Abstract
Excessive production of interleukin-4 impairs clearance of the fungal pathogen Histoplasma capsulatum in mice lacking the chemokine receptor CCR2. An increase in the interleukin-4 level is associated with decreased recruitment of dendritic cells to lungs; therefore, we investigated the possibility that these cells influence interleukin-4 production. Adoptive transfer of wild-type or CCR2−/− bone marrow-derived dendritic cells loaded with heat-killed yeast cells to infected CCR2−/− mice suppressed interkeukin-4 transcription. Surprisingly, transfer of cells did not reduce the fungal burden despite the fact that it limited interleukin-4 transcription. Yeast cell-loaded bone marrow-derived dendritic cell-mediated regulation of interleukin-4 transcription was dependent on major histocompatibility complex II antigen presentation to CD4+ T cells. We previously showed that CD4+ T cells were a source of interleukin-4 in infected CCR2−/− mice, but their contribution to the TH2 phenotype was unclear. Here we demonstrated that these cells were functionally important since elimination of them prior to infection, but not elimination of them at the time of infection, reduced the interleukin-4 level in infected CCR2−/− mice. However, the fungal burden was reduced only in CD4-depleted CCR2−/− mice that received yeast cell-loaded bone marrow-derived dendritic cells. Taken together, the data indicate that generation of excess interleukin-4 in lungs of H. capsulatum-infected CCR2−/− mice is at least partially a consequence of decreased recruitment of dendritic cells capable of antigen presentation. Furthermore, CD4+ T cells had a deleterious impact on immunity in infected CCR2−/− mice.
Infection by the intracellular fungal pathogen Histoplasma capsulatum causes a fatal disseminated infection in immunocompromised individuals (14). In mice, impaired immunity results from a diminished TH1 immune response and/or an increased TH2 immune response (3-5, 8, 16). Production of gamma interferon (IFN-γ), a TH1 cytokine, promotes resolution of an infection by activating macrophages (Mφ) harboring yeast cells (4, 51). These Mφ, termed classically activated Mφ, produce nitric oxide (NO), resulting in yeast cell degradation (7). Tumor necrosis factor alpha (TNF-α) signaling also promotes production of NO and protective immunity (5). In contrast, excessive production of interleukin-4 (IL-4), a TH2 cytokine, is associated with alternative Mφ activation (43) and dampens the protective immune response (2, 3, 8, 43).
A principal source of IL-4 is the CD4+ T cells (25, 35, 46). Basophils also produce IL-4 and induce CD4+ T cells to generate this cytokine in an antigen-dependent manner upon helminth infection or allergic inflammation of the airways (35, 41, 50). In addition, presentation of microbial antigen by dendritic cells (DC) influences T cell polarization, but the mechanisms that determine whether TH1 or TH2 cells are dominant are not fully understood. In the presence of IL-4, DC stimulate CD4+ T cells to transcribe IL-4, resulting in an amplified TH2 response (31). Increased surface expression of the Notch ligand Jagged 1 on human DC (29) or of Jagged 2 on murine DC (48) and decreased expression of the maturation marker CD40 (22) promote a TH2 phenotype as well. In contrast, pathogens that induce production of IL-12 by DC promote TH1 polarization and simultaneously inhibit IL-4 production (30).
The chemokine receptor CCR2 contributes to DC recruitment to inflamed tissues. This receptor is necessary for monocyte egress from bone marrow (40); hence, a lack of CCR2 results in reduced numbers of monocytes and monocyte-derived DC in infected tissues and lymph nodes (1, 20, 28, 37). In some infections and autoimmunity models, CCR2 is required to maintain a TH1 response (19, 33, 36, 38, 45). Diminished recruitment of monocyte-derived DC to lymph nodes in CCR2−/− mice immunized with ovalbumin (OVA)-incomplete Freund's adjuvant or influenza virus-infected mice results in decreased IL-12 and TH1 cytokine production (33). Impaired recruitment of DC to lungs of Cryptococcus neoformans-infected CCR2−/− mice is associated with a detrimental shift from a TH1 dominant response to a TH2 dominant response (34, 44, 45), and in H. capsulatum-infected CCR2−/− mice there is a similar decrease in the number of DC that correlates with elevated IL-4 levels and impaired immunity despite significant production of TH1 cytokines (43).
We investigated whether decreased recruitment of DC to lungs of H. capsulatum-infected CCR2−/− mice influences regulation of IL-4 production. Adoptive transfer of bone marrow-derived dendritic cells (BMDC) to CCR2−/− mice not exposed to yeast (Ag-free BMDC) did not suppress IL-4 transcription and exacerbated infection. However, transfer of H. capsulatum antigen-exposed BMDC (Ag-BMDC) suppressed IL-4 production. Expression of CCR2 or in vitro maturation of Ag-BMDC was not required for IL-4 regulation. We investigated the mechanism by which Ag-BMDC decreases IL-4 generation in lungs. Ag-BMDC expression of major histocompatibility complex class II (MHCII) was necessary for limiting IL-4 generation, suggesting that suppression of production was due to interaction with MHCII-restricted CD4+ T cells. Furthermore, depletion of CD4+ T cells beginning prior to infection reduced IL-4. The immunity-dampening effects of CD4+ T cells were demonstrated in CD4-depleted CCR2−/− mice that received Ag-BMDC. These mice contained an increased number of CD8+ T cells, which was associated with a decrease in the fungal burden and with survival.
MATERIALS AND METHODS
Mice.
Male C57BL/6, B6.SJL-Ptprc (CD45.1), and MHCII−/− mice and breeding pairs of CCR2−/− mice with a C57BL/6 background were purchased from Jackson Laboratories (Bar Harbor, ME). CCR2−/− mice were backcrossed for >9 generations. Animals were housed in isolator cages and were maintained by the Department of Laboratory Animal Medicine, University of Cincinnati, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. All animal experiments were performed in accordance with the Animal Welfare Act guidelines of the National Institutes of Health, and all protocols were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati.
Preparation of H. capsulatum and infection of mice.
H. capsulatum yeast strain G217B was grown for 72 h at 37°C as previously described (4). To produce infection in mice, animals were inoculated intranasally (i.n.) with 2 × 106 H. capsulatum yeast cells in ∼30 μl of Hanks balanced salt solution (HBSS) (Mediatech, Manassas, VA). To heat kill H. capsulatum, we incubated yeast cells at 60°C for 30 min. G217B expressing green fluorescent protein (GFP) was generated as described previously (10).
Generation of BMDC.
Bone marrow was isolated from the hind tibias and femurs of 5- to 6-week-old mice by flushing the bones with HBSS. Isolated cells were added at a concentration of 2 × 105 cells/ml to 50 ml of RPMI-1640 supplemented with 10% fetal bovine serum, 0.1% gentamicin sulfate, and 0.005% 2-mercaptoethanol. Cells were treated with 10 ng/ml of mouse granulocyte-Mφ colony-stimulating factor (GM-CSF) (Peprotech, Rocky Hill, NJ) to stimulate differentiation and were cultured at 37°C in the presence of 5% CO2. Bone marrow cells were fed using an additional 50 ml of medium and 10 ng/ml GM-CSF at days 3 and 6 after isolation. FMS-related tyrosine kinase 3 (FLT3)-expanded BMDC were obtained just like GM-CSF-derived BMDC, except that the cells were cultured with 300 ng/ml FLT3. At days 8 to 10, the nonadherent CD11c+ BMDC were enriched with CD11c+ microbeads (Miltenyi Biotec, Auburn, CA). The enriched cells were >80% CD11c+.
Adoptive transfer of BMDC.
BMDC from wild-type (WT) or CCR2−/− mice were isolated as described above. On days 7, 5, and 3 before infection 1 × 106 to 2 × 106 BMDC were administered i.n. in ∼30 μl of HBSS to CCR2−/− mice. For experiments in which CCR2−/− mice were given matured and yeast cell-exposed BMDC, the cells were treated with 5 μg/ml anti-CD40 (clone 3/23; BD Biosciences, San Jose, CA) and 1 μg/ml lipopolysaccharide (LPS) (23) to induce maturation plus heat-killed H. capsulatum at a ratio of yeast cells to DC of 8:1 for 4 h. As a control, DC were treated with LPS and anti-CD40 but were not exposed to yeast cells; these cells were considered mature. Immature DC were not exposed to LPS, anti-CD40, or yeast cells. For experiments in which H. capsulatum soluble cell wall membrane and cell wall (CW/M), heat shock protein 60 (HSP60), or ovalbumin (OVA) (Sigma-Aldrich, St. Louis, MO) was used as a source of antigen, the concentration used was 5 μg/ml. CW/M and HSP60 from H. capsulatum were prepared as previously described (17, 39); CW/M is composed principally of glycoproteins.
CD4+ T-cell depletion.
For experiments in which CD4+ T cells were depleted at the start of infection, mice were given 100 μg of anti-CD4 (clone GK1.5) in 500 μl HBSS at the time of infection and 3 days postinfection. Depletion prior to infection was accomplished by administering the monoclonal antibody (MAb) 7, 5, and 3 days prior to infection and at the time of infection. This treatment resulted in >95% depletion of CD4+ cells from wild-type and CCR2−/− mice when they were analyzed at day 7. The level of depletion at day 7 was independent of the day that treatment was initiated.
Organ culture for H. capsulatum.
Organs were homogenized in sterile HBSS and then serially diluted and plated onto Mycosel agar (Becton Dickinson) plates containing 5% sheep blood and 5% glucose. The plates were incubated at 30°C for 1 week. The limit of detection was 1 × 102 CFU.
RNA isolation and cDNA synthesis.
Total RNA was isolated from whole lungs of mice using TRIzol (Invitrogen, Carlsbad, CA). Oligo(dT)-primed cDNA was prepared by using the reverse transcriptase system (Promega, Madison, WI) according to the manufacturer's instructions.
Quantitative real-time PCR.
Quantitative real-time PCR for analysis of cytokine transcription was performed using TaqMan master mixture and primers obtained from Applied Biosystems (Foster City, CA). Samples were analyzed with an ABI Prism 7500 (Applied Biosystems). For IL-12, we assessed transcription of IL-12p35. In each experiment, the hypoxanthine phosphoribosyl transferase housekeeping gene was used as an internal control. The conditions used for amplification were 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min.
Measurement of IL-4 by ELISA.
Lungs were homogenized in 5 ml of HBSS and centrifuged. IL-4 was quantified by using an enzyme-linked immunosorbent assay (ELISA) kit, which was purchased from Endogen (Cambridge, MA).
Isolation of lung leukocytes.
Lungs were homogenized in HBSS using a gentleMACS dissociator (Miltenyi Biotec, Auburn, CA). The resulting preparation was filtered through 60-μm nylon mesh (Spectrum Laboratories Inc., Rancho Dominguez, CA) and washed with HBSS. Leukocytes were isolated by Lympholyte M (Cederlane Laboratories, Ontario, Canada) separation.
Flow cytometry.
The phenotype of cells from mouse lungs was determined by incubating lung leukocytes with the antibodies indicated below and CD16/32 to limit nonspecific binding. Leukocytes were stained at 4°C for 15 min in HBSS containing 1% bovine serum albumin (BSA) and 0.01% sodium azide. Cells were stained with combinations of the following antibodies: phycoerythrin-conjugated I-Ab CD40, CD80, CD86, and CD8; peridinin chlorophyll protein (PerCP)-conjugated CD11b, CD11c, and CD4; and allophycocyanin (APC)-conjugated CD45.1, CD11c, and CD3 from BD Biosciences (San Jose, CA). Cells were washed and resuspended in 1% paraformaldehyde to fix them. Experiments with appropriate isotype controls were performed in parallel. The analysis was performed utilizing a FACSCaliber (BD Biosciences) flow cytometer, and the results were analyzed with the FCS Express software.
Statistics.
Analysis of variance (ANOVA) with the Tukey test was used to compare multiple groups, while Student's t test was used to compare two groups. Survival was analyzed using log rank. A P value of <0.05 was considered statistically significant.
RESULTS
IL-4 transcription in lungs of H. capsulatum-infected mice subsequent to adoptive transfer of BMDC.
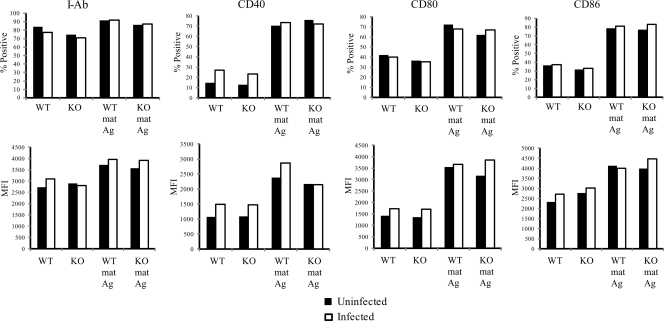
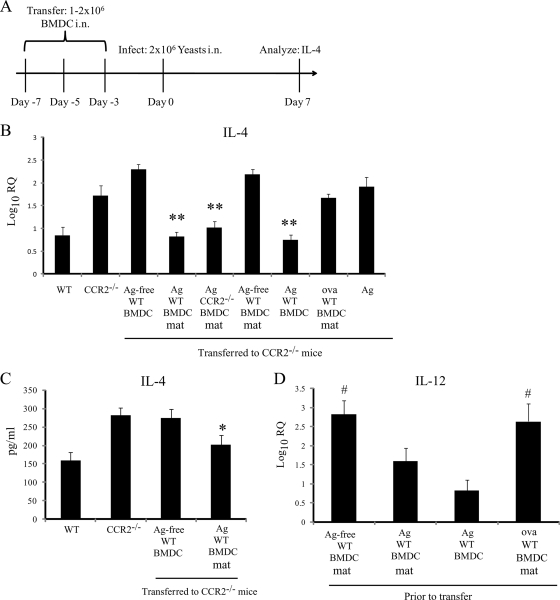
Our previous data demonstrated that an elevated IL-4 level in lungs of H. capsulatum-infected mice deficient in CCR2 or CCL7 and CCL2 impairs fungal clearance (43). An increase in the IL-4 level in these mice was associated with a profound decrease in the proportion of conventional DC (CD8− cDC) in the lungs. In contrast, IL-4 production, the percentage of CD8− cDC in lungs, and survival were not affected by the absence of CCL2, suggesting that recruitment of DC is important for regulation of IL-4 and resolution of infection. To determine if an increase in the number of DC in the lungs could influence IL-4 generation, we adoptively transferred BMDC loaded with yeast cells (Ag-BMDC) i.n. to CCR2−/− mice prior to infection. Ag-BMDC were fully matured in vitro before they were transferred with anti-CD40 and LPS to promote generation of proinflammatory cytokine-producing BMDC capable of restricting IL-4 production, as described previously (48). These cells exhibited upregulation of I-Ab, CD40, CD80, and CD86 (Fig. 1). The chronology of the transfer of DC is shown in Fig. 2A. Transfer of mature wild-type (WT) Ag-BMDC (treated with LPS and anti-CD40), but not transfer of immature Ag-free BMDC (not exposed to LPS and anti-CD40), suppressed IL-4 transcription in lungs (Fig. 2B). CCR2−/− Ag-BMDC also decreased the IL-4 level in the lungs when they were was transferred into CCR2−/− mice (Fig. 2B). As a control, we assessed the ability of Ag alone to limit IL-4 production and found that exposure to heat-killed H. capsulatum without BMDC had no effect (Fig. 2B). We confirmed that transfer of WT Ag-BMDC decreased IL-4 protein levels and suppressed transcription (Fig. 2C).
FIG. 1.
Expression of costimulatory molecules on the surface of BMDC. WT or CCR2−/− BMDC were not treated or exposed to LPS, anti-CD40, and heat-killed H. capsulatum for 4 h. Subsequently, a portion of the BMDC were infected with viable H. capsulatum yeast cells at a ratio of yeast cells to BMDC of 2:1. mat, matured (treated with LPS and anti-CD40); Ag, heat-killed H. capsulatum; KO, knockout. The data are data from one of three similar experiments.
FIG. 2.
Adoptive transfer of Ag-BMDC to CCR2−/− mice suppresses IL-4 production. (A) Schematic diagram of adoptive transfer experiments. (B) Log10 relative quantification (RQ) of IL-4 transcription from whole lungs 7 days postinfection compared to uninfected lung expression. Prior to infection BMDC were not treated (Ag-free) or were matured (mat) and exposed to heat-killed H. capsulatum (Ag) or ovalbumin (ova) (n = 6 to 9). (C) Levels of IL-4 protein in whole-lung homogenates as determined by ELISA (n = 6). (D) IL-12 transcription by WT BMDC prior to transfer compared to IL-12 transcription by untreated WT BMDC. **, P = 0.002 compared to CCR2−/−; *, P < 0.05 compared to CCR2−/−; #, P < 0.05 compared to Ag-BMDC that were not matured. The data are the means and SEM of two or three experiments.
We questioned if transfer of Ag-BMDC could reduce the IL-4 level in WT mice, which have relatively low levels of IL-4 compared to CCR2−/− mice. The transcription of IL-4 7 days postinfection in the lungs of WT mice that received Ag-BMDC was decreased (P < 0.05) compared to the transcription in WT mice or WT mice that received Ag-free BMDC (Table 1).
TABLE 1.
Ag-BMDC reduce the IL-4 level in wild-type mice infected with H. capsulatum for 7 days
| Cells transferred | IL-4 level (log10 RQ)a |
|---|---|
| None | 1.07 ± 0.10 |
| Ag-BMDC | 0.56 ± 0.05b |
| Ag-free BMDC | 1.13 ± 0.16 |
The data are means ± SEM (n = 6) and are pooled data from two experiments. IL-4 transcription was normalized to transcription in uninfected lungs.
P < 0.05 compared to no cells or to mice that received Ag-free BMDC.
Requirements for stimulating donor bone marrow-derived cells to suppress IL-4.
We investigated how antigen exposure and maturation contributed to IL-4 regulation. Transfer of WT BMDC matured with LPS and anti-CD40 to CCR2−/− mice did not reduce (P > 0.05) IL-4 transcription in the lungs of these mice (mean log10 relative quantification [RQ] ± standard error of the mean [SEM], 2.18 ± 0.12; n = 3) compared to IL-4 transcription in CCR2−/− controls (mean log10 RQ ± SEM, 1.90 ± 0.19; n = 6). In contrast, Ag-BMDC not exposed to LPS and anti-CD40 still limited IL-4 transcription (mean log10 RQ ± SEM, 0.80 ± 0.17; n = 6). To determine if BMDC had to be exposed specifically to H. capsulatum antigen, we treated BMDC with OVA (OVA-BMDC) prior to transfer. Inoculation of CCR2−/− mice with OVA-BMDC did not reduce the IL-4 level (Fig. 2B). We questioned whether BMDC differentiated with FLT3 could also limit IL-4 transcription since these BMDC have a different phenotype than GM-CSF-expanded BMDC (12). Transfer of these Ag-BMDC reduced (P < 0.05) IL-4 transcription in CCR2−/− mice compared to the transcription in untreated CCR2−/− mice (Table 2).
TABLE 2.
Transfer of FLT3-generated DC into CCR2−/− mice modifies the IL-4 level, but not the fungal burden, at day 7 of infection
| Cells transferred | IL-4 level (log10 RQ)a | No. of CFU (log10)a |
|---|---|---|
| None | 2.23 ± 0.07 | 6.73 ± 0.18 |
| Ag-BMDC | 0.80 ± 0.17b | 6.49 ± 0.11c |
The data are means ± SEM for 6 to 8 mice per group and are pooled data from two independent experiments. IL-4 transcription was normalized to the transcription in uninfected lungs.
P < 0.05.
P > 0.05.
We asked if the H. capsulatum CW/M (17) could mimic the effect of heat-killed yeast cells. BMDC exposed to CW/M for 24 h prior to transfer suppressed (P < 0.05) IL-4 transcription in lungs of CCR2−/− mice (mean log10 RQ ± SEM, 0.95 ± 0.10; n = 6) compared to the IL-4 transcription in lungs of CCR2−/− mice that did not receive BMDC (mean log10 RQ ± SEM, 1.72 ± 0.22; n = 6). We postulated that the immunodominant H. capsulatum antigen HSP60 (39) may be responsible for IL-4 regulation; however, BMDC exposed to HSP60 alone did not limit IL-4 generation in lungs of CCR2−/− mice (mean log10 RQ ± SEM, 1.50 ± 0.18; n = 6).
IL-12 production by DC is associated with a decrease in IL-4 generation (24, 26, 30); therefore, IL-4 regulation in CCR2−/− mice that receive BMDC could be a result of IL-12 production rather than DC-mediated Ag presentation. To address this possibility, we measured IL-12 transcription by BMDC prior to transfer. Mature OVA-BMDC and mature untreated BMDC that did not reduce the level of IL-4 in vivo actually transcribed the largest amounts of IL-12 (Fig. 2D).
Distinguishing endogenous and donor BMDC in lungs.
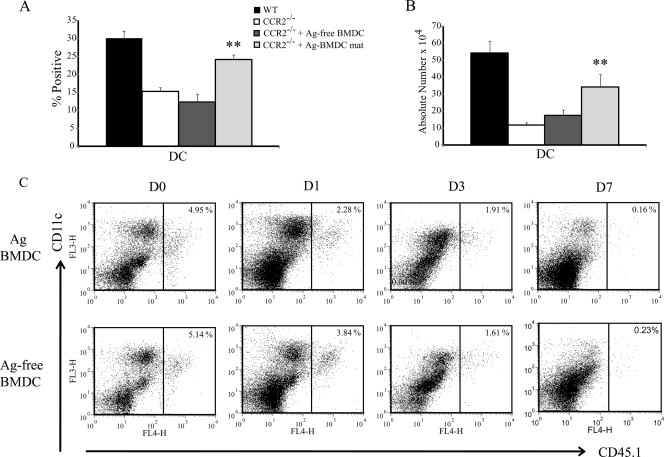
An increase in the number of DC in the lungs of adoptively transferred CCR2−/− mice correlated with a reduction in the IL-4 level. CCR2−/− mice that received WT Ag-BMDC exhibited increases in the relative percentage and absolute number of lung DC 7 days postinfection, whereas transfer of WT Ag-free BMDC did not increase the number of DC beyond the number in CCR2−/− controls (Fig. 3A and 3B). We asked if the increased number of lung DC were donor Ag-BMDC or host lung DC. The percentage of transferred CD45.1 Ag-BMDC or CD45.1 Ag-free BMDC detected in lungs was greatest prior to infection. Between days 1 and 7 postinfection the percentage of BMDC transferred to other leukocytes was reduced. At day 7, the donor Ag-BMDC in lungs represented less than 0.5% of the total lung leukocytes (Fig. 3C).
FIG. 3.
IL-4 regulation is associated with an increase in the number of lung DC. (A) Percentage of lung CD11c+ CD11b+ I-Abhi DC 7 days postinfection (n = 8 or 9). mat, matured (treated with LPS and anti-CD40). (B) Absolute number of lung DC at day 7 (n = 8 or 9). **, P < 0.001 compared to CCR2−/−. The data represent the means and SEM of two or three experiments. (C) Representative FACS plots of isolated lung leukocytes stained with CD11c and CD45.1 to identify transferred donor Ag-BMDC and Ag-free BMDC prior to infection (D0) and at days 1 (D1), 3 (D3), and 7 (D7) postinfection. CD45.1 transfer experiments were performed two times with three or four mice per group.
Phenotype of endogenous CCR2−/− lung DC after H. capsulatum infection.
We hypothesized that the lung DC in CCR2−/− mice may differ functionally from the DC present in lungs of WT mice, thus exaggerating the dysregulation of IL-4. Our previous studies revealed that MHCII expression is decreased on lung CD8− cDC from infected-CCR2−/− mice; thus, there is at least one phenotypic difference (43). Utilizing GFP-expressing H. capsulatum, we examined the percentage of infected DC expressing CD40 in lungs of CCR2−/− mice. Decreased expression of CD40 can promote TH2 polarization upon antigen-dependent engagement of CD4+ T cells (22). The percentage of GFP+ DC that were CD40+ in lungs of CCR2−/− mice was decreased (P < 0.05) at days 1 and 3 but not at day 7 compared to the percentage in lungs of WT mice (Table 3).
TABLE 3.
Percentage of GFP+ DC that express CD40 in infected wild-type and CCR2−/− mice
| Mice | % of GFP+ DC that express CD40 ata: |
||
|---|---|---|---|
| Day 1 | Day 3 | Day 7 | |
| Wild type | 53.5 ± 3.4 | 87.3 ± 3.7 | 98.1 ± 1.2 |
| CCR2−/− | 21.5 ± 1.2b | 61.1 ± 3.9b | 94.4 ± 1.7 |
The data are means ± SEM for 7 to 8 mice and are pooled data from at least two independent experiments.
P < 0.05 compared to the wild type.
Fungal burden in CCR2−/− mice immunized with BMDC.
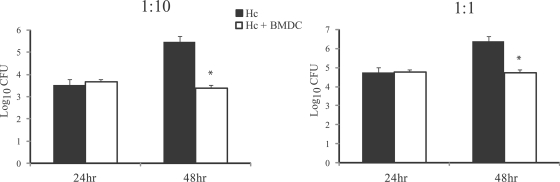
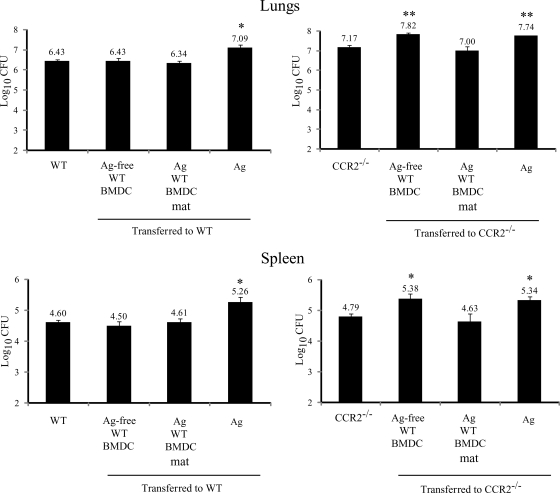
Human DC are capable of degrading H. capsulatum yeast cells in vitro (15). This led us to hypothesize that transfer of BMDC may improve the clearance of H. capsulatum in mice. Like human DC, BMDC restricted the growth of H. capsulatum in vitro (Fig. 4). Transfer of Ag-free BMDC had no effect in WT mice and increased the fungal burden in the lungs and spleens of CCR2−/− mice (Fig. 5). Unexpectedly, addition of Ag-BMDC did not decrease the fungal burden in WT or CCR2−/− mice (Fig. 5) despite lower IL-4 levels. Transfer of FLT3-derived Ag-BMDC did not reduce (P > 0.05) the fungal burden in the lungs compared to the fungal burden in the lungs of CCR2−/− mice (Table 2). We addressed the possibility that exposure to heat-killed H. capsulatum had a deleterious effect that negated any Ag-BMDC-mediated yeast clearance since heat-killed yeast cells suppress Mφ (32) and DC IL-12 generation (Fig. 5). Exposure to heat-killed yeast cells alone prior to infection impaired the clearance of H. capsulatum in WT and CCR2−/− mice (Fig. 5).
FIG. 4.
BMDC kill H. capsulatum in vitro. BMDC (105 cells) were infected at a ratio of yeast cells to BMDC of 1:10 or 1:1. After 24 and 48 h, samples were plated on medium to enumerate the viable organisms. The data are the means and SEM of three experiments. *, P < 0.05. Hc, H. capsulatum.
FIG. 5.
Transfer of Ag-free BMDC impairs yeast clearance. The fungal burdens in lungs and spleens were determined 7 days postinfection (n = 6). **, P < 0.001; *, P < 0.005. The data are the means and SEM of two experiments. mat, matured (treated with LPS and anti-CD40).
Analysis of infected populations in CCR2−/− mice that received BMDC.
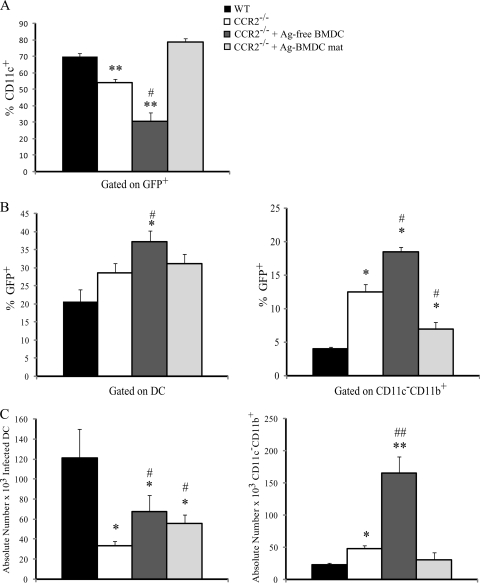
We investigated how the absence of CCR2 and adoptive transfer of BMDC affected the distribution of yeast cells among phagocytes since this could help identify populations that provide a reservoir for yeast growth. At day 7 postinfection the majority of GFP+ H. capsulatum cells were associated with DC in the lungs of WT or CCR2−/− mice that received Ag-BMDC, whereas in CCR2−/− mice the proportion of yeast cells within DC was decreased (Fig. 6A). In CCR2−/− mice inoculated with Ag-free BMDC there was a further reduction in the percentage of yeast cells within DC (Fig. 6A). We next examined the percentage of DC and CD11c− CD11b+ cells that were infected in the lungs. This population was comprised of CD11c− CD11b+ Ly6Ghigh neutrophils and CD11c− CD11b+ Mac3+ tissue Mφ (43). We found that the percentage of DC associated with yeast cells was increased in the lungs of CCR2−/− mice immunized with Ag-free BMDC compared to the lungs of WT and CCR2−/− mice (Fig. 6B). The percentage of infected CD11c− CD11b+ phagocytes was increased in CCR2−/− mice compared to WT mice and was increased further in CCR2−/− mice that received Ag-free BMDC (Fig. 6B). Additionally, a remarkable increase in the number of infected CD11c− CD11b+ phagocytes was observed in CCR2−/− mice that received Ag-free BMDC compared to WT or CCR2−/− mice (Fig. 4C). Both CCR2−/− mice immunized with Ag-BMDC and CCR2−/− mice immunized with Ag-free BMDC exhibited an increase in the absolute number of infected DC compared to CCR2−/− mice but not compared to WT mice (Fig. 6C).
FIG. 6.
Transfer of Ag-free BMDC to CCR2−/− mice results in altered distribution of yeast cells within phagocyte populations. Flow cytometry was performed to determine the relative percentage of lung leukocytes infected with GFP-labeled H. capsulatum 7 days postinfection (A), the percentage of CD11c+ CD11b+ I-Abhigh lung DC infected with GFP-labeled H. capsulatum (B), and the percentage of GFP-labeled H. capsulatum distributed in DC 7 days postinfection (C) (n = 8 or 9). **, P < 0.001 compared to WT mice; *, P < 0.05 compared to WT mice; ##, P < 0.001 compared to CCR2−/− mice; #, P < 0.05 compared to CCR2−/− mice. The data are the means and SEM of three experiments. mat, matured (treated with LPS and anti-CD40).
Although fluorescence-activated cell sorting (FACS) analysis allowed us to examine the number and types of infected cells, the number of yeast cells per cell could be different in different infected populations, indicating cells in which yeast degradation may have been impaired. To determine the number of yeast cells per cell, we sorted GFP+ phagocytes from lungs of CCR2−/− and WT mice 7 days postinfection. Analysis of the sorted samples revealed that DC and CD11c− Mac3+ tissue Mφ from CCR2−/− mice contained a higher (P < 0.05) number of yeast cells per cell than the corresponding WT cell populations contained, whereas the numbers of yeast cells in CD11c+ I-Abint alveolar Mφ and neutrophils were similar (P > 0.05) (Table 4).
TABLE 4.
Number of yeast cells per phagocyte at day 7 of infection
| Source of infected cells | No. of GFP+ yeast cells/phagocytea |
|||
|---|---|---|---|---|
| DCb | Tissue Mφb | Alveolar Mφb | Neutrophilsb | |
| WT | 2.08 ± 0.14 | 1.90 ± 0.18 | 3.06 ± 0.25 | 1.48 ± 0.08 |
| CCR2−/− | 3.19 ± 0.18c | 4.11 ± 0.57c | 3.00 ± 0.19d | 1.63 ± 0.10d |
The data are means ± SEM for at least 100 cells counted. The experiment was performed twice.
DC were CD11c+ CD11b+ I-Abhigh; tissue Mφ were CD11c− Mac-3+; alveolar Mφ were CD11c+ I-Abintermediate; and neutrophils were CD11c− CD11b+ Ly6-Ghigh.
P < 0.05.
P > 0.05.
Cytokine production in lungs of BMDC-immunized CCR2−/− mice.
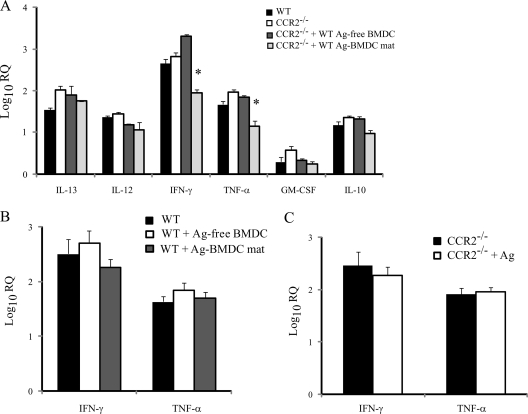
We assessed proinflammatory cytokine production in lungs of mice that received BMDC since a reduction in this production could contribute to yeast persistence. Transfer of Ag-BMDC resulted in decreased levels of TNF-α and IFN-γ in the lungs of CCR2−/− mice compared to the lungs of untreated WT or CCR2−/− mice at 7 days postinfection (Fig. 7A). Transfer of Ag-free BMDC to CCR2−/− mice did not alter generation of these cytokines (Fig. 7A). Transcription of IL-10, which is deleterious to host resistance (9), was not modulated in immunized mice, and despite the ability of Ag-BMDC to suppress IL-4 transcription, transcription of the TH2 cytokine IL-13 was not reduced in the lungs of CCR2−/− mice that received Ag-BMDC (Fig. 7A).
FIG. 7.
Transfer of Ag-BMDC suppresses IFN-γ and TNF-α production. The data indicate the log10 RQ cytokine transcription 7 days postinfection in lungs of CCR2−/− mice immunized with BMDC (A), WT mice immunized with BMDC (B), and CCR2−/− mice immunized with Ag (C) (n = 6). *, P < 0.01. The data are the means and SEM of two experiments. mat, matured (treated with LPS and anti-CD40).
We examined TNF-α and IFN-γ levels in WT mice given Ag-BMDC to ascertain if a decrease in the TH1 cytokine response was attributable to the absence of CCR2. The TNF-α and IFN-γ levels were not decreased in the lungs of WT mice that received Ag-BMDC or Ag-free BMDC (Fig. 7B). To determine if decreased TNF-α or IFN-γ levels were an effect of exposure to heat-killed H. capsulatum Ag, we measured cytokine production in Ag-immunized mice that did not receive BMDC. Ag alone did not decrease cytokine production in CCR2−/− mice (Fig. 7C).
DC interaction with CD4+ T cells is critical for reducing the IL-4 level.
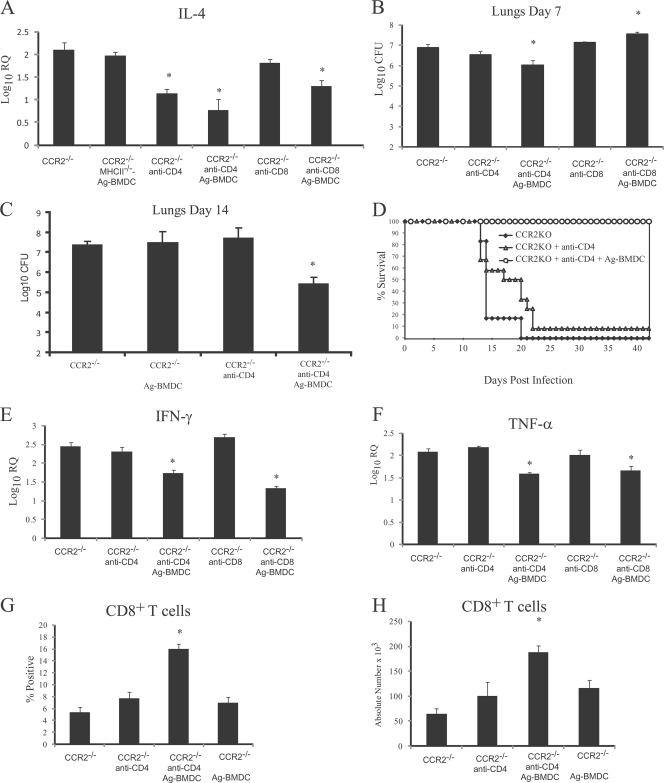
DC interaction with CD4+ T cells through MHCII antigen binding to the T-cell receptor (TCR) activates T cells and regulates cytokine production (21, 30, 33). To determine if DC-mediated regulation of IL-4 was dependent on MHCII antigen presentation, we transferred MHCII−/− Ag-BMDC into CCR2−/− mice. Ag-BMDC lacking MHCII did not suppress IL-4 production (Fig. 8A). We then asked if DC interaction with CD4+ T cells was necessary for restriction of IL-4 production. We previously demonstrated that CD4+ T cells from CCR2−/− mice transcribe more IL-4 than WT CD4+ T cells transcribe after H. capsulatum infection (43). CD4+ T cell depletion in CCR2−/− mice starting at the time of infection did not suppress (P > 0.05) IL-4 transcription in the lungs (mean log10 RQ ± SEM for rat IgG-treated CCR2−/− mice, 2.21 ± 0.33; mean log10 RQ ± SEM for CD4-depleted CCR2−/− mice, 1.97 ± 0.06) or, as demonstrated previously, decrease the fungal burden (43). However, if depletion was started prior to infection, the IL-4 level was significantly reduced (Fig. 8A). Subsequently, we examined how quickly CD4+ T cells are depleted from lungs after MAb treatment in CCR2−/− mice. After 24 h, the number of CD4+ T cells was reduced by ∼50%, whereas by 48 h the number of CD4+ T cells was reduced by >90% (n = 4).
FIG. 8.
Depletion of CD4+ T cells, but not depletion of CD8+ T cells, prior to infection in CCR2−/− mice that received Ag-BMDC suppressed IL-4 production and decreased the fungal burden. (A) Log10 IL-4 transcription in lungs compared to transcription in uninfected lungs 7 days postinfection (n = 6). (B and C) Fungal burden in lungs at 7 days (n = 6) and 14 days (n = 8) postinfection. (D) Survival after infection with 2 × 106 yeast cells (n = 12, except for CCR2−/− [n = 6]). (E and F) Transcription of IFN-γ and TNF-α in mice (n = 8 to 10). (G and H) Percentages and absolute numbers of CD8+ T cells 7 days postinfection (n = 6). *, P < 0.01 compared to CCR2−/− mice. The data are the means and SEM of two experiments.
Although CD4 depletion prior to infection reduced the IL-4 level in CCR2−/− mice, the fungal burden was not significantly reduced (Fig. 8B and 8C). Conversely, addition of Ag-BMDC to CCR2−/− mice in which CD4+ T cells were depleted prior to infection reduced the IL-4 level and the fungal burden in the lungs at days 7 and 14 compared to the results for untreated CCR2−/− mice (Fig. 8A to 8C), and all mice survived (Fig. 8D). At day 42 postinfection the fungal burden in the lungs was sharply reduced to 3.39 ± 0.1 log10 CFU (n = 6). As a control, we investigated if an interaction between DC and CD8+ T cells regulated IL-4 production. Depletion of CD8+ T cells in CCR2−/− mice did not reduce the level of IL-4, whereas in CD8-depleted CCR2−/− mice that received Ag-BMDC the level of IL-4 was reduced compared to the level in untreated CCR2−/− mice (Fig. 8A). The fungal burden was elevated in CD8-depleted CCR2−/− mice immunized with Ag-BMDC compared to the fungal burden in CCR2−/− mice (Fig. 8B).
CD4+ T cells are critical for resolution of H. capsulatum infection (6); thus, the finding that fungal clearance was increased in CD4-depleted CCR2−/− mice injected with Ag-BMDC was not expected. We investigated the mechanisms responsible for improved immunity. Transcription of IFN-γ and TNF-α was reduced in CD4-depleted CCR2−/− mice that received Ag-BMDC compared to CCR2−/− mice (Fig. 8E and 8F), but a decrease in the fungal burden was associated with increases in the proportion and number of CD8+ T cells in the lungs (Fig. 8G and 8H). WT mice that received Ag-BMDC in which CD4+ T cells were depleted prior to infection exhibited an increase in the number of CD8+ T cells in the lungs, but the difference was not statistically significant (P > 0.05) when the data were compared to data for untreated WT or CD4-depleted WT mice on day 7 postinfection (data not shown). In WT mice in which CD4+ T cells were depleted, as well as CD4-depleted WT mice that received Ag-BMDC, there was reduced (P < 0.05) IL-4 transcription in the lungs at day 7 compared to the transcription in the lungs of the WT mice (Table 5). The fungal burdens in the lungs of these groups were similar (P > 0.05) to the fungal burden in the lungs of untreated WT mice (Table 5).
TABLE 5.
IL-4 transcription and numbers of CFU in wild-type mice depleted of CD4+ cells and given Ag-BMDC
| Group | IL-4 level (log10 RQ)a | Log10 CFUa |
|---|---|---|
| Wild-type + rat IgG | 0.94 ± 0.10 | 6.17 ± 0.12 |
| Wild-type + anti-CD4 | −0.43 ± 0.11b | 6.32 ± 0.02c |
| Wild-type + anti-CD4 + Ag-BMDC | −0.77 ± 0.21b | 6.44 ± 0.07c |
The data are means ± SEM for four mice. Transcription of IL-4 was normalized to the transcription in uninfected lungs.
P < 0.05 compared to controls.
P > 0.05 compared to controls.
DISCUSSION
In this study, we demonstrated that the level of IL-4 in the lungs of H. capsulatum-infected CCR2−/− mice was reduced sharply by adoptive transfer of H. capsulatum-exposed DC. However, transfer of these cells did not improve fungal clearance. The failure to improve fungal elimination was associated with slightly diminished TH1 cytokine production, which may have contributed to the inability to reduce the fungal burden despite reduced IL-4 levels. In contrast, transfer of Ag-free BMDC actually increased the IL-4 level and exacerbated infection in CCR2−/− mice. The increased fungal burden in CCR2−/− mice that received Ag-free BMDC was associated with a higher number of infected CD11c− CD11b+ phagocytes. Ag-BMDC-mediated regulation of IL-4 was dependent on MHCII antigen presentation to CD4+ T cells. Accelerated fungal clearance in CCR2−/− mice was observed only for mice that received Ag-BMDC and in which CD4+ T cells were depleted prior to infection. These results indicate that in CCR2−/− mice, this population of T cells inhibits protective immunity.
The absence of CCR2 decreases recruitment of DC to infected tissues in mice exposed to H. capsulatum or other organisms (20, 33, 34, 43, 45). In lungs of CCR2−/− mice infected with C. neoformans or H. capsulatum, elevated IL-4 levels accompany decreased DC infiltration. These results imply that the number of DC at the site of infection is important for cytokine regulation (34, 43, 45). Suppression of IL-4 production required an increase in the number of BMDC that bore antigen. These results suggest that antigen presentation must occur for IL-4 regulation to occur. The failure of Ag-free BMDC, OVA-BMDC, or MHCII−/− Ag-BMDC to limit IL-4 production supports this contention.
One obvious possible explanation for the decrease in the IL-4 level is that IL-12 generation was elevated in Ag-BMDC. This possibility is unlikely since OVA-BMDC and Ag-free BMDC produced the same amount of IL-12 or more IL-12, yet the level of IL-4 was not decreased. Moreover, transfer of Ag-BMDC did not increase the level of IL-12 in vivo (Fig. 5). Collectively, the data support the contention that decreased recruitment of DC capable of antigen presentation promotes type 2 immunity in CCR2−/− mice.
The ability of BMDC to limit the IL-4 level was dependent on the presence of exposure to antigen. We principally employed heat-killed H. capsulatum yeast cells as a source of antigen to a priori provide the broadest array of antigens to DC. We heat killed organisms at 60°C for 30 min, and it is unlikely that this treatment altered the structure of antigenic peptides that were potentially presented. Interestingly, our crude extract, CW/M, which was largely composed of glycoproteins (17), mimicked the effect of heat-killed yeast cells, but the protective antigen HSP60 did not. These results indicate that although HSP60 is useful as a vaccine, it does not drive the suppression of IL-4 production by Ag-pulsed DC.
IL-4 regulation by Ag-BMDC was associated with an increase in the number of lung DC at the peak of infection. Donor Ag-BMDC constituted only a small proportion of these cells at this time. The fact that host DC were more prominent may have been a result of inefficient transfer or cell death of donor Ag-BMDC before day 7. Alternatively, yeast-laden DC may have trafficked to lymph nodes. Indeed, donor CD45.1+ DC were detected in mediastinal lymph nodes by day 3 postinfection (data not shown). We previously demonstrated that IL-4 neutralization in CCR2−/− mice does not alter cell recruitment (43); therefore, the increased number of DC in lungs of CCR2−/− mice inoculated with Ag-BMDC cannot be attributed strictly to reduced IL-4 production. However, decreased TNF-α production in lungs of these mice may promote DC survival since this cytokine induces apoptosis during H. capsulatum infection (2). An additional possibility is that the increased number of CD8+ T cells affects the number of DC. Some evidence suggests that T cell interaction with mature, Ag-presenting DC is critical for preventing DC death (11).
Expression of CCR2 on Ag-BMDC was not required for limiting IL-4 generation. Previous in vitro studies indicated that CCR2+ DC produce more IL-4 than their CCR2− counterparts and induce T cells to generate more IL-4 (13). Our findings unequivocally demonstrate that CCR2 expression was dispensable for IL-4 regulation. The data support the hypothesis that CCR2 regulation of IL-4 production is mediated indirectly through recruited DC.
DC induce IL-4 production by CD4+ T cells and NK cells (24, 29, 42, 48). DC in lungs of infected CCR2−/− mice exhibited a phenotype consistent with TH2 cytokine instruction. A decreased proportion of CD8− cDC expressed CD40, a costimulatory molecule that restricts IL-4 production by CD4+ T cells (22). In addition, DC from CCR2−/− mice bear less MHCII than control DC (43). Taken together, these alterations may contribute to increased IL-4 production by CD4+ cells (24). The increase in IL-4 production in infected CCR2−/− mice is also attributable to greater production by CD8− cDC than by WT DC (43). The significance of this finding is that excess IL-4 amplifies signaling via IL-4Rα to promote DC-mediated TH2 polarization (47).
Inoculation with Ag-BMDC limited IL-4 production without promoting the TH1 immune response in WT and CCR2−/− mice. This finding differs from the results for Leishmania major infection, in which transfer of mature, Ag-specific BMDC to BALB/c mice decreased IL-4 production, increased IFN-γ production, and improved infection (48). Surprisingly, the levels of IFN-γ and TNF-α, two cytokines critical for resolution of H. capsulatum infection (3, 51), were reduced in CCR2−/− recipients of Ag-BMDC but not in WT recipients of Ag-BMDC. The decreases in the levels of these two pivotal cytokines could explain, in part, why the fungal burden was not reduced in CCR2−/− mice despite a reduction in IL-4 production. The decreases in the TNF-α and IFN-γ levels were not caused by T cell-mediated suppression. Both CD4-depleted and CD8-depleted CCR2−/− mice that received Ag-BMDC exhibited similar reductions in these cytokines.
The higher fungal burden in CCR2−/− mice that received Ag-free BMDC implies that an elevated IL-4 level creates an environment permissive for yeast growth. Uncontrolled yeast replication in untreated CCR2−/− mice and CCR2−/− mice that received Ag-free BMDC was associated with an increase in the number of infected CD11c− CD11b+ phagocytes compared to the number in WT mice. This population contains CD11c− Mac3+ tissue Mφ that in CCR2−/− mice harbor more yeast cells per Mφ than the corresponding WT population. The increase in the yeast cell burden in Mφ from infected CCR2−/− mice was attributable to the presence of alternatively activated Mφ (43).
Similar to the findings for H. capsulatum, transfer of immature BMDC to C. neoformans-infected mice increased the fungal burden (18), demonstrating that donor BMDC can dampen immunity; however, H. capsulatum infection differs from C. neoformans infection in that transfer of Ag-free BMDC, but not transfer of Ag-BMDC, to CCR2−/− mice resulted in a worse condition. Other differences between these two fungi should be stressed. C. neoformans resides extracellularly as well as intracellularly, it is larger, and it secretes an immunomodulatory capsule. Unlike the findings for transfer of Ag-exposed BMDC to C. neoformans-infected mice, a further increase in the IL-4 level was not observed in lungs and therefore cannot account for the higher fungal burden. Possibly, Ag-free BMDC provide a reservoir for yeast replication early in infection in an environment where there is excess IL-4. In CCR2−/− mice there was an increase in the number of yeast cells per DC, suggesting that DC can promote yeast survival when the IL-4 level is elevated.
CD4+ T cells, as well as phagocytes, contribute to elevated IL-4 levels in lungs of CCR2−/− mice at the peak of infection (43). Our data described here provide evidence that CD4+ T cell production of IL-4 early in infection promotes production at later time points. IL-4 levels were reduced in CCR2−/− mice by depletion of CD4+ T cells prior to infection but not at the time of infection. Complete elimination of CD4+ T cells required 48 h following MAb administration. Thus, mice treated at the time of infection still have IL-4-producing CD4+ T cells that amplify the type 2 response early after exposure to the fungal pathogen. The timing of CD4+ T cell elimination was critical. Within the narrow 48-h window there was sufficient stimulus to propagate and maintain the type 2 immune response.
CD4+ T cells are required for resolution of primary H. capsulatum infection in WT mice (6); thus, it was found that Ag-BMDC reduced the fungal burden in CCR2−/− mice devoid of CD4+ cells. The increase in the number of CD8+ T cells in the lungs of these mice correlated with control of yeast replication. Although CD8+ T cells are dispensable for protective immunity in immunocompetent mice, they can be activated independent of CD4+ cells and can confer protection in MHCII−/− mice and in a vaccination model (27, 49). In the absence of an inhibitory CD4+ cell population, the interaction between Ag-BMDC and CD8+ T cells promotes activation of the latter cells and accelerated clearance of the fungus.
In conclusion, addition of Ag-presenting BMDC can suppress excessive production of IL-4 by CD4+ T cells in lungs of CCR2−/− mice infected with H. capsulatum; thus, reduced recruitment of DC to the lungs at least partially impairs IL-4 regulation. Addition of Ag-BMDC in conjunction with the loss of CD4+ T cells improved immunity, demonstrating that CD4+ T cells can inhibit the resolution of infection, possibly by suppressing DC-dependent CD8+ T cell functions. These findings highlight the opposing roles of CD4+ T cells in immunity to H. capsulatum infection and demonstrate the pivotal functions of DC in regulation of IL-4 and immunity.
Acknowledgments
We thank Reta Gibbons, Michael Winters, and Danielle Kroetz for their assistance with preparation of the manuscript.
This work was supported by a merit review grant from the Department of Veterans Affairs and by grants AI-073337 and AI-083313 from the National Institutes of Health.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 1 March 2010.
REFERENCES
- 1.Aldridge, J. R., Jr., C. E. Moseley, D. A. Boltz, N. J. Negovetich, C. Reynolds, J. Franks, S. A. Brown, P. C. Doherty, R. G. Webster, and P. G. Thomas. 2009. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc. Natl. Acad. Sci. U. S. A. 106:5306-5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, H. L., and G. S. Deepe, Jr. 2005. Apoptosis modulates protective immunity to the pathogenic fungus Histoplasma capsulatum. J. Clin. Invest. 115:2875-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allendoerfer, R., and G. S. Deepe, Jr. 1998. Blockade of endogenous TNF-α exacerbates primary and secondary pulmonary histoplasmosis by differential mechanisms. J. Immunol. 160:6072-6082. [PubMed] [Google Scholar]
- 4.Allendoerfer, R., and G. S. Deepe, Jr. 1997. Intrapulmonary response to Histoplasma capsulatum in gamma interferon knockout mice. Infect. Immun. 65:2564-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allendoerfer, R., and G. S. Deepe, Jr. 2000. Regulation of infection with Histoplasma capsulatum by TNFR1 and -2. J. Immunol. 165:2657-2664. [DOI] [PubMed] [Google Scholar]
- 6.Allendorfer, R., G. D. Brunner, and G. S. Deepe, Jr. 1999. Complex requirements for nascent and memory immunity in pulmonary histoplasmosis. J. Immunol. 162:7389-7396. [PubMed] [Google Scholar]
- 7.Brummer, E., and D. A. Stevens. 1995. Antifungal mechanisms of activated murine bronchoalveolar or peritoneal macrophages for Histoplasma capsulatum. Clin. Exp. Immunol. 102:65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deepe, G. S., Jr., R. Gibbons, and E. Woodward. 1999. Neutralization of endogenous granulocyte-macrophage colony-stimulating factor subverts the protective immune response to Histoplasma capsulatum. J. Immunol. 163:4985-4993. [PubMed] [Google Scholar]
- 9.Deepe, G. S., Jr., and R. S. Gibbons. 2003. Protective and memory immunity to Histoplasma capsulatum in the absence of IL-10. J. Immunol. 171:5353-5362. [DOI] [PubMed] [Google Scholar]
- 10.Deepe, G. S., Jr., R. S. Gibbons, and A. G. Smulian. 2008. Histoplasma capsulatum manifests preferential invasion of phagocytic subpopulations in murine lungs. J. Leukoc. Biol. 84:669-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Smedt, T., B. Pajak, G. G. Klaus, R. J. Noelle, J. Urbain, O. Leo, and M. Moser. 1998. Antigen-specific T lymphocytes regulate lipopolysaccharide-induced apoptosis of dendritic cells in vivo. J. Immunol. 161:4476-4479. [PubMed] [Google Scholar]
- 12.Dunne, P. J., B. Moran, R. C. Cummins, and K. H. Mills. 2009. CD11c+ CD8α+ dendritic cells promote protective immunity to respiratory infection with Bordetella pertussis. J. Immunol. 183:400-410. [DOI] [PubMed] [Google Scholar]
- 13.Fiorina, P., M. Jurewicz, A. Vergani, A. Augello, J. Paez, V. Ricchiuti, V. Tchipachvili, M. H. Sayegh, and R. Abdi. 2008. Phenotypic and functional differences between wild-type and CCR2−/− dendritic cells: implications for islet transplantation. Transplantation 85:1030-1038. [DOI] [PubMed] [Google Scholar]
- 14.Frank, K. M., D. K. Hogarth, J. L. Miller, S. Mandal, P. J. Mease, R. J. Samulski, G. A. Weisgerber, and J. Hart. 2009. Investigation of the cause of death in a gene-therapy trial. N. Engl. J. Med. 361:161-169. [DOI] [PubMed] [Google Scholar]
- 15.Gildea, L. A., G. M. Ciraolo, R. E. Morris, and S. L. Newman. 2005. Human dendritic cell activity against Histoplasma capsulatum is mediated via phagolysosomal fusion. Infect. Immun. 73:6803-6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gildea, L. A., R. Gibbons, F. D. Finkelman, and G. S. Deepe, Jr. 2003. Overexpression of interleukin-4 in lungs of mice impairs elimination of Histoplasma capsulatum. Infect. Immun. 71:3787-3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomez, A. M., J. C. Rhodes, and G. S. Deepe, Jr. 1991. Antigenicity and immunogenicity of an extract from the cell wall and cell membrane of Histoplasma capsulatum yeast cells. Infect. Immun. 59:330-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herring, A. C., N. R. Falkowski, G. H. Chen, R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2005. Transient neutralization of tumor necrosis factor alpha can produce a chronic fungal infection in an immunocompetent host: potential role of immature dendritic cells. Infect. Immun. 73:39-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izikson, L., R. S. Klein, I. F. Charo, H. L. Weiner, and A. D. Luster. 2000. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR) 2. J. Exp. Med. 192:1075-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia, T., N. V. Serbina, K. Brandl, M. X. Zhong, I. M. Leiner, I. F. Charo, and E. G. Pamer. 2008. Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. J. Immunol. 180:6846-6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kadowaki, N. 2007. Dendritic cells: a conductor of T cell differentiation. Allergol. Int. 56:193-199. [DOI] [PubMed] [Google Scholar]
- 22.Karimi, M. H., P. Ebadi, A. A. Pourfathollah, Z. S. Soheili, S. Samiee, Z. Ataee, S. Z. Tabei, and S. M. Moazzeni. 2009. Immune modulation through RNA interference-mediated silencing of CD40 in dendritic cells. Cell. Immunol. 259:74-81. [DOI] [PubMed] [Google Scholar]
- 23.Kulkarni, H., B. K. Agan, V. C. Marconi, R. J. O'Connell, J. F. Camargo, W. He, J. Delmar, K. R. Phelps, G. Crawford, R. A. Clark, M. J. Dolan, and S. K. Ahuja. 2008. CCL3L1-CCR5 genotype improves the assessment of AIDS risk in HIV-1-infected individuals. PLoS One 3:e3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamhamedi-Cherradi, S. E., R. E. Martin, T. Ito, F. Kheradmand, D. B. Corry, Y. J. Liu, and M. Moyle. 2008. Fungal proteases induce Th2 polarization through limited dendritic cell maturation and reduced production of IL-12. J. Immunol. 180:6000-6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Launois, P., I. Maillard, S. Pingel, K. G. Swihart, I. Xenarios, H. Acha-Orbea, H. Diggelmann, R. M. Locksley, H. R. MacDonald, and J. A. Louis. 1997. IL-4 rapidly produced by Vβ 4 Vα 8 CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity 6:541-549. [DOI] [PubMed] [Google Scholar]
- 26.Launois, P., K. G. Swihart, G. Milon, and J. A. Louis. 1997. Early production of IL-4 in susceptible mice infected with Leishmania major rapidly induces IL-12 unresponsiveness. J. Immunol. 158:3317-3324. [PubMed] [Google Scholar]
- 27.Lin, J. S., C. W. Yang, D. W. Wang, and B. A. Wu-Hsieh. 2005. Dendritic cells cross-present exogenous fungal antigens to stimulate a protective CD8 T cell response in infection by Histoplasma capsulatum. J. Immunol. 174:6282-6291. [DOI] [PubMed] [Google Scholar]
- 28.Lin, K. L., Y. Suzuki, H. Nakano, E. Ramsburg, and M. D. Gunn. 2008. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J. Immunol. 180:2562-2572. [DOI] [PubMed] [Google Scholar]
- 29.Liotta, F., F. Frosali, V. Querci, A. Mantei, L. Fili, L. Maggi, B. Mazzinghi, R. Angeli, E. Ronconi, V. Santarlasci, T. Biagioli, L. Lasagni, C. Ballerini, P. Parronchi, A. Scheffold, L. Cosmi, E. Maggi, S. Romagnani, and F. Annunziato. 2008. Human immature myeloid dendritic cells trigger a TH2-polarizing program via Jagged-1/Notch interaction. J. Allergy Clin. Immunol. 121:1000-1005 e1008. [DOI] [PubMed] [Google Scholar]
- 30.Macatonia, S. E., N. A. Hosken, M. Litton, P. Vieira, C. S. Hsieh, J. A. Culpepper, M. Wysocka, G. Trinchieri, K. M. Murphy, and A. O'Garra. 1995. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J. Immunol. 154:5071-5079. [PubMed] [Google Scholar]
- 31.Maroof, A., M. Penny, R. Kingston, C. Murray, S. Islam, P. A. Bedford, and S. C. Knight. 2006. Interleukin-4 can induce interleukin-4 production in dendritic cells. Immunology 117:271-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marth, T., and B. L. Kelsall. 1997. Regulation of interleukin-12 by complement receptor 3 signaling. J. Exp. Med. 185:1987-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakano, H., K. L. Lin, M. Yanagita, C. Charbonneau, D. N. Cook, T. Kakiuchi, and M. D. Gunn. 2009. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat. Immunol. 10:394-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osterholzer, J. J., J. L. Curtis, T. Polak, T. Ames, G. H. Chen, R. McDonald, G. B. Huffnagle, and G. B. Toews. 2008. CCR2 mediates conventional dendritic cell recruitment and the formation of bronchovascular mononuclear cell infiltrates in the lungs of mice infected with Cryptococcus neoformans. J. Immunol. 181:610-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perrigoue, J. G., S. A. Saenz, M. C. Siracusa, E. J. Allenspach, B. C. Taylor, P. R. Giacomin, M. G. Nair, Y. Du, C. Zaph, N. van Rooijen, M. R. Comeau, E. J. Pearce, T. M. Laufer, and D. Artis. 2009. MHC class II-dependent basophil-CD4+ T cell interactions promote TH2 cytokine-dependent immunity. Nat. Immunol. 10:697-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters, W., H. M. Scott, H. F. Chambers, J. L. Flynn, I. F. Charo, and J. D. Ernst. 2001. Chemokine receptor 2 serves an early and essential role in resistance to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 98:7958-7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robben, P. M., M. LaRegina, W. A. Kuziel, and L. D. Sibley. 2005. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J. Exp. Med. 201:1761-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato, N., S. K. Ahuja, M. Quinones, V. Kostecki, R. L. Reddick, P. C. Melby, W. A. Kuziel, and S. S. Ahuja. 2000. CC chemokine receptor (CCR) 2 is required for langerhans cell migration and localization of T helper cell type 1 (Th1)-inducing dendritic cells. Absence of CCR2 shifts the Leishmania major-resistant phenotype to a susceptible state dominated by Th2 cytokines, B cell outgrowth, and sustained neutrophilic inflammation. J. Exp. Med. 192:205-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheckelhoff, M., and G. S. Deepe, Jr. 2002. The protective immune response to heat shock protein 60 of Histoplasma capsulatum is mediated by a subset of Vβ 8.1/8.2+ T cells. J. Immunol. 169:5818-5826. [DOI] [PubMed] [Google Scholar]
- 40.Serbina, N. V., and E. G. Pamer. 2006. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7:311-317. [DOI] [PubMed] [Google Scholar]
- 41.Sokol, C. L., N. Q. Chu, S. Yu, S. A. Nish, T. M. Laufer, and R. Medzhitov. 2009. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat. Immunol. 10:713-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinfelder, S., J. F. Andersen, J. L. Cannons, C. G. Feng, M. Joshi, D. Dwyer, P. Caspar, P. L. Schwartzberg, A. Sher, and D. Jankovic. 2009. The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1). J. Exp. Med. 206:1681-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szymczak, W. A., and G. S. Deepe, Jr. 2009. The CCL7-CCL2-CCR2 axis regulates IL-4 production in lungs and fungal immunity. J. Immunol. 183:1964-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Traynor, T. R., A. C. Herring, M. E. Dorf, W. A. Kuziel, G. B. Toews, and G. B. Huffnagle. 2002. Differential roles of CC chemokine ligand 2/monocyte chemotactic protein-1 and CCR2 in the development of T1 immunity. J. Immunol. 168:4659-4666. [DOI] [PubMed] [Google Scholar]
- 45.Traynor, T. R., W. A. Kuziel, G. B. Toews, and G. B. Huffnagle. 2000. CCR2 expression determines T1 versus T2 polarization during pulmonary Cryptococcus neoformans infection. J. Immunol. 164:2021-2027. [DOI] [PubMed] [Google Scholar]
- 46.Voehringer, D., K. Shinkai, and R. M. Locksley. 2004. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity 20:267-277. [DOI] [PubMed] [Google Scholar]
- 47.Webb, D. C., Y. Cai, K. I. Matthaei, and P. S. Foster. 2007. Comparative roles of IL-4, IL-13, and IL-4Rα in dendritic cell maturation and CD4+ Th2 cell function. J. Immunol. 178:219-227. [DOI] [PubMed] [Google Scholar]
- 48.Wiethe, C., A. Debus, M. Mohrs, A. Steinkasserer, M. Lutz, and A. Gessner. 2008. Dendritic cell differentiation state and their interaction with NKT cells determine Th1/Th2 differentiation in the murine model of Leishmania major infection. J. Immunol. 180:4371-4381. [DOI] [PubMed] [Google Scholar]
- 49.Wuthrich, M., H. I. Filutowicz, T. Warner, G. S. Deepe, Jr., and B. S. Klein. 2003. Vaccine immunity to pathogenic fungi overcomes the requirement for CD4 help in exogenous antigen presentation to CD8+ T cells: implications for vaccine development in immune-deficient hosts. J. Exp. Med. 197:1405-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshimoto, T., K. Yasuda, H. Tanaka, M. Nakahira, Y. Imai, Y. Fujimori, and K. Nakanishi. 2009. Basophils contribute to TH2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat. Immunol. 10:706-712. [DOI] [PubMed] [Google Scholar]
- 51.Zhou, P., M. C. Sieve, J. Bennett, K. J. Kwon-Chung, R. P. Tewari, R. T. Gazzinelli, A. Sher, and R. A. Seder. 1995. IL-12 prevents mortality in mice infected with Histoplasma capsulatum through induction of IFN-γ. J. Immunol. 155:785-795. [PubMed] [Google Scholar]