Abstract
Listeriolysin O (LLO), an hly-encoded cytolysin of Listeria monocytogenes, plays an essential role in the entry of L. monocytogenes into the host cell cytoplasm. L. monocytogenes-infected macrophages produce various proinflammatory cytokines, including interleukin-1α (IL-1α), that contribute to the host immune response. In this study, we have examined IL-1α production in macrophages infected with wild-type L. monocytogenes or a nonescaping mutant strain deficient for LLO (Δhly). Expression of IL-1α mRNA and accumulation of pro-IL-1α in the cytoplasm were induced by both strains. In contrast, the secretion of the mature form of IL-1α from infected macrophages was observed in infection with wild-type L. monocytogenes but not with the Δhly mutant. A recovery of the ability to induce IL-1α secretion was shown in a mutant strain complemented with the hly gene. The Toll-like receptor (TLR)/MyD88 signaling pathway was exclusively required for the expression of pro-IL-1α, independently of LLO-mediated cytoplasmic entry of L. monocytogenes. The LLO-dependent secretion of mature IL-1α was abolished by addition of calcium chelators, and only LLO-producing L. monocytogenes strains were able to induce elevation of the intracellular calcium level in infected macrophages. A calcium-dependent protease, calpain, was implicated in the maturation and secretion of IL-1α induced by LLO-producing L. monocytogenes strains based on the effect of calpain inhibitor. Functional activation of calpain was detected in macrophages infected with LLO-producing L. monocytogenes strains but not with a mutant strain lacking LLO. These results clearly indicated that LLO-mediated cytoplasmic entry of bacteria could induce the activation of intracellular calcium signaling, which is essential for maturation and secretion of IL-1α in macrophages during L. monocytogenes infection through activation of a calcium-dependent calpain protease. In addition, recombinant LLO, when added to macrophages infected with the Δhly strain, could induce calcium influx and IL-1α secretion at doses exhibiting cytolytic activity, suggesting that LLO produced by intracellular L. monocytogenes may be implicated in induction of calcium influx through pore formation.
Listeria monocytogenes is an intracellular parasitic bacterial pathogen that causes life-threatening infections, such as meningitis and septicemia, in immunocompromised individuals, newborns, elderly persons, and animals (17, 48). After invasion into the host tissue, L. monocytogenes can survive and replicate within a variety of cells, including epithelial cells, hepatocytes, fibroblasts, and macrophages (25, 47). Intracellular survival of L. monocytogenes is supported by an array of virulence factors, many of which are encoded by the genes in Listeria pathogenicity island 1 (LIPI-1). Among them, listeriolysin O (LLO), a 56-kDa cytolysin encoded by the hly gene, plays an essential role in bacterial escape from the phagosome and entry into the cytoplasm. This enables bacterial replication even in the professional phagocytes highly capable of intracellular killing, such as macrophages (9). In addition to its essential role in the entry of L. monocytogenes into the macrophage cytoplasm, LLO has also been shown to be involved in the activation of macrophages and endothelial and epithelial cells, resulting in the secretion of cytokines and chemokines (29, 30, 37, 41).
Macrophages recognize bacterial components via pattern recognition receptors (PRRs), including Toll-like receptors (TLRs) and Nod-like receptors (NLRs), and the downstream signaling events induce the expression of cytokine genes (1, 2, 42). Upon infection with L. monocytogenes, macrophages secrete a variety of cytokines, including tumor necrosis factor alpha (TNF-α), interleukin 1α/β (IL-1α/β), IL-6, IL-12, and IL-18, all of which play critical roles in the innate immune response of the host (15, 53). Among these cytokines, IL-1 was initially characterized as a factor that causes fever and augments the lymphocyte response (14). Recent studies have shown that IL-1α plays a role as a key mediator of the inflammatory response and necrotic cell death (7, 19). Probably due to its multiple biological activities, IL-1 has been reported to participate in the host defense against L. monocytogenes in wild-type (wt) and SCID mice (45). A number of reports have shown that recombinant IL-1α enhances the resistance of mice to infection with L. monocytogenes (8, 11-13, 28, 33).
IL-1α, IL-1β, and IL-18 are the cytokines belonging to the same IL-1 superfamily (3). Recent studies have elucidated the molecular mechanism of IL-1β and IL-18 secretion in response to various stimuli (1, 2, 34). Upon infection with L. monocytogenes, the expression of both IL-1β and IL-18 genes is induced via activation of the TLR/MyD88 signaling pathway, and these cytokines are produced after processing by caspase-1, which is activated through inflammasome formation (42, 51). In this regard, we have found that the Δhly strain, an LLO-nonproducing mutant strain of L. monocytogenes, is incapable of inducing the production of IL-18 due to its inability for caspase-1 activation, in spite of the effective induction of IL-18 mRNA expression in infected macrophages (26, 27). These findings indicated that LLO plays an important role in the production of these cytokines through caspase-1 activation. In contrast to IL-1β and IL-18, a few early studies reported that IL-1α can be induced in macrophages and processed, possibly by a calcium-dependent protease (6, 32). However, the precise mechanism of production of the mature form of IL-1α in bacterial infection remained to be analyzed.
Based on increasing evidence regarding the modulation of the macrophage response by LLO, we hypothesized that the production of IL-1α is also supported by some LLO-dependent mechanism, like LLO-dependent caspase-1 activation, resulting in the secretion of mature IL-1β or IL-18 belonging to the same IL-1 superfamily. In the present study, we have analyzed the molecular basis for the L. monocytogenes-induced IL-1α secretion in mouse macrophages with special reference to the role of hly-encoded LLO, and we have found that the LLO-mediated cytoplasmic entry of bacteria can induce the activation of calcium signaling, resulting in the maturation and secretion of IL-1α via a calcium-dependent cysteine protease, calpain.
MATERIALS AND METHODS
Reagents.
EDTA and EGTA were purchased from Nacalai Tesque Inc. (Kyoto, Japan). BAPTA-AM, an intracellular calcium chelator, and A23187 were obtained from Sigma Aldrich (St. Louis, MO.). Calpain inhibitors, carbobenzoxy-valyl-phenylalanial (MDL28170, calpain inhibitor III), N-acetyl-l-leucyl-l-leucyl-l-norleucinal (ALLN) (calpain inhibitor I), (2S,3S)-trans-epoxysuccinyl-l-leucylamido-3-methylbutane ethyl ester (EST) (E-64d), N-benzyloxycarbonyl l-leucyl-l-leucyl-l-tyrosyl fluoromethyl ketone (calpain inhibitor IV), calpastatin peptide (CS peptide), 3-(4-iodophenyl)-2-mercapto-(Z)-2-propenoic acid (PD150606), and 3-(5-fluoro-3-indolyl)-2-mercapto-(Z)-2-propenoic acid (PD151746) were obtained from Calbiochem (Darmstadt, Germany). Recombinant LLO (>95% purity) was purchased from Abcam (Cambridge, United Kingdom).
Mice.
Female C57BL/6 mice (wt) were purchased from Japan SLC (Hamamatsu, Japan). Myeloid differentiation primary response gene 88-deficient mice (MyD88−/− mice on a C57BL/6 background) were obtained from Oriental Bio Service Inc. (Kyoto, Japan). Caspase-1−/− mice and mice deficient for apoptosis-associated speck-like protein containing a CARD (ASC−/− mice) on a C57BL/6 background were kindly provided from Tsutsui (Hyogo College of Medicine, Nishinomiya, Japan). Mice were maintained under specific-pathogen-free conditions and used at 7 to 9 weeks of age. All animal experimental procedures were approved by the Animal Ethics and Research Committee of Kyoto University Graduate School of Medicine.
Bacteria.
A hemolytic and virulent strain of Listeria monocytogenes, EGD (serovar 1/2a), was used as the wild type (wt). An L. monocytogenes mutant deficient for the hly gene (Δhly) and an hly-complemented strain (Δhly::hly) were constructed from the wt using the homologous recombination method as described previously (26). Bacteria were grown overnight in brain heart infusion broth (BHI broth; Eiken Chemical Co., Ltd., Tokyo, Japan) at 37°C with shaking. One volume of the overnight culture was added to 100 volumes of fresh BHI broth and cultured for 4 to 5 h. Bacteria were washed, suspended in phosphate-buffered saline (PBS) supplemented with 10% glycerol, and stored in aliquots at −80°C. The concentration of bacteria was determined by plating 10-fold serial dilutions of bacterial suspension on tryptic soy agar (Eiken Chemical) plates and counting the colonies after cultivation for 24 h at 37°C.
Infection and cytokine assay.
Mice were injected intraperitoneally (i.p.) with 3% thioglycolate broth, and peritoneal exudate cells (PECs) were collected 4 days later. Whole PECs, seeded at 2.5 × 105 cells/well in 48-well plates, and nonadherent cells were removed after 2 h of incubation at 37°C. Latex-bead loading revealed that more than 98% of the adherent cells were phagocytic cells. Adherent macrophages were infected with L. monocytogenes strains at a multiplicity of infection (MOI) of 5 for 1 h at 37°C. The culture was continued for 18 h in the presence of 25 μg/ml gentamicin, and the culture supernatant was collected to monitor the production of cytokines. In some experiments, cell lysates were prepared by treatment of infected macrophages with 1% Triton X-100 solution. The levels of pro-IL-1α in the cell lysate and a mature form of IL-1α in the culture supernatant were determined by the OptEIA mouse IL-1α enzyme-linked immunosorbent assay (ELISA) set (BD Bioscience Pharmingen, San Diego, CA). In the experiments to examine the effects of 1 mM EGTA, 1 mM EDTA, 10 to 20 μM BAPTA-AM, 10 to 40 μM MDL28170, 10 μM A23187, and various calpain inhibitors, the reagents were added 3 h after infection in the presence of gentamicin and the culture was continued for a further 15 h. We used these reagents at a range of concentrations exhibiting no cytotoxic effects. To determine the effect of bafilomycin A1, macrophages were treated with 0.5 μM bafilomycin A1 30 min prior to infection and the cytokine production was measured 18 h after infection. In addition, we infected macrophages with the Δhly mutant and added 1 to 500 ng/ml of recombinant LLO 3 h later. The culture was continued for 15 h, and IL-1α production was measured. TNF-α and IL-1β were quantified using a mouse TNF-α ELISA and a mouse IL-1β ELISA (eBioscience Inc., San Diego, CA), respectively. IL-18 was detected using a pair of biotin-labeled and unlabeled monoclonal antibodies specific for IL-18 (MBL, Nagoya, Japan). IL-12p70 was measured using a pair of antibodies specific for IL-12p70 (Endogen, Woburn, MA).
Detection of LDH.
For detection of lactate dehydrogenase (LDH), macrophages were treated with 10 μM A23157 or 1 to 500 ng/ml recombinant LLO for 18 h. The culture supernatant was collected, and LDH activity was measured using an LDH cytotoxicity detection kit (TaKaRa BIO). The percentage of LDH release was calculated by using the following formula: percentage of LDH release = 100 × (experimental LDH release − spontaneous LDH release)/(maximal LDH release − spontaneous LDH release). To obtain the value of maximal LDH release, cells were disrupted in 1% Triton X-100 and LDH activity in the supernatant was measured.
Quantitative real-time PCR.
Total cellular RNA was extracted from macrophages at 0, 3, or 6 h after infection with L. monocytogenes strains by using the Nucleospin RNA cleanup kit (Macherey-Nagel, Düren, Germany). The collected RNA (0.2 μg) was treated with RNase-free DNase (Promega) to eliminate contaminating DNA and then subjected to reverse transcription using the SuperScript III first-strand synthesis system for reverse transcription-PCR (RT-PCR) (Invitrogen, Carlsbad, CA). Quantitative real-time RT-PCR was performed on an ABI Prism 7000 instrument (Applied Biosystems, Foster City, CA) using Platinum SYBR green qPCR SuperMix-UDG (Invitrogen) according to the manufacturer's instructions. The level of mRNA expression of each cytokine was normalized on the basis of β-actin mRNA expression, and the results were analyzed with ABI Prism 7000 SDS software. The DNA sequences of PCR primers are follows: Il-1α, 5′-CTCTAGAGCACCATGCTACAGAC-3′ (forward) and 5′-TGGAATCCAGGGGAAACACTG-3′ (reverse); β-actin, 5′-TGGAATCCTGTGGCATCCATGAAAC-3′ (forward) and 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′ (reverse).
Immunoblotting.
Macrophages were infected with L. monocytogenes strains for 1 h at a MOI of 5, and gentamicin (25 μg/ml) was added to the culture. Cells were incubated for 18 h in the presence or absence of 10 to 40 μM MDL28170, and the culture supernatant was collected. The cell lysate of infected macrophages was prepared by treatment with 10 mM HEPES buffer (pH 7.5) containing 150 mM NaCl, 1% Nonidet P-40, 10% glycerol, and 1 mM EDTA with protease and phosphatase inhibitor cocktail (Nacalai Tesque Inc., Kyoto, Japan). The culture supernatant and the cell lysate were subjected to SDS-PAGE and transferred to a polyvinylidene difluoride (PVDF) membrane by electroblotting. To detect pro-IL-1α and a mature form of IL-1α, the membrane was treated sequentially with biotinylated goat anti-IL-1α polyclonal antibody (Genzyme, Cambridge, MA) and streptavidin-horseradish peroxidase (HRP) conjugate (Invitrogen). After a brief incubation of the membrane in ECL Plus (GE Healthcare Bio-Sciences AB, Uppsala, Sweden), the bands representative of pro-IL-1α and mature IL-1α were detected by a LAS-4000 mini imager (Fuji Film, Tokyo, Japan). In order to evaluate the activation of calpain in infected macrophages, we monitored the amount of proteolytic fragment of α-fodrin, an internal substrate of calpain, by Western blotting. Macrophages were infected with L. monocytogenes strains for 1 h and cultured in the presence of 25 μg/ml gentamicin. At indicated time points after infection, the culture supernatant was collected and the cell lysate was prepared. Samples were subjected to SDS-PAGE and electroblotted to a PVDF membrane. The membrane was treated with mouse anti-α-fodrin monoclonal antibody (Chemicon International, Temecula, CA) and HRP-conjugated rabbit anti-mouse IgG1 antibody (Invitrogen). β-Actin was employed as a loading control for the cell lysates and detected by mouse anti-β-actin antibody (A5441; Sigma-Aldrich).
Immunofluorescence detection.
Macrophages were seeded in 24-well plate at 2.5 × 105 cells/well for 2 h, washed, and treated with 0.5 μM bafilomycin A1 (Invitrogen) or left untreated for 30 min. Cells were infected with the wt strain for 1 h (MOI = 5) and cultured for 5 h in the presence of 25 μg/ml gentamicin. After several washes, cells were fixed in 3% paraformaldehyde solution at 4°C overnight and permeabilized with 0.1% saponin (Nacalai Tesque) for 10 min. To visualize F-actin (comet tail) and bacterial cells in infected macrophages, cells were stained with Alexa Fluor 488-phalloidin (Invitrogen) and rabbit anti-Listeria polyclonal antibody (VitroStat Inc., Portland, ME), followed by Alexa Fluor 594-conjugated anti-rabbit IgG antibody (Invitrogen), respectively. The fluorescence image was taken under a fluorescent microscope.
Measurement of intracellular calcium level.
Macrophages (1.25 × 105 cells) were plated in 96-well black microplates for 2 h and infected with L. monocytogenes strains for 1 h at a MOI of 5. Cells were cultured for 2 h in the presence of 25 μg/ml gentamicin. After washes, the intracellular calcium concentration was monitored using the Fluo-4 NW calcium assay kit (Invitrogen) according to the manufacturer's instructions. In some experiments, macrophages were treated with 1 to 500 ng/ml recombinant LLO or 10 μM A23187 for 18 h, and the calcium level was determined.
Statistical analysis.
Statistical analysis was performed using Student's t test. Values of <0.05 were considered to be statistically significant.
RESULTS
Escape-dependent and -independent production of proinflammatory cytokines in macrophages infected with L. monocytogenes strains.
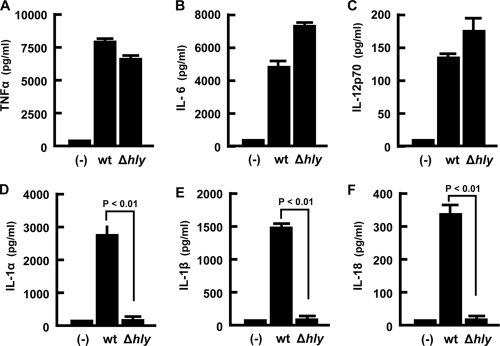
First, we determined the production of proinflammatory cytokines during macrophage infection with the wt and the nonescaping mutant L. monocytogenes Δhly strain. There was no significant difference in the level of production of TNF-α, IL-6, and IL-12p70 between macrophages infected with the wt and Δhly strains (Fig. 1A, B, and C). In contrast, three cytokines belonging to the same IL-1 superfamily, IL-1α, IL-1β, and IL-18, were produced exclusively when macrophages were infected with the wt strain but not with the Δhly strain (Fig. 1D, E, and F). The results suggested that LLO also plays a role in the secretion of IL-1α and in caspase-1-dependent secretion of IL-1β and IL-18.
FIG. 1.
Production of various proinflammatory cytokines by macrophages infected with LLO-producing or LLO-nonproducing L. monocytogenes strains. Adherent macrophages were infected with each L. monocytogenes strain at a MOI of 5 for 1 h. Cells were cultured in the presence of gentamicin, and supernatants were collected at 18 h after infection. The amount of each cytokine was determined by ELISA. Data represent the means and the standard deviations of results from triplicate assays. Similar results were obtained in three independent experiments. (-), no infection.
Kinetics of gene expression, production, and secretion of IL-1α.
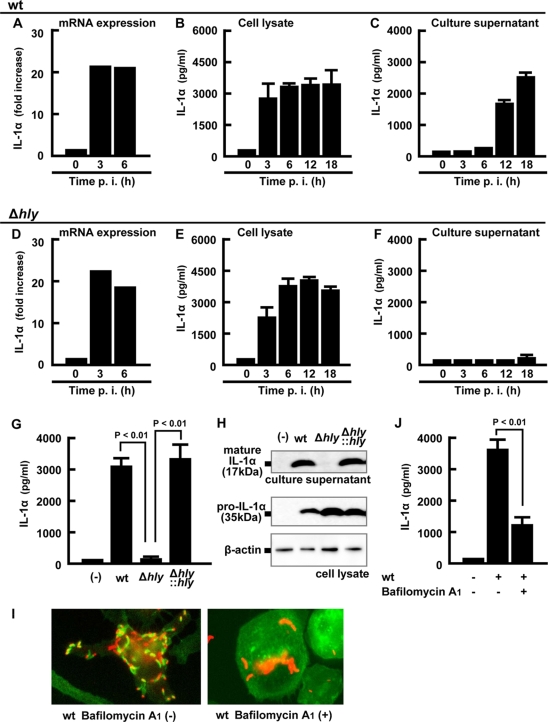
IL-1α/β and IL-18 are known to be synthesized as the proform and then processed to the mature form for secretion (3). In order to examine which stage of IL-1α production and secretion is dependent on the cytoplasmic entry of L. monocytogenes, we analyzed the kinetics of IL-1α mRNA expression, pro-IL-1α production inside macrophages, and secretion in the culture supernatant at different time points after infection with the wt or Δhly strain. As early as 3 h after infection, a significant level of IL-1α expression by a more than 20-fold increase was observed in both macrophage cultures infected with the wt and Δhly strains (Fig. 2A and D). No significant difference was noted in the accumulation of intracellular IL-1α between infection with the wt and Δhly strains (Fig. 2B and E). However, no secretion of IL-1α was detected in this early phase of infection (<6 h). In spite of the similar pattern in the time-dependent transcription and translation of IL-1α inside infected macrophages of both groups, a time-dependent secretion of mature IL-1α was detected only after infection with the wt strain but not after infection with the Δhly strain (Fig. 2C and F) during 18 h of observation.
FIG. 2.
LLO-dependent entry into the cytoplasm is required for the secretion of IL-1α. Macrophages were infected with the wt (A to C) or Δhly strain (D to F) for 1 h, and total cellular RNA, the cell lysate, and the culture supernatant were prepared at the indicated time points. Expression of IL-1α mRNA was quantified by real-time RT-PCR (A and D). The levels of mature IL-1α in the cell lysates (B and E) and the culture supernatants (C and F) were determined by ELISA. Macrophages were infected with the wt, Δhly, or hly-complemented (Δhly::hly) strain for 1 h and cultured for 18 h in the presence of gentamicin. The amount of mature IL-1α in the culture supernatant and pro-IL-1α in the cell lysate was quantified by ELISA (G) and Western blotting (H). β-Actin is utilized as a loading control. Macrophages were treated with 0.5 μM bafilomycin A1 for 30 min and infected with L. monocytogenes for 5 h, fixed, and stained with rabbit anti-L. monocytogenes antibody (Ab), Alexa Fluor 594-anti-rabbit IgG Ab, and Alexa Fluor 488-phalloidin. L. monocytogenes (red) and F-actin (green) are visualized using fluorescence microscopy. L. monocytogenes associating with F-actin represents a bacterium that has escaped from a phagosome (I). Macrophages were treated with 0.5 μM bafilomycin A1 for 30 min, infected with L. monocytogenes for 1 h, and cultured for 18 h in the presence of gentamicin. The supernatant was collected, and the IL-1α concentration was determined by ELISA (J). Three independent experiments were done, and similar results were obtained. (−), no infection.
In order to confirm that the observed difference in IL-1α secretion can be attributed solely to the in-frame deletion of hly, a mutant strain complemented with hly (Δhly::hly) was examined for recovery in the ability to induce IL-1α secretion. The abolished activity was completely restored by gene complementation as determined by ELISA detection of the secreted form of IL-1α (Fig. 2G). A Western blot analysis showed a result consistent with that determined by ELISA (Fig. 2H). While a band of 35 kDa corresponding to pro-IL-1α was detected similarly in the lysates of macrophages infected with L. monocytogenes strains, the 17-kDa band representing the processed, mature form of IL-1α was detected only in macrophages infected with the wt and Δhly::hly strains, the two LLO-producing strains.
Since LLO is indispensable for the escape of L. monocytogenes from phagosome and entry into the cytosol, the above findings suggested the requirement of bacterial entry into the cytosolic space for an effective induction of IL-1α secretion. In order to test this possibility, macrophage culture was pretreated with bafilomycin A1 and infected with the wt. Formation of comet tail, which is indicative of bacterial entry into the cytosol, was mostly suppressed, as shown by the absence of F-actin (Fig. 2I), and under this condition, IL-1α secretion induced by the wt was significantly affected (Fig. 2J). These data indicated that LLO-dependent entry of bacteria into the cytoplasm is essential for mature IL-1α secretion, although mRNA expression and pro-IL-1α synthesis occur independently of bacterial escape.
Involvement of the MyD88 signaling pathway in IL-1α gene expression.
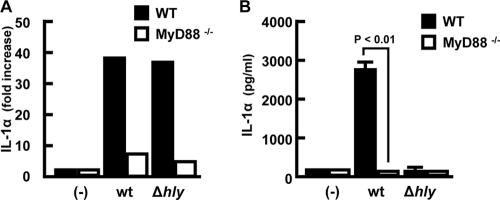
L. monocytogenes-induced production of several cytokines, including TNF-α, IL-12, IL-6, and IL-1β, occurs through the activation of the TLR/MyD88-dependent signaling pathway (18, 41, 45). Therefore, we next examined the involvement of MyD88 in the production of IL-1α during L. monocytogenes infection by using macrophages obtained from MyD88−/− mice. IL-1α mRNA expression was observed at the same level in wt macrophages after infection with the wt and Δhly strains but was not observed in the absence of MyD88 (Fig. 3A). Due to the absence of mRNA expression, secretion of IL-1α was not observed in MyD88−/− macrophages infected with the wt (Fig. 3B). Again, IL-1α secretion was not observed in Δhly-infected macrophages irrespective of the presence or absence of the MyD88 signaling pathway. These results indicated that the MyD88 signaling pathway contributes to the induction of IL-1α mRNA expression, resulting in accumulation of pro-IL-1α in macrophages infected with L. monocytogenes, and that this process is not dependent on LLO-mediated bacterial escape.
FIG. 3.
Dependency of MyD88 signaling pathway on pro-IL-1α synthesis. Macrophages derived from wt (black column) or MyD88−/− (white column) mice were infected with the wt or Δhly strain at a MOI of 5 for 1 h. After addition of gentamicin, the culture was continued and expression of IL-1α mRNA was measured by real-time RT-PCR at 3 h after infection (A). The levels of mature IL-1α in the culture supernatants were determined by ELISA at 18 h after infection (B). Data represent the means and standard deviations of results from triplicate assays. Similar results were obtained in three independent experiments. (-), no infection.
Absence of involvement of caspase-1 and ASC in L. monocytogenes-induced secretion of IL-1α.
Although caspase-1 is required for processing of IL-1β and IL-18 (3, 14), Keller et al. showed that it is also implicated in secretion of IL-1α from macrophages (31). In addition, it has been shown that caspase-1 is activated by formation of the inflammasome and ASC is a critical component of the inflammasome (34). Therefore, we examined the possible involvement of caspase-1 and ASC in LLO-dependent IL-1α secretion induced by L. monocytogenes infection. A high level of IL-1α mRNA expression and pro-IL-1α production was observed even in caspase-1−/− and ASC−/− macrophages at almost the same level as in normal macrophages (Fig. 4A). The significant level of IL-1α secretion induced after infection with the wt was observed not only in wt macrophages but also in caspase-1−/− and ASC−/− macrophages without any significant reduction (Fig. 4B). Western blot analysis also confirmed that there was no reduction in the level of the 17-kDa mature form of IL-1α in the supernatant from caspase-1−/− and ASC−/− macrophages compared to that from normal macrophages (Fig. 4C). It was clear that neither caspase-1 nor ASC is involved in the secretion of IL-1α from macrophages after infection with L. monocytogenes.
FIG. 4.
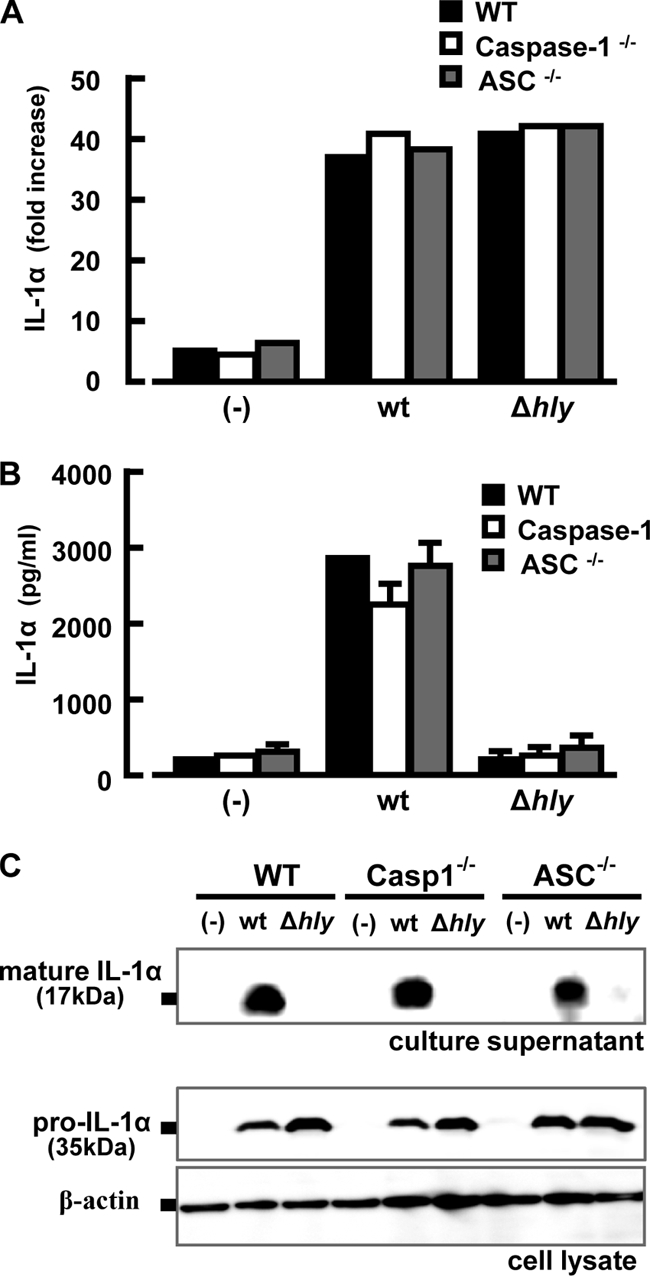
Caspase-1 and ASC are dispensable for L. monocytogenes-induced IL-1α secretion. Macrophages derived from wt (black column), caspase-1−/− (white column), or ASC−/− (gray column) mice were infected with the L. monocytogenes wt or Δhly strain at a MOI of 5 for 1 h. After addition of gentamicin, the culture was continued, and expression of IL-1α mRNA was measured at 3 h after infection (A). The amount of IL-1α in the culture supernatant was determined at 18 h after infection (B). In addition, Western blotting was carried out to detect pro-IL-1α in the cell lysate and mature IL-1α in the culture supernatant at 18 h after infection (C). Similar results were obtained in three independent experiments. (-), no infection.
Involvement of calpain activation in processing of IL-1α.
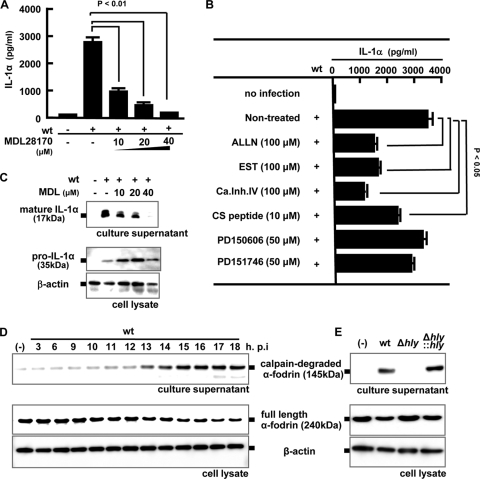
A few previous reports suggested that calpain, a calcium-dependent cysteine protease, contributes to the maturation of IL-1α in response to lipopolysaccharide (LPS) plus a calcium ionophore, A23187 (6, 32). Therefore, we next examined the possible involvement of calpain protease in the processing of IL-1α after infection with L. monocytogenes by using a calpain-specific inhibitor, MDL28170. As shown in Fig. 5A, secretion of IL-1α induced by the wt was inhibited by treatment with MDL28170 in a dose-dependent manner, whereas the level of escape-independent cytokine, TNF-α, was not affected (data not shown). The inhibitory effect of MDL28170 was confirmed by Western blotting in which the intensity of the band corresponding to 17-kDa mature IL-1α was diminished with increasing concentrations of MDL23170 (Fig. 5B). Several broad-spectrum calpain inhibitors (ALLN, EST, calpain inhibitor IV, and CS peptide) partially inhibited IL-1α secretion (Fig. 5C). However, PD150606 and PD151746, specific inhibitors for calpain I and calpain II, marginally inhibited cytokine secretion. These data suggested that a member of calpain proteases other than calpain I or calpain II is possibly involved in the processing and secretion of IL-1α during L. monocytogenes infection.
FIG. 5.
Involvement of calpain activation in the processing and secretion of IL-1α. Macrophages were infected with the wt (MOI = 5), and MDL28170 (MDL) was added to the culture 3 h later at the indicated concentrations. Macrophages were then cultured for 18 h, and the IL-1α level in the culture supernatant was determined by ELISA (A). The amounts of mature IL-1α in the culture supernatant and pro-IL-1α in the cell lysate were determined by Western blotting (B). Macrophages were infected with the wt for 1 h, and other calpain inhibitors (ALLN, EST, calpain inhibitor IV, CS peptide, PD150606, and PD151746) were added 3 h after infection. Cells were further cultured for 15 h, and mature IL-1α production in the culture supernatant was measured by ELISA (C). Macrophages were infected with the wt at a MOI of 5 for 1 h and cultured in the presence of gentamicin for the indicated periods. The full-length form and the proteolytic fragment of α-fodrin were detected by Western blotting in the cell lysate and the culture supernatant, respectively (D). The activity of calpain was monitored by measuring the amount of degraded fragment of α-fodrin. β-Actin was utilized as a loading control. Similarly, macrophages were infected with the wt, Δhly, or Δhly::hly strain for 1 h. Eighteen h after infection, Western blotting was performed to evaluate the activity of calpain (E). Similar results were obtained in three independent experiments. (-), no infection.
Next, to confirm whether the activation of calpain occurs during L. monocytogenes infection, we examined the time course of degradation of α-fodrin, a 240-kDa cytoskeletal protein. Degradation of α-fodrin into a 145-kDa fragment is considered characteristic of calpain activation (20, 23). As shown in Fig. 5D, infection with the wt induced the degradation of α-fodrin starting from 13 h after infection. This timing was consistent with the kinetics of secretion of mature IL-1α (Fig. 2C). When macrophages were infected with the Δhly strain, such a significant level of degradation product of α-fodrin was not detected even after 18 h of infection, whereas the degradation induced by the Δhly::hly strain was similar to that of the wt (Fig. 5E).
Requirement of elevation of the intracellular calcium level for IL-1α processing.
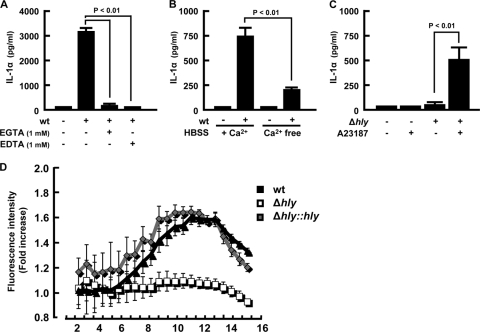
Since calpain is a calcium-dependent protease and an increase in the intracellular calcium level triggers the activation of calpain, it appears that the L. monocytogenes wild type but not the Δhly strain is capable of inducing calcium influx. To make this point clear, we examined the effect of calcium chelators on IL-1α secretion after infection with L. monocytogenes. In the presence of EGTA and EDTA, wt-induced secretion of IL-1α was almost completely abrogated (Fig. 6A). We also found that there was a significant reduction in IL-1α secretion when L. monocytogenes infection was done in calcium-free medium (Fig. 6B). Furthermore, the inability of the Δhly strain to induce IL-1α secretion was restored by additional treatment with A23187 at 3 h postinfection (Fig. 6C), suggesting that an elevation in the level of intracellular calcium is essential for secretion of IL-1α accumulated in cells.
FIG. 6.
Involvement of intracellular calcium elevation in IL-1α secretion. Macrophages were infected with L. monocytogenes for 1 h, and EGTA or EDTA was added to the cultures at 3 h after infection (A). Alternatively, macrophages were infected with the wt for 1 h and cultured in calcium-free (Ca2+ free) or calcium-containing (+ Ca2+) RPMI1640 medium (B). The culture supernatants were collected at 18 h after infection, and IL-1α production was determined by ELISA. Macrophages were infected with the Δhly strain for 1 h and treated with 10 μM A23187 3 h later. The culture supernatant was collected 18 h after infection, and IL-1α production was measured (C). Macrophages were infected with the wt, Δhly, or Δhly::hly strain (MOI = 5) for 1 h, and the culture medium was replaced with dye-loading solution containing Fluo-4 NW at 2 h after infection. Following further incubation for 30 min, the fluorescence intensity was measured for 16 h at 30-min intervals. Results are expressed as fold increases in the fluorescence intensity over levels for nonstimulated controls. Data represent the averages and standard deviations of a quadruplet assay. Similar results were obtained in two independent experiments. HBSS, Hanks balanced salt solution.
Since calpain activation occurred only during macrophage infection with LLO-producing L. monocytogenes strains, it was likely that L. monocytogenes infection causes a change in intracellular calcium levels. To test this hypothesis, we measured the kinetic change in the cytoplasmic calcium level by using a calcium-dependent fluorescent probe after infection with L. monocytogenes strains. Infection with the wt resulted in a significant increase in the fluorescence intensity of macrophages compared to that of noninfected cells (Fig. 6D). It is of interest that the level of fluorescent peak was not so high but was maintained from 9 h to 13 h after infection, corresponding to the initiation of the secretion of IL-1α in the culture supernatants. In contrast, the Δhly strain never induced an increase in the fluorescence intensity. Infection with the Δhly::hly strain exhibited an increase in fluorescence intensity, and the kinetics was similar to that induced by wt infection. These data showed that infection with LLO-producing L. monocytogenes strains increased the intracellular calcium level, which is necessary for the secretion of IL-1α from infected macrophages. Taken together, our results clearly indicated that LLO-mediated bacterial entry into the cytoplasm triggered an increase in the intracellular calcium level, followed by calpain activation and IL-1α secretion.
Ability of recombinant LLO to induce calcium influx and IL-1α secretion.
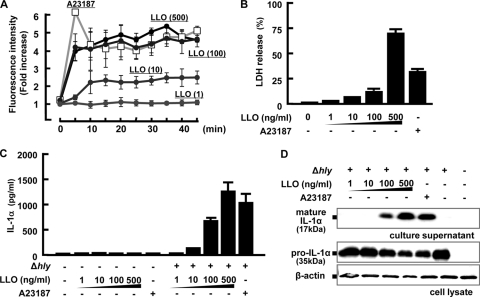
Repp. et al. showed that LLO forms calcium-permeable pores in HEK293 cells (43). We also showed that LLO participates in calcium influx through pore formation and IL-6 production in Caco-2 cells (49). These reports raised a possibility that LLO produced by L. monocytogenes in the cytoplasm of macrophages may contribute to calcium influx by pore formation in the cytoplasmic membrane. To address this question, we first incubated macrophages with graded doses of recombinant LLO and determined the intracellular calcium concentration by measuring the fluorescence intensity. Upon stimulation with LLO, a rapid increase in the fluorescence intensity was observed, and the activity was almost similar to that of A23187 (Fig. 7A). Furthermore, the response was dependent on doses of LLO, and the dose of LLO which induced the highest LDH release from macrophages exhibited the highest activity for calcium influx (Fig. 7B).
FIG. 7.
Calcium influx and IL-1α secretion after treatment with recombinant LLO. Macrophages were treated with the indicated concentrations of recombinant LLO or 10 μM A23187, and the fluorescence intensity of Fluo-4 was measured to evaluate kinetics of the intracellular calcium concentration (A). Macrophages were cultured with various concentrations of LLO or 10 μM A23187 for 18 h, and the amount of LDH released from macrophages were measured (B). Macrophages were infected with the Δhly strain for 1 h and treated with various concentrations of LLO or 10 μM A23187 3 h later. The culture was continued for 15 h, and the amount of IL-1α in the culture supernatant was measured (C). In addition, the amounts of mature IL-1α in the culture supernatant and pro-IL-1α in the cell lysate were investigated by Western blotting (D).
Next, we determined the effect of recombinant LLO or A23187 on the secretion of IL-1α from macrophages infected with the Δhly strain. As expected, due to the lack of synthesis of pro-IL-1α, IL-1α secretion was not detectable when macrophages were stimulated only with LLO or A23187 (Fig. 7C). On the other hand, treatment with either A23187 or higher doses of LLO leads to the secretion of mature IL-1α from macrophages infected with the Δhly strain. Western blots also showed that pro-IL-1α was synthesized after infection with the Δhly strain whereas mature IL-1α was detected only when cells were stimulated with higher doses of LLO. These results suggest that LLO may be involved in not only bacterial escape from phagosomes but also induction of calcium influx through the formation of calcium-permeable pores.
DISCUSSION
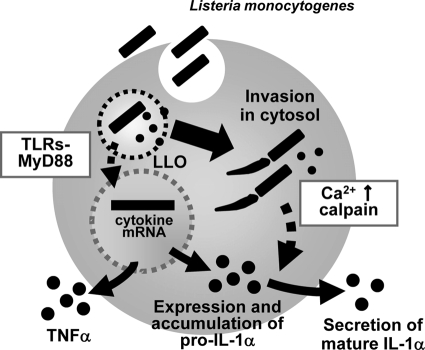
In this study, we have shown that the following occur during infection of macrophages with L. monocytogenes: (1) an extracellular bacterial signal-dependent pathway is activated, leading to IL-1α gene expression and accumulation of pro-IL-1α in macrophages at an early stage of L. monocytogenes infection; (ii) secretion of the mature form of IL-1α at a late stage of L. monocytogenes infection is critically dependent on LLO-mediated bacterial escape from the phagosome; and (iii) entry of bacteria into the cytoplasm induces an increase in the intracellular calcium levels, resulting in the activation of a calpain protease that is necessary for the processing and secretion of mature IL-1α (Fig. 8).
FIG. 8.
Proposed model of IL-1α secretion in L. monocytogenes-infected macrophages. Upon L. monocytogenes infection, activation of the TLR/MyD88 signaling pathway induces IL-1α mRNA expression and pro-IL-1α synthesis independently of bacterial entry into the cytoplasm. LLO-induced cytoplasmic entry of bacteria is essential for secretion of mature IL-1α without any involvement of inflammasome activation. Entry of L. monocytogenes into the host cytoplasm leads to an elevation in the intracellular calcium level and activation of calpain protease, involved in the processing and secretion of mature IL-1α.
The TLR/MyD88-dependent signaling pathway is known to play a critical role in the induction of a variety of proinflammatory cytokines, including TNF-α, IL-12, IL-6, and IL-1β (18, 46). In the present study, we show that this extracellular pattern recognition receptor system can induce gene expression of IL-1α. However, this signal is unable to induce maturation of accumulated pro-IL-1α and secretion of mature IL-1α. Since MyD88 is an adaptor protein indispensable for signaling through TLRs, one or more of the TLRs may be involved in this process. Although TLR2 is known to be the major receptor recognizing Gram-positive L. monocytogenes, we found that IL-1α secretion was not completely abolished in macrophages from TLR2−/− mice (data not shown). It seemed that a number of TLRs, including TLR2, are engaged in the recognition of ligands of L. monocytogenes, resulting in the induction of IL-1α mRNA expression.
The requirement for a further processing step has been well documented in the maturation and secretion of cytokines belonging to the IL-1 superfamily (3, 14, 34). We have previously shown that LLO-dependent bacterial escape is critically important for the activation of caspase-1, required for the maturation and secretion of IL-18 (26). The present study also indicated that LLO-dependent bacterial escape is essential for the maturation of IL-1α. On the other hand, Keller et al. showed that active caspase-1 contributes to secretion and processing of IL-1α in macrophages stimulated with LPS and ATP (31). However, we did not detect any defect in the secretion of IL-1α in caspase-1−/− and ASC−/− macrophages after L. monocytogenes infection. The discrepancy might be due to differences in the properties of stimuli. In their study, IL-1α was secreted from macrophages stimulated with LPS and ATP in a caspase-1-dependent manner, while to some extent, IL-1α was secreted caspase-1 independently. It appears that L. monocytogenes possesses an ability to induce caspase-1-independent IL-1α secretion, although the molecular mechanism remains to be solved.
Compared to extensive studies on caspase-1 activation followed by processing and secretion of IL-18 or IL-1β in macrophages infected with L. monocytogenes, the available information on IL-1α secretion has been limited. The results of our study have clearly indicated that IL-1α secretion in L. monocytogenes-infected macrophages is not dependent on the formation of an ASC-containing inflammasome but is due to calcium-dependent protease activation, which was highly dependent on cytoplasmic entry of L. monocytogenes. Calpain was described more than 40 years ago as a calcium-activated neutral protease originally detected in various tissues, including brain (24) and skeletal muscles (35). Now calpain is defined as an intracellular calcium-dependent cysteine protease, which is ubiquitously distributed, and more than 15 calpain proteins comprise the calpain family (23). Calpain is reported to have two distinct domains that are similar to papain-like protease and calmodulin-like calcium-binding protein (39). In experimental systems other than bacterial infection, there is increasing evidence that calpains participate in a variety of signal transduction pathways and function in important cellular processes (23, 10). Calpain gene disruption has revealed an essential role of calpain in embryonic development (4, 5). Mutation in a particular human calpain has been implicated in the progression of neuromuscular degeneration (40, 44). Thus, calpains appear to be involved in a variety of processes in mammalian development. So far, very few biochemical studies have proposed the involvement of a calcium-dependent calpain protease as the enzyme required for the processing of pro-IL-1α (32, 6). In this regard, our present finding may have added a novel insight into the process of L. monocytogenes-induced maturation of IL-1α. There is no doubt that LLO-dependent cytoplasmic entry is critically involved in the elevation of the intracellular calcium level resulting in calpain activation. In addition, specific inhibitors for calpain I and calpain II exhibited only marginal effect, suggesting that a member of the calpain family other than calpain I or calpain II may be involved in this process. However, we cannot exclude the possibility that other calcium-dependent cysteine or cathepsin proteases may contribute to the processing of IL-1α either alone or in combination with calpain protease.
A number of previous studies have documented the elevation of intracellular calcium levels, especially at the very early stage of infection with LLO-expressing L. monocytogenes strains in mast cells, HEK cells, and J774 cells (16, 21, 22, 43, 49, 50). In all these reports, a quick and transient response in the increase in intracellular calcium was observed in a system employing in vitro infection with an extremely high number of L. monocytogenes cells (MOI = 100). Since pro-IL-1α has not yet accumulated immediately after the L. monocytogenes infection, it is unlikely that this early calcium signaling actually contributes to the maturation of IL-1α that was observed in the later phase of infection. Under the present experimental condition using a MOI of 5, it was not possible to observe such a quick response as that reported, but instead, we have observed a significant response at the later stage. This long-lasting elevation of the intracellular calcium level at the later stage has not been described yet. After infection of macrophages in vitro with L. monocytogenes at MOI of 5, the entry of L. monocytogenes into the cytosol was observed as early as 60 min when determined by comet tail staining under a fluorescence microscope. Thereafter, a gradual bacterial multiplication was noted (data not shown). Taking the into consideration that a long-lasting elevation of the intracellular calcium level started about 13 h after infection, it is likely that the accumulation of bacterial ligands inside the cytoplasmic space initiates the effective cellular response for calcium elevation. The absence of IL-1α processing in an experiment using bafilomycin A1 and the mutant Δhly strain indicated that bacterial entry into the cytoplasm is a prerequisite for the increase in the intracellular calcium level at the later stage. We also found that IL-1α secretion was reduced when L. monocytogenes infection was done in the presence of chloramphenicol (data not shown). This suggests that either intracellular bacterial growth or protein synthesis is essential for this process. It is not yet clear whether LLO itself is required or bacterial components other than the LLO molecule also contribute to the elevation of the calcium level, since bacteria never enter and multiply inside the cytoplasm in infection with the mutant strain and the wt in the presence of bafilomycin A1. Although our results demonstrated an LLO-dependent increase in the intracellular calcium level at the late stage, the precise mechanisms of the activation of this cytoplasmic entry-dependent calcium signaling remain to be analyzed. It has been reported that LLO forms a calcium-permeable pore, leading to intracellular calcium oscillations in mast cells and HEK cells (21, 43). We here showed that membrane perturbation induced by LLO initiated a calcium influx necessary for the processing and secretion of IL-1α. Recently Gekara et al. reported that LLO-expressing L. monocytogenes infection induces calcium influx from the extracellular milieu and the release of calcium from intracellular stores by injury to the endoplasmic reticulum (21). However, again these mechanisms were reported to be involved in an early increase in intracellular calcium levels. Since the LLO is known to be activated in an acidic environment in phagosomes but is not activated at a neutral or weakly alkaline pH in the cytosol of host macrophages (38), other bacterial factors and processes after cytoplasmic entry may play a role in the elevation of calcium in this late stage of infection. Further studies are currently under way to elucidate the exact mechanisms involved. It will be intriguing to determine in the further study whether such a late-stage increase in intracellular calcium levels plays a role in other calcium-dependent cellular processes, which are reported to occur with cholesterol-dependent cytolysins/pore-forming toxins (8, 52).
There are several articles on the contribution of calpain to the host cell response to extracellular bacteria. Muller et al. reported that neisserial porin caused a rapid calcium influx and apoptosis in target cells through calpain activation (36). A similar finding on the induction of calcium influx and apoptosis through calpain activation was found during group B Streptococcus infection (20). Based on these reports, the possibility may be raised that calpain-dependent host cell death, if any, is involved in the secretion of mature IL-1α after processing in L. monocytogenes-infected cells. Though we have not monitored the changes in membrane permeability or membrane damage in infected macrophages since the main focus of the present study was on calpain activation for IL-1α processing, such a possibility could be ruled out because we never detected the presence of the processed mature form of IL-1α in the cell lysate by Western blotting in macrophages which do not secrete mature IL-1α. Processing of pro-IL-1α into the mature form of IL-1α and the secretion of mature IL-1α seem to occur fundamentally as a single event governed by an activated calpain protease(s).
In conclusion, our study clearly indicated that L. monocytogenes-induced IL-1α secretion depends on two steps, LLO-independent expression/production of pro-IL-1α and processing/secretion of mature IL-1α that is induced by LLO-mediated cytoplasmic entry-dependent elevation of intracellular calcium, resulting in calpain activation. These findings may provide further insight into the interaction between the virulence factor of L. monocytogenes and the host cellular response. The overall effect of this mechanism observed in vitro in the context of the in vivo response needs to be addressed in a future study.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Culture, and Sports of Japan, Grants-in-Aid for Scientific Research from the Japan Society for Promotion of Science, a Grant-in-Aid for Research on Emerging and Re-emerging Infectious Diseases from the Ministry of Health, Labor, and Welfare of Japan, and the Waksman Foundation of Japan.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 1 March 2010.
REFERENCES
- 1.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1 and IL-18-mediated function. Immunity 9:143-150. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. [DOI] [PubMed] [Google Scholar]
- 3.Arend, W. P., G. Palmer, and C. Gabay. 2008. IL-1, IL-18 and IL-33 families of cytokines. Immunol. Rev. 223:20-38. [DOI] [PubMed] [Google Scholar]
- 4.Arthur, J. S. C., J. S. Elce, C. Hegadorn, K. Williams, and P. A. Greer. 2000. Disruption of the murine calpain small subunit gene, Capn4: calpain is essential for embryonic development but not for cell growth and division. Mol. Cell. Biol. 20:4474-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao, Y., H. Zhao, and H. Grunz. 2001. XCL-2 is a novel m-type calpain and disrupts morphogenetic movements during embryogenesis in Xenopus laevis. Dev. Growth differ. 43:563-571. [DOI] [PubMed] [Google Scholar]
- 6.Carruth, L. M., S. Demezuk, and S. B. Mizel. 1991. Involvement of a calpain-like protease in the processing of the murine interleukin 1α precursor. J. Biol. Chem. 266:12162-12167. [PubMed] [Google Scholar]
- 7.Chen, C., H. Kono, D. Golenbock, G. Reed, S. Akira, and K. L. Rock. 2007. Identification of a key pathway for the sterile inflammatory response triggered by dying cells. Nat. Med. 13:851-856. [DOI] [PubMed] [Google Scholar]
- 8.Chu, J., L. M. Thomas, S. C. Watkins, L. Franchi, G. Nunez, and R. D. Salter. 12 August 2009. Cholesterol-dependent cytolysins induce rapid release of mature IL-1 (beta) from murine macrophages in a NLRP3 inflammasome and cathepsin B-dependent manner. J. Leukoc. Biol. [Epub ahead of print.] [DOI] [PMC free article] [PubMed]
- 9.Cossart, P., M. F. Vicente, J. Mengaud, F. Baquero, J. C. Perez-Diaz, and P. Berche. 1989. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect. Immun. 57:3629-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Croall, D. E., and K. Ersfeld. 2007. The calpains: modular designs and functional diversity. Genome Biol. 8:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czuprynski, C. J., and J. F. Brown. 1987. Purified human and recombinant murine IL-1α induced accumulation of inflammatory peritoneal neutrophils and mononuclear phagocytes: possible contributions to antibacterial resistance. Microb. Pathog. 3:377-386. [DOI] [PubMed] [Google Scholar]
- 12.Czuprynski, C. J., and J. F. Brown. 1987. Recombinant murine interleukin-1α enhancement of nonspecific antibacterial resistance. Infect. Immun. 55:2061-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czuprynski, C. J., J. F. Brown, K. M. Young, J. Cooley, and R. S. Kurtz. 1988. Effects of murine recombinant interleukin 1α on the host response to bacterial infection. J. Immunol. 140:962-968. [PubMed] [Google Scholar]
- 14.Dinarello, C. A. 1994. The interleukin-1 family: 10 years of discovery. FASEB J. 8:1314-1325. [PubMed] [Google Scholar]
- 15.Dongou, L. 2008. Handbook of Listeria monocytogenes, 1st ed. CRC Press. London, United Kingdom.
- 16.Dramsi, S., and P. Cossart. 2003. Listeriolysin O-mediated calcium influx potentiates entry of Listeria monocytogenes into the human Hep-2 epithelial cell line. Infect. Immun. 71:3614-3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drevets, D. A., and M. S. Bronze. 2008. Listeria monocytogenes: epidemiology, human disease, and mechanisms of brain invasion. FEMS Immunol. Med. Microbiol. 53:151-165. [DOI] [PubMed] [Google Scholar]
- 18.Edelson, B. T., and E. R. Unanue. 2002. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophage listericidal activity. J. Immunol. 169:3869-3875. [DOI] [PubMed] [Google Scholar]
- 19.Elkon, K. B. 2007. IL-1α responds to necrotic cell death. Nat. Med. 13:778-780. [DOI] [PubMed] [Google Scholar]
- 20.Fettucciari, K., I. Fetriconi, R. Mannucci, I. Nicoletti, A. Bartoli, S. Coaccioli, and P. Marconi. 2006. Group B Streptococcus induces macrophage apoptosis by calpain activation. J. Immunol. 176:7542-7556. [DOI] [PubMed] [Google Scholar]
- 21.Gekara, N. O., K. Westphal, M. Bin, M. Rohde, L. Groebe, and S. Weiss. 2007. The multiple mechanisms of Ca2 signaling by listeriolysin O, the cholesterol-dependent cytolysin of Listeria monocytogenes. Cell Microbiol. 9:2008-2021. [DOI] [PubMed] [Google Scholar]
- 22.Goldfine, H., and S. J. Wadsworth. 2002. Macrophage intracellular signaling induced by Listeria monocytogens. Microbes Infect. 4:1335-1342. [DOI] [PubMed] [Google Scholar]
- 23.Goll, D. E., V. F. Thompson, H. Li, W. Wei, and J. Cong. 2003. The calpain system. Physiol. Rev. 83:731-801. [DOI] [PubMed] [Google Scholar]
- 24.Guroff, G. 1964. A neutral, calcium activated proteinase from the soluble fraction of the rat brain. J. Biol. Chem. 239:149-155. [PubMed] [Google Scholar]
- 25.Hamon, M., B. Helene, and P. Cossart. 2006. Listeria monocytogenes: a multifaceted model. Nat. Rev. Microbiol. 4:423-434. [DOI] [PubMed] [Google Scholar]
- 26.Hara, H., K. Tsuchiya, T. Nomura, I. Kawamura, S. Shoma, and M. Mitsuyama. 2008. Dependency of caspase-1 activation induced in macrophages by Listeria monocytogenes on cytolysin, listeriolysin O, after evasion from phagosome into the cytoplasm. J. Immunol. 180:7859-7868. [DOI] [PubMed] [Google Scholar]
- 27.Hara, H., I. Kawamura, T. Nomura, T. Tominaga, K. Tsuchiya, and M. Mitsuyama. 2007. Cytolysin-dependent escape of the bacterium from the phagosome is required but not sufficient for induction of the Th1 immune response against Listeria monocytogenes infection: distinct role of listeriolysin O determined by cytolysin gene replacement. Infect. Immun. 75:3791-3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Igarashi, K., M. Mitsuyama, K. Muramori, H. Tsukada, and K. Nomoto. 1990. Interleukin-1-induced promotion of T-cell differentiation in mice immunized with killed Listeria monocytogenes. Infect. Immun. 58:3973-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kayal, S., A. Lilienbaum, O. Join-Lambert, X. Li, A. Israel, and P. Berche. 2002. Listeriolysin O secreted by Listeria monocytogenes induces NF-κB signalling by activating the IκB kinase complex. Mol. Microbiol. 44:1407-1419. [DOI] [PubMed] [Google Scholar]
- 30.Kayal, S., A. Lilienbaum, C. Poyart, S. Memet, A. Israel, and P. Berche. 1999. Listeriolysin O-dependent activation of endothelial cells during infection with Listeria monocytogenes: activation of NF-κB and upregulation of adhesion molecules and chemokines. Mol. Microbiol. 31:1709-1722. [DOI] [PubMed] [Google Scholar]
- 31.Keller, M., A. Ruegg, S. Werner, and H. Beer. 2008. Active caspase-1 is a regulator of unconventional protein secretion. Cell 132:818-831. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi, Y., K. Yamamoto, T. Saido, H. Kawasaki, J. J. Oppenheim, and K. Matsushima. 1990. Identification of calcium-activated neutral protease as a processing enzyme of human interleukin 1α. Proc. Natl. Acad. Sci. U. S. A. 87:5548-5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurtz, R. S., K. M. Young, and C. J. Czuprynski. 1989. Separate and combined effects of recombinant interleukin-1α and gamma interferon on antibacterial resistance. Infect. Immun. 57:553-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mariathasan, S., and D. M. Monack. 2007. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat. Rev. Immunol. 7:31-40. [DOI] [PubMed] [Google Scholar]
- 35.Meyer, W. L., E. H. Fischer, and E. G. Krebs. 1964. Activation of skeletal muscle phosphorylase B kinase by CA. Biochemistry 3:1033-1039. [DOI] [PubMed] [Google Scholar]
- 36.Muller, A., D. Gunther, F. Dux, M. Naumann, T. F. Meyer, and T. Rudel. 1999. Neisserial porin (PorB) causes rapid calcium influx in target cells and induces apoptosis by the activation of cysteine proteases. EMBO J. 18:339-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nomura, T., I. Kawamura, K. Tsuchiya, C. Kohda, H. Baba, Y. Ito, T. Kimoto, I. Watanabe, and M. Mitsuyama. 2002. Essential role of interleukin-12 (IL-12) and IL-18 for gamma interferon production induced by listeriolysin O in mouse spleen cells. Infect. Immun. 70:1049-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nomura, T., I. Kawamura, C. Kohda, H. Baba, Y. Ito, T. Kimoto, I. Watanabe, and M. Mitsuyama. 2007. Irreversible loss of membrane-binding activity of Listeria-derived cytolysins in non-acidic conditions: a distinct difference from allied cytolysins produced by other Gram-positive bacteria. Microbiology 153:2250-2258. [DOI] [PubMed] [Google Scholar]
- 39.Ohno, S., Y. Emori, S. Imajoh, H. Kawasaki, M. Kisaragi, and K. Suzuki. 1984. Evolutionary origin of a calcium-dependent protease by fusion of genes for a thiol protease and a calcium-binding protein? Nature 312:566-570. [DOI] [PubMed] [Google Scholar]
- 40.Ono, Y., H. Shimada, H. Sorimachi, I. Richard, T. C. Saido, J. S. Beckmann, S. Ishiua, and K. Suzuki. 1998. Functional defects of a muscle-specific calpain, p94, caused by mutations associated with limb-girdle muscular dystrophy type 2A. J. Biol. Chem. 273:17073-17078. [DOI] [PubMed] [Google Scholar]
- 41.O'Riordan, M., C. H. Yi, R. Gonzales, K. Lee, and D. A. Portnoy. 2002. Innate recognition of bacteria by a macrophage cytosolic surveillance pathway. Proc. Natl. Acad. Sci. U. S. A. 99:13861-13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozoren, N., J. Masumoto, L. Franchi, T. D. Kanneganti, M. Body-Malapel, I. Erturk, R. Jagirdar, L. Zhu, N. Inohara, J. Bertin, A. Coyle, E. P. Grant, and G. Nunez. 2006. Distinct roles of TLR2 and the adaptor ASC in IL-1β/IL-18 secretion in response to Listeria monocytogenes. J. Immunol. 176:4337-4342. [DOI] [PubMed] [Google Scholar]
- 43.Repp, H., Z. Pamukci, A. Koschinski, E. Domann, A. Darji, J. Birringer, D. Brockmeier, T. Chakraborty, and F. Dreyer. 2002. Listeriolysin of Listeria monocytogens forms Ca2+-permeable pores leading to intracellular Ca2+ oscillations. Cell Microbiol. 4:483-491. [DOI] [PubMed] [Google Scholar]
- 44.Richard, I., O. Broux, V. Allamand, F. Fougerousse, N. Chiannilkulchai, N. Bourg, L. Brenguier, C. Devaud, P. Pasturaud, and C. Roudaut. 1995. Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell 81:27-40. [DOI] [PubMed] [Google Scholar]
- 45.Rogers, H. W., K. C. F. Sheehan, L. M. Brunt, S. K. Dower, E. M. Unanue, and R. D. Schreiber. 1992. Interleukin-1 participates in the development of anti-Listeria responses in normal and SCID mice. Proc. Natl. Acad. Sci. U. S. A. 89:1011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seki, E., H. Tsutsui, N. M. Tsuji, N. Hayashi, K. Adachi, H. Nakano, S. Futatsugi-Yumikura, O. Takeuchi, K. Hoshino, S. Akira, J. Fujimoto, and K. Nakanishi. 2002. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J. Immunol. 169:3863-3868. [DOI] [PubMed] [Google Scholar]
- 47.Seveau, S., J. Pizarro-Cerda, and P. Cossart. 2007. Molecular mechanisms exploited by Listeria monocytogenes during host cell invasion. Microbes Infect. 9:1167-1175. [DOI] [PubMed] [Google Scholar]
- 48.Swaminathan, B., and P. Gerner-Smidt. 2007. The epidemiology of human listeriosis. Microbes Infect. 9:1236-1243. [DOI] [PubMed] [Google Scholar]
- 49.Tsuchiya, K., I. Kawamura, A. Takahashi, T. Nomura, C. Kohda, and M. Mitsuyama. 2005. Listeriolysin O-induced membrane permeation mediates persistent interleukin-6 production in Caco-2 cells during Listeria monocytogenes infection in vitro. Infect. Immun. 73:3869-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wadsworth, S. J., and H. Goldfine. 1999. Listeria monocytogenes phospholipase C-dependent calcium signaling modulates bacterial entry into J774 macrophage-like cells. Infect. Immun. 67:1770-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Warren, S. E., D. P. Mao, A. E. Rodriguez, E. A. Miao, and A. Aderem. 2008. Multiple Nod-like receptors activate caspase-1 during Listeria monocytogenes infection. J. Immunol. 180:7558-7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiles, T. J., B. K. Dhakal, D. S. Eto, and M. A. Mulvey. 2008. Inactivation of host Akt/protein kinase B signaling by bacterial pore-forming toxins. Mol. Biol. Cell 19:1427-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zenewicz, L. A., and H. Shen. 2007. Innate and adaptive immune responses to Listeria monocytogenes: a short overview. Microbes Infect. 9:1208-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]