Abstract
Major impediments to developing a Chlamydia vaccine lie in identifying immunologically relevant T-cell antigens and delivery in a manner to stimulate protective immunity. Using an immunoproteomic approach, we previously identified three immunodominant Chlamydia T-cell antigens (PmpG-1, PmpE/F-2, and RplF). Because RplF has high homology to a human ortholog, it may not be suitable for human vaccine development. Therefore, in this study, we evaluated protection against Chlamydia infection in the genital tract in C57BL/6 mice immunized with Chlamydia-specific membrane proteins PmpG-1, PmpE/F-2, and major outer membrane protein (MOMP; as a reference) or a combination of them formulated with one of three adjuvants, CpG oligodeoxynucleotide (CpG-ODN), AbISCO-100 (AbISCO), or DDA/TDB (dimethyldioctadecylammonium bromide/d-(+)-trehalose 6,6′-dibehenate). The results show that immunization with the CpG-ODN formulation failed to provide protection against Chlamydia infection; the AbISCO formulation conferred moderate protection, and the DDA/TDB formulation showed the highest degree of protective efficacy. The combination of PmpG-1, PmpE/F-2, and MOMP proteins formulated with DDA/TDB exhibited the greatest degree of protection among all vaccine groups studied. Moreover, this vaccine combination also engendered significant protection in BALB/c mice, which have a different major histocompatibility complex (MHC) background. We measured cell-mediated immune cytokine responses in mice immunized with PmpG-1 mixed with each of the three adjuvants. The results demonstrate that mice immunized with the DDA/TDB formulation induced the strongest gamma interferon (IFN-γ) and interleukin-17 (IL-17) responses, characterized by the highest frequency of IFN-γ/tumor necrosis factor alpha (TNF-α) and IFN-γ/IL-17 double-positive CD4+ T cells. In conclusion, a Chlamydia vaccine based on the recombinant proteins PmpG-1, PmpE/F-2, and MOMP delivered in a DDA/TDB adjuvant conferred protection against infection that correlated with IFN-γ/TNF-α and IFN-γ/IL-17 double-positive CD4+ T cells.
Chlamydia trachomatis is a major cause of sexually transmitted bacterial disease, with over 92 million new cases of C. trachomatis-related disease annually occurring worldwide (39). C. trachomatis infects epithelial cells lining the reproductive tract and causes severe complications such as pelvic inflammatory disease, ectopic pregnancy, and infertility in women. Public health programs to prevent and control Chlamydia based on early case identification and antibiotic treatment appear to be failing, as case rates continued to rise during the past decade (6). Thus, the development of an effective vaccine is of paramount importance.
Early vaccination trials involved both human and nonhuman primates immunized with whole, inactivated C. trachomatis elementary bodies (EBs). The results demonstrated incomplete and short-lived protection, with even worse inflammation postvaccination among infected subjects (12, 42), and thus, current C. trachomatis vaccine research has focused on the production of subunit vaccines that are based on individual protective C. trachomatis proteins (7, 38). To date, the Chlamydia major outer membrane protein (MOMP) has been the most widely investigated subunit vaccine. Although immune responses against MOMP can be elicited in animal models, studies indicate that vaccines based on MOMP alone do not afford complete protection (18, 32-34).
Immunity to Chlamydia is currently understood as being mediated by T cells and mainly relies on tissue trafficking CD4+ T cells producing gamma interferon (IFN-γ) (28, 40, 41). One major impediment to developing a vaccine against this intracellular pathogen lies in identifying relevant T-cell antigens (Ags). Recently, we used a combination of affinity chromatography and tandem mass spectrometry to successfully identify 8 major histocompatibility complex (MHC) class II (I-Ab)-bound Chlamydia peptides eluted from C. muridarum-infected dendritic cells (DCs) from C57BL/6 mice. Adoptive transfer of DCs pulsed with a pool of the 8 peptides partially protected mice against challenge infection (19). Further study demonstrated that 3 of the 8 Chlamydia antigens, polymorphic membrane protein G (PmpG-1), polymorphic membrane protein F (PmpE/F-2), and ribosomal protein L6 (RplF) were immunodominant, and vaccination with DCs individually transfected with these proteins induced significant protective immunity against lung and genital tract infections (49).
Another challenge in developing an effective Chlamydia vaccine is to identify suitable adjuvants and the appropriate routes and methods of delivery of the vaccine (15). CpG oligodeoxynucleotide (CpG-ODN) is a potent Th1-polarizing adjuvant which activates antigen-presenting cells through Toll-like receptor 9 (TLR9), which leads to interleukin-12 (IL-12) production, driving Th1 immune responses (21). AbISCO-100 (AbISCO), an effective immunostimulating complex (ISCOM) adjuvant generates a balanced immune response with both Th1 and Th2 characteristics. Compared with other adjuvants such as alum, Freund's incomplete adjuvant (FIA), and a synthetic monophosphoryl lipid A adjuvant (Ribi), AbISCO generated the highest antibody titers, the highest proliferation index and gave consistently higher production levels of IFN-γ, IL-4, and IL-5. Moreover, animal studies demonstrated that AbISCO adjuvant induced greater protection against challenge infection with human immunodeficiency virus (HIV)/simian immunodeficiency virus (SIV), herpesvirus, and influenza virus (24, 27). The adjuvant DDA/TDB consists of cationic liposome DDA (dimethyldioctadecylammonium bromide) and an immunomodulator, TDB [d-(+)-trehalose 6,6′-dibehenate], a less-toxic, synthetic mycobacterial cord factor analogue (31, 36), inserted into liposomes. DDA/TDB was found to be a particularly promising adjuvant capable of inducing both strong Th1 responses and high antibody titers. It was reported that DDA/TDB makes an effective tuberculosis subunit vaccine adjuvant with the ability to raise high levels of protective memory immunity, comparable to that found in Mycobacterium bovis BCG-vaccinated mice. The specific Th1-type immune response was characterized by substantial production of IFN-γ and high levels of IgG2b isotype antibodies. The lymphocyte subset releasing IFN-γ was identified as CD4 T cells (11). A recent study demonstrated that mice vaccinated with Chlamydia MOMP protein formulated with DDA/TDB generated high titers of IgG2b, IFN-γ, and tumor necrosis factor alpha (TNF-α) and had significantly reduced vaginal Chlamydia shedding relative to control mice (13).
Among the three immunodominant Chlamydia antigens PmpG-1, RplF, and PmpE/F-2, RplF has high homology to the human ortholog and therefore may be not suitable for development as a human vaccine. In the present study, we evaluated the protective effect against Chlamydia genital tract infection in mice immunized with individual PmpG-1, PmpE/F-2, and MOMP proteins or a combination of them formulated with the adjuvant CpG-ODN, AbISCO, or DDA/TDB. We observed that the protein antigen combination of PmpG-1, PmpE/F-2, and MOMP formulated with DDA/TDB exhibited the greatest degree of protective immunity among all groups tested. The AbISCO formulation conferred moderate protection against challenge, whereas the CpG-ODN formulation failed to induce protection. To explore correlates of protection, we measured cell-mediated immune cytokine responses in mice immunized with PmpG-1 mixed with the three adjuvants. The results demonstrated that mice immunized with the DDA/TDB formulation had the strongest IFN-γ and IL-17 responses, characterized by the highest frequency of double-positive IFN-γ/TNF-α and IFN-γ/IL-17 CD4+ T cells.
MATERIALS AND METHODS
Chlamydia.
Chlamydia muridarum mouse pneumonitis (MoPn)-causing strain Nigg was used in this study. This MoPn-causing strain was grown in HeLa 229 cells, and elementary bodies (EBs) were purified by discontinuous density gradient centrifugation as previously described (8). Purified EBs were aliquoted and stored at −80°C in sucrose-phosphate-glutamic acid buffer and thawed immediately before use. The infectivity of the stock Chlamydia EBs was determined by infection of HeLa 229 cells and enumeration of inclusions that were stained by anti-EB mouse polyclonal antibody followed by biotinylated anti-mouse IgG (Jackson ImmunoResearch) and DAB (3,3-diaminobenzidine) substrate (Vector Laboratories) (47). Inactivation of EBs was carried out by heating to 56°C for 30 min.
Chlamydia proteins.
The production and purification of Chlamydia recombinant proteins PmpG-1 and PmpE/F-2 have been described previously (49). Briefly, pmpG-1 and pmpE/F-2 gene fragments (representing amino acids 25 to 500 and 25 to 575, respectively) from C. muridarum were generated by PCR and cloned into vector pET32a (Novagen) for expression. The omp-1 gene characteristically encodes the MOMP protein without first 22 amino acids, was cloned as BamHI/XhoI fragment into the vector pET30a. Plasmids containing pmpG-1, pmpE/F-2, and omp-1 were transformed into Escherichia coli strain BL21(DE3) (Stratagene). The expressed PmpG-1, PmpE/F-2, and MOMP proteins, with an N-terminal His tag, were purified with a nickel column by using the His-Bind purification system (Qiagen). Lipopolysaccharide (LPS) removal was carried out by adding 0.1% Triton X-114 in the wash buffers during purification.
Adjuvants.
Three adjuvants (CpG-ODN 1826, AbISCO-100, and DDA/TDB) were evaluated in the present study. CpG-ODN 1826 (5′-TCCATGACGTTCCTGACGTT-3′, phosphorothioate modified; Integrated DNA Technologies, Inc.) was used in either a free form (free CpG) or a form conjugated with liposomal nanoparticles (LN-CpG). AbISCO-100 adjuvant (ISCONOVA Sweden) is a selection of purified fractions of quillaja saponins formulated with a mixture of cholesterol (ovine wool) and phosphatidyl choline (egg). DDA (dimethyldioctadecylammonium bromide [product no. 890810P]) and TDB [d-(+)-trehalose 6,6′-dibehenate (product no. 890808P)] were purchased from Avanti Polar Lipids (Alabaster, AL). For the DDA/TDB formulation (11, 14), DDA was mixed into 10 mM Tris buffer at pH 7.4 to a concentration of 1.67 mg/ml, heated to 80°C while being stirred continuously on a magnetic hot plate for 20 min, and then cooled to room temperature. TDB was suspended in distilled water (dH2O) containing 2% dimethyl sulfoxide to a concentration of 5 mg/ml by repeated passaging through a fine-tipped pipette followed by 30 s of vortexing. This step was repeated three times before the solution was frozen at −20°C until use. A 5-ml volume of TDB (1 mg/ml) was added to 15 ml DDA (1.67 mg/ml). The resulting solution was then vortexed briefly and stored at 4°C until use. The final concentration of DDA was 1.25 mg/ml, and the final concentration of TDB was 0.25 mg/ml. Each inoculation dose of 200 μl for immunization contained 250 μg DDA and 50 μg TDB.
Mice.
Female C57BL/6 mice and BALB/c mice (8 to 10 weeks old) were purchased from Charles River Canada (Saint Constant, Canada). The mice were maintained and used in strict accordance with University of British Columbia guidelines for animal care.
Mouse immunization.
Four mouse trials were conducted in this study. All mice except the live-EB group were immunized three times subcutaneously (s.c.) in the base of the tail at 2-week intervals. Mice intranasally infected with 1,500 inclusion-forming units (IFU) of live C. muridarum (EBs) were used as positive controls.
In the first trial, groups of six C57BL/6 mice were immunized with 20 μg Chlamydia protein (PmpG-1 or MOMP) mixed with 700 μg LN-CpG or 700 μg free CpG. Groups of mice immunized with LN-CpG alone and phosphate-buffered saline (PBS) were used as negative controls. In the second trial, groups of eight C57BL/6 mice were immunized with 5 μg individual Chlamydia proteins PmpG-1, PmpE/F-2, and MOMP or a combination (1.67 μg for each protein) formulated with AbISCO-100 (12 μg) or DDA/TDB (250 μg DDA-50 μg TDB) as follows: (i) PmpG-1 plus AbISCO-100 (PmpG+AbISCO), (ii) PmpE/F-2 plus AbISCO-100 (PmpF+AbISCO), (iii) MOMP plus AbISCO-100 (MOMP+AbISCO), (iv) PmpG-1 plus PmpE/F-2 plus MOMP plus AbISCO-100 (G+F+M+AbISCO), (v) AbISCO-100 alone (AbISCO alone), (vi) PmpG-1 plus DDA/TDB (PmpG+DDA/TDB), (vii) PmpE/F-2 plus DDA/TDB (PmpF+DDA/TDB), (viii) MOMP plus DDA/TDB, (ix) PmpG-1 plus PmpE/F-2 plus MOMP plus DDA/TDB (G+F+M+DDA/TDB), (x) DDA/TDB alone, (xi) PBS, or (xii) EBs. In the third trial, three groups of eight BALB/c mice were immunized with (i) G+F+M+DDA/TDB, (ii) DDA/TDB alone, or (iii) EBs. Two weeks after the final immunization, the mice in the three animal trials described above were then challenged with live EBs for protection evaluation.
In the fourth trial, groups of 16 C57BL/6 mice were immunized with 5 μg PmpG-1 formulated with DDA/TDB (250 μg DDA-50 μg TDB), AbISCO-100 (12 μg), or CpG (20 μg). Two weeks after the last immunization, half of the mice in each group were sacrificed to isolate splenocytes for lymphocyte multicolor flow cytometry, enzyme-linked immunosorbent assay (ELISA), and enzyme-linked immunospot (ELISPOT) assays; the other half of the mice were challenged with live EBs and sacrificed seven days later to isolate splenocytes and iliac lymph nodes for multicolor flow cytometry.
Genital tract infection and determination of Chlamydia titer.
One week after the last immunization, mice were injected s.c. with 2.5 mg of medroxyprogesterone acetate (Depo-Provera; Pharmacia and Upjohn). One week after medroxyprogesterone acetate treatment, mice were challenged intravaginally with 1,500 IFU of C. muridarum. Cervicovaginal washes were taken at selected dates after infection and stored at −80°C for titration on HeLa cells as described previously (5).
ELISPOT assay.
The cytokine-specific ELISPOT assay was performed as described previously (16). Briefly, 96-well MultiScreen-HA filtration plates (Millipore) were coated overnight at 4°C with 2 μg/ml of murine-IFN-γ-specific monoclonal antibody (clone R4-6A2; BD PharMingen) or murine-IL-17-specific monoclonal antibody (clone TC11-18H10.1; BioLegend). Splenocytes isolated from mice in complete RPMI 1640 medium (Sigma-Aldrich) were added to the coated plates at 106 cells per well in the presence of PmpG-1 protein (1 μg/ml) or heat-killed EBs (HK-EBs; 5 × 105 IFU/ml). After 20 h of incubation at 37°C and 5% CO2, the plates were washed and then incubated with biotin anti-mouse IFN-γ (clone XMG1.2; BD PharMingen) or biotin anti-mouse IL-17 (clone TC11-8H4; BioLegend) at 2 μg/ml. This was followed by incubation with streptavidin-alkaline phosphatase (BD PharMingen) at a 1:1,000 dilution. The spots were visualized with a substrate consisting of 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium (Sigma-Aldrich).
Cytokine measurement.
The culture supernatants of the splenocytes stimulated with PmpG-1 protein or HK-EBs for 48 h were collected and analyzed with respect to TNF-α production with a sandwich ELISA using corresponding specific capture and detection antibodies (BD PharMingen). TNF-α levels were calculated using a standard curve constructed with recombinant murine TNF-α (BD PharMingen).
Multiparameter flow cytometry.
Two weeks after the last immunization or seven days after live-EB challenge, the mice from specified groups were sacrificed and the cells harvested from spleen and iliac lymph nodes (after challenge) were stimulated with 2 μg/ml antibody to CD28 and PmpG-1 protein (1 μg/ml) or HK-EBs (5 × 105 IFU/ml) in complete RPMI 1640 for 4 h at 37°C. Brefeldin A was added at a final concentration of 1 μg/ml, and cells were incubated for an additional 12 h before intracellular cytokine staining. Cells were surface stained for CD3, CD4, and CD8 as well as with the viability dye, red fluorescent reactive dye (RViD) (L23102; Molecular Probes), followed by staining for IFN-γ, TNF-α, and IL-17 by using the BD Cytoperm kit according to the manufacturer's instructions. Finally, the cells were resuspended in a 4% formaldehyde solution. All antibodies and all reagents for intracellular cytokine staining were purchased from BD PharMingen except where noted. We acquired 200,000 live lymphocytes per sample by using an Aria flow cytometer and analyzed the data by using FlowJo software (Tree Star).
Statistical analysis.
All data were analyzed with the aid of the GraphPad Prism software program. The Kruskal-Wallis test was performed to analyze data for IFU (Chlamydia shedding) from multiple groups, and the Mann-Whitney U test was used to compare medians between pairs. Two-way repeated-measure analysis of variance (ANOVA) was used to compare IFU values over the course of infection in BALB/c mice. Comparison of cytokine productions as determined by ELISPOT assay, ELISA, and flow cytometry in multiple groups were analyzed using one-way ANOVA followed by the Tukey posttest. P values of <0.05 were considered significant. Data are presented as means ± standard errors of the means (SEM). No statistic analyses were done for those data sets generated from lymph nodes, as they were pooled from each group.
RESULTS
Multiple Chlamydia antigens formulated with DDA/TDB exhibit protection against challenge with live C. muridarum.
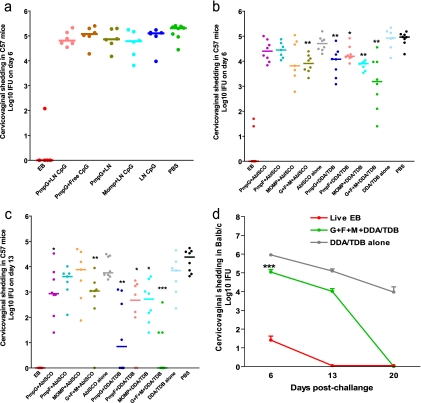
In order to discover a Th1-polarizing adjuvant that efficiently delivers Chlamydia antigens, we first tested mouse-specific CpG-ODN 1826. It was reported that immunostimulation by CpG-ODN was enhanced when the compound was encapsulated and delivered in lipid particles (29). In the current trial, mice were immunized with PmpG-1 or MOMP protein formulated with either a free form of CpG-ODN 1826 (free CpG) or a liposomal nanoparticle-conjugated form (LN-CpG). Mice immunized with PmpG-1 plus liposomal nanoparticles only (PmpG+LN), LN-CpG only, or PBS were used as negative controls, and mice who recovered from previous intranasal infection served as positive controls. Two weeks after the final immunization, mice were challenged vaginally with C. muridarum. Protection against intravaginal infection was assessed by isolation of Chlamydia from cervicovaginal wash and the determination of the number of IFU recovered from each experimental group at day 6 postinfection. As shown in Fig. 1a, mice immunized with live EBs exhibited excellent protection against infection, as indicated by very low-level Chlamydia detection or no Chlamydia detection. However, there were no significant differences between levels of cervicovaginal shedding of C. muridarum among all other groups (Fig. 1a), demonstrating that CpG-ODN formulated with PmpG-1 or MOMP failed to induce protection against Chlamydia infection.
FIG. 1.
Vaccine-elicited protection against Chlamydia genital tract infection. Mice were intravaginally challenged with 1,500 IFU of live C. muridarum after immunization with a variety of vaccine formulations. Cervicovaginal washes were taken at selected dates after infection, and bacterial titers were measured on HeLa 229 cells. *, **, and *** indicate P values of <0.05, <0.01, and <0.001, respectively, in comparison to the corresponding adjuvant-alone group. (a) Failure to induce protection after vaccination with PmpG-1 or MOMP protein formulated with CpG-ODN. (b and c) Resistance to Chlamydia infection in C57BL/6 (C57) mice immunized with PmpG-1, PmpE/F-2, and MOMP proteins or a combination of them formulated with adjuvant AbISCO-100 or DDA/TDB. Cervicovaginal washes were taken at day 6 (b) and day 13 (c) after infection. (d) Resistance to Chlamydia infection in BALB/c mice (n = 8) immunized with the combination of PmpG-1, PmpE/F-2, and MOMP proteins formulated with the adjuvant DDA/TDB.
In the next trial, we evaluated protection against Chlamydia infection in C57BL/6 mice immunized with individual PmpG-1, PmpE/F-2, and MOMP proteins or a combination of them formulated with adjuvant AbISCO-100 or DDA/TDB. After the genital challenge, we tested the Chlamydia inclusion titers in cervicovaginal washes taken at day 6 and day 13. Overall, the results indicate that DDA/TDB provided better protection than AbISCO. As shown in Fig. 1b, mice immunized with individual PmpG-1, PmpE/F-2, and MOMP proteins or a combination formulated with DDA/TDB demonstrated a significant reduction in Chlamydia titer at day 6 compared to that of the group with DDA/TDB adjuvant alone (P values of <0.01 for the PmpG+DDA/TDB group, <0.05 for the PmpF+DDA/TDB group, <0.01 for the MOMP+DDA/TDB group, and <0.01 in the G+F+M+DDA/TDB group). The antigen combination group tended to develop greater protection than individual antigen group, as indicated by much lower Chlamydia titers detected in some mice of the G+F+M+DDA/TDB group. Significant protection induced by AbISCO was observed only in the antigen combination group (P value of <0.01 in comparison to AbISCO alone) but not in the individual antigen groups. Data at day 13 (Fig. 1c) further confirmed the results obtained at day 6. Mice immunized with individual PmpG-1 protein or the combination of three Chlamydia proteins formulated with AbISCO exhibited significant protection at day 13 compared to the group with adjuvant alone (P values of <0.05 for the PmpG+AbISCO group and <0.01 for the G+F+M+AbISCO group). On the other hand, all DDA/TDB-formulated Chlamydia antigens conferred significant protection at day 13 compared to DDA/TDA alone, and vaccination with G+F+M+DDA/TDB provided the greatest degree of protective immunity among all the groups tested. Of interest, five out of eight mice vaccinated with G+F+M+DDA/TDB completely resolved the infection, and the other three mice in this group showed very low Chlamydia loads at day 13 (P values of <0.01 for the PmpG+DDA/TDB group, <0.05 for the PmpF+DDA/TDB group, <0.05 for the MOMP+DDA/TDB group, and <0.001 for the G+F+M+DDA/TDB group).
Since all the protection results described above were observed in C57BL/6 mice, the strain in which the antigens were originally discovered by use of immunoproteomics, we challenged mice with a different MHC genetic background to determine if immunization with multiple Chlamydia protein antigens and DDA/TDB conferred protection. BALB/c mice were immunized with G+F+M+DDA/TDB or DDA/TDB alone, and mice infected with live EBs were used as positive controls. Chlamydia inclusion titers in the cervicovaginal washes were detected postchallenge. As shown in Fig. 1d, BALB/c mice immunized with live EBs demonstrated excellent protection against infection, as indicated by very low bacterial loads at day 6 and no chlamydiae detected at day 13 and day 20. Vaccination with G+F+M+DDA/TDB in BALB/c mice significantly decreased the Chlamydia load in the cervicovaginal washes compared with DDA/TDB alone (P < 0.001). At day 20 after challenge, all BALB/c mice vaccinated with G+F+M+DDA/TDB completely resolved infection.
Among the three tested adjuvants CpG-ODN 1826, AbISCO-100, and DDA/TDB, the CpG-ODN formulation was not able to engender protection against Chlamydia infection at any level in vaccinated mice. The AbISCO formulation conferred moderate protection, while the DDA/TDB formulation showed the greatest efficacy. The combination of PmpG-1, PmpE/F-2, and MOMP formulated with DDA/TDB generated an additive or synergistic effect that exhibited the greatest degree of protective immunity among all groups studied. Moreover, G+F+M+DDA/TDB vaccination also gave a one-log reduction of infection burden at day 6 and a 5-log reduction at day 20 in BALB/c mice, with an MHC background different from that of C57BL/6 mice.
PmpG-1 formulated with DDA/TDB induced strong IFN-γ, TNF-α, and IL-17 responses, characterized by a high frequency of double-positive IFN-γ/TNF-α and IFN-γ/IL-17 CD4+ T cells in immunized mice.
In order to explore the cellular immune mechanisms for different degrees of protection induced by the three adjuvants, C57BL/6 mice were immunized with PmpG-1 formulated with DDA/TDB, AbISCO-100, and CpG-ODN 1826 and then challenged with live C. muridarum. The magnitude and quality of T cells producing IFN-γ, TNF-α, and IL-17 were assessed before and after challenge using an ELISPOT assay, ELISA, and multiparameter flow cytometry.
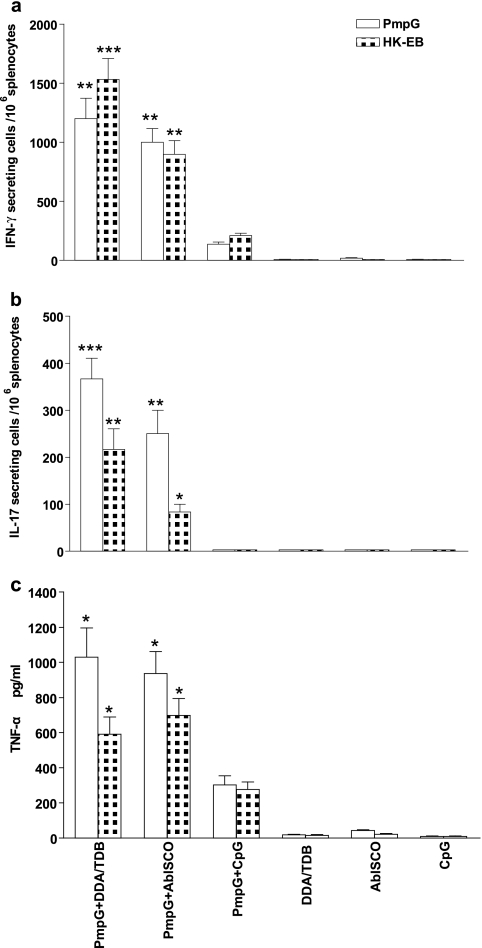
In this study, the ELISPOT assay was performed to detect IFN-γ- and IL-17-producing cells in immune splenocytes stimulated with PmpG-1 protein or HK-EBs. ELISA was performed to measure the TNF-α level in the supernatant of stimulated immune splenocytes.
After immunization with PmpG-1 formulated with DDA/TDB, AbISCO-100, or CpG-ODN 1826, splenocytes exhibited markedly different levels of IFN-γ (Fig. 2a), TNF-α (Fig. 2c), and IL-17 (Fig. 2b) responses. The PmpG+DDA/TDB immune splenocytes exposed to PmpG-1 protein or HK-EBs developed the highest numbers of IFN-γ- and IL-17-secreting cells; the PmpG+AbISCO immune splenocytes demonstrated less-strong IFN-γ and IL-17 responses but similar levels of TNF-α compared with those corresponding to PmpG+DDA/TDB immunization; and the weakest IFN-γ/TNF-α response (as well as an absent IL-17 response) was found following PmpG+CpG immunization. In addition, splenocytes from mice immunized with adjuvant alone, which served as negative controls, showed nearly blank background levels, indicating that cytokine responses detected in the experimental system are all Chlamydia Ag specific. The differing levels of IFN-γ and IL-17 responses in mice immunized with different adjuvants are remarkably consistent with the differing degrees of protection against challenge infection (Fig. 1), suggesting that a correlate of vaccine-mediated protection against Chlamydia is the magnitude of specific cytokine responses.
FIG. 2.
Chlamydia antigen-specific cytokine responses after immunization with PmpG-1 protein formulated with DDA/TDB, AbISCO, and CpG adjuvants. Two weeks after the final immunization, mouse splenocytes from different vaccine groups were harvested and stimulated with 1 μg/ml PmpG-1 protein or 5 × 105 inclusion-forming units (IFU)/ml HK-EBs. Adjuvants consisting of DDA/TDB alone, AbISCO alone, or CpG alone were used as negative controls. The results represent the averages of duplicate wells and are expressed as the means ± SEM for groups of six mice. *, **, and *** indicate P values of <0.05, <0.01, and <0.001, respectively, in comparison to the PmpG+CpG group. (a) IFN-γ responses to PmpG-1 or HK-EBs detected by ELISPOT assay. (b) IL-17 responses to PmpG-1 or HK-EBs detected by ELISPOT assay. (c) TNF-α response to PmpG-1 or HK-EBs detected by ELISA.
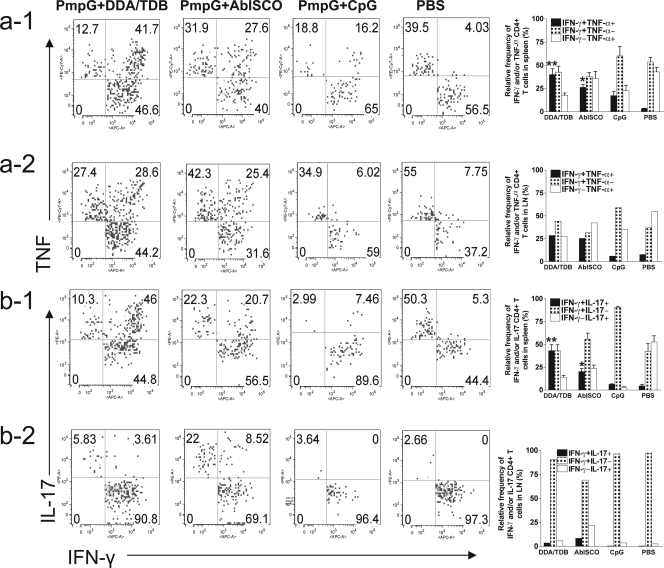
To characterize the distinct populations of Th1 and Th17 responses, multiparameter flow cytometry was used to simultaneously analyze multiple cytokines at the single-cell level. As shown in Fig. 3a, a seven-color flow cytometry panel and gating strategy was used to identify IFN-γ-, TNF-α-, and IL-17-producing CD4+ T cells in splenocytes from a representative mouse immunized with PmpG+DDA/TDB. Since an individual responding cell could be present in more than one of the total cytokine gates, we used Boolean combinations of the cytokine gates to discriminate responding cells based on their functionality or quality of IFN-γ/TNF-α (Fig. 3b) and IFN-γ/IL-17 (Fig. 3c) production.
FIG. 3.
Functional characterization of distinct populations of Chlamydia antigen-specific cytokine responses after immunization. Splenocytes from different vaccine groups before challenge were analyzed by multiparameter flow cytometry as described in Materials and Methods. Three or four mice were in each group. Shown are results representative of two experiments. * and ** indicate P values of <0.05 and <0.01, respectively, in comparison to the PmpG+CpG group. A, area; W, width; FSC, forward scatter; SSC, side scatter; PerCP, peridinin chlorophyll protein; APC, allophycocyanin; PE, phycoerythrin. (a) The staining panel and gating strategy used to identify IFN-γ-, TNF-α-, and IL-17-producing CD4+ T cells in the splenocytes from a representative mouse immunized with PmpG+DDA/TDB. (b) Comparison of the levels of quality of CD4+ IFN-γ/TNF-α responses to PmpG-1 protein (b-1) or HK-EBs (b-2) in different vaccine groups. (c) Comparison of the levels of quality of CD4+ IFN-γ/IL-17 responses to PmpG-1 protein (c-1) or HK-EBs (c-2) in different vaccine groups.
Using the Boolean combination of IFN-γ gating and TNF-α gating, frequencies of three distinct populations (IFN-γ positive/TNF-α negative [IFN-γ+ TNF-α−], IFN-γ− TNF-α+, and IFN-γ+ TNF-α+) from immune splenocytes stimulated with PmpG-1 and HK-EBs are shown in Fig. 3b-1 and -2, respectively. The results demonstrate that the response after immunization with PmpG+DDA/TDB was dominated by IFN-γ and TNF-α double-positive cells, and about half of the response in the PmpG+AbISCO group was IFN-γ and TNF-α double positive, whereas the PmpG+CpG vaccine induced the weakest IFN-γ and TNF-α double-positive response, with the single-positive response dominating. Importantly, this analysis also showed a correlation between the frequency of multifunctional (IFN-γ and TNF-α double positive) CD4+ T cells and the degree of protection in mice vaccinated with PmpG+DDA/TDB, PmpG+AbISCO, and PmpG+CpG.
The protective effects of the newly identified lineage of Th17 in host defense against bacterial, mycobacterial, and fungal mucosal pathogens have been reported previously (4, 44). In this study, the quality of IFN-γ/IL-17 cytokine response from immune splenocytes stimulated with PmpG (Fig. 3c-1) or HK-EBs (Fig. 3c-2) was also evaluated by multiparameter flow cytometry. Quantifying the fraction of IFN-γ/IL-17 response, we found that over half of the response in the best-protected group (PmpG+DDA/TDB) was IFN-γ and IL-17 double positive; the PmpG+AbISCO group had a moderate IFN-γ and IL-17 double-positive response. The no-protection group (PmpG+CpG) did not develop a detectable IL-17 response. These data also indicate a correlation between the degree of protection in vaccinated mice and the frequency of IFN-γ and IL-17 double-positive CD4+ T cells.
The magnitude and quality of IFN-γ, TNF-α, and IL-17 responses in spleens and lymph nodes after challenge infection.
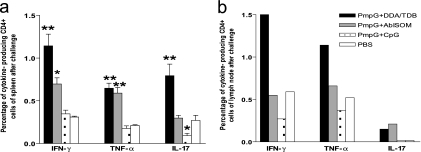
To define the magnitude of the response on day 7 after C. muridarum challenge, the frequencies of the total PmpG-specific CD4+ T-cell cytokine responses comprising IFN-γ-, TNF-α-, and IL-17-producing cells in spleens (Fig. 4a) and draining lymph nodes (iliac lymph nodes) (Fig. 4b) from each vaccine group are presented. The results demonstrate that among spleen cells, immunization with PmpG+DDA/TDB induced statistically higher frequencies of IFN-γ- and IL-17-producing CD4+ T cells. The PmpG+AbISCO vaccine induced a frequency of TNF-α-producing cells similar to that of the PmpG+DDA/TDB vaccine but lower frequencies of IFN-γ- and IL-17-producing cells. The PmpG+CpG and PBS groups developed similar frequencies of IFN-γ- and TNF-α-producing cells, but these were the lowest frequencies. Notably, PmpG+CpG induced an extremely weak IL-17 response, while PBS induced a response about one-third of the magnitude of the IL-17 response of the PmpG+DDA/TDB group (Fig. 4a).
FIG. 4.
The magnitude of Chlamydia antigen-specific cytokine responses in spleens and draining lymph nodes after challenge. Splenocytes and pooled draining lymph nodes (iliac lymph node) from different vaccine groups after challenge were stimulated with PmpG-1 and analyzed by multiparameter flow cytometry as described in Materials and Methods. Four mice were studied in each group. * and ** indicate P values of <0.05 and <0.01, respectively, in comparison to the PBS group. Shown are results representative of two experiments. (a) The percentage of IFN-γ-, TNF-α-, or IL-17-producing CD4+ T cells in spleens. (b) The percentage of IFN-γ-, TNF-α-, or IL-17-producing CD4+ T cells in pooled iliac lymph nodes.
The data from pooled regional draining lymph node cells following genital challenge showed that prior immunization with PmpG+DDA/TDB resulted in strong IFN-γ and TNF-α responses. The PmpG+AbISCO and PBS groups developed similar moderate IFN-γ and TNF-α responses. The PmpG+CpG group had the weakest IFN-γ and TNF-α responses. Surprisingly, and contrary to the spleen cell results, the IL-17 responses in lymph nodes were very low in the PmpG+DDA/TDB and PmpG+AbISCO groups, and no IL-17-producing cells were observed in the PmpG+CpG and PBS groups (Fig. 4b).
We further analyzed the quality of cytokine-producing cells in spleens and iliac lymph nodes from immunized mice at day 7 following genital challenge. Immunization with PmpG+DDA/TDB induced the strongest IFN-γ and TNF-α double-positive response (Fig. 5a -1) in spleens and an IFN-γ and TNF-α double-positive response similar to that induced by PmpG+AbISCO (Fig. 5a-2) in lymph nodes. We found very few or no IFN-γ and TNF-α double-positive cells in the PmpG+CpG group and in the PBS group (Fig. 5a-1 and -2). Analysis of the IFN-γ/IL-17 response in spleen (Fig. 5b-1) after challenge revealed the strongest IFN-γ and IL-17 double-positive response in the PmpG+DDA/TDB group, a moderate response in the PmpG+AbISCO group, and the weakest response in the PmpG+CpG group. These findings show a pattern similar to what was seen prechallenge (Fig. 3). However, low-level-IL-17-producing cells, and especially few IFN-γ/IL-17 double-positive cells, were detected in the lymph nodes (Fig. 5b-2) after challenge. The PBS group developed IFN-γ, TNF-α, and IL-17 responses after challenge, although all three types of cytokine-producing cells in this group were singly positive in both spleens and lymph nodes (Fig. 4a and b).
FIG. 5.
The quality of Chlamydia antigen-specific cytokine responses in spleen and draining lymph node after challenge. Splenocytes and pooled draining lymph nodes (iliac lymph nodes) from different vaccine groups after challenge were stimulated with PmpG-1 and analyzed by multiparameter flow cytometry as described in Materials and Methods. Four mice were studied in each group. * and ** indicate P values of <0.05 and <0.01, respectively, in comparison to the PBS group. Shown are results representative of two experiments. (a) Comparison of the levels of quality of CD4+ IFN-γ/TNF-α responses to PmpG-1 protein in spleens (a-1) and iliac lymph nodes (a-2) from different vaccine groups. (b) Comparison of the levels of quality of CD4+ IFN-γ/IL-17 responses to PmpG-1 protein in spleens (b-1) and pooled iliac lymph nodes (b-2) from different vaccine groups.
DISCUSSION
Studies in animal models and during human infection have established that Chlamydia-specific CD4+ T cells producing IFN-γ are critically involved in the clearance of infection. Therefore, selection of protective T-cell antigens that stimulate CD4+ Th1 cells for a subunit vaccine is central to the current vaccine design effort. Using an immunoproteomic approach, we identified three immunodominant Chlamydia T-cell antigens, PmpG-1, PmpE/F-2, and RplF, and vaccination with DCs transfected with these three Chlamydia proteins induced significant protection against Chlamydia infection. For the development of a feasible vaccine, the search for an optimal adjuvant that efficiently delivers Chlamydia antigens is essential. In the present study, we tested three adjuvants, DDA/TDB, AbISCO, and CpG, and found that the combination of PmpG-1, PmpE/F-2, and MOMP proteins formulated with DDA/TDB elicited the greatest degree of protection among all groups studied. The CpG formulation with different antigens, including MOMP, did not give any protection. This was surprising, since Pal et al. previously showed that CpG-Montanide ISA 720, when combined with MOMP, gave protection from infection and pathology (32). The G+F+M+DDA/TDB formulation also provided protection in BALB/c mice, indicating that T-cell antigens identified by immunoproteomics in C57BL/6 mice were also recognized by Chlamydia-specific T cells with a different MHC genetic background. The superior protection conferred onto the protein combination group relative to that of the individual protein groups suggests that a successful Chlamydia vaccine will need to be composed of multiple proteins in order to provide broad coverage in an outbred population and to cross-protect against multiple variants of C. trachomatis.
It is commonly assumed that Chlamydia vaccine studies require Th1 responses as measured by the frequency and magnitude of IFN-γ-producing cells as the primary immune correlate of protection. Although IFN-γ is clearly necessary, using it as a single immune parameter may not be sufficient to predict protection. Recently, a number of reports dealing with various disease targets in different animal models have demonstrated a correlation between protection and high-quality T cells that coexpress multiple cytokines (10, 46). TNF is another effector cytokine produced by Th1 cells that can mediate control of intracellular infection, including that due to Chlamydia (23, 30, 35). In the present study, we assessed antigen-specific IFN-γ- and TNF-α-producing CD4+ T cells in vaccinated mice. Our data demonstrated that the PmpG+DDA/TDA group that conferred the greatest protection showed the highest frequency of double-positive IFN-γ and TNF-α CD4+ T cells as well as the highest magnitude of IFN-γ production. These data suggest that there is an association between IFN-γ and TNF-α double-positive CD4+ T cells and protection against Chlamydia infection. IFN-γ and TNF-α double-positive CD4+ T cells may provide a more accurate predictor of Chlamydia vaccine-primed protective immunity than solely IFN-γ-producing CD4+ T cells.
Th17 cells are known to play important roles in mucosal defense against infection with extracellular bacterial and fungal pathogens by recruiting neutrophils into local sites of infection. Recently, several studies also indicated that Th17 augments host defense against intracellular bacteria like Mycobacterium tuberculosis, Listeria monocytogenes, and Salmonella enterica (20, 26, 37). In the present study, we investigated Th17 cells in Chlamydia vaccine-primed protective immunity. We found that the PmpG+DDA/TDA group, which exhibited the greatest protection among the three adjuvant formulations, also showed the highest magnitude of IL-17 production before and after challenge. Importantly, this group also developed the highest frequency of IFN-γ and IL-17 double-positive CD4+ T cells, whereas the CpG group or the PBS group, with no protection, induced very low levels of IFN-γ and IL-17 double-positive CD4+ T cells or none. These results indicate that IL-17 may play cooperative roles with IFN-γ in vaccine-primed protective immunity against Chlamydia. Our data also suggest a potential protective role for Chlamydia-specific IFN-γ and IL-17 double-positive CD4+ T cells as effector T cells against Chlamydia infection.
Generally, Th1 and Th17 cells represent separate and distinct CD4 T-cell lineages that individually produce IFN-γ and IL-17, respectively. To date, most in vitro studies demonstrated that cytokines that promote CD4 T-cell differentiation into each lineage not only strongly reinforce differentiation of other cells into the same lineage but also potently inhibit differentiation into other CD4 lineages. In this study, however, we demonstrated that IFN-γ and IL-17 were coexpressed in single cells in a manner similar to what has been found in two other studies that observed IFN-γ and IL-17 double-positive cells. One study on human Th17 identified the existence of a remarkable number of double-positive (IFN-γ/IL-17) freshly derived cells or T-cell clones. The incubation of Th17 clones with IL-12 allowed these cells to produce IFN-γ in addition to IL-17, and this effect was associated with reduced Th17 transcription factor RORγt and increased Th1 transcription factor T-bet expression, suggesting the existence of flexibility between Th1 and Th17 cells and a possible developmental relationship between the two cell types (2). A recent study using a rodent model of autoimmune encephalomyelitis observed IFN-γ and IL-17 double-positive cells and demonstrated that T-bet expression was critical for cell pathogenicity, regardless of cytokine expression by the encephalitogenic T cells. These data suggest that encephalitogenicity of myelin-specific T cells appears to be mediated by a pathway dependent on T-bet and not necessarily pathway-specific cytokine end products, such as interferon and IL-17 (48). In the present study, even though we observed a correlation between double-positive cells and protection, we are unclear whether there is cause-effect relationship between them.
Recent studies on Chlamydia showed the importance of IL-17 in both innate immunity and adaptive immunity. The mechanism of IL-17-mediated protection against Chlamydia infection involves neutrophil induction to infected sites and regulation of DC function and Th1 responses through cytokines and chemokines induced by Th17 or other IL-17-producing cells (3, 50). Since these studies were conducted using the murine lung model, it is uncertain whether the same protective role for IL-17-producing T cells will be seen in the genital tract model. In a vaccination model targeting a protective antigen from Mycobacterium tuberculosis, it was demonstrated that mycobacterial Ag-specific Th17 responses enhance protective immunity through induction of chemokines (CXCR3 ligands CXCL9, CXCL10, and CXCL11) that recruit mycobacterial Ag-specific protective Th1 T cells into the infected lung and inhibit bacterial growth (20). Therefore, vaccination that induces both IFN-γ and IL-17 could represent a new strategy to prevent Chlamydia infection. However, uncontrolled production of IL-17 may also lead to immune pathology in infected organs (51). We need further information on the regulation of Th17, innate IL-17-producing cells, and the effects of IL-17 itself to establish safe and effective methods to utilize IL-17.
It is of interest that we did not observe IL-17-producing T cells in lymph nodes. This may be due to proliferative and trafficking kinetics differing between IL-17- and IFN-γ-producing T cells. We observed that in immune and immunized mice, the number of IL-17-secreting cells in spleens as determined by intracellular staining declined dramatically 4 h after antigen stimulation in vitro. In contrast, the number of IFN-γ-secreting cells continued to increase even after 24 h following antigen stimulation (unpublished data). Since cells in the draining lymph nodes harvested after challenge had already contacted Chlamydia antigens for 7 days in vivo, we may have been unable to detect IL-17-secreting cells in vitro because of these kinetic differences. The other possible explanation may be that IL-17-producing cells directly trafficked to the mucosal surface of the oviduct.
DDA/TDB is a recently discovered adjuvant that consists of liposomes and a synthetic mycobacterial cord factor as an immune modulator. The small cationic molecule DDA forms liposomes acting as an antigen “depot” to ensure the long-term release of antigen. Incorporation of TDB into the liposome bilayers effectively stabilizes the DDA liposomes (11). When injected subcutaneously, DDA forms depots that last up to 60 days in mice without any evidence of overt toxicity. One recent study reported that TDB selectively activates the FcRγ-Syk-Card9 pathway in antigen-presenting cells to induce a unique innate immune activation program that directs protective Th1 and Th17 immunity after subunit Mycobacterium tuberculosis vaccination (45), demonstrating that the TDB adjuvant with this mode of action is distinct from TLR-triggering adjuvants engaging Myd88 (CpG and IC31) (1, 22) or Trif (monophosphoryl lipid A) (25). Ishikawa et al. recently reported that the monocyte-inducible C-type lectin (Mincle) is the essential cell receptor for TDB (17). According to the current concept of murine and human Th17 differentiation, TDB likely operates through the combined induction of IL-1β, -6, and -23 from antigen-presenting cells, whereas CpG may inhibit Th17 differentiation through IL-12 and IFN-γ (9, 43). This may explain why the Th17 Chlamydia antigen-specific response was not found in mice vaccinated with the CpG formulation.
Since PmpG-1 is an outer membrane protein, we tested PmpG-1-specific antibody responses, and the results showed that all adjuvant formulations (DDA/TDB, ABISCO, and CpG) induced very high total IgG, IgG1, and IgG2a responses to recombinant PmpG-1 antigen. Using these sera, we failed to detect neutralizing antibody responses by using C. muridarum in HeLa cell culture (data not shown). These data suggest that protection is not correlated with antibody responses.
While the vaccine formulated with the three Chlamydia antigens and DDA/TDB adjuvant generated statistically significant protection against infection, it remains important to evaluate protection against oviductal pathology. We therefore have initiated experiments in mice immunized with G+F+M/DDA-TDB in order to examine for pathology as determined by visually apparent hydrosalpinx in one or both oviducts. Pathological results similar to those reported by Hansen et al., using the same adjuvant with MOMP as an antigen (13), were observed in the majority of mice 60 days after challenge infection (data not shown). Importantly, mice primed by a prior intranasal infection were completely protected against the development of oviductal pathology following intravaginal challenge with Chlamydia. It remains important to discover the mechanism behind the different outcomes for oviductal pathology following infection-induced immunity and vaccine-induced immunity. Clearly, an ongoing challenge for Chlamydia vaccine research remains the discovery of strategies that maximize the protective effects of immune T cells while simultaneously preventing such cells from causing immune response-mediated tissue damage.
In conclusion, our study demonstrated that a Chlamydia vaccine based on the multiple purified recombinant proteins (PmpG-1, PmpE/F-2, and MOMP) formulated with a liposome adjuvant, DDA/TDB, efficiently protects against vaginal infection with C. muridarum. This protection correlates with strong IFN-γ, TNF-α, and IL-17 responses characterized by the high frequency of IFN-γ/TNF-α and IFN-γ/IL-17 double-positive CD4+ T cells.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (grant no. R01AI076483).
We are grateful to Lindsey Laycock, David Ko, and Gayle Thornbury in the Terry Fox Laboratory at the British Columbia Cancer Research Centre for providing flow cytometry services and technical assistance and to Norbert Maurer and Kaley D. Wilson at the Centre for Drug Research and Development, Vancouver, for providing liposomal nanoparticle-conjugated CpG.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 15 March 2010.
REFERENCES
- 1.Agger, E. M., I. Rosenkrands, A. W. Olsen, G. Hatch, A. Williams, C. Kritsch, K. Lingnau, A. von Gabain, C. S. Andersen, K. S. Korsholm, and P. Andersen. 2006. Protective immunity to tuberculosis with Ag85B-ESAT-6 in a synthetic cationic adjuvant system IC31. Vaccine 24:5452-5460. [DOI] [PubMed] [Google Scholar]
- 2.Annunziato, F., L. Cosmi, V. Santarlasci, L. Maggi, F. Liotta, B. Mazzinghi, E. Parente, L. Fili, S. Ferri, F. Frosali, F. Giudici, P. Romagnani, P. Parronchi, F. Tonelli, E. Maggi, and S. Romagnani. 2007. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 204:1849-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai, H., J. Cheng, X. Gao, A. G. Joyee, Y. Fan, S. Wang, L. Jiao, Z. Yao, and X. Yang. 2009. IL-17/Th17 promotes type 1 T cell immunity against pulmonary intracellular bacterial infection through modulating dendritic cell function. J. Immunol. 183:5886-5895. [DOI] [PubMed] [Google Scholar]
- 4.Bettelli, E., T. Korn, and V. K. Kuchroo. 2007. Th17: the third member of the effector T cell trilogy. Curr. Opin. Immunol. 19:652-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilenki, L., S. Wang, J. Yang, Y. Fan, A. G. Joyee, and X. Yang. 2005. NK T cell activation promotes Chlamydia trachomatis infection in vivo. J. Immunol. 175:3197-3206. [DOI] [PubMed] [Google Scholar]
- 6.Brunham, R. C., B. Pourbohloul, S. Mak, R. White, and M. L. Rekart. 2005. The unexpected impact of a Chlamydia trachomatis infection control program on susceptibility to reinfection. J. Infect. Dis. 192:1836-1844. [DOI] [PubMed] [Google Scholar]
- 7.Brunham, R. C., and J. Rey-Ladino. 2005. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat. Rev. Immunol. 5:149-161. [DOI] [PubMed] [Google Scholar]
- 8.Caldwell, H. D., J. Kromhout, and J. Schachter. 1981. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect. Immun. 31:1161-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu, R. S., O. S. Targoni, A. M. Krieg, P. V. Lehmann, and C. V. Harding. 1997. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J. Exp. Med. 186:1623-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darrah, P. A., D. T. Patel, P. M. De Luca, R. W. Lindsay, D. F. Davey, B. J. Flynn, S. T. Hoff, P. Andersen, S. G. Reed, S. L. Morris, M. Roederer, and R. A. Seder. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13:843-850. [DOI] [PubMed] [Google Scholar]
- 11.Davidsen, J., I. Rosenkrands, D. Christensen, A. Vangala, D. Kirby, Y. Perrie, E. M. Agger, and P. Andersen. 2005. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6′-dibehenate)-a novel adjuvant inducing both strong CMI and antibody responses. Biochim. Biophys. Acta 1718:22-31. [DOI] [PubMed] [Google Scholar]
- 12.Grayston, J. T., and S. P. Wang. 1978. The potential for vaccine against infection of the genital tract with Chlamydia trachomatis. Sex. Transm. Dis. 5:73-77. [DOI] [PubMed] [Google Scholar]
- 13.Hansen, J., K. T. Jensen, F. Follmann, E. M. Agger, M. Theisen, and P. Andersen. 2008. Liposome delivery of Chlamydia muridarum major outer membrane protein primes a Th1 response that protects against genital chlamydial infection in a mouse model. J. Infect. Dis. 198:758-767. [DOI] [PubMed] [Google Scholar]
- 14.Holten-Andersen, L., T. M. Doherty, K. S. Korsholm, and P. Andersen. 2004. Combination of the cationic surfactant dimethyl dioctadecyl ammonium bromide and synthetic mycobacterial cord factor as an efficient adjuvant for tuberculosis subunit vaccines. Infect. Immun. 72:1608-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Igietseme, J. U., F. O. Eko, and C. M. Black. 2003. Contemporary approaches to designing and evaluating vaccines against Chlamydia. Expert Rev. Vaccines 2:129-146. [DOI] [PubMed] [Google Scholar]
- 16.Ioannou, X. P., P. Griebel, R. Hecker, L. A. Babiuk, and S. van Drunen Littel-van den Hurk. 2002. The immunogenicity and protective efficacy of bovine herpesvirus 1 glycoprotein D plus Emulsigen are increased by formulation with CpG oligodeoxynucleotides. J. Virol. 76:9002-9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikawa, E., T. Ishikawa, Y. S. Morita, K. Toyonaga, H. Yamada, O. Takeuchi, T. Kinoshita, S. Akira, Y. Yoshikai, and S. Yamasaki. 2009. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J. Exp. Med. 206:2879-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kari, L., W. M. Whitmire, D. D. Crane, N. Reveneau, J. H. Carlson, M. M. Goheen, E. M. Peterson, S. Pal, L. M. de la Maza, and H. D. Caldwell. 2009. Chlamydia trachomatis native major outer membrane protein induces partial protection in nonhuman primates: implication for a trachoma transmission-blocking vaccine. J. Immunol. 182:8063-8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karunakaran, K. P., J. Rey-Ladino, N. Stoynov, K. Berg, C. Shen, X. Jiang, B. R. Gabel, H. Yu, L. J. Foster, and R. C. Brunham. 2008. Immunoproteomic discovery of novel T cell antigens from the obligate intracellular pathogen Chlamydia. J. Immunol. 180:2459-2465. [DOI] [PubMed] [Google Scholar]
- 20.Khader, S. A., G. K. Bell, J. E. Pearl, J. J. Fountain, J. Rangel-Moreno, G. E. Cilley, F. Shen, S. M. Eaton, S. L. Gaffen, S. L. Swain, R. M. Locksley, L. Haynes, T. D. Randall, and A. M. Cooper. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8:369-377. [DOI] [PubMed] [Google Scholar]
- 21.Klinman, D. M., D. Currie, I. Gursel, and D. Verthelyi. 2004. Use of CpG oligodeoxynucleotides as immune adjuvants. Immunol. Rev. 199:201-216. [DOI] [PubMed] [Google Scholar]
- 22.Kwissa, M., R. R. Amara, H. L. Robinson, B. Moss, S. Alkan, A. Jabbar, F. Villinger, and B. Pulendran. 2007. Adjuvanting a DNA vaccine with a TLR9 ligand plus Flt3 ligand results in enhanced cellular immunity against the simian immunodeficiency virus. J. Exp. Med. 204:2733-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liew, F. Y., Y. Li, and S. Millott. 1990. Tumor necrosis factor-alpha synergizes with IFN-gamma in mediating killing of Leishmania major through the induction of nitric oxide. J. Immunol. 145:4306-4310. [PubMed] [Google Scholar]
- 24.Martina, B. E., M. W. van de Bildt, T. Kuiken, G. van Amerongen, and A. D. Osterhaus. 2003. Immunogenicity and efficacy of recombinant subunit vaccines against phocid herpesvirus type 1. Vaccine 21:2433-2440. [DOI] [PubMed] [Google Scholar]
- 25.Mata-Haro, V., C. Cekic, M. Martin, P. M. Chilton, C. R. Casella, and T. C. Mitchell. 2007. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science 316:1628-1632. [DOI] [PubMed] [Google Scholar]
- 26.Miyamoto, M., M. Emoto, Y. Emoto, V. Brinkmann, I. Yoshizawa, P. Seiler, P. Aichele, E. Kita, and S. H. Kaufmann. 2003. Neutrophilia in LFA-1-deficient mice confers resistance to listeriosis: possible contribution of granulocyte-colony-stimulating factor and IL-17. J. Immunol. 170:5228-5234. [DOI] [PubMed] [Google Scholar]
- 27.Mooij, P., I. G. Nieuwenhuis, C. J. Knoop, R. W. Doms, W. M. Bogers, P. J. Ten Haaft, H. Niphuis, W. Koornstra, K. Bieler, J. Kostler, B. Morein, A. Cafaro, B. Ensoli, R. Wagner, and J. L. Heeney. 2004. Qualitative T-helper responses to multiple viral antigens correlate with vaccine-induced immunity to simian/human immunodeficiency virus infection. J. Virol. 78:3333-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison, S. G., H. Su, H. D. Caldwell, and R. P. Morrison. 2000. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4(+) T cells but not CD8(+) T cells. Infect. Immun. 68:6979-6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mui, B., S. G. Raney, S. C. Semple, and M. J. Hope. 2001. Immune stimulation by a CpG-containing oligodeoxynucleotide is enhanced when encapsulated and delivered in lipid particles. J. Pharmacol. Exp. Ther. 298:1185-1192. [PubMed] [Google Scholar]
- 30.Njau, F., U. Wittkop, M. Rohde, H. Haller, A. Klos, and A. D. Wagner. 2009. In vitro neutralization of tumor necrosis factor-alpha during Chlamydia pneumoniae infection impairs dendritic cells maturation/function and increases chlamydial progeny. FEMS Immunol. Med. Microbiol. 55:215-225. [DOI] [PubMed] [Google Scholar]
- 31.Olds, G. R., L. Chedid, E. Lederer, and A. A. Mahmoud. 1980. Induction of resistance to Schistosoma mansoni by natural cord factor and synthetic lower homologues. J. Infect. Dis. 141:473-478. [DOI] [PubMed] [Google Scholar]
- 32.Pal, S., E. M. Peterson, and L. M. de la Maza. 2005. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect. Immun. 73:8153-8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pal, S., E. M. Peterson, R. Rappuoli, G. Ratti, and L. M. de la Maza. 2006. Immunization with the Chlamydia trachomatis major outer membrane protein, using adjuvants developed for human vaccines, can induce partial protection in a mouse model against a genital challenge. Vaccine 24:766-775. [DOI] [PubMed] [Google Scholar]
- 34.Pal, S., I. Theodor, E. M. Peterson, and L. M. de la Maza. 2001. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein can elicit a protective immune response against a genital challenge. Infect. Immun. 69:6240-6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perry, L. L., H. Su, K. Feilzer, R. Messer, S. Hughes, W. Whitmire, and H. D. Caldwell. 1999. Differential sensitivity of distinct Chlamydia trachomatis isolates to IFN-gamma-mediated inhibition. J. Immunol. 162:3541-3548. [PubMed] [Google Scholar]
- 36.Pimm, M. V., R. W. Baldwin, J. Polonsky, and E. Lederer. 1979. Immunotherapy of an ascitic rat hepatoma with cord factor (trehalose-6, 6′-dimycolate) and synthetic analogues. Int. J. Cancer 24:780-785. [DOI] [PubMed] [Google Scholar]
- 37.Schulz, S. M., G. Kohler, C. Holscher, Y. Iwakura, and G. Alber. 2008. IL-17A is produced by Th17, gammadelta T cells and other CD4-lymphocytes during infection with Salmonella enterica serovar Enteritidis and has a mild effect in bacterial clearance. Int. Immunol. 20:1129-1138. [DOI] [PubMed] [Google Scholar]
- 38.Stanberry, L. R., and S. L. Rosenthal. 2005. Progress in vaccines for sexually transmitted diseases. Infect. Dis. Clin. North Am. 19:477-490, xi. [DOI] [PubMed] [Google Scholar]
- 39.Starnbach, M. N., and N. R. Roan. 2008. Conquering sexually transmitted diseases. Nat. Rev. Immunol. 8:313-317. [DOI] [PubMed] [Google Scholar]
- 40.Su, H., and H. D. Caldwell. 1995. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect. Immun. 63:3302-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang, S., Y. Fan, R. C. Brunham, and X. Yang. 1999. IFN-gamma knockout mice show Th2-associated delayed-type hypersensitivity and the inflammatory cells fail to localize and control chlamydial infection. Eur. J. Immunol. 29:3782-3792. [DOI] [PubMed] [Google Scholar]
- 42.Wang, S. P., J. T. Grayston, and E. R. Alexander. 1967. Trachoma vaccine studies in monkeys. Am. J. Ophthalmol. 63(Suppl.):1615-1630. [DOI] [PubMed] [Google Scholar]
- 43.Weaver, C. T., L. E. Harrington, P. R. Mangan, M. Gavrieli, and K. M. Murphy. 2006. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity 24:677-688. [DOI] [PubMed] [Google Scholar]
- 44.Weaver, C. T., R. D. Hatton, P. R. Mangan, and L. E. Harrington. 2007. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 25:821-852. [DOI] [PubMed] [Google Scholar]
- 45.Werninghaus, K., A. Babiak, O. Gross, C. Holscher, H. Dietrich, E. M. Agger, J. Mages, A. Mocsai, H. Schoenen, K. Finger, F. Nimmerjahn, G. D. Brown, C. Kirschning, A. Heit, P. Andersen, H. Wagner, J. Ruland, and R. Lang. 2009. Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRgamma-Syk-Card9-dependent innate immune activation. J. Exp. Med. 206:89-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wille-Reece, U., B. J. Flynn, K. Lore, R. A. Koup, A. P. Miles, A. Saul, R. M. Kedl, J. J. Mattapallil, W. R. Weiss, M. Roederer, and R. A. Seder. 2006. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J. Exp. Med. 203:1249-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang, X., K. T. HayGlass, and R. C. Brunham. 1996. Genetically determined differences in IL-10 and IFN-gamma responses correlate with clearance of Chlamydia trachomatis mouse pneumonitis infection. J. Immunol. 156:4338-4344. [PubMed] [Google Scholar]
- 48.Yang, Y., J. Weiner, Y. Liu, A. J. Smith, D. J. Huss, R. Winger, H. Peng, P. D. Cravens, M. K. Racke, and A. E. Lovett-Racke. 2009. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. J. Exp. Med. 206:1549-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu, H., X. Jiang, C. Shen, K. P. Karunakaran, and R. C. Brunham. 2009. Novel Chlamydia muridarum T cell antigens induce protective immunity against lung and genital tract infection in murine models. J. Immunol. 182:1602-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang, X., L. Gao, L. Lei, Y. Zhong, P. Dube, M. T. Berton, B. Arulanandam, J. Zhang, and G. Zhong. 2009. A MyD88-dependent early IL-17 production protects mice against airway infection with the obligate intracellular pathogen Chlamydia muridarum. J. Immunol. 183:1291-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou, X., Q. Chen, J. Moore, J. K. Kolls, S. Halperin, and J. Wang. 2009. Critical role of the interleukin-17/interleukin-17 receptor axis in regulating host susceptibility to respiratory infection with Chlamydia species. Infect. Immun. 77:5059-5070. [DOI] [PMC free article] [PubMed] [Google Scholar]