Abstract
Immunization with live Plasmodium sporozoites under chloroquine prophylaxis (Spz plus CQ) induces sterile immunity against sporozoite challenge in rodents and, more importantly, in humans. Full protection is obtained with substantially fewer parasites than with the classic immunization with radiation-attenuated sporozoites. The sterile protection observed comprised a massive reduction in the hepatic parasite load and an additional effect at the blood stage level. Differences in the immune responses induced by the two protocols occur but are as yet little characterized. We have previously demonstrated that in mice immunized with irradiated sporozoites, immune responses against the circumsporozoite protein (CSP), the major component of the sporozoite's surface and the leading malaria vaccine candidate, were not essential for sterile protection. Here, we have employed transgenic Plasmodium berghei parasites in which the endogenous CSP was replaced by that of Plasmodium yoelii, another rodent malaria species, to assess the role of CSP in the sterile protection induced by the Spz-plus-CQ protocol. The data demonstrated that this role was minor because sterile immunity was obtained irrespective of the origin of CSP expressed by the parasites in this model of protection. The immunity was obtained through a single transient exposure of the host to the immunizing parasites (preerythrocytic and erythrocytic), a dose much smaller than that required for immunization with radiation-attenuated sporozoites.
Plasmodium species undergo an obligatory initial developmental stage in the liver that leads to the pathogenic erythrocytic phase of the infection. Effective inhibition of sporozoites (Spz) and hepatic parasites, the preerythrocytic (PE) stages, prevents blood infection and consequently disease and transmission. However, immunity against PE parasites must be total, because productive infection of a single hepatocyte can lead to a patent blood infection. Three vaccination protocols have been shown to confer sterile immunity (absence of blood stage parasites after challenge with sporozoites) against PE stages. First, immunization with radiation-attenuated sporozoites (RAS) has long been the gold standard for the induction of sterile immunity in rodents, monkeys, and humans (12, 19). Second, immunization with a smaller number of live sporozoites under chloroquine prophylaxis (Spz plus CQ) equally conferred sterile protection against sporozoite challenge in mice (2, 3). Recently, full protection was also obtained in human volunteers immunized by the bites of 15 Plasmodium falciparum-infected mosquitoes while under chloroquine prophylaxis (23). Finally, immunization with genetically attenuated sporozoites (GAS) was shown to be effective at fully protecting mice from sporozoite infections (16, 31). In hosts immunized with irradiated sporozoites, persistence of developmentally arrested early liver stages is thought to be important, because primaquine treatment abolishes protection (25). For vaccination with live sporozoites under drug cover, few if any hepatic parasites are expected to persist, but still, the liver parasites are necessary for the induction of immunity (3). In vaccination with GAS, liver parasite numbers waned rapidly, becoming undetectable 4 to 5 days postinoculation (16). In all three models of protection, antigens expressed during the liver stages appeared to be crucial for the induction and maintenance of sterile immunity. The circumsporozoite protein (CSP), a protein expressed by sporozoites and early liver forms, has long been considered the antigen that contributes most to the protective responses induced, justifying its status as the major vaccine candidate (reviewed in reference 18). Nonetheless, two recent studies demonstrated that protection induced by RAS could be obtained in the absence of significant responses against CSP (10, 15). We wished to characterize the immune responses induced against CSP in mice immunized by Spz plus CQ and to ascertain whether they played an important role in the sterile protection obtained. In order to do so, we used two rodent malaria parasite species, Plasmodium berghei and Plasmodium yoelii, and a transgenic line of P. berghei in which the endogenous csp gene had been replaced by csp of P. yoelii (P. berghei[PyCS]). In this manner, the contribution of CSP alone to protection could be assessed.
MATERIALS AND METHODS
Mice and Plasmodium sporozoites.
BALB/c J female mice were purchased from Harlan Laboratories (Gannat, France) and were housed in a pathogen-free rodent barrier facility. All experiments and procedures involving mice were approved by the Direction Départementale des Service Vétérinaires de Paris, France (authorization no. 75-129) and performed in compliance with the regulations of the French Ministry of Agriculture for animal experimentation (1987). A P. berghei ANKA cloned line was used to derive P. berghei[PyCS] (29), and the line of P. berghei ANKA expressing the green fluorescent protein line used in this study was submitted to the same selection procedures that were used to obtain the P. berghei[PyCS] parasites used (7). The infectivity and development of P. berghei[PyCS] sporozoites were similar to those of P. berghei sporozoites in the mosquito and in the mouse, as described previously (29). Anopheles stephensi mosquitoes were fed on infected mice and maintained for 15 days at 24°C in the case of the P. yoelii yoelii 17XNL clone 1.1 line and for 21 days at 21°C for P. berghei strains before dissection of the salivary glands to isolate the sporozoites.
Immunization and challenge.
Mice were injected intravenously (i.v.) with one dose of 20,000 sporozoites. One hundred microliters of an 8-mg/ml chloroquine hydrochloride (CQ) (Sigma) solution in phosphate-buffered saline (PBS) was injected intraperitoneally for 10 consecutive days, starting the same day as sporozoite inoculation, into both immunized and control mice. This regimen induced sterile immunity in 80 to 100% of the mice (3). Control and immunized mice were challenged intravenously with 100 P. yoelii Spz or 5,000 P. berghei Spz at least 15 days after the last injection of CQ. Because of differences in the infectivities of sporozoites from these Plasmodium species, the doses were chosen to induce infection in all control mice. Successful infection was determined by the presence of parasites in Giemsa-stained blood smears prepared daily from days 4 to 10 postchallenge, and parasitemia was determined by counting the number of infected red blood cells (RBC) per 1,000 erythrocytes.
Peptides.
All peptides were obtained from Neosystems (Strasbourg, France) and were produced according to the amino acid sequence shown in Fig. S1 in the supplemental material. Peptides Py3 [(QGPGAP)3] and Pb2 [(DPPPPNPN)2], corresponding, respectively, to the repeat regions of CSPs of P. yoelii and P. berghei (PyCSP and PbCSP) were used in enzyme-linked immunosorbent assays (ELISAs) as previously described (8). The following long peptides corresponding to NH2-terminal (Nt) and COOH-terminal (Ct) parts of the two different CSPs (kindly given by Giampietro Corradin, Institute of Biochemistry, University of Lausanne, Lausanne, Switzerland) were used in ELISA and enzyme-linked immunospot (ELISPOT) assays: PyCSP long peptides, PyNt (N-terminal region, amino acid segment 20 to 138: PGYGQNKSVQAQRNNLYENNLHLSNGKINRNIVNRLLGDANGKPEEKKDDPPKDGNKDDLPKEEKKDLPKEEKKD DPPKDPKKDDPPKNED) and PyCt (C-terminal region, amino acid segment 277 to 345: NEDSYVPSAEQILEFVKQISSQLTEEWSQCSVTCGSGVRVKRKNVNKQPENLTLEDIDTEICKMDKCS); PbCSP long peptides, PbNt (amino acid segment 21 to 91: YGQNKSIQAQRNLNELCYNEGNDNKLYHVLNSKNGKIYIRNTVNRLLADAPEGKKNEKKNKIERNNKLK) and PbCt (amino acid segment 242 to 310: NDDSYIPSAEKILEFVKQIRDSITEEWSQCNVTCGSGIRVRKRKGSNKKAEDLTLEDIDTEICKMDKCS) (14). Lyophilized material was resuspended in sterile distilled water at 10 mg/ml, aliquoted, and stored at −20°C until it was used.
ELISA.
Antibodies to P. yoelii and P. berghei CSP peptides (PyCSP and PbCSP) were detected by ELISA, as previously described (10). Briefly, 96-well flat-bottom plates (Maxisorp; Nunc, Roskilde, Denmark) were coated with 1 μg/ml of peptide in PBS, pH 7.8, by overnight incubation at 4°C. After extensive washes and a 1-h incubation with 200 μl of PBS containing 0.05% Tween and 1% bovine serum albumin (BSA), the wells were incubated for 1 h at 37°C with 100 μl of mouse sera diluted 1/100 in PBS-Tween-BSA. After two washes, the wells were incubated for 45 min at room temperature, either with goat IgG anti-mouse IgM (Invitrogen) or with a biotinylated goat anti-mouse IgG (Jackson ImmunoResearch) diluted in PBS-Tween. The wells containing the goat IgG anti-IgM antibody were washed and further incubated with a biotinylated rabbit anti-goat IgG (Sigma-Aldrich) diluted in PBS-Tween for 45 min at room temperature. The wells were then washed and incubated with extravidin-coupled alkaline phosphatase (Sigma-Aldrich) diluted in PBS-Tween for 1 h at room temperature. Phosphatase activity was measured using 4-methylumbelliferyl phosphate (Sigma-Aldrich) as a substrate, and the fluorescence at 355/460 nm was measured using a spectrophotometer (Victor 1420; Wallac Oy).
Immunofluorescence antibody test (IFAT).
Pooled sera from each group of mice immunized with Spz plus CQ were individually tested by immunofluorescence on wet sporozoites from the different Plasmodium lines to detect surface antigens, as previously described (21).
ELISPOT assay.
Polyvinylidene difluoride (PVDF) microplates (Millipore, Bedford, MA) were coated overnight at 4°C with 15 μg/ml of an anti-mouse gamma interferon (IFN-γ) rat monoclonal antibody (MAb) (clone AN18; Mabtech AB, Sophia Antipolis, France) diluted in PBS. After extensive washes and 2 h of incubation at 37°C with RPMI medium containing 10% fetal calf serum, 3 × 105 spleen cells isolated at least 15 days after the last CQ injection were incubated overnight with the different peptides (final concentration, 10 μg/ml) and with 30 U/ml of recombinant human interleukin 2 (IL-2). The plates were then washed, incubated with 2 μg/ml of biotinylated anti-mouse IFN-γ rat monoclonal antibody (clone R4-6A2; Mabtech AB) diluted in PBS containing 0.5% bovine serum albumin for 2 h at 37°C and then overnight at 4°C. The plates were subsequently incubated with extravidin-coupled alkaline phosphatase (Sigma-Aldrich) diluted in PBS. After the 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium (BCIP-NBT) substrate (Sigma-Aldrich) was added, IFN-γ spot-forming cells were counted under a stereomicroscope and expressed as the number of spots per million tested cells.
Quantification of the parasite loads in the livers of sporozoite-infected mice.
Quantification of the parasite loads in the livers of sporozoite-infected mice was performed as described previously (15). Briefly, mice were injected i.v. with 40,000 to 60,000 sporozoites at least 15 days after the last chloroquine injection. DNA was extracted from liver biopsy specimens collected 42 to 44 h after the sporozoite injection, and 50 to 100 ng of each DNA sample was used as a template in a real-time quantitative PCR assay, using specific primers for P. yoelii and P. berghei as described previously (15).
Standard curves were generated using a 10-fold dilution series (from 106 to 1 parasite nuclei/μl) of DNA solution purified from P. yoelii or P. berghei blood stages obtained from a sample in which the number of parasite nuclei per microliter was determined accurately by microscopy examination of Giemsa-stained blood smears and calculation of the number of RBC/μl of blood. Genomic DNA, rather than a plasmid bearing a single-stranded rRNA (ssrRNA) gene, was used to generate the standard curve, because it reflects more accurately the multiple targets amplified, since there are more than 5 different ssrRNA genes in the genome of Plasmodium. One liver parasite load unit corresponds to the log number of parasite nuclei/μg of liver DNA. Log numbers were deduced from the linear portion of the standard calibration curve derived using incremental 10-fold amounts of genomic DNA. Statistical analyses of data were performed using a one-way analysis of variance (ANOVA) test, followed by Bonferroni's posttest. Inhibition of liver stages was expressed as a percentage derived from the actual numbers of parasite nuclei/μg of liver DNA calculated from the log values.
RESULTS
The efficacy of the Spz-plus-CQ immunization protocol was initially demonstrated using the rodent parasite P. yoelii 265 BY in BALB/c mice (3). The transgenic parasites we generated were P. berghei ANKA parasites expressing the P. yoelii 1.1 CSP. In preliminary experiments, we established that full protection (80 to 100%) was also obtained in BALB/c mice after a single Spz-plus-CQ inoculation with 20,000 sporozoites of P. berghei (data not shown) or of P. yoelii (3).
Characterization of immune responses to CSP and to whole sporozoites.
PyCSP and PbCSP display significant homology outside the B-cell immunodominant central repeat region (see Fig. S1 in the supplemental material). Therefore, it was important to assess cross-reactive responses between the two heterologous CSPs, as well as the responses induced against the homologous CSP by Spz-plus-CQ immunization. Three groups of mice (n = 5) were immunized with Spz plus CQ using either P. berghei, P. yoelii, or transgenic P. berghei[PyCS] parasites. The resulting humoral responses were assessed by IFAT on whole sporozoites and by ELISA using CSP species-specific long peptides, one spanning the N terminus (Nt) and the other the C terminus (Ct), and short peptides representing the repeat region. T-cell responses were assessed in an IFN-γ ELISPOT assay using splenocytes restimulated by CSP long peptides containing all potential CD4+ and CD8+ epitopes (10).
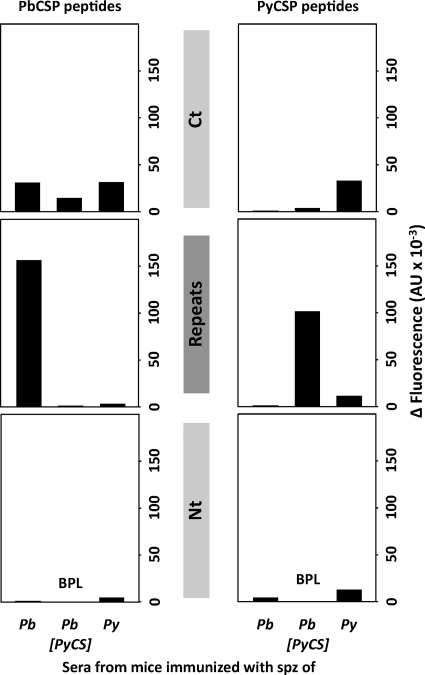
ELISA showed that the IgG responses were mainly directed against the CSP repeat region and, to a lesser extent, against the C terminus, whereas they were barely detectable against the CSP N terminus (Fig. 1). It was interesting that the responses against the PyCSP repeat region were higher in mice immunized with P. berghei[PyCS] than in those immunized with P. yoelii. Some cross-reactive responses were found against the PbCSP C terminus in sera from mice immunized with a parasite expressing PyCSP, but cross-reactivity was not detected against PyCSP in the sera from P. berghei-immunized mice (Fig. 1, right). A similar profile was observed when IgM responses were assayed, except that in this case only low levels of antibodies reactive with the repeat region were found (see Fig. S2 in the supplemental material).
FIG. 1.
Anti-CSP antibody responses are species specific. Pooled sera from each group of 5 mice immunized with either P. berghei (Pb), P. berghei[PyCS], or P. yoelii (Py) Spz plus CQ were tested by ELISA to determine P. berghei (left) or P. yoelii (right) CSP-specific IgG reactivity, using long peptides spanning the N-terminal (PyNt or PbNt) or the C-terminal (PyCt or PbCt) domain of CSP, as well as peptides partially covering the central species-specific repeats (Repeats). The background reactivity of naive mouse serum was subtracted. The data are expressed as differences (Δ) of arbitrary units of fluorescence (AU). BPL, below positivity level. The data are representative of two or three independent experiments.
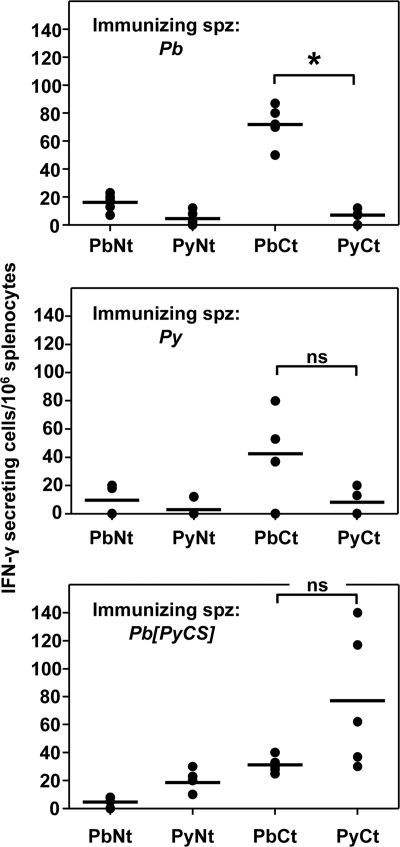
Cellular responses in mice (n = 5) against both the N and C termini of the homologous PbCSP were induced by immunization with P. berghei, with levels considerably higher against the C terminus (Fig. 2, top). In contrast, responses against the heterologous PyCSP long peptides were very low. Cellular responses in mice (n = 5) immunized with P. yoelii against both homologous N- and C-terminal P. yoelii CSP long peptides were detected but were low (Fig. 2, middle). Although cross-reactive responses against the PbCt peptide were found in some mice immunized with P. yoelii Spz, they did not significantly differ from those against the homologous P. yoelii peptides. In mice immunized with P. berghei[PyCS], cellular responses were induced against the homologous PyCSP long peptides, with those against the C terminus higher than those against the N terminus (Fig. 2, bottom). It is interesting that the responses against the PyCt were higher in these mice than in those immunized with P. yoelii sporozoites (P < 0.05; Kruskal-Wallis test). In the P. berghei[PyCS]-immunized mice, cross-reactive responses were detected against only the PbCt peptide. Overall, the cellular responses induced against CSP were mainly directed against the C terminus and were low.
FIG. 2.
CSP-specific T-cell responses. Groups of BALB/c mice were immunized by Spz plus CQ with either P. berghei, P. berghei[PyCS], or P. yoelii. The frequency of epitope-specific T cells in the spleen of each immunized animal was determined by IFN-γ ELISPOT using long peptides spanning the C-terminal (PyCt or PbCt) or the N-terminal (PyNt or PbNt) domain of PyCSP and PbCSP, respectively. The background responses of nonstimulated splenocytes were subtracted. The scatter plots show the number of responding T cells from each mouse spleen (4 or 5 mice per group). The horizontal bars represent the median for each group. *, P < 0.05 between the two data sets; ns, nonsignificant difference, as calculated by a Kruskal-Wallis test followed by Dunn's multiple-comparison test.
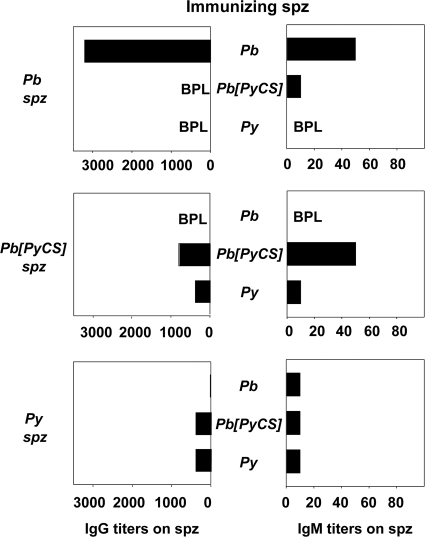
Given that CSP covers the sporozoite surface, we assessed the abilities of the different sera to recognize whole sporozoites from the three parasite lines (Fig. 3). IgG antibody responses against the homologous sporozoite induced by immunization with P. yoelii or P. berghei[PyCS] had a 4- to 5-fold-lower titer (≤1/800) than those induced following P. berghei immunization (>1/3,000). Although IgM responses were substantially less pronounced, they showed a broadly similar profile of reactivities (Fig. 3, right). Cross-reactive responses against the sporozoite surface were minimal. The fact that reactivity to the sporozoite surface was correlated with the type of CSP expressed strongly indicated that the bulk of antibody reactivities to the sporozoite surface were directed against CSP. For example, pooled sera from P. berghei-immunized animals (n = 5) recognized the P. berghei sporozoites with high titers (>1/3,000), whereas pooled sera from P. berghei[PyCS]-immunized animals (n = 5) failed to react with the P. berghei sporozoites.
FIG. 3.
Antibody responses against whole sporozoites in Spz-plus-CQ-immunized mice. Pooled sera from 5 mice immunized with either P. berghei, P. berghei[PyCS], or P. yoelii Spz plus CQ were tested by IFAT on P. berghei (top), P. berghei[PyCS] (middle), or P. yoelii (bottom) sporozoites to measure IgG (left) or IgM (right) reactivity. The titers correspond to the highest dilution of the pooled sera tested in duplicate that gave positive staining. BPL, below positivity level. The data are representative of two independent experiments with similar results.
Protection experiments.
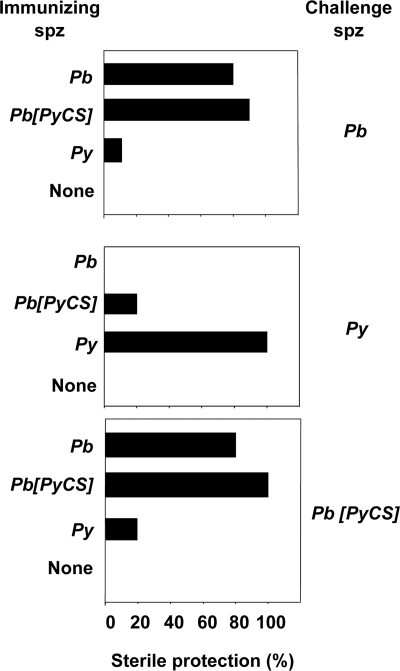
Having established that the humoral responses induced in the homologous combinations were predominantly directed against CSP and that anti-CSP T-cell responses were present, at least for P. berghei-immunized animals, we wanted to measure their effects on the outcome of challenges with live sporozoites. If the CSP responses induced were central to the sterile protection seen in this model, we would expect the mice immunized/challenged with sporozoites bearing identical CSPs to be equally fully protected irrespective of the parasite species of the challenge sporozoites, whereas immunization/challenge using sporozoites expressing the heterologous CSP would not lead to full protection. When these experiments were carried out, protection from infection was observed equally in all animal groups (5 mice per group) immunized with P. berghei or P. berghei[PyCS] sporozoites whether they were challenged with P. berghei sporozoites expressing the homologous or the heterologous CSP (Fig. 4). When challenged with P. yoelii sporozoites, 20% or fewer of the mice immunized with P. berghei or P. berghei[PyCS] sporozoites were protected. Mice immunized with P. yoelii sporozoites were fully protected from challenge with homologous sporozoites, whereas only 10% to 20% of those challenged with P. berghei or P. berghei[PyCS] sporozoites were protected (Fig. 4). These patterns of protection cannot be accounted for by the pattern of CSP cross-reactive humoral or cellular responses induced by immunization. For example, similar levels of cross-reactive cellular responses were observed against the PbCt in mice immunized with P. yoelii or P. berghei[PyCS] (Fig. 2), but on challenge with P. berghei sporozoites, little protection was observed in the former and full protection in the latter (Fig. 4). When P. yoelii was the challenge parasite, we observed no cross-species protection in P. berghei-immunized mice. In mice immunized with P. berghei[PyCS] that bore the homologous CSP, 20% were protected from P. yoelii challenge, indicating at best a modest effect of CSP. Thus, acquisition of sterile immunity in 80% to 100% of the immunized mice can be achieved with no major implication of CSP-specific cellular and humoral responses induced by the Spz-plus-CQ immunization.
FIG. 4.
The role of CSP in sterile protection. Groups of 5 BALB/c mice, each immunized by Spz plus CQ with either P. berghei, P. berghei[PyCS], or P. yoelii, were challenged with either 100 sporozoites (P. yoelii) or 5,000 sporozoites (P. berghei or P. berghei[PyCS]) at least 25 days after immunization. The presence of parasites in the blood was monitored in each animal by microscopic examination of Giemsa-stained blood smears. All control mice treated with chloroquine alone (None) developed patent blood stage parasitemia after sporozoite challenge. Protection was defined as the percentage of mice that did not develop patent blood stage parasitemia over 10 days postchallenge. The data are the cumulative results of two experiments with 5 mice per group and per experiment.
Preerythrocytic-stage immunity.
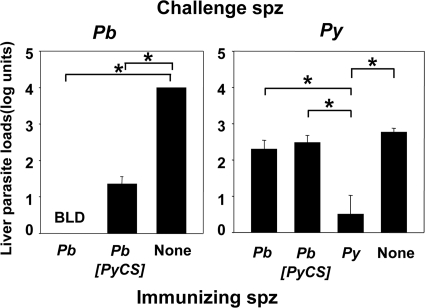
The protection described above was due to a combination of immune responses against sporozoites, hepatic and erythrocytic parasites, as already described (3). We wished to evaluate in vivo the specific contribution to protection of the responses against sporozoites and liver forms, the two parasite stages where CSP is expressed. This was achieved by quantifying the effect of immunization on liver parasite loads after sporozoite challenge (Fig. 5). In animals challenged with P. berghei sporozoites, liver parasite loads were nearly completely abrogated whether the mice were immunized with P. berghei or with P. berghei[PyCS] (Fig. 5, left; 100% and 99% reduction, respectively). Drastic reduction in hepatic loads was also seen for animals immunized with P. yoelii and challenged with P. yoelii sporozoites (Fig. 5, right; 99% reduction when the arithmetic values derived from the log liver load units were compared). In contrast, reduction in liver loads of animals immunized with P. berghei or P. berghei[PyCS] sporozoites and challenged with P. yoelii sporozoites was much less pronounced and did not differ significantly for the two groups (Fig. 5, right; P > 0.05; one-way ANOVA, followed by the Bonferroni posttest). Thus, inhibition of preerythrocytic-parasite development does not seem to depend primarily on the immune responses induced against CSP by Spz-plus-CQ immunization.
FIG. 5.
Liver stage quantification. Groups of 5 BALB/c mice were immunized with either P. berghei, P. berghei[PyCS], or P. yoelii Spz plus CQ. The control groups received injections of CQ alone (None). The results were expressed as mean liver parasite load log units ± standard error of the mean of n = 5 mice. The percent reduction of the liver parasite load was then calculated using the arithmetic values derived from the log liver load units. (Left) Liver parasite loads in immunized mice challenged with 60,000 P. berghei sporozoites. The percent reductions versus the control group were 100% in mice immunized with P. berghei and 99% in P. berghei[PyCS]-immunized mice. (Right) Liver parasite loads in immunized mice challenged with 40,000 P. yoelii sporozoites. The percent reductions versus the control group were 99% in mice immunized with P. yoelii, 48% in P. berghei-immunized mice, and 66% in mice immunized with P. berghei[PyCS] Spz plus CQ. *, P < 0.05 using one-way ANOVA followed by the Bonferroni posttest. BLD, below level of detection.
DISCUSSION
All immunization protocols based on live sporozoites (attenuated or otherwise) have led to the induction of sterile protection. These observations, initially made for RAS in mice more than 40 years ago (19) and more recently for Spz plus CQ (2, 3) and GAS (16, 31), gained in importance and relevance to vaccine development when they were repeated in humans in the 1970s for RAS (4, 12, 22) and last year for Spz plus CQ (23). Clinical trials to test the efficacy of immunization with recently produced P. falciparum GAS (30) are imminent. In RAS-immunized hosts, the immunodominance of the humoral responses to the major sporozoite surface protein CSP (13) and the crucial cellular responses (18) directed at the hepatic parasite in which CSP is also expressed during the early stages have justified the selection and subsequent concerted focus on CSP as a vaccine candidate. The immunodominance of CSP was also observed in mice immunized with GAS. Here, we show that humoral responses against CSP are also dominant in animals immunized by Spz plus CQ.
The immunodominance of CSP notwithstanding, we have previously demonstrated, using a parasite of one species made transgenic for CSP of another species, that immune responses specifically induced against the CS by RAS immunization in mice did not contribute to the consequent sterile protection (10, 15). Here, we have demonstrated that the full protection induced by Spz plus CQ in inbred BALB/c mice is obtained with a minimal role for responses against CSP. In a single experiment, where outbred CD1 mice (5 mice per group) were immunized with P. berghei or P. berghei[PyCS] Spz and challenged with Spz of P. berghei, substantial sterile protection (60%) was observed in both groups, suggesting that the mouse background did not influence the outcome. This supports the conclusion that CSP is minimally involved in the protection induced by immunization with Spz plus CQ.
Cross-reactive responses against the different CSPs were induced in some combinations, as expected from the relative homology between the conserved domains of the gene in the two parasite species, but their presence could not account for the protection observed. It must be noted that we did not detect cross-species sterile protection in the Spz-plus-CQ model, as observed for the models of RAS (15, 26) or GAS (6). This clearly suggests that the immune mechanisms involved in protection differ between the models but are nevertheless independent of CSP.
In the course of this work, we observed that the levels of immune responses against CSP depended in part on the parasite genetic background in which the antigen was presented (the same CSP gene in P. berghei sporozoites or P. yoelii sporozoites induced different levels of T-cell responses and antibody titers). This phenomenon, though of immunological interest, does not affect the conclusions discussed above.
The demonstration that induced responses to CSP do not lead to full protection should not be taken as inimical to efforts to develop vaccines based on CSP. Indeed, such vaccine formulations have invariably shown some protective efficacy, culminating in RTS, S, where the association of CSP-containing particles with a powerful new-generation adjuvant has significantly reduced malaria morbidity in African adults and children (1) despite short-lived maintenance of sterile immunity (27). By comparing the results of this study with our previously published data using the same parasites but the RAS immunization protocol (15), it appears that CSP plays a less important role in the Spz-plus-CQ immunization protocol than in that based on RAS. A factor that we consider most likely to account for this difference concerns the levels and duration of exposure of CSP to the immune system, particularly when in the liver. CSP is continuously expressed by the Spz, whether normal or radiation attenuated, and by liver stage parasites, but not by blood stage parasites. In contrast to Spz that develop normally, liver stages derived from radiation-attenuated sporozoites can persist for long periods, during which they continue to express CSP (25). We suggest that this allows the induction of responses to CSP higher than those induced by a shorter, transient expression of the antigen.
Ultimately, the main conclusion from this work and our previous observations using another immunization scheme that also induces sterile immunity against sporozoite infection (10, 15) is that one or more antigens other than CSP are actually responsible for the induction of sterile immunity against preerythrocytic malaria parasites. Although other preerythrocytic antigens have been studied (TRAP, STARP, SALSA, LSA1, and LSA3 for P. falciparum) (11), the identities and natures of most antigens expressed by the sporozoites and, possibly more pertinently, by the hepatic parasite have remained inaccessible to detailed investigations until recently (9, 24, 28, 32). The implication of other preerythrocytic antigens in our Spz-plus-CQ immunization model would be important to investigate, using an approach similar to ours and those of others (5, 17, 20). Nevertheless, studies of preerythrocytic parasites remain technically challenging, and knowledge of the biology and immunology of these stages has consequently lagged behind that of the erythrocytic stages.
Supplementary Material
Acknowledgments
This work was supported by intramural grants from the Agency for Science, Technology and Research (A*STAR) and Institut National de la Sante et de la Recherche Medicale (INSERM) and by a grant from the European community (MALINV contract number LSH-CT-2005-01299) (L.R.).
A.C.G and L.R. equally directed this work. A.C.G., G.S., L.R., M.M., and R.T. conceived and designed the experiments. A.C.G., E.L., M.K., M.M., and N.D. performed the experiments. R.T. constructed the transgenic parasites. A.C.G., G.S., J-.M.C., and M.M. produced or provided parasites and materials. A.C.G., G.S., L.R., and M.M. analyzed the data. A.C.G., G.S., L.R., and M.M. wrote the paper. All authors read and corrected the manuscript. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Editor: J. H. Adams
Footnotes
Published ahead of print on 1 March 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Ballou, W. R. 2009. The development of the RTS, S malaria vaccine candidate: challenges and lessons. Parasite Immunol. 31:492-500. [DOI] [PubMed] [Google Scholar]
- 2.Beaudoin, R. L., C. P. Strome, F. Mitchell, and T. A. Tubergen. 1977. Plasmodium berghei: immunization of mice against the ANKA strain using the unaltered sporozoite as an antigen. Exp. Parasitol. 42:1-5. [DOI] [PubMed] [Google Scholar]
- 3.Belnoue, E., F. T. Costa, T. Frankenberg, A. M. Vigario, T. Voza, N. Leroy, M. M. Rodrigues, I. Landau, G. Snounou, and L. Rénia. 2004. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J. Immunol. 172:2487-2495. [DOI] [PubMed] [Google Scholar]
- 4.Clyde, D. F., V. C. McCarthy, R. M. Miller, and W. E. Woodward. 1975. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am. J. Trop. Med. Hyg. 24:397-401. [DOI] [PubMed] [Google Scholar]
- 5.de Koning-Ward, T. F., R. A. O'Donnell, D. R. Drew, R. Thomson, T. P. Speed, and B. S. Crabb. 2003. A new rodent model to assess blood stage immunity to the Plasmodium falciparum antigen merozoite surface protein 119 reveals a protective role for invasion inhibitory antibodies. J. Exp. Med. 198:869-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douradinha, B., M. R. Van Dijk, R. Ataide, G. J. van Gemert, J. Thompson, J. F. Franetich, D. Mazier, A. J. F. Luty, R. W. Sauerwein, C. J. Janse, A. P. Waters, and M. M. Mota. 2007. Genetically attenuated P36p-deficient Plasmodium berghei sporozoites confer long-lasting and partial cross-species protection. Int. J. Parasitol. 37:1511-1519. [DOI] [PubMed] [Google Scholar]
- 7.Franke-Fayard, B., H. Trueman, J. Ramesar, J. Mendoza, M. van der Keur, R. van der Linden, R. E. Sinden, A. P. Waters, and C. J. Janse. 2004. A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol. Biochem. Parasitol. 137:23-33. [DOI] [PubMed] [Google Scholar]
- 8.Grillot, D., M. Michel, I. Muller, C. Tougne, L. Rénia, D. Mazier, G. Corradin, P. H. Lambert, J. A. Louis, and G. Del Guidice. 1990. Immune responses to defined epitopes of the circumsporozoite protein of the murine malaria parasite, Plasmodium yoelii. Eur. J. Immunol. 20:1215-1222. [DOI] [PubMed] [Google Scholar]
- 9.Grüner, A. C., S. Hez-Deroubaix, G. Snounou, N. Hall, C. Bouchier, F. Letourneur, I. Landau, and P. Druilhe. 2005. Insights into the P. y. yoelii hepatic stage transcriptome reveal complex transcriptional patterns. Mol. Biochem. Parasitol. 142:184-192. [DOI] [PubMed] [Google Scholar]
- 10.Grüner, A. C., M. Mauduit, R. Tewari, J. F. Romero, N. Depinay, M. Kayibanda, E. Lallemand, J. M. Chavatte, A. Crisanti, P. Sinnis, D. Mazier, G. Corradin, G. Snounou, and L. Rénia. 2007. Sterile protection against malaria is independent of immune responses to the circumsporozoite protein. PLoS One 2:e1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grüner, A. C., G. Snounou, K. Brahimi, F. Letourneur, L. Rénia, and P. Druilhe. 2003. Pre-erythrocytic antigens of Plasmodium falciparum: from rags to riches? Trends Parasitol. 19:74-78. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman, S. L., L. M. Goh, T. C. Luke, I. Schneider, T. P. Le, D. L. Doolan, J. Sacci, P. de la Vega, M. Dowler, C. Paul, D. M. Gordon, J. A. Stoute, L. W. Church, M. Sedegah, D. G. Heppner, W. R. Ballou, and T. L. Richie. 2002. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J. Infect. Dis. 185:1155-1164. [DOI] [PubMed] [Google Scholar]
- 13.Kumar, K. A., G. Sano, S. Boscardin, R. S. Nussenzweig, M. C. Nussenzweig, F. Zavala, and V. Nussenzweig. 2006. The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature 444:937-940. [DOI] [PubMed] [Google Scholar]
- 14.Lopez, J. A., M. A. Roggero, O. Duombo, J. M. Gonzalez, R. Tolle, O. Koita, M. Arevalo-Herrera, S. Herrera, and G. Corradin. 1996. Recognition of synthetic 104-mer and 102-mer peptides corresponding to N- and C-terminal nonrepeat regions of the Plasmodium falciparum circumsporozoite protein by sera from human donors. Am. J. Trop. Med. Hyg. 55:424-429. [DOI] [PubMed] [Google Scholar]
- 15.Mauduit, M., A. C. Gruner, R. Tewari, N. Depinay, M. Kayibanda, J. M. Chavatte, J. F. Franetich, A. Crisanti, D. Mazier, G. Snounou, and L. Renia. 2009. A role for immune responses against non-CS components in the cross-species protection induced by immunization with irradiated malaria sporozoites. PLoS One 4:e7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mueller, A. K., M. Labaied, S. H. Kappe, and K. Matuschewski. 2005. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature 433:164-167. [DOI] [PubMed] [Google Scholar]
- 17.Murhandarwati, E. E., L. Wang, H. D. de Silva, C. Ma, M. Plebanski, C. G. Black, and R. L. Coppel. 2009. Growth-inhibitory antibodies are not necessary for protective immunity to malaria infection. Infect. Immun. 78:680-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nardin, E. H., and R. S. Nussenzweig. 1993. T cell responses to pre-erythrocytic stages of malaria: role in protection and vaccine development against pre-erythrocytic stages. Annu. Rev. Immunol. 11:687-727. [DOI] [PubMed] [Google Scholar]
- 19.Nussenzweig, R. S., J. Vanderberg, G. L. Spitalny, C. I. Rivera, C. Orton, and H. Most. 1972. Sporozoite-induced immunity in mammalian malaria. A review. Am. J. Trop. Med. Hyg. 21:722-728. [DOI] [PubMed] [Google Scholar]
- 20.O'Donnell, R. A., A. Saul, A. F. Cowman, and B. S. Crabb. 2000. Functional conservation of the malaria vaccine antigen MSP-119 across distantly related Plasmodium species. Nat. Med. 6:91-95. [DOI] [PubMed] [Google Scholar]
- 21.Rénia, L., F. Miltgen, Y. Charoenvit, T. Ponnudurai, J. P. Verhave, W. E. Collins, and D. Mazier. 1988. Malaria sporozoite penetration. A new approach by double staining. J. Immunol. Methods 112:201-205. [DOI] [PubMed] [Google Scholar]
- 22.Rieckmann, K. H., P. E. Carson, R. L. Beaudoin, J. S. Cassells, and K. W. Sell. 1974. Sporozoite induced immunity in man against an Ethiopian strain of Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 68:258-259. [DOI] [PubMed] [Google Scholar]
- 23.Roestenberg, M., M. McCall, J. Hopman, J. Wiersma, A. J. Luty, G. J. van Gemert, M. van de Vegte-Bolmer, B. van Schaijk, K. Teelen, T. Arens, L. Spaarman, Q. de Mast, W. Roeffen, G. Snounou, L. Rénia, A. van der Ven, C. C. Hermsen, and R. Sauerwein. 2009. Protection against a malaria challenge by sporozoite inoculation. N. Engl. J. Med. 361:468-477. [DOI] [PubMed] [Google Scholar]
- 24.Sacci, J. B., Jr., J. M. Ribeiro, F. Huang, U. Alam, J. A. Russell, P. L. Blair, A. Witney, D. J. Carucci, A. F. Azad, and J. C. Aguiar. 2005. Transcriptional analysis of in vivo Plasmodium yoelii liver stage gene expression. Mol. Biochem. Parasitol. 142:177-183. [DOI] [PubMed] [Google Scholar]
- 25.Scheller, L. F., and A. F. Azad. 1995. Maintenance of protective immunity against malaria by persistent hepatic parasites derived from irradiated sporozoites. Proc. Natl. Acad. Sci. U. S. A. 92:4066-4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sedegah, M., W. W. Weiss, and S. L. Hoffman. 2007. Cross-protection between attenuated Plasmodium berghei and P. yoelii sporozoites. Parasite Immunol. 29:559-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snounou, G., A. C. Grüner, C. D. Müller-Graf, D. Mazier, and L. Renia. 2005. The Plasmodium sporozoite survives RTS,S vaccination. Trends Parasitol. 21:456-461. [DOI] [PubMed] [Google Scholar]
- 28.Tarun, A. S., X. Peng, R. F. Dumpit, Y. Ogata, H. Silva-Rivera, N. Camargo, T. M. Daly, L. W. Bergman, and S. H. Kappe. 2008. A combined transcriptome and proteome survey of malaria parasite liver stages. Proc. Natl. Acad. Sci. U. S. A. 105:305-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tewari, R., D. Rathore, and A. Crisanti. 2005. Motility and infectivity of Plasmodium berghei sporozoites expressing avian Plasmodium gallinaceum circumsporozoite protein. Cell Microbiol. 7:699-707. [DOI] [PubMed] [Google Scholar]
- 30.VanBuskirk, K. M., M. T. O'Neill, P. De La Vega, A. G. Maier, U. Krzych, J. Williams, M. G. Dowler, J. B. Sacci, Jr., N. Kangwanrangsan, T. Tsuboi, N. M. Kneteman, D. G. Heppner, Jr., B. A. Murdock, S. A. Mikolajczak, A. S. Aly, A. F. Cowman, and S. H. Kappe. 2009. Preerythrocytic, live-attenuated Plasmodium falciparum vaccine candidates by design. Proc. Natl. Acad. Sci. U. S. A. 106:13004-13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Dijk, M. R., B. Douradinha, B. Franke-Fayard, V. Heussler, M. W. van Dooren, B. van Schaijk, G. J. van Gemert, R. W. Sauerwein, M. M. Mota, A. P. Waters, and C. J. Janse. 2005. Genetically attenuated, P36p-deficient malarial sporozoites induce protective immunity and apoptosis of infected liver cells. Proc. Natl. Acad. Sci. U. S. A. 102:12194-12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang, Q., S. Brown, D. S. Roos, V. Nussenzweig, and P. Bhanot. 2004. Transcriptome of axenic liver stages of Plasmodium yoelii. Mol. Biochem. Parasitol. 137:161-168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.