD40 signaling. Infect. Immun. 75:4799-4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.