Abstract
The spirochete Leptospira interrogans is a highly invasive pathogen of worldwide public health importance. Studies from our laboratories and another have demonstrated that L. interrogans can acquire host plasminogen on its surface. Exogenous plasminogen activators can then convert bound plasminogen into the functionally active protease plasmin. In this study, we extend upon those observations and report that leptospiral endostatin-like protein A (LenA) binds human plasminogen in a dose-dependent manner. LenA-plasminogen interactions were significantly inhibited by the lysine analog ξ-aminocaproic acid, suggesting that the lysine-binding sites on the amino-terminal kringle portion of the plasminogen molecule play a role in the binding. Previous studies have shown that LenA also binds complement regulator factor H and the extracellular matrix component laminin. Plasminogen competed with both factor H and laminin for binding to LenA, which suggests overlapping ligand-binding sites on the bacterial receptor. Finally, LenA-bound plasminogen could be converted to plasmin, which in turn degraded fibrinogen, suggesting that acquisition of host-derived plasmin by LenA may aid bacterial dissemination throughout host tissues.
Leptospirosis is a highly prevalent zoonotic disease of humans throughout many parts of the world and is an important emerging disease within the United States and other temperate regions. Leptospira interrogans and other pathogenic members of the Leptospira genus are the causative agents of leptospirosis. The prevalence of leptospirosis in many parts of the world is due to chronic kidney infections in a wide variety of domestic, peridomestic, and wild reservoir mammals, including dogs, cattle, horses, pigs, rodents, raccoons, and skunks. Colonization of the renal tubules of carrier animals results in the urinary shedding of virulent leptospires, which persist in freshwater until infection of a new host occurs (7, 15-17, 22, 29, 30, 33, 48).
Leptospires are capable of infecting a variety of hosts by penetrating intact mucous membranes or abraded skin. Once inside the body, leptospires disseminate via the bloodstream and invade internal organs. The spirochete must overcome barriers imposed by epithelial tissues and extracellular matrices in order to spread and colonize host organs. To do so, leptospires require either endogenous proteases or acquisition of host proteolytic enzymes for degrading components of extracellular matrices.
Plasminogen, a single-chain, 92-kDa glycoprotein, is the key component of the host fibrinolytic system. This proenzyme is found in plasma and extracellular fluids at concentrations of approximately 2 μM or 180 to 200 μg/ml (14). Host plasminogen activators, such as tissue-type plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA), cleave plasminogen at Arg560-Val561 into its two-chain active form, plasmin, which has a serine protease-active site in the C-terminal region (35). Plasmin has the ability to degrade a broad spectrum of substrates, including fibrin clots, connective tissue, components of extracellular matrices, precursors of matrix metalloproteases, and immunoglobulins (10, 14, 35).
Several pathogens of medical importance, including Streptococcus pyogenes (5, 9, 32, 37), Staphylococcus aureus (25, 26), Yersinia pestis (28), Neisseria meningitidis, Neisseria gonorrhoeae (44), Pseudomonas aeruginosa (24), Candida albicans (34), Borrelia burgdorferi (8, 11, 12, 21, 23), Borrelia hermsii (39), and Borrelia recurrentis (19), acquire plasminogen on their surfaces and utilize the proteolytic activity of plasmin to disseminate to various host tissues/organs. Some pathogens have an endogenous mechanism for activating surface-bound plasminogen, while others utilize host-derived plasminogen activators (5, 9, 32, 37). To address the question of whether leptospires may also be capable of a similar mechanism to invade host tissues, we examined their ability to bind plasminogen and in the process also identified a leptospiral surface receptor for plasminogen. During the preparation of this report, another research group published results that also indicated that L. interrogans binds and activates plasminogen on its surface (47). In this report, we further present data on the identification and biophysical characterization of a plasminogen-binding surface protein of Leptospira interrogans.
MATERIALS AND METHODS
Leptospira.
Leptospira interrogans serovar Pomona type kennewicki (strain JEN4) was grown in Johnson-Harris bovine serum albumin (BSA)-Tween 80 medium (Bovuminar PLM-5 microbiological medium; Intergen, Purchase, NY) at 30°C, and genomic DNA was isolated from 5-ml JEN4 cultures, as previously described (1).
Recombinant LenA.
Production of recombinant LenA (also known as LfhA and Lsa24) has been described previously (2, 44, 46). Recombinant protein was purified from cleared lysates using MagneHis nickel-conjugated magnetic beads (Promega, Madison, WI). The purity of recombinant protein was assessed by SDS-PAGE, followed by staining with Coomassie brilliant blue. Recombinant protein was dialyzed overnight against phosphate-buffered saline (PBS) by using 7,000-molecular-weight-cutoff Slide-A-Lyzer cassettes (Pierce, Rockford, IL) at 4°C. Concentrations of protein preparations were determined by the bicinchoninic acid assay (Pierce, Rockford, IL) and, if required, concentrated using a centrifugal filter device, Amicon Ultra (Millipore, Billerica, MA). Polyclonal antiserum was produced in two New Zealand White rabbits against a synthetic peptide specific for LenA in a commercial facility (Proteintech Group, Inc., Chicago, IL).
Plasminogen binding assays.
For studies of interactions between intact leptospires and plasminogen, approximately 108 L. interrogans JEN4 bacteria from mid-exponential-phase cultures were washed with PBS. Bacteria were incubated with 10 μg/ml plasminogen in the presence or absence of 100 mM trans-4-(aminomethyl)cyclohexanecarboxylic acid (tranexamic acid; Sigma-Aldrich, St. Louis, MO) for 2 h at 30°C. As a control, intact leptospires were also incubated with 10 μg/ml of BSA. Leptospires were washed twice with PBS and then subjected to 12.5% SDS-PAGE and electrotransferred to nitrocellulose membranes. Membranes were blocked and probed with a monoclonal antiplasminogen antibody (Santa Cruz Biotechnology, Santa Cruz, CA; catalog no. sc-73708), diluted 1:500 in Tris-buffered saline-Tween 20 (TBS-T; 20 mM Tris [pH 7.5], 150 mM NaCl, 0.05% Tween 20). Membranes were washed with TBS-T and incubated for 1 h at room temperature with goat anti-mouse IgG-horseradish peroxidase (HRP; Santa Cruz Biotechnology), diluted 1:20,000 in TBS-T. Membranes were then developed with SuperSignal West Pico enhanced chemiluminescence substrate (Pierce), and bands were visualized with BioMax Light film (Kodak).
For enzyme-linked immunosorbent assay (ELISA)-based binding assays, wells of Maxisorp 96-well plates (Nalge Nunc, Rochester, NY) were coated overnight with 10 μg/ml human plasminogen (Sigma-Aldrich), 10 μg/ml recombinant LenA, or 10 μg/ml BSA in 50 mM NaCO3 (pH 9.6) at 4°C. Plates were brought to room temperature and washed thrice with PBS supplemented with 0.5% Tween 20 (PBS-T). Wells were blocked for 2 h at room temperature with 2% BSA in PBS-T and washed three times with PBS-T. Afterwards, wells were incubated with 100 μl/well of either recombinant LenA (10 μg/ml), plasminogen (10 μg/ml; Sigma-Aldrich), factor H (Sigma-Aldrich), or Engelbreth-Holm-Swarm (EHS) mouse sarcoma laminin (Sigma-Aldrich) for 2 h at 37°C. Wells were washed three times with PBS-T and then incubated for 1 h at room temperature with either LenA-specific rabbit antiserum (diluted 1:250 in PBS-T), goat anti-human plasminogen (Novus Biologicals, Littleton, CO; catalog no. NB600-930), goat anti-human factor H (Calbiochem, San Diego, CA; catalog no. 341276), or an affinity-isolated rabbit anti-EHS laminin polyclonal antiserum (Sigma-Aldrich; catalog no. L9393). Plates were washed three times with PBS-T and incubated for 1 h at room temperature with horseradish peroxidase-conjugated protein G (HRP-ProG; Invitrogen), diluted 1:5,000. Wells were washed five times with PBS-T, and 100 μl/well of ready-to-use 3,3′,5,5′-tetramethyl benzidine (TMB) substrate solution (1-Step Turbo TMB-ELISA; Thermo Scientific, Rockford, IL) was added. Reactions were stopped by the addition of 2 N H2SO4, 50 μl/well. Absorbance was read at 450 nm using a SpectraMax plate reader and SoftMax Pro (Molecular Devices, Sunnyvale, CA). Statistical analyses were performed using analysis of variance or Student's t test, assuming unequal variances.
Role of lysine in LenA-plasminogen interactions.
Essentially the same ELISA protocol as that described above was followed for determining the role of lysines in plasminogen-LenA interactions, except that the lysine analog ξ-aminocaproic acid (final concentration, 1 mM; Sigma-Aldrich) was added with LenA (10 μg/ml) to plasminogen-coated wells (10 μg/ml).
The role of kringle domains of plasminogen in binding with LenA was assessed using plasminogen lysine-binding site I (LBS I)-coated wells (10 μg/ml; Sigma-Aldrich). After blocking, graded molar concentrations of LenA (0.125 μM, 0.25 μM, and 0.5 μM) were added and incubated for 1 h at 37°C. Bound LenA was detected by LenA-specific antiserum as described above.
Effects of salt and heparin on LenA-plasminogen interactions.
For experiments examining the role of ionic interactions in LenA binding to plasminogen, increasing concentrations of NaCl (62.5 mM, 125 mM, 250 mM, and 500 mM) were added to the buffer with LenA. For experiments analyzing the role of heparin-binding domains in the LenA-plasminogen interaction, porcine heparin (0 to 50 μM; Sigma-Aldrich) was added to the plasminogen-coated wells in the binding buffer, along with the recombinant LenA. Bound LenA was detected by LenA-specific antiserum as described above.
Plasminogen activation assay.
To assay the processing of plasminogen on the surfaces of intact leptospires, approximately 108 L. interrogans JEN4 bacteria were washed with PBS and incubated with 10 μg/ml plasminogen in the presence or absence of 100 mM trans-4-(aminomethyl)cyclohexanecarboxylic acid (tranexamic acid; Sigma-Aldrich) for 2 h at 30°C. Controls included intact leptospires incubated with 10 μg/ml of BSA. Leptospires were washed twice with PBS and resuspended in the reaction buffer containing 64 mM Tris, 350 mM NaCl, and 0.15% Triton X-100 (pH 7.5). Fifty microliters of resuspended bacteria was transferred to each assay well of a microtiter plate, and 4 ng/well uPA (Chemicon, Temecula, CA) as well as the plasmin-specific substrate d-valyl-leucyl-lysine-p-nitroanilide dihydrochloride (final concentration, 0.3 mM; Sigma-Aldrich) was added. Plates were incubated at 37°C, and absorbance was read at 405 nm.
Similarly, Maxisorp 96-well plates (Nalge Nunc) were coated overnight with 10 μg/ml recombinant LenA or 10 μg/ml BSA at 4°C. After three washings with PBS-T, wells were blocked for 1 h at 37°C with PBS with 2% (mass/vol) BSA and then washed three times with PBS-T. Afterwards, 100 μl/well of human plasminogen (10 μg/ml) was added and incubated for 2 h at 37°C. Unbound plasminogen was removed by washing wells three times with PBS-T, and then 4 ng/well of human uPA (Chemicon) was added. Afterwards, the plasmin-specific substrate d-valyl-leucyl-lysine-p-nitroanilide dihydrochloride (Sigma-Aldrich) was added at a final concentration of 0.3 mM in the reaction buffer containing 64 mM Tris, 350 mM NaCl, and 0.15% Triton X-100 (pH 7.5). The plates were incubated at 37°C, and absorbance was read at 405 nm after 24 h.
Fibrinogen degradation assay.
Microtiter plate wells were coated with recombinant LenA (10 μg/ml) and incubated with 10 μg/ml plasminogen for 2 h at 37°C. After the wells were washed thrice with PBS-T, 500 ng fibrinogen (Cabiochem) and 10 ng uPA were added and incubated at 37°C for 24 h. Reaction mixtures were then separated by SDS-PAGE, transferred to a nitrocellulose membrane, and probed with a polyclonal antifibrinogen serum (1:500). Bound antibodies were detected with horseradish peroxidase-conjugated protein G (Invitrogen; diluted 1:20,000), and membranes were developed with SuperSignal West Pico enhanced chemiluminescence substrate (Pierce) using BioMax Light film (Kodak).
RESULTS
Leptospira interrogans binds human plasminogen.
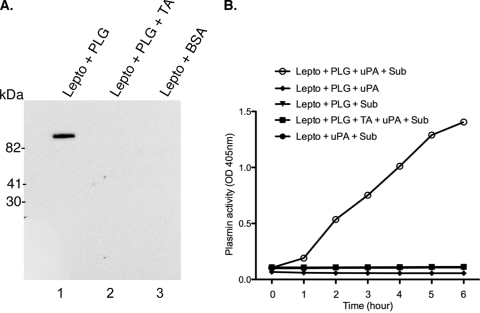
Several invasive bacteria, including the related spirochete B. burgdorferi, utilize their hosts' plasminogen activation system for dissemination. To test if a similar mechanism might be used by the pathogenic leptospires, we assayed binding of plasminogen to intact L. interrogans. Leptospires were incubated with plasminogen, washed and separated by SDS-PAGE, electrotransferred to a nitrocellulose membrane, and probed with a monoclonal antibody. Intact leptospires bound plasminogen (Fig. 1A, lane 1), while addition of tranexamic acid, a lysine analog, resulted in a complete inhibition of binding.
FIG. 1.
Leptospira interrogans serovar Pomona strain JEN4 binds and processes plasminogen on its surface. (A) Intact leptospires preincubated with either plasminogen (PLG; 10 μg/ml), plasminogen (10 μg/ml) plus tranexamic acid (TA; 100 mM), or BSA alone were subjected to SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was probed with a monoclonal antibody to plasminogen (1:500; catalog no. sc-73708). (B) Intact leptospires were incubated with plasminogen (10 μg/ml) in the absence or presence of 100 mM tranexamic acid for 2 h at 30°C and washed twice, and the pellet was reconstituted in the reaction buffer. Fifty microliters of resuspended leptospires was transferred to each microwell, and plasminogen activator (uPA; 4 ng/well) was added. The plasmin activity was measured using a chromogenic substrate, d-valyl-leucyl-lysine-p-nitroanilide dihydrochloride (Sub), and the optical density (OD) was measured at 405 nm. Plasmin conversion was inhibited by tranexamic acid or when uPA was not added to the reaction buffer.
Next, we examined if plasminogen bound to L. interrogans can be processed to its active form, plasmin, by exogenously provided plasminogen activators. In the presence of urokinase-type plasminogen activator (uPA), bound plasminogen on the surfaces of leptospires was processed to plasmin, as measured by conversion of the chromogenic substrate (Fig. 1B). While this report was being prepared, another research group reported similar findings for another serovar of L. interrogans (47).
LenA is a receptor for human plasminogen.
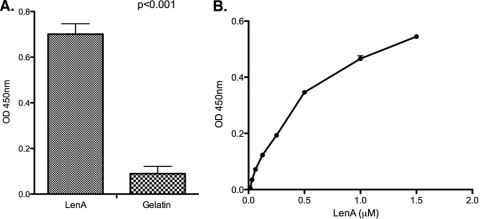
Some factor H-binding proteins of other pathogens also interact with plasminogen (8, 19, 24, 34, 37, 39). LenA, a leptospiral outer membrane protein known to bind factor H, was therefore tested for its ability to bind plasminogen. Recombinant LenA and gelatin were individually immobilized onto microwell plates and incubated with human plasminogen, and their interactions were assessed. Significant binding of plasminogen was detected for LenA relative to that for the control gelatin (Fig. 2A). To test the plasminogen-binding ability of LenA in solution, immobilized plasminogen was incubated with graded molar concentrations of LenA (Fig. 2B). LenA bound immobilized plasminogen in a dose-dependent manner (Fig. 2B), which is in agreement with the reported dose-dependent binding of plasminogen with intact leptospires (47).
FIG. 2.
LenA binds plasminogen. (A) ELISA results showing binding of immobilized LenA (10 μg/ml) to soluble plasminogen (10 μg/ml). Bound plasminogen was detected by sequential addition of an antiplasminogen (1:2,500; catalog no. NB600-930), HRP-conjugated protein G (1:5,000), and 3,3′,5,5′-tetramethyl benzidine substrate solution. The optical density was measured at 450 nm. Gelatin was used as a negative control. (B) ELISA results showing binding of increasing concentrations of LenA to immobilized plasminogen (10 μg/ml). Bound LenA was detected by a LenA-specific antiserum (1:250), followed by HRP-conjugated protein G and the substrate, as described for panel A. Results are representative of at least two independent experiments with 3 or more repeats. P values were calculated by Student's t test, assuming unequal variances.
Role of lysines in LenA-plasminogen interactions.
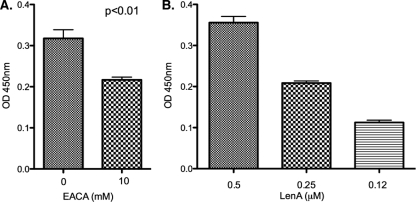
The role of plasminogen kringle domains in interactions with lysine residues of receptors on the surface of host cells is well characterized (35). The ability of intact leptospires to bind plasminogen decreased significantly in the presence of a lysine analog, indicating a role for lysines in plasminogen binding (47). Several plasminogen binding bacterial proteins interact through their lysine residues (8, 19, 49). LenA has several lysine residues, mostly spread on its amino-terminal two-thirds (42). The addition of a synthetic lysine analog ξ-aminocaproic acid significantly reduced the LenA-plasminogen binding, indicating a role for lysines in this interaction (Fig. 3A).
FIG. 3.
Role of lysine residues in LenA-plasminogen interactions. (A) Binding of LenA (10 μg/ml) to immobilized plasminogen (10 μg/ml) in the absence or presence of ξ-aminocaproic acid (EACA; 10 mM) was analyzed by ELISA. (B) ELISA results showing binding of graded concentrations of LenA to the immobilized lysine binding domain of plasminogen. In both panels A and B, bound LenA was detected by sequential addition of a LenA-specific antiserum (1:250), HRP-conjugated protein G (1:5,000), and 3,3′,5,5′-tetramethyl benzidine substrate solution. The optical density was measured at 450 nm. BSA was used as a negative control. Results are representative of at least two independent experiments with 3 or more repeats. P values were calculated by Student's t test, assuming unequal variances.
To further assess the ability of LenA to interact with the lysine-binding sites of plasminogen, a microwell plate was coated with a fragment of plasminogen which contains the lysine-binding domain and incubated with different molar concentrations of LenA. LenA bound this fragment of plasminogen in a dose-dependent manner, further supporting a role for the lysines of LenA in its interactions with plasminogen (Fig. 3B).
Effects of salt and heparin on LenA binding to plasminogen.
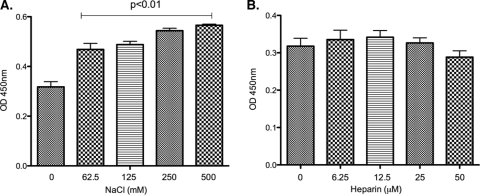
To assess the role of ionic interactions in LenA-plasminogen interactions, assays were performed in the presence of various concentrations of sodium chloride or the polyanion heparin. Intriguingly, the addition of sodium chloride (62.5 mM, 125 mM, 250 mM, and 500 mM) to PBS increased binding of LenA to plasminogen (Fig. 4A). Heparin did not significantly affect LenA binding to plasminogen (Fig. 4B).
FIG. 4.
Ionic interactions in LenA binding to plasminogen. Role of ionic interactions in LenA binding to plasminogen was analyzed by ELISA. ELISA plate wells were coated with plasminogen (10 μg/ml) and incubated with LenA (10 μg/ml) in the presence of increasing concentrations of NaCl (A) or heparin (B). In both panels A and B, bound LenA was detected using a LenA-specific antiserum (1:250), followed by incubation with HRP-conjugated protein G (1:5,000). Plates were developed using 3,3′,5,5′-tetramethyl benzidine substrate solution, and the optical density was measured at 450 nm. Results are representative of at least two independent experiments with 3 or more repeats. BSA was used as a negative control.
Plasminogen competes with factor H and laminin for binding to LenA.
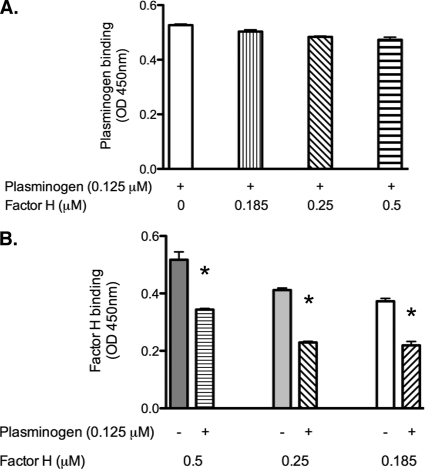
LenA also binds host complement regulator factor H and host laminin (2, 46). To assess if factor H and plasminogen share binding sites on LenA, the binding of plasminogen to LenA was assayed in the presence of increasing concentrations of factor H. In normal human sera, the molar ratio of plasminogen to factor H is 1:1.4. At experimental ratios of 1:1.5, 1:2, and 1:4, a significant decrease in factor H binding to LenA was observed (Fig. 5B). These data suggest that these two host proteins bind to overlapping sites of LenA. LenA appears to preferentially bind plasminogen, as binding of that ligand was not significantly affected by the addition of factor H (Fig. 5A).
FIG. 5.
Plasminogen competes with factor H for binding to LenA. ELISA plate wells were coated with LenA (10 μg/ml) and incubated with plasminogen (0.125 μM) in the absence or presence of 0.185 μM (1:1.5), 0.25 μM (1:2), or 0.5 μM (1:4) factor H. (A) Plasminogen binding to LenA was detected by antiplasminogen (1:2,500; catalog no. NB600-930). (B) Factor H binding to immobilized LenA was detected using anti-factor H (1:2,500; catalog no. 341276). In both panels A and B, HRP-conjugated protein G (1:5,000) was used as the secondary antibody. Plates were developed with 3,3′,5,5′-tetramethyl benzidine substrate solution. The optical density was measured at 450 nm. An asterisk indicates a P value of less than 0.05.
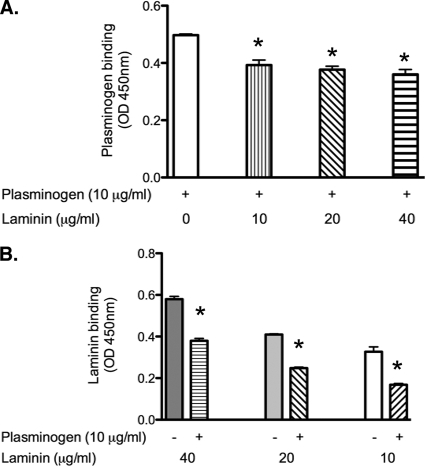
The effects of laminin on plasminogen-LenA interactions were similarly studied. Binding of plasminogen to LenA was assayed in the presence of increasing concentrations of laminin. At ratios of 1:1, 1:2, and 1:4, a significant decrease in the binding of plasminogen (Fig. 6A) and laminin (Fig. 6B) to LenA was observed.
FIG. 6.
Plasminogen and laminin compete for binding to LenA. ELISA plate wells were coated with LenA (10 μg/ml) and incubated with plasminogen (0.125 μM) in the absence or presence of 10 μg/ml, 20 μg/ml, or 40 μg/ml of laminin. (A) Plasminogen binding to LenA was detected by antiplasminogen (1:2,500; catalog no. NB600-930). (B) Laminin binding to immobilized LenA was detected using antilaminin (1:2,500; L9393). In both panels A and B, HRP-conjugated protein G (1:5,000) was used as the secondary antibody. Plates were developed with 3,3′,5,5′-tetramethyl benzidine substrate solution. The optical density was measured at 450 nm. An asterisk indicates a P value of less than 0.05.
Activation of LenA-bound plasminogen to functionally active plasmin.
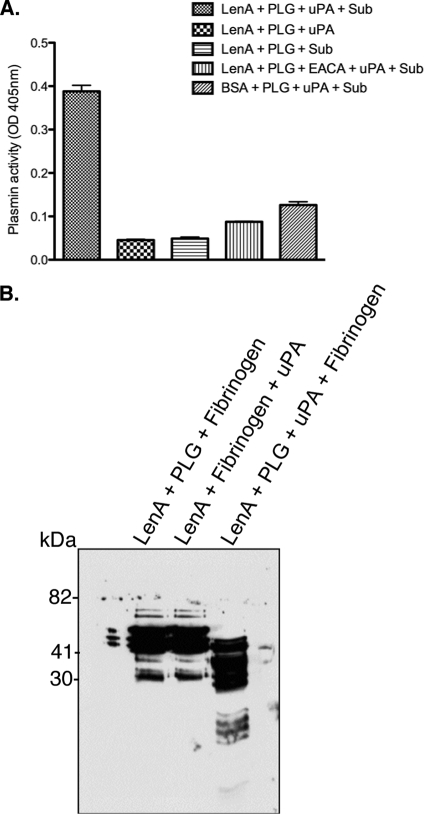
Leptospira spp. acquire and convert plasminogen to its active form, plasmin, on their surfaces (47). To assess whether LenA-bound plasminogen can similarly be processed to plasmin, recombinant LenA-coated plates were incubated with plasminogen. Subsequently, unbound plasminogen was washed off and wells were incubated with the exogenous human plasminogen activator uPA and a chromogenic substrate specific for plasmin. Plasminogen bound to LenA was processed to its active form, plasmin (Fig. 7A). Significantly lower plasmin activity was observed when plasminogen was incubated in the presence of the lysine analog ξ-aminocaproic acid. These results indicate that LenA-bound plasminogen can be processed to plasmin.
FIG. 7.
LenA-bound plasminogen is processed to functionally active plasmin. (A) LenA (10 μg/ml), immobilized to microtiter plate wells, was incubated with plasminogen. After extensive washing, uPA was added and the plasmin activity was measured using a chromogenic substrate, d-valyl-leucyl-lysine-p-nitroanilide dihydrochloride. The optical density was measured at 405 nm. Presented data are representative of at least two independent experiments with 3 or more repeats. (B) LenA (10 μg/ml), immobilized to microtiter plate wells, was incubated with plasminogen (10 μg/ml). Subsequently, fibrinogen and uPA were added and incubated at 37°C for 24 h. The reaction mixtures were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and probed with a rabbit polyclonal fibrinogen antibody.
The proteolytic activity of LenA-bound plasmin was further analyzed by its ability to cleave fibrinogen, one of its physiological substrates. When incubated with LenA-bound plasmin, fibrinogen was degraded to low-molecular-mass fragments, as assessed by Western blotting (Fig. 7B). In the absence of plasminogen or uPA, no fibrinogen degradation was observed.
DISCUSSION
Invasive pathogens must overcome host tissue barriers to reach circulation and disseminate to various organs. Proteolytic activity is therefore key to enhancing bacterial spread within a host. Some bacterial pathogens secrete proteases (18, 20, 43). Other bacteria express receptors that bind host plasminogen onto their outer surfaces and then activate it to the broad-spectrum serine protease, plasmin, through endogenous or host plasminogen activators (12, 23, 36, 37, 47). The bacterial plasminogen activators streptokinase and staphylokinase form a complex with plasminogen, leading to changes in conformation and specificity of plasminogen, and then act as plasminogen activators (6, 13, 36). In contrast, the Pla surface protease of Yersinia pestis acts like mammalian plasminogen activators by demonstrating limited proteolysis at Arg560-Val561 (28, 40). Many other bacterial pathogens, including the Lyme disease spirochete Borrelia burgdorferi, require host plasminogen activators to activate plasminogen on their surfaces (23). Irrespective of the activation mechanism, plasminogen receptors and activators provide bacteria a host-derived proteolytic system for their translocation and dissemination within the host.
L. interrogans serovar Pomona bound plasminogen and processed the proenzyme to its active form in the presence of an exogenous plasminogen activator. During the preparation of this report, another group published work drawing the same conclusions (47). Although Vieira and colleagues showed that outer-membrane-rich detergent-phase and aqueous-phase fractions of L. interrogans contributed to plasminogen acquisition, no specific receptor was identified in that study (47). Here, we present results showing that the leptospiral outer surface protein LenA is a receptor for plasminogen and binds plasminogen in a dose-dependent manner. LenA is expressed during mammalian infection and has previously been shown to bind complement regulator factor H and the extracellular matrix component laminin (2, 42, 46). There are several examples of plasminogen-binding bacterial proteins that play important roles in immune evasion, adhesion, and dissemination of bacteria. For example, the M protein of Streptococcus pyogenes (37), ErpA, ErpC, and ErpP of B. burgdorferi (8), FhbA of B. hermsii (39), and HcpA of B. recurrentis (19) bind complement regulator factor H as well as plasminogen. However, unlike ErpP, FhbA, and HcpA, which bind factor H and plasminogen through discrete regions, LenA appears to bind plasminogen and factor H through overlapping domains, since factor H and plasminogen competed for binding to LenA. Pla, a plasminogen binding protein of Y. pestis, also binds laminin and collagen type IV. Pla-mediated adhesion of Y. pestis to the extracellular matrix proteins has been shown to promote the focusing of the acquired proteolytic activity to regions of bacterial colonization and help in subsequent dissemination (3, 4, 31).
Lysine analogs ξ-aminocaproic acid and tranexamic acid inhibited plasminogen binding to intact leptospires, indicating the importance of lysines in plasminogen binding to leptospires (Fig. 1A) (47). LenA has 13 lysine residues positioned in the amino-terminal two-thirds of the protein. Our results show that lysine residues in LenA are involved in its interaction with plasminogen, as lysine analogs significantly decreased LenA-plasminogen binding. In addition, LenA also bound in a dose-dependent manner to the lysine-binding domain (kringles 1, 2, and 3) of plasminogen. Lysine residues of several bacterial receptors of plasminogen are involved in those interactions. Plasminogen binding of group A streptococcal M protein is localized to its amino terminus, where replacing a single lysine residue, Lys69, with Ala specifically abolished plasminogen binding (49). Similar mutagenesis studies of LenA are under way.
The roles of ionic interactions in the LenA-plasminogen binding were studied by measuring LenA-plasminogen binding in the presence of polyanionic heparin or sodium chloride. The addition of heparin or sodium chloride did not have any inhibitory effect on the binding. On the contrary, the addition of salt increased the binding, presumably by stabilizing interactions between the receptor and the ligand.
Recombinant LenA binds plasminogen, and upon processing to enzymatically active plasmin by human uPA, the bound protease degrades physiological substrate fibrinogen. Although plasmin generated on or activated by several medically important pathogens has been shown to degrade mammalian extracellular matrices and aid in bacterial migration (27), plasmin/plasminogen acquisition has also been shown to interfere with a proper functioning of the components of the immune system. Staphylococcus aureus utilizes host plasmin to degrade surface-bound IgG and C3b (38). Likewise, Pla of Y. pestis degrades complement proteins C3, C3b, and C4b, resulting in reduced opsonophagocytosis and chemotaxis (41). Another plasminogen-binding protein, enolase, expressed by Streptococcus sobrinus, increases interleukin-10 production, leading to immunosuppression in mice (45).
In conclusion, we identified LenA as a leptospiral receptor for plasminogen and characterized receptor-ligand interactions. We also showed that bound plasminogen can be processed to a functionally active form that is capable of degrading host fibrinogen. Results from this and our previous studies suggest that LenA is a multifunctional bacterial surface protein and a virulence factor that is involved in adhesion, immune evasion, and invasion by pathogenic leptospires. Hence, further characterization of LenA and its interactions with host ligands will provide important insights into the pathogenic mechanisms of L. interrogans.
Acknowledgments
This study was supported by National Institutes of Health grant AI-78111 to B. Stevenson.
We thank Claire Adams, Logan Burns, Alicia Chenail, and Brandon Jutras for helpful comments and technical assistance.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 16 February 2010.
REFERENCES
- 1.Artiushin, S., J. F. Timoney, J. Nally, and A. Verma. 2004. Host-inducible immunogenic sphingomyelinase-like protein, Lk73.5, of Leptospira interrogans. Infect. Immun. 72:742-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbosa, A. S., P. A. E. Abreu, F. O. Neves, M. V. Atzingen, M. M. Watanabe, M. L. Vieira, Z. M. Morais, S. A. Vasconcellos, and A. L. T. O. Nascimento. 2006. A newly identified leptospiral adhesin mediates attachment to laminin. Infect. Immun. 74:6356-6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benedek, O., J. Bene, B. Melegh, and L. Emody. 2003. Mapping of possible laminin binding sites of Y. pestis plasminogen activator (Pla) via phage display. Adv. Exp. Med. Biol. 529:101-104. [DOI] [PubMed] [Google Scholar]
- 4.Benedek, O., A. S. Khan, G. Schneider, G. Nagy, R. Autar, R. J. Pieters, and L. Emody. 2005. Identification of laminin-binding motifs of Yersinia pestis plasminogen activator by phage display. Int. J. Med. Microbiol. 295:87-98. [DOI] [PubMed] [Google Scholar]
- 5.Berge, A., and U. Sjobring. 1993. PAM, a novel plasminogen-binding protein from Streptococcus pyogenes. J. Biol. Chem. 268:25417-25424. [PubMed] [Google Scholar]
- 6.Bergmann, S., and S. Hammerschmidt. 2007. Fibrinolysis and host response in bacterial infections. J. Thromb. Haemost. 98:512-520. [PubMed] [Google Scholar]
- 7.Bharti, A. R., J. E. Nally, J. N. Ricaldi, M. A. Matthias, M. M. Diaz, M. A. Lovett, P. N. Levett, R. H. Gilman, M. R. Willig, E. Gotuzzo, and J. M. Vinetz. 2003. Leptospirosis: a zoonotic disease of global importance. Lancet Infect. Dis. 3:757-771. [DOI] [PubMed] [Google Scholar]
- 8.Brissette, C. A., K. Haupt, D. Barthel, A. E. Cooley, A. Bowman, C. Skerka, R. Wallich, P. F. Zipfel, P. Kraiczy, and B. Stevenson. 2009. Borrelia burgdorferi infection-associated surface proteins ErpP, ErpA, and ErpC bind human plasminogen. Infect. Immun. 77:300-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broeseker, T. A., M. D. P. Boyle, and R. Lottenberg. 1988. Characterization of the interaction of human plasmin with its specific receptor on a group A streptococcus. Microb. Pathog. 5:19-27. [DOI] [PubMed] [Google Scholar]
- 10.Chu, C. T., G. C. Howard, U. K. Misra, and S. V. Pizzo. 1994. Alpha 2-macroglobulin: a sensor for proteolysis. Ann. N. Y. Acad. Sci. 737:291-307. [DOI] [PubMed] [Google Scholar]
- 11.Coleman, J. L., J. A. Gebbia, J. Piesman, J. L. Degen, T. H. Bugge, and J. L. Benach. 1997. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell 89:1111-1119. [DOI] [PubMed] [Google Scholar]
- 12.Coleman, J. L., T. J. Sellati, J. E. Testa, R. R. Kew, M. B. Furie, and J. L. Benach. 1995. Borrelia burgdorferi binds plasminogen, resulting in enhanced penetration of endothelial monolayers. Infect. Immun. 63:2478-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collen, D. 1998. Staphylokinase: a potent, uniquely fibrin-selective thrombolytic agent. Nat. Med. 4:279-284. [DOI] [PubMed] [Google Scholar]
- 14.Danø, K., P. A. Andreasen, J. Grondahl-Hansen, P. Kristensen, L. S. Nielsen, and L. Skriver. 1985. Plasminogen activators, tissue degradation, and cancer. Adv. Cancer Res. 44:139-266. [DOI] [PubMed] [Google Scholar]
- 15.Faine, S., B. Adler, C. Bolin, and P. Perolat. 1999. Leptospira and leptospirosis. MediSci, Melbourne, Australia.
- 16.Feigin, R. D., L. A. Lobes, D. Anderson, and L. Pickering. 1973. Human leptospirosis from immunized dogs. Ann. Intern. Med. 79:777-785. [DOI] [PubMed] [Google Scholar]
- 17.Fraser, D. W., J. W. Glosser, D. P. Francis, C. J. Phillips, J. C. Feeley, and C. R. Sulzer. 1973. Leptospirosis caused by serotype Fort-Bragg: a suburban outbreak. Ann. Intern. Med. 79:786-789. [DOI] [PubMed] [Google Scholar]
- 18.Goguen, J. D., N. P. Hoe, and Y. V. B. K. Subrahmanyam. 1995. Proteases and bacterial virulence: a view from the trenches. Infect. Agents Dis. 4:47-54. [PubMed] [Google Scholar]
- 19.Grosskinsky, S., M. Schott, C. Brenner, S. J. Cutler, P. Kraiczy, P. F. Zipfel, M. M. Simon, and R. Wallich. 2009. Borrelia recurrentis employs a novel multifunctional surface protein with anti-complement, anti-opsonic and invasive potential to escape innate immunity. PLoS One 4:e4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrington, D. J. 1996. Bacterial collagenases and collagen-degrading enzymes and their potential role in human diseases. Infect. Immun. 64:1885-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu, L. T., S. D. Pratt, G. Perides, L. Katz, R. A. Rogers, and M. S. Klempner. 1997. Isolation, cloning and expression of a 70-kilodalton plasminogen binding protein of Borrelia burgdorferi. Infect. Immun. 65:4989-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz, A. R., V. E. Ansdell, P. V. Effler, C. R. Middleton, and D. M. Sasaki. 2001. Assessment of the clinical presentation and treatment of 353 cases of laboratory-confirmed leptospirosis in Hawaii, 1974-1998. Clin. Infect. Dis. 33:1834-1841. [DOI] [PubMed] [Google Scholar]
- 23.Klempner, M. S., R. Noring, M. P. Epstein, B. McCloud, R. Hu, S. A. Limentani, and R. A. Rogers. 1995. Binding of human plasminogen and urokinase-type plasminogen activator to the Lyme disease spirochete, Borrelia burgdorferi. J. Infect. Dis. 171:1258-1265. [DOI] [PubMed] [Google Scholar]
- 24.Kunert, A., J. Losse, C. Gruszin, M. Huhn, K. Kaendler, S. Mikkat, D. Volke, R. Hoffmann, T. S. Jokiranta, H. Seeberger, U. Moellmann, J. Hellwage, and P. F. Zipfel. 2007. Immune evasion of the human pathogen Pseudomonas aeruginosa: elongation factor Tuf is a factor H and plasminogen-binding protein. J. Immunol. 179:2979-2988. [DOI] [PubMed] [Google Scholar]
- 25.Kuusela, P., and O. Saksela. 1990. Binding and activation of plasminogen at the surface of Staphylococcus aureus: increase in affinity after conversion to the Lys form of the ligand. Eur. J. Biochem. 193:759-765. [DOI] [PubMed] [Google Scholar]
- 26.Kuusela, P., M. Ullberg, G. Kronvall, T. Tervo, A. Tarkkanen, and O. Saksela. 1992. Surface-associated activation of plasminogen on gram-positive bacteria: effect of plasmin on the adherence of Staphylococcus aureus. Acta Ophthalmol. 70:42-46. [DOI] [PubMed] [Google Scholar]
- 27.Lähteenmäaki, K., P. Kuusela, and T. K. Korhonen. 2001. Bacterial plasminogen activators and receptors. FEMS Microbiol. Rev. 25:531-552. [DOI] [PubMed] [Google Scholar]
- 28.Lähteenmäki, K., R. Virkola, A. Sarén, L. Emödy, and T. K. Korhonen. 1998. Expression of plasminogen activator Pla of Yersinia pestis enhances bacterial attachment to the mammalian extracellular matrix. Infect. Immun. 66:5755-5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee, S. H., D. A. Levy, G. F. Craun, M. J. Beach, and R. L. Calderon. 2002. Surveillance for waterborne-disease outbreaks—United States, 1999-2000. MMWR Surveill. Summ. 51:1-47. [PubMed] [Google Scholar]
- 30.Levett, P. N. 2001. Leptospirosis. Clin. Microbiol. Rev. 14:296-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lobo, L. A. 2006. Adhesive properties of the purified plasminogen activator Pla of Yersinia pestis. FEMS Microbiol. Lett. 262:158-162. [DOI] [PubMed] [Google Scholar]
- 32.Lottenberg, R., C. C. Broder, and M. D. P. Boyle. 1987. Identification of a specific receptor for plasmin on a group A streptococcus. Infect. Immun. 55:1914-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meites, E., M. T. Jay, S. Deresinski, W. J. Shieh, S. R. Zaki, L. Tompkins, and D. S. Smith. 2004. Reemerging leptospirosis, California. Emerg. Infect. Dis. 10:406-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poltermann, S., A. Kunert, M. von der Heide, R. Eck, A. Hartmann, and P. F. Zipfel. 2007. Gpm1p is a factorH, FHL-1 and plasminogen-binding surface protein of Candida albicans. J. Biol. Chem. 282:37537-37544. [DOI] [PubMed] [Google Scholar]
- 35.Ponting, C. P., J. M. Marshall, and S. A. Cederholm-Williams. 1992. Plasminogen: a structural review. Blood Coagul. Fibrinolysis 3:605-614. [PubMed] [Google Scholar]
- 36.Reddy, K. N., and G. Markus. 1972. Mechanism of activation of human plasminogen by streptokinase. Presence of active center in streptokinase-plasminogen complex. J. Biol. Chem. 247:1683-1691. [PubMed] [Google Scholar]
- 37.Ringdahl, U., and U. Sjobring. 2000. Analysis of plasminogen-binding M proteins of Streptococcus pyogenes. Methods 21:143-150. [DOI] [PubMed] [Google Scholar]
- 38.Rooijakkers, S. H., W. J. van Wamel, M. Ruyken, K. P. van Kessel, and J. A. van Strijp. 2005. Anti-opsonic properties of staphylokinase. Microbes Infect. 7:476-484. [DOI] [PubMed] [Google Scholar]
- 39.Rossmann, E., P. Kraiczy, P. Herzberger, C. Skerka, M. Kirschfink, M. M. Simon, P. F. Zipfel, and R. Wallich. 2007. Dual binding specificity of a Borrelia hermsii-associated complement regulator-acquiring surface protein for factor H and plasminogen discloses a putative virulence factor of relapsing fever spirochetes. J. Immunol. 178:7292-7301. [DOI] [PubMed] [Google Scholar]
- 40.Sodeinde, O. A., A. K. Sample, R. R. Brubaker, and J. D. Goguen. 1988. Plasminogen activator/coagulase gene of Yersinia pestis is responsible for degradation of plasmid-encoded outer membrane proteins. Infect. Immun. 56:2749-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sodeinde, O. A., Y. V. B. K. Subrahmanyam, K. Stark, T. Quan, Y. Bao, and J. D. Goguen. 1992. A surface protease and the invasive character of plague. Science 258:1004-1007. [DOI] [PubMed] [Google Scholar]
- 42.Stevenson, B., H. A. Choy, M. Pinne, M. L. Rotondi, M. C. Miller, E. DeMoll, P. Kraiczy, A. E. Cooley, T. P. Creamer, M. A. Suchard, C. A. Brissette, A. Verma, and D. A. Haake. 2007. Leptospira interrogans endostatin-like outer membrane proteins bind host fibronectin, laminin and regulators of complement. PLoS One 2:e1188. doi: 10.1371/journal/pone.0001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Travis, J., J. Potempa, and H. Maeda. 1995. Are bacterial proteinases pathogenic factors? Trends Microbiol. 3:405-407. [DOI] [PubMed] [Google Scholar]
- 44.Ullberg, M., P. Kuusela, B.-E. Kristensen, and G. Kronvall. 1992. Binding of plasminogen to Neisseria meningitidis and Neisseria gonorrhoeae and formation of surface-associated plasmin. J. Infect. Dis. 166:1329-1334. [DOI] [PubMed] [Google Scholar]
- 45.Veiga-Malta, I., M. Duarte, M. Dinis, A. Tavares, A. Videira, and P. Ferreira. 2004. Enolase from Streptococcus sobrinus is an immunosuppressive protein. Cell Microbiol. 6:79-88. [DOI] [PubMed] [Google Scholar]
- 46.Verma, A., J. Hellwage, S. Artiushin, P. F. Zipfel, P. Kraiczy, J. F. Timoney, and B. Stevenson. 2006. LfhA, a novel factor H-binding protein of Leptospira interrogans. Infect. Immun. 74:2659-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vieira, M. L., S. A. Vasconcellos, A. P. Gonçales, Z. M. de Morais, and A. L. T. O. Nascimento. 2009. Plasminogen acquisition and activation at the surface of Leptospira species lead to fibronectin degradation. Infect. Immun. 77:4092-4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vinetz, J. M. 1997. Leptospirosis. Curr. Opin. Infect. Dis. 10:357-361. [DOI] [PubMed] [Google Scholar]
- 49.Wistedt, A. C., U. Ringdahl, W. Muller-Esterl, and U. Sjobring. 1995. Identification of a plasminogen-binding motif in PAM, a bacterial surface protein. Mol. Microbiol. 18:569-578. [DOI] [PubMed] [Google Scholar]