Abstract
Food-borne infections caused by Salmonella enterica species are increasing globally, and pregnancy poses a high risk. Pregnant mice rapidly succumb to S. enterica serovar Typhimurium infection. To determine the mechanisms involved, we addressed the role of inflammation and bacterial burden in causing placental and systemic disease. In vitro, choriocarcinoma cells were a highly conducive niche for intracellular S. Typhimurium proliferation. While infection of mice with S. Typhimurium wild-type (WT) and mutant (ΔaroA and ΔinvA) strains led to profound pathogen proliferation and massive burden within placental cells, only the virulent WT S. Typhimurium infection evoked total fetal loss and adverse host outcome. This correlated with substantial placental expression of granulocyte colony-stimulating factor (G-CSF), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α) and increased serum inflammatory cytokines/chemokines, such as G-CSF, IL-6, CCL1, and KC, evoked by WT S. Typhimurium infection. In contrast, infection with high doses of S. Typhimurium ΔaroA, despite causing massive placental infection, resulted in reduced inflammatory cellular and cytokine response. While S. Typhimurium WT bacteria were dispersed in large numbers across all regions of the placenta, including the deeper labyrinth trophoblast, S. Typhimurium ΔaroA bacteria localized primarily to the decidua. This correlated with the widespread placental necrosis accompanied by neutrophil infiltration evoked by the S. Typhimurium WT bacteria. Thus, the ability of Salmonella to localize to deeper layers of the placenta and the nature of inflammation triggered by the pathogen, rather than bacterial burden, profoundly influenced placental integrity and host survival.
Typhoid fever in humans, caused by Salmonella enterica serovar Typhi, is widespread in developing nations with poor hygienic conditions, whereas nontyphoidal intestinal disease caused by S. enterica serovar Typhimurium, a food-borne pathogen, is of global concern (17). Vaccines against typhoid fever offer variable protection against different serovars and have had limited use (24). Salmonella species are also becoming more resistant to antibiotics (19). Young, elderly, pregnant, and HIV-infected individuals form the high-risk groups for Salmonella infections (7). The mouse model of S. Typhimurium infection mimics human typhoid, causing disseminated disease. The T-cell response to S. Typhimurium is generally detectable only beyond 7 to 14 days (26, 37). Thus, innate immunity and inflammatory cytokines are critical in controlling the early primary infection (47).
Salmonella species can cause pregnancy complications such as chorioamnionitis, transplacental fetal infection, abortions, and neonatal and maternal septicemia (15, 44). Intracellular infections in general can lead to pregnancy complications such as preterm labor and preeclampsia (12, 29, 38). Infections with many chronic intracellular pathogens may also be exacerbated during pregnancy and/or pose a risk of reactivation postpartum (3, 21, 31). However, the specific mechanisms by which different infections trigger placental pathology and/or alter maternal immunity are not clear.
Previously, we showed that S. Typhimurium infection initiated during pregnancy in normally resistant mice results in exacerbated maternal disease due to reduced systemic innate immunity (33). Herein, we addressed the issue of whether the severity of systemic disease is related to preferential expansion of Salmonella in the placental environment and consequent inflammatory response. We demonstrate that the placental cells provide a niche for S. Typhimurium proliferation and that the virulent bacterium triggers overt local inflammation, which, rather than the absolute bacterial burden, triggers placental necrosis and fatal host outcome.
MATERIALS AND METHODS
Mice, mating, and pregnancy.
129.B6F1 mice were bred in-house by crossing 129X1Sv/J females with C57BL6/J males obtained from the Jackson Laboratory (Bar Harbor, ME). These mice develop a chronic S. Typhimurium infection in the nonpregnant state. Mice were maintained according to the guidelines of the Canadian Council on Animal Care. For mating, male and female mice were caged overnight, and detection of vaginal plugs the following morning was considered day 0 of pregnancy. Fetal resorptions were identified as resorbing necrotic scars in the uterus. The percent resorption rate was calculated using the formula R/(R + V) × 100, where R is the number of resorbing fetuses and V is the number of viable fetuses per animal.
Bacteria and in vivo infection.
S. Typhimurium wild-type (WT) strain SL1344, ΔinvA strain SL1344, and ΔaroA strain SL3261 were grown in brain heart infusion (BHI) medium as described previously (41). Nonpregnant, age-matched midterm pregnant mice (days 11 to 13) were inoculated with organisms suspended in 200 μl of 0.9% NaCl intravenously via the lateral tail vein. Organs of infected mice were homogenized in 0.9% NaCl, and CFU were determined by plating 100 μl of serial 10-fold dilutions on BHI agar plates (Difco). For determining the localization of the bacteria to particular regions of the placenta, mice were infected with WT S. Typhimurium and S. Typhimurium ΔaroA expressing green fluorescent protein (GFP).
Cell lines and in vitro infection.
HeLa (human cervical adenocarcinoma) and JEG-3 cells (human choriocarcinoma) (ATCC) were maintained in RPMI 1640 plus 8% fetal bovine serum (R8) in 8% CO2 at 37°C. Cells in 24-well plates (1 × 105 cells/ml) were infected at a multiplicity of infection (MOI) of 10. After 30 min of exposure to the bacteria to allow cellular entry, cells were incubated for 2 h in media containing 50 μg/ml gentamicin to remove extracellular bacteria, washed several times, and then incubated at 37°C in R8 medium containing 10 μg/ml gentamicin. Representative duplicate wells were sampled at various times, and cells were lysed and plated onto BHI agar plates for enumeration of intracellular bacteria. Intracellular doubling time was calculated using the formula G = t/(3.3 × log b/B), where G is the generation time, t is the time elapsed, B is the CFU at the start time, and b is the CFU at the end time.
Cytokine profiling array.
Levels of 40 different mouse cytokines/chemokines in the serum were detected using a proteome profiler cytokine array from R&D Systems (Minneapolis, MN). Briefly, the kit comprised nitrocellulose membranes containing anticytokine antibodies printed in duplicate. Membranes were incubated with serum samples and processed according to the manufacturer's protocols, followed by chemiluminescent detection. Pixel density for each spot was captured by a Fluorochem 8900 (Alpha Innotech) imager.
Assessment of placental cytokine expression by Q-RT-PCR.
Quantitative reverse transcription-PCR (Q-RT-PCR) for specific cytokines using high-efficiency primers was carried out on RNA extracted from tissue of individual mice as described previously (33). Relative quantitative expression was extrapolated from the standard curve. Fold change in cytokine expression level relative to β-actin expression in the same sample was then calculated.
Histological studies and immunofluorescence staining.
Placentas/uteroplacental units were fixed for 24 h in 4% paraformaldehyde. Hematoxylin and eosin (H&E) and myeloperoxidase (MPO) staining was done on transverse sections (5 μm) cut from paraffin-embedded tissue. MPO staining was done as described by Pinkus and Pinkus (34). Briefly, sections were toluene treated and rehydrated with ethanol. After antigen retrieval, sections were treated with 3% H2O2 to remove endogenous peroxidase, stained with anti-MPO antibody, and detected with diaminobenzidine (DAB; 0.02%) counterstained with Harris hematoxylin.
Frozen sections were used for immunofluorescence staining. These were prechilled in methanol at room temperature for 10 min and then permeabilized using phosphate-buffered saline (PBS) containing 0.1% TritonX-100 for 5 min at room temperature. Blocking was done for 1 h at room temperature in a PBS buffer containing 10% goat serum, 0.1% TritonX-100, and anti-CD16 (Fc block). Then sections were stained with Alexa647-conjugated anti-mouse Gr1 (1:100 dilution; eBiosciences) and the nuclear stain Hoechst 33342 and viewed under a fluorescence microscope (Olympus IX81).
Histological scoring.
H&E-stained slides were evaluated in a blind manner for pathology and assigned a histology score after viewing 8 to 10 fields for each placental sample at 3-mm distance based on a random sampling protocol. Each individual placenta was scored, and a total of 2 to 4 individual mice for each group (12 to 28 placentas) per group were evaluated. For necrosis, the scoring guide used was as follows: 0, no necrosis; 1, mild necrosis limited to the decidual area; 2, moderate necrosis extending into the junctional zone; and 3, severe necrosis throughout the tissue. Neutrophils were identified by their classical polymorphonuclear appearance, and the scoring guide was as follows: 0, no neutrophils discerned; 1, neutrophils limited to the decidua; 2, moderate neutrophil infiltration extending into the junctional zone; and 3, massive neutrophil infiltration throughout the tissue.
Statistical analysis.
The nonparametric Mann-Whitney U test, Student's t test, and analysis of variance (ANOVA), as appropriate and as stated in the figure legends, were used to determine the statistical significance of the experimental data.
RESULTS
Virulent WT S. Typhimurium but not attenuated S. Typhimurium ΔaroA infection is exacerbated in pregnant hosts.
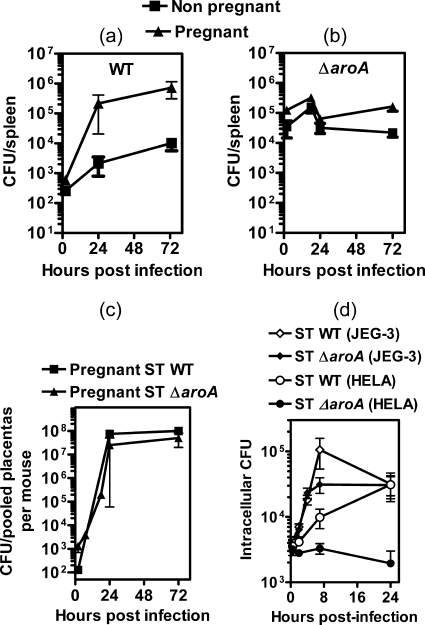
Infection of pregnant 129.B6F1 mice with 103 CFU of WT S. Typhimurium resulted in massively increased splenic and placental bacterial burden 3 days postinfection relative to nonpregnant controls (Fig. 1a and c). This led to nearly 100% fetal loss (Fig. 1d). The ΔinvA strain has a mutation in the Salmonella pathogenicity island 1 (Spi-1) type III secretion system (17). Systemic infection of pregnant mice with 103 CFU of S. Typhimurium ΔinvA also resulted in significantly increased splenic bacterial burden relative to nonpregnant controls (Fig. 1a) and profound placental infection (Fig. 1c). The median resorption rates were slightly lower than those for pregnant WT S. Typhimurium-infected hosts but significantly higher than those for the noninfected pregnant group (Fig. 1d).
FIG. 1.
S. Typhimurium infection in pregnancy. (a and b) Splenic bacterial burden in 129.B6F1 mice on day 3 after infection with 103 CFU of WT S. Typhimurium or the ΔinvA or ΔaroA mutant (a) or 106 CFU of S. Typhimurium ΔaroA (b). **, splenic bacterial burden in pregnant mice infected with WT S. Typhimurium or the ΔinvA mutant is significantly higher than that in the nonpregnant group by Mann-Whitney U test (P < 0.01). (c and d) Placental bacterial burden (c) and fetal resorption rates (d) on day 3 postinfection. Fetal resorption rates evoked by WT S. Typhimurium and S. Typhimurium ΔinvA are significantly higher than that for the noninfected pregnant group by Mann-Whitney U test; **, P < 0.001; *, P < 0.05. Each data point indicates individual mice, and the mean value for each group is indicated by a horizontal line.
In contrast, infection with 103 CFU of S. Typhimurium ΔaroA, an auxotrophic mutant (16), resulted in low splenic and placental bacterial burden (Fig. 1a and c) and no significant fetal resorption (Fig. 1d). A 1,000-fold-higher infection dose of S. Typhimurium ΔaroA (106 CFU), while increasing bacterial burden massively, resulted in similar splenic bacterial numbers for both pregnant and nonpregnant mice (Fig. 1b). Interestingly, profound placental colonization (Fig. 1c) similar to WT infection occurred. Despite this high placental bacterial burden and fetal infection (data not shown), even a high dose of S. Typhimurium ΔaroA did not evoke resorptions (Fig. 1d).
Thus, pregnant hosts were highly susceptible to virulent WT S. Typhimurium infection, whereas mutant/attenuated strains were less deleterious. Furthermore, taken together, the above results indicated that pathogen burden alone does not influence fetal resorption rates. The high dose of S. Typhimurium ΔaroA provided a clear contrast to the WT S. Typhimurium infection, wherein both evoked similar placental bacterial burdens yet only the latter evoked placental lesions. Thus, further studies to delineate the role of inflammation versus bacterial burden in maternal immunity were carried out with these two strains.
S. Typhimurium preferentially expands in placental tissue.
We compared the in vivo growth kinetics of S. Typhimurium infection. After 2 h of infection with 103 CFU of WT S. Typhimurium, ∼10% of the bacteria trafficked to the various organs. In the spleens of pregnant mice, WT S. Typhimurium expanded to >105 CFU by 24 h and >106 CFU by 48 h (Fig. 2a), whereas the S. Typhimurium ΔaroA strain exhibited little increase in splenic numbers after 24 h (Fig. 2b). In remarkable contrast to results for the spleen, both WT S. Typhimurium and the ΔaroA strain showed preferential expansion in the placenta. Relative to the number of bacteria that trafficked to the placenta 2 h after infection, an ∼5-log in vivo expansion occurred by 24 h (Fig. 2c), with an in vivo doubling time of <2 h for both strains.
FIG. 2.
Growth kinetics of S. Typhimurium (ST). (a, b, and c) Bacterial burden in organs after infection of mice with 103 CFU of WT S. Typhimurium or 106 CFU of S. Typhimurium ΔaroA. Data indicate means ± standard errors of the means (SEM) for 4 to 6 mice per time point. (d) In vitro intracellular growth of S. Typhimurium at an MOI of 10 within HeLa and human choriocarcinoma cells (JEG-3).
S. Typhimurium is a facultative intracellular bacterium, and in vivo growth may be accounted for by extracellular and intracellular replication. We therefore evaluated its intracellular growth in vitro in cell lines. The doubling time for WT S. Typhimurium in HeLa cells was ∼4 h, whereas WT S. Typhimurium proliferated substantially in JEG-3 human choriocarcinoma cells, with a placental doubling time of 1 h (Fig. 2d). Even the S. Typhimurium ΔaroA mutant showed growth in JEG-3 cells, with a doubling time of 2.2 h, despite its inability to grow within HeLa cells (doubling time of 22 h). Overall, it appears that the placental cells provide a unique niche to support the growth of S. Typhimurium.
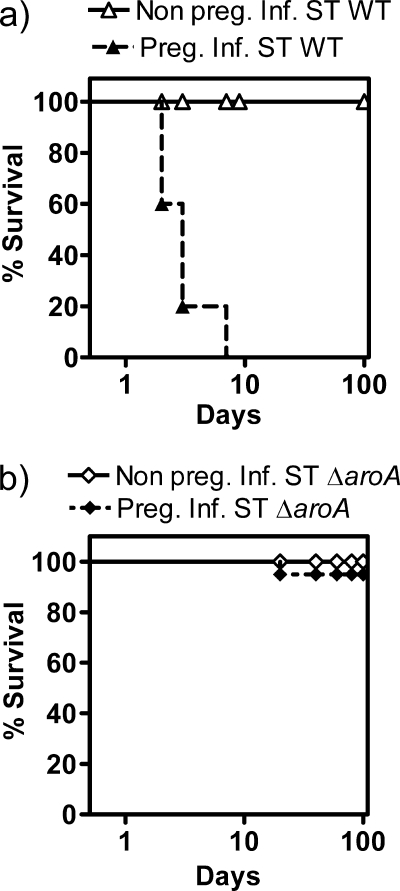
Pregnant hosts succumb to WT S. Typhimurium but not S. Typhimurium ΔaroA infection.
Nonpregnant 129.B6F1 mice infected with 103 CFU of WT S. Typhimurium did not succumb to infection (Fig. 3a), although they developed a chronic infection that lasted >50 days (26). In contrast, ∼80% of the pregnant mice infected with WT S. Typhimurium succumbed by 4 days (Fig. 3a). Neither the nonpregnant nor pregnant mice succumbed to even a high dose (106 CFU) of S. Typhimurium ΔaroA (Fig. 3b). Furthermore, despite invasive fetal infection (data not shown), the infected mothers had normal term delivery. S. Typhimurium ΔaroA infection in pregnant mice followed a chronic infection pattern similar to that in nonpregnant control mice and was cleared beyond 30 to 40 days (data not shown).
FIG. 3.
Host survival kinetics following S. Typhimurium (ST) infection. Nonpregnant and pregnant 129.B6F1 mice were infected with 103 CFU of WT S. Typhimurium (a) or 106 CFU of S. Typhimurium ΔaroA (b). Survival curves are based on 6 mice/group. WT S. Typhimurium-infected mice were euthanized once they showed 2 or 3 of the following clinical signs of infection: morbidity, piloerection, slow/retarded movement, or >20% weight loss.
WT S. Typhimurium infection results in overt serum inflammatory response in pregnant hosts.
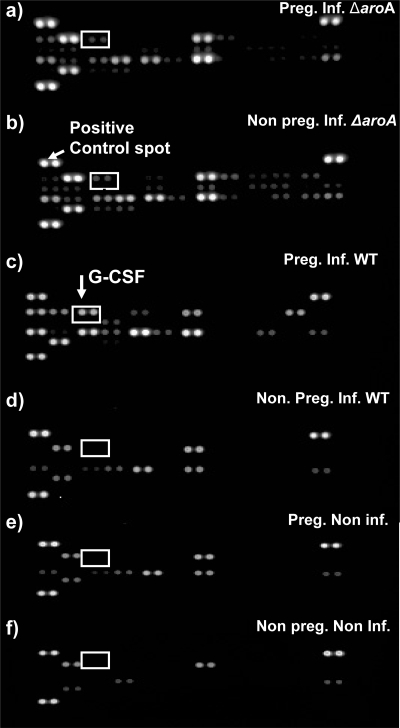
Cytokines are often crucial regulators of host immune response to infection. In order to ascertain a holistic picture of serum cytokine/chemokine levels following S. Typhimurium infection, levels of 40 cytokines were profiled using a cytokine array. Figure 4 shows a representative chemiluminescence blot obtained from the analysis of individual serum samples of 6 different groups of mice: nonpregnant and pregnant mice infected with S. Typhimurium ΔaroA, nonpregnant and pregnant mice infected with WT S. Typhimurium, nonpregnant noninfected (naive) mice, and pregnant noninfected (healthy) mice. Each cytokine was represented in duplicate on the blot, and the intensity of each spot was quantified using densitometric software to derive the pixel level for each spot relative to that for the positive control spots on each blot (Fig. 4). Fold change for each cytokine relative to the levels in respective noninfected controls was calculated. For example, the fold change for nonpregnant infected mice was obtained by comparison with nonpregnant noninfected mice. Similarly, pregnant infected mice were compared to pregnant noninfected mice.
FIG. 4.
Cytokine proteome profiling blot. Representative chemiluminescence cytokine blots carried out on serum of individual mice in the indicated 6 groups. Infections were with 106 (a and b) or 103 (c and d) CFU of S. Typhimurium ΔaroA or WT S. Typhimurium, respectively. The spot intensity is proportional to the amount of cytokine bound. Each cytokine is represented as duplicate spots, and there are 3 sets of positive control spots on each blot. The intensity of spots was quantified relative to the positive control spot based on pixel density.
In response to both WT S. Typhimurium and S. Typhimurium ΔaroA infection, certain serum chemokines such as IP10, CCL2, and CXCL9 rapidly increased in both pregnant and nonpregnant hosts (Fig. 5). However, the levels of several other inflammatory cytokines/chemokines, namely, interleukin-6 (IL-6), BLC (B-lymphocyte chemoattractant)/CXCL13, granulocyte colony-stimulating factor (G-CSF), IL-1R antagonist (IL-1Ra), I-309/CCL1, KC, CCL12, and MIP-2, were selectively elevated 5- to 30-fold in the serum of pregnant WT S. Typhimurium-infected animals (Fig. 5). Levels of other groups were not elevated. All groups exhibited similar levels of several other cytokines/chemokines (Table 1). Therefore, WT S. Typhimurium, but not ΔaroA mutant, infection evoked a massive inflammatory signature in pregnant hosts.
FIG. 5.
Profile of serum inflammatory cytokines/chemokines. 129.B6F1 mice were infected with either WT S. Typhimurium (ST) (103 CFU) or S. Typhimurium ΔaroA (106 CFU). Fold changes in analyte levels in the serum on day 3 of infection relative to the respective noninfected control group are indicated. Data represent mean changes ± SEM. Values were obtained for analyzed serum from individual mice (n = 3 to 5/group). *, P < 0.05 in comparison with the nonpregnant WT S. Typhimurium-infected group by Mann-Whitney U test.
TABLE 1.
Serum cytokines/chemokines that remain unchanged in pregnant WT S. Typhimurium-infected mice relative to nonpregnant and pregnant S. Typhimurium ΔaroA-infected hosts
| Cytokine/chemokineb | Fold changea (mean ± SEM) in mice infected with: |
|||
|---|---|---|---|---|
| WT S. Typhimurium |
S. Typhimurium ΔaroA |
|||
| Nonpregnant | Pregnant | Nonpregnant | Pregnant | |
| C5a (complement component 5a) | 0.98 ± 0.57 | 1.13 ± 0.45 | 0.77 ± 0.48 | 1 ± 0.73 |
| GM-CSF | 1.16 ± 0.49 | 3.95 ± 0.98 | 2.59 ± 1.43 | 3.12 ± 1.28 |
| Eotaxin | 1.18 ± 0.48 | 4.2 ± 1.62 | 2.4 ± 1.31 | 3.55 ± 1.71 |
| sICAM-1 (CD54) | 1.02 ± 0.17 | 1.26 ± 0.24 | 0.78 ± 0.48 | 0.92 ± 0.62 |
| IFN-γ | 1.2 ± 0.6 | 1.58 ± 0.36 | 1.53 ± 0.92 | 1.56 ± 0.63 |
| IL-1α | 0.92 ± 0.41 | 2.35 ± 0.64 | 1.92 ± 1.34 | 2.05 ± 0.75 |
| IL-1β | 1.07 ± 0.41 | 2.29 ± 0.58 | 2.25 ± 1.67 | 1.97 ± 0.75 |
| IL-2 | 1.26 ± 0.59 | 2.25 ± 0.58 | 1.75 ± 0.72 | 1.70 ± 0.68 |
| IL-3 | 1.07 ± 0.49 | 1.40 ± 0.47 | 1.62 ± 0.89 | 1.10 ± 0.38 |
| IL-4 | 0.98 ± 0.31 | 1.53 ± 0.22 | 1.49 ± 0.67 | 1.56 ± 0.60 |
| IL-5 | 1.06 ± 0.67 | 3.98 ± 1.75 | 2.49 ± 1.43 | 3.07 ± 1.55 |
| IL-7 | 1.18 ± 0.37 | 2.26 ± 0.39 | 1.69 ± 0.79 | 2.20 ± 0.89 |
| IL-10c | 1.24 ± 0.58 | 6.33 ± 0.94 | 2.28 ± 1.20 | 4.34 ± 1.73 |
| IL-13 | 0.97 ± 0.37 | 1.46 ± 0.30 | 1.30 ± 0.66 | 1.69 ± 0.93 |
| IL12p70 | 0.95 ± 0.51 | 3.77 ± 2.51 | 2.46 ± 1.40 | 4.42 ± 2.48 |
| IL-16 | 1.01 ± 0.31 | 1.88 ± 0.36 | 1.68 ± 0.77 | 1.76 ± 0.57 |
| IL-17 | 1.06 ± 0.31 | 2.61 ± 1.08 | 1.85 ± 1.30 | 1.84 ± 0.82 |
| IL-23 | 1.04 ± 0.49 | 2.86 ± 0.78 | 2.22 ± 1.31 | 2.52 ± 1.18 |
| IL-27 | 1.45 ± 0.53 | 2.08 ± 1.07 | 1.80 ± 0.94 | 1.65 ± 0.79 |
| I-TAC (CXCL11) | 1.48 ± 0.69 | 2.59 ± 0.82 | 2.28 ± 1.34 | 2.31 ± 1.27 |
| M-CSF | 1.11 ± 0.48 | 1.34 ± 0.60 | 0.91 ± 0.55 | 0.89 ± 0.52 |
| MIP-1α (CCL3) | 1.10 ± 0.38 | 2.16 ± 0.36 | 2.18 ± 1.15 | 2.56 ± 0.99 |
| MIP-1β (CCL4) | 0.97 ± 0.46 | 4.36 ± 2.31 | 2.67 ± 1.92 | 3.63 ± 2.32 |
| RANTES (CCL5) | 1.44 ± 0.67 | 2.81 ± 0.68 | 2.97 ± 1.98 | 2.3 ± 0.75 |
| SDF-1 (CXCL12) | 0.65 ± 0.21 | 1.00 ± 0.44 | 0.57 ± 0.31 | 0.79 ± 0.42 |
| TARC (CCL17) | 1.10 ± 0.59 | 2.80 ± 0.82 | 2.40 ± 0.93 | 2.85 ± 1.30 |
| TIMP-1 | 1.60 ± 0.65 | 3.09 ± 0.56 | 1.94 ± 1.26 | 1.54 ± 0.98 |
| TNF-α | 1.22 ± 0.37 | 2.65 ± 0.51 | 1.97 ± 0.75 | 2.24 ± 0.45 |
| TREM-1 | 1.19 ± 0.51 | 3.1 ± 0.60 | 1.97 ± 0.89 | 2.03 ± 0.76 |
Data for each infected group are relative to the mean serum values for the respective noninfected nonpregnant or pregnant groups. There were 4 mice per group.
GM-CSF, granulocyte-macrophage colony-stimulating factor; M-CSF, macrophage colony-stimulating factor.
Values for IL-10 are significantly increased in the pregnant infected (WT S. Typhimurium and S. Typhimurium ΔaroA) animals relative to the nonpregnant infected group by Mann-Whitney U test.
WT S. Typhimurium infection results in overt placental inflammation.
Placentas of pregnant WT S. Typhimurium-infected mice showed very high levels of G-CSF (∼400-fold), tumor necrosis factor alpha (TNF-α) (∼1,000-fold), IL-6 (∼200-fold), and gamma interferon (IFN-γ) mRNA expression compared to pregnant noninfected mice. In contrast, the expression of these cytokines in the placentas of S. Typhimurium ΔaroA-infected mice was similar to levels in healthy controls and significantly less than levels in pregnant WT S. Typhimurium-infected mice (Fig. 6). Expression levels of IL-10, transforming growth factor β (TGF-β), and IL-12p40 in infected and noninfected placentas were found to be similar (data not shown). Taken together, these results indicate that, while the placental bacterial burdens of WT S. Typhimurium and the ΔaroA mutant were similar, the expression levels of key inflammatory cytokines were profoundly different.
FIG. 6.
Placental cytokine expression. Shown is cytokine expression determined by Q-RT-PCR of placentas obtained from noninfected and WT S. Typhimurium (ST) (103 CFU)- or S. Typhimurium ΔaroA (106 CFU)-infected mice on day 3 postinfection. Relative mRNA expression is normalized to β-actin. Each data point indicates the expression level in pooled placental tissue of individual mice, and the mean expression level for each group is indicated by a horizontal line. *, expression levels for WT S. Typhimurium-infected mice are significantly higher (P < 0.05) than those for healthy noninfected placentas; ★, expression levels in the placentas of S. Typhimurium ΔaroA-infected mice are significantly lower (P < 0.05) than those in the WT S. Typhimurium-infected group. Data were analyzed by Mann-Whitney U test.
WT S. Typhimurium infection evokes massive placental infiltration of polymorphonuclear cells.
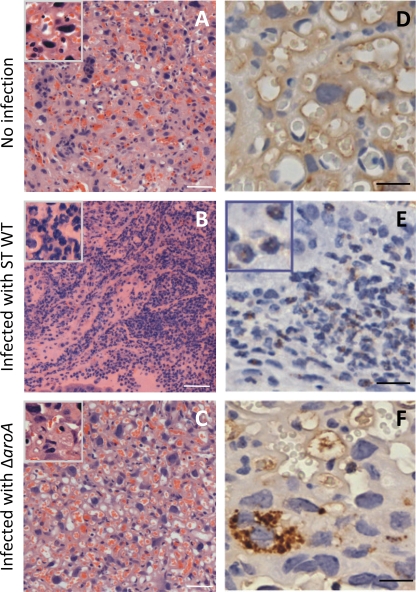
Figure 7 depicts H&E and MPO staining of transverse placental sections representative of 8 to 10 fields viewed at 3 mm from each other in accordance with a systematic uniform random sampling protocol (27). These sections depict the trophoblast labyrinth region, as evident from the high vasculature of the healthy noninfected placenta (Fig. 7A). By 72 h of WT S. Typhimurium infection placental tissue layers were often indiscernible and necrotic (Fig. 7B). Under higher magnification, astounding numbers of typical polymorphonucleated cells were visible, suggesting neutrophil infiltration (Fig. 7B). In contrast, the S. Typhimurium ΔaroA-infected placental tissues had a healthy morphology (Fig. 7C) similar to those of noninfected placentas (Fig. 7A), with clear maternal artery and cellular appearance. H&E-stained placental tissue was also assigned a histology score based on extent of necrosis and neutrophil infiltration (Table 2). Data quantified from 2 to 4 individual mice for each group clearly showed positive correlation between WT S. Typhimurium infection and rapid placental damage. Additionally, granulocyte-specific MPO (34) was obvious in the WT S. Typhimurium-infected placenta (Fig. 7E) as opposed to the pregnant noninfected (Fig. 7D) and S. Typhimurium ΔaroA-infected (Fig. 7F) samples. Placentas infected with S. Typhimurium ΔaroA showed few very large cells positive for MPO staining, which from their size and morphological presentation appear to be macrophages that may have engulfed dying neutrophils (Fig. 7F). Further investigation is required to determine the implication of this observation. Moreover, a diffuse background MPO staining was found in the noninfected healthy placenta and S. Typhimurium ΔaroA-infected samples, which may be due to reactivity of intact placental cells to the MPO antibody.
FIG. 7.
H&E and MPO staining of placental tissue. Histology of placental tissue infected with WT S. Typhimurium (ST) (103 CFU) or S. Typhimurium ΔaroA (106 CFU) was determined on day 3 postinfection. (A to C) H&E staining of noninfected (A) and WT S. Typhimurium (B)- and S. Typhimurium ΔaroA-infected (C) placentas. Scale bars, 100 μm. Insets show tissue at higher magnification. (D to F) MPO staining of noninfected (D) and WT S. Typhimurium (E)- and S. Typhimurium ΔaroA-infected (F) placentas. Scale bar, 20 μm. The inset (E) shows polymorphonuclear cell-specific MPO production. Images are representative of tissues processed from 3 mice per group.
TABLE 2.
Placental histological scorea
| Group | Mean scoreb ± SEM for: |
|
|---|---|---|
| Necrosis | Neutrophil infiltration | |
| Pregnant noninfected | 0.12 ± 0.06 | 0.08 ± 0.08 |
| Pregnant WT infected (48 h) | 1.95 ± 0.61 | 1.95 ± 1.06 |
| Pregnant WT infected (72 h) | 2.72 ± 0.66* | 2.61 ± 0.76* |
| Pregnant ΔaroA mutant infected (48 h) | 0.56 ± 0.44 | 0 |
| Pregnant ΔaroA mutant infected (72 h) | 0.58 ± 0.28 | 0.46 ± 0.14 |
H&E-stained placental tissue was assigned a histology score between 0 and 3 based on extent of necrosis and neutrophil infiltration. For necrosis the following scoring guide was used: 0, no necrosis; 1, mild necrosis limited to the decidual area; 2, moderate necrosis extending into the junctional zone; and 3, severe necrosis throughout the tissue. For neutrophil infiltration the following scoring guide was used: 0, no neutrophils discerned; 1, neutrophils limited to the decidua; 2, moderate neutrophil infiltration extending into the junctional zone; and 3, massive neutrophil infiltration throughout the tissue.
Data were derived from evaluation of all the placentas processed from 2 to 4 individual mice for each group (12 to 28 placentas per group). *, value significantly different by one-way ANOVA or by Dunnett's post hoc test (P < 0.01) from that for control noninfected placentas.
The presence of neutrophils was additionally confirmed by Gr1-positive staining. In the pregnant noninfected placenta, Gr1-positive cells denoting neutrophils were spotted along the blood vessels (Fig. 8A). However, in the WT S. Typhimurium-infected placenta, Gr1-positive cells were encountered in large numbers throughout the tissue (Fig. 8B), beyond the vicinity of the blood vasculature. In contrast, in the S. Typhimurium ΔaroA-infected placenta, Gr1-positive cells remained restricted to the blood vessel lining (Fig. 8C). Thus, virulent WT S. Typhimurium infection evoked neutrophil-mediated placental pathology.
FIG. 8.
Gr1-positive staining of placental tissue. Shown are fluorescent microscopic images of frozen placental sections from noninfected tissue (A) and tissue infected with WT S. Typhimurium (103 CFU) (B) or S. Typhimurium ΔaroA (106 CFU) (C) stained with Alexa647-conjugated anti-GR1 (red) and Hoechst 33342 (blue). Scale bar, 20 μm. Images are representative of placentas obtained from 3 mice per group.
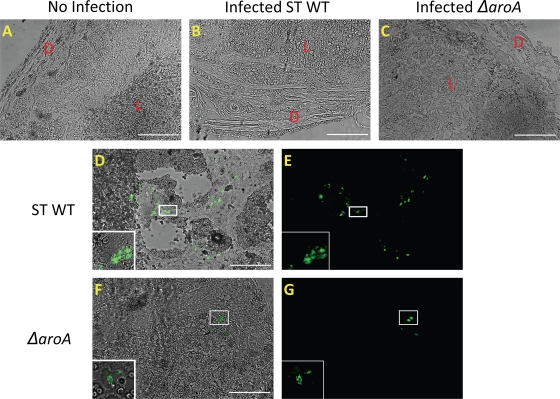
WT S. Typhimurium bacteria are widely dispersed throughout the placental tissue.
In order to determine the localization of the bacteria to particular regions of the placenta, mice were infected with WT S. Typhimurium and S. Typhimurium ΔaroA expressing GFP. Seventy-two hours postinfection, tissues were frozen, sectioned, and viewed microscopically. In the uninfected placenta, the deciduas, junctional zone, and labyrinth layers were clearly distinguished (Fig. 9A). In contrast, in WT S. Typhimurium-infected tissue, the decidual layer was highly reduced and the labyrinth layer was necrotic (Fig. 9B). S. Typhimurium ΔaroA-infected tissue retained placental layer integrity (Fig. 9C). When the bacteria were visualized based of GFP green fluorescence, in the WT S. Typhimurium-infected placental tissue, bacteria were dispersed in large numbers throughout the necrotic labyrinth trophoblast (Fig. 9D and E). On the other hand, S. Typhimurium ΔaroA bacteria were localized to outer decidual layers (Fig. 9F and G), with few bacteria encountered in the deeper labyrinth regions in some selected samples (data not shown). Overall these results indicate that WT S. Typhimurium rapidly pervades the entire placental tissue.
FIG. 9.
Localization of WT S. Typhimurium (ST) and S. Typhimurium ΔaroA bacteria to distinct regions of the placenta. Shown are images of frozen placental sections from mice infected with WT S. Typhimurium (103 CFU) or S. Typhimurium ΔaroA (106 CFU) expressing GFP 72 h postinfection. (A, B, and C) Morphology of the placentas from uninfected mice and mice infected with WT S. Typhimurium or S. Typhimurium ΔaroA, respectively. D, decidual region; L, labyrinth region. (D and E) Localization of WT S. Typhimurium-GFP in the highly necrotic labyrinth trophoblast. (F and G) Localization of S. Typhimurium ΔaroA-GFP near the decidual region of the placenta. The presence of bacteria was confirmed by observing the marked areas using a 60× oil immersion objective, and the higher-magnification images of bacteria are shown in insets. The images were captured using an Olympus IX81 fluorescence microscope. Scale bars, 200 μm. Images are representative of tissues processed from 3 mice per group.
DISCUSSION
Infections caused by food- and waterborne pathogens are on the rise, and these can get exacerbated in immunocompromised hosts. While it makes biological sense that pregnancy does not result in compromised immunity against all pathogens, it is not clear why some pathogens, including Salmonella, pose an increased threat. The interplay between pregnancy, pathogen virulence, pathogen burden, inflammation, and the consequent host survival is not clear. In this study using a murine model of S. Typhimurium, we have detailed the differential interaction of virulent and avirulent strains with the fetomaternal cellular environment. We have shown that profound pathogen proliferation in the placenta triggers a virulence-dependent inflammatory signature that adversely affects fetal and maternal survival.
The placenta is highly permissive to S. Typhimurium proliferation and indeed even supports the growth of the ΔaroA strain, which requires extraneous aromatic amino acids for replication (16). As the placenta is a metabolically active tissue, aromatic amino acids may have been easily available, providing a compensatory mechanism for ΔaroA strain replication. S. Typhimurium has a rapid extracellular doubling time of ∼25 min (26). However, we show that S. Typhimurium exhibits contrasting intracellular replication rates depending on the cell type, doubling in ∼1 h in choriocarcinoma cells and 4 h within HeLa cells in vitro (Fig. 2d). Even the ΔaroA strain doubled rapidly within choriocarcinoma cells (2.2 h). Furthermore, in vivo, in the placental milieu, burdens of both WT S. Typhimurium and the ΔaroA strain rapidly increased, reaching >108 bacteria within 24 h. These differential replication rates of S. Typhimurium strains in various environments may be an evolutionary adaptation for evading host immune systems. As both S. Typhimurium strains invade the placenta and cause fetal infection, they may strategically survive within specialized cells at the fetomaternal interface. Indeed, many pathogens such Listeria, Toxoplasma, cytomegalovirus, and Plasmodium are known to preferentially colonize the placenta (2, 22). In the case of pregnancy-associated malaria, Plasmodium-infected erythrocytes sequester in the placenta, as chondroitin sulfate A serves as an adhesion receptor for the parasite (39). Listeria monocytogenes exploits ActA to gain accesses to the placenta (22). It will be of further interest to explore the specific trophoblast and/or other cell types that provide a unique reservoir for S. Typhimurium at the fetomaternal interface.
It has been reported that the intracellular replication of S. Typhimurium is essential for its virulence (23). Pathogen virulence is a highly complex phenomenon, and proliferation is among the mechanisms that contribute to virulence. Virulent mycobacteria were reported to have faster doubling times in vivo (8). However, faster doubling times may not be the only mechanism that enables virulent pathogens to escape the developing immune response. Virulent, but not avirulent, mycobacteria were shown to selectively overcome the growth-inhibitory action of a Th1-dependent, NOS2-independent immune response (18). Previously we have shown that S. Typhimurium ΔssaR, a mutant defective in Salmonella pathogenicity island 2 genes, is less deleterious to pregnancy than the WT (33). Herein, a ΔinvA mutant defective in Salmonella pathogenicity island 1 type III secretion genes caused slightly reduced fetal loss relative to WT S. Typhimurium. Furthermore, with a 1,000-fold-higher infection dose (106 CFU) of S. Typhimurium ΔaroA, the bacterial load increased and was comparable to that achieved with WT S. Typhimurium, but this did not adversely affect fetal and/or maternal survival. Therefore, bacterial burden alone does not appear to adversely affect host survival.
The nature of inflammation evoked by the pathogen appears to be the key mechanism that influences pathogenesis and host survival profoundly, and this correlated with virulence. Given the high placental bacterial burden of WT S. Typhimurium and S. Typhimurium ΔaroA, at least some of the same bacterial pathogen recognition events may be expected, and this is supported by the similar increases for both infections in levels of chemoattractants for monocytes (4) such as IP10, CCL2, and CXCL9. In contrast, cytokines/chemokines that were specifically elevated in the serum of WT S. Typhimurium-infected pregnant hosts are known to be neutrophil chemoattractants (30, 43). Additionally, cytokines, such as IL-6, evoked by WT S. Typhimurium infection can have pathological effects such as those implicated in rheumatoid arthritis and inflammatory bowel disease (9, 40). High levels of cytokines such as G-CSF can also evoke complications like preterm labor and chorioamnionitis (12, 46). The lack of placental lesions following S. Typhimurium ΔaroA infection, despite high bacterial burden, correlated with the reduced inflammation evoked by this mutant. For example, placental TNF-α levels were very low in the pregnant S. Typhimurium ΔaroA-infected hosts, whereas they were considerably higher (∼100-fold) in the pregnant WT S. Typhimurium-infected group. The toxic effects of TNF-α have been associated with immunopathology and mortality due to septic shock (36). Moreover, it has been suggested that attenuated strains such as S. Typhimurium ΔaroA may not require host TNF-α production for clearance and hence may fail to evoke their production by host immune cells as well (35). However, it is still unclear what difference between the ΔaroA mutant and WT S. Typhimurium results in a weaker inflammatory response. Recently, it has been reported that the ΔaroA mutant has defects in cell envelope biosynthesis, leading to weaker outer membrane integrity (45). Whether such structural differences between these mutants translate into differential expression of other innate immune ligands and/or interactions with host immune cells needs further investigation.
Infections and associated inflammation are considered to play a pivotal role in premature birth (12, 38) and placental pathologies such as preeclampsia and chorioamnionitis (6, 42). Inflammatory cytokines such as IFN-γ, TNF-α, and IL-12 can induce resorptions, and the Th2/anti-inflammatory bias during pregnancy counteracts their detrimental effects (5). For example, Porphyromonas gingivalis infection in mice augmented maternal TNF-α production while suppressing maternal IL-10, leading to increased fetal resorptions (25). Trypanosoma cruzi infection in mice induced high levels of maternal TNF-α, resulting in increased fetal mortality (28). The placental Th1 response against Leishmania infection resulted in increased fetal resorptions in infected mice (20). Similarly, S. Typhimurium may have disrupted the Th2 bias in pregnancy. However, gross differences in the levels of placental Th2 and/or regulatory cytokines such as TGF-β between noninfected pregnant and pregnant WT S. Typhimurium-infected mice were not observed (data not shown). Therefore, it is likely that the toxic cytokines such as IFN-γ and TNF-α evoked by S. Typhimurium directly and rapidly inflict placental damage.
The infiltration of WT S. Typhimurium bacteria deep in the labyrinth region appears to have ultimately caused placental necrosis. In contrast, the less-damaging S. Typhimurium ΔaroA mutant was mainly localized to the decidua. This dichotomous localization of bacteria to distinct regions of the placenta may have contributed to the differential inflammation. Indeed, in human first and second trimester placentas, the expression of Toll-like receptors (TLR) is restricted to inner layers of the cytotrophoblast. Thus, only pathogens that breach the outer TLR-negative syncytiotrophoblast are able to evoke inflammatory damage (12). In mice, enhanced TLR4 expression in the labyrinthine trophoblast following maternal infection has been reported (1). Thus, the slower penetration of S. Typhimurium ΔaroA into the labyrinthine trophoblast may have offset the placental inflammation.
Ultimately, the loss of placental integrity evoked by WT S. Typhimurium correlated with widespread polymorphonuclear cell infiltration. Activation of neutrophils can induce DNA-containing neutrophil extracellular traps (NETs) for trapping and killing bacteria. However, neutrophil activation has also been implicated in the pathogenesis of preeclampsia by leading to deficient placental perfusion (13). An increase in myeloperoxidase has also been observed in the placenta and systemic circulation of women suffering from preeclampsia (11). While neutrophils are considered vital for defense against S. Typhimurium until adaptive immunity develops (10), their overt activation resulted in deleterious effects in the maternal host.
Many studies have reported Salmonella-induced clinical complications during pregnancy (14, 15, 32, 44). Although antibiotics may control Salmonella infections, our study highlights the risk to pregnant mothers, given the rising incidence of such food-borne infections. Our model provides a convenient reproducible tool to decipher the role of infections and inflammation at the fetomaternal interface. Studies such as ours that address the mechanisms of host susceptibility to infection may provide insights into management and treatment of epidemics.
Acknowledgments
This work was supported in part by Canadian Institutes of Health Research grant XNE-87055 to S.S., L.K., and B.B.F.
We gratefully acknowledge the technical assistance provided by Komal Gurnani, Renu Dudani, and Ahmed Zafer.
This is NRC publication number 42532.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 1 March 2010.
REFERENCES
- 1.Arce, R. M., S. P. Barros, B. Wacker, B. Peters, K. Moss, and S. Offenbacher. 2009. Increased TLR4 expression in murine placentas after oral infection with periodontal pathogens. Placenta 30:156-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barragan, A., and L. D. Sibley. 2003. Migration of Toxoplasma gondii across biological barriers. Trends Microbiol. 11:426-430. [DOI] [PubMed] [Google Scholar]
- 3.Beeson, J. G., and P. E. Duffy. 2005. The immunology and pathogenesis of malaria during pregnancy. Curr. Top. Microbiol. Immunol. 297:187-227. [DOI] [PubMed] [Google Scholar]
- 4.Carr, M. W., S. J. Roth, E. Luther, S. S. Rose, and T. A. Springer. 1994. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc. Natl. Acad. Sci. U. S. A. 91:3652-3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaouat, G., E. Menu, D. A. Clark, M. Dy, M. Minkowski, and T. G. Wegmann. 1990. Control of fetal survival in CBA x DBA/2 mice by lymphokine therapy. J. Reprod. Fertil. 89:447-458. [DOI] [PubMed] [Google Scholar]
- 6.Conrad, K. P., T. M. Miles, and D. F. Benyo. 1998. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am. J. Reprod. Immunol. 40:102-111. [DOI] [PubMed] [Google Scholar]
- 7.Doffinger, R., S. Patel, and D. S. Kumararatne. 2005. Human immunodeficiencies that predispose to intracellular bacterial infections. Curr. Opin. Rheumatol. 17:440-446. [DOI] [PubMed] [Google Scholar]
- 8.Dunn, P. L., and R. J. North. 1995. Virulence ranking of some Mycobacterium tuberculosis and Mycobacterium bovis strains according to their ability to multiply in the lungs, induce lung pathology, and cause mortality in mice. Infect. Immun. 63:3428-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fielding, C. A., R. M. McLoughlin, L. McLeod, C. S. Colmont, M. Najdovska, D. Grail, M. Ernst, S. A. Jones, N. Topley, and B. J. Jenkins. 2008. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J. Immunol. 181:2189-2195. [DOI] [PubMed] [Google Scholar]
- 10.Fierer, J. 2001. Polymorphonuclear leukocytes and innate immunity to Salmonella infections in mice. Microbes Infect. 3:1233-1237. [DOI] [PubMed] [Google Scholar]
- 11.Gandley, R. E., J. Rohland, Y. Zhou, E. Shibata, G. F. Harger, A. Rajakumar, V. E. Kagan, N. Markovic, and C. A. Hubel. 2008. Increased myeloperoxidase in the placenta and circulation of women with preeclampsia. Hypertension 52:387-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldenberg, R. L., J. C. Hauth, and W. W. Andrews. 2000. Intrauterine infection and preterm delivery. N. Engl. J. Med. 342:1500-1507. [DOI] [PubMed] [Google Scholar]
- 13.Gupta, A. K., P. Hasler, W. Holzgreve, and S. Hahn. 2007. Neutrophil NETs: a novel contributor to preeclampsia-associated placental hypoxia? Semin. Immunopathol. 29:163-167. [DOI] [PubMed] [Google Scholar]
- 14.Gyang, A., and M. Saunders. 2008. Salmonella Mississippi: a rare cause of second trimester miscarriage. Arch. Gynecol. Obstet. 277:437-438. [DOI] [PubMed] [Google Scholar]
- 15.Hedriana, H. L., J. L. Mitchell, and S. B. Williams. 1995. Salmonella typhi chorioamnionitis in a human immunodeficiency virus-infected pregnant woman. A case report. J. Reprod. Med. 40:157-159. [PubMed] [Google Scholar]
- 16.Hoiseth, S. K., and B. A. Stocker. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291:238-239. [DOI] [PubMed] [Google Scholar]
- 17.Jones, B. D., and S. Falkow. 1996. Salmonellosis: host immune responses and bacterial virulence determinants. Annu. Rev. Immunol. 14:533-561. [DOI] [PubMed] [Google Scholar]
- 18.Jung, Y. J., R. LaCourse, L. Ryan, and R. J. North. 2002. Virulent but not avirulent Mycobacterium tuberculosis can evade the growth inhibitory action of a T helper 1-dependent, nitric oxide synthase 2-independent defense in mice. J. Exp. Med. 196:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufmann, S. H., B. Raupach, and B. B. Finlay. 2001. Introduction: microbiology and immunology: lessons learned from Salmonella. Microbes Infect. 3:1177-1181. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan, L., L. J. Guilbert, T. G. Wegmann, M. Belosevic, and T. R. Mosmann. 1996. T helper 1 response against Leishmania major in pregnant C57BL/6 mice increases implantation failure and fetal resorptions. Correlation with increased IFN-gamma and TNF and reduced IL-10 production by placental cells. J. Immunol. 156:653-662. [PubMed] [Google Scholar]
- 21.Laibl, V. R., and J. S. Sheffield. 2005. Tuberculosis in pregnancy. Clin. Perinatol. 32:739-747. [DOI] [PubMed] [Google Scholar]
- 22.Le Monnier, A., N. Autret, O. F. Join-Lambert, F. Jaubert, A. Charbit, P. Berche, and S. Kayal. 2007. ActA is required for crossing of the fetoplacental barrier by Listeria monocytogenes. Infect. Immun. 75:950-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung, K. Y., and B. B. Finlay. 1991. Intracellular replication is essential for the virulence of Salmonella typhimurium. Proc. Natl. Acad. Sci. U. S. A. 88:11470-11474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine, M. M., C. Ferreccio, R. E. Black, C. O. Tacket, and R. Germanier. 1989. Progress in vaccines against typhoid fever. Rev. Infect. Dis. 11(Suppl. 3):S552-S567. [DOI] [PubMed] [Google Scholar]
- 25.Lin, D., M. A. Smith, C. Champagne, J. Elter, J. Beck, and S. Offenbacher. 2003. Porphyromonas gingivalis infection during pregnancy increases maternal tumor necrosis factor alpha, suppresses maternal interleukin-10, and enhances fetal growth restriction and resorption in mice. Infect. Immun. 71:5156-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luu, R. A., K. Gurnani, R. Dudani, R. Kammara, H. van Faassen, J. C. Sirard, L. Krishnan, and S. Sad. 2006. Delayed expansion and contraction of CD8+ T cell response during infection with virulent Salmonella typhimurium. J. Immunol. 177:1516-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayhew, T. M. 2006. Stereology and the placenta: where's the point?—a review. Placenta 27(Suppl. A):S17-S25. [DOI] [PubMed] [Google Scholar]
- 28.Mjihdi, A., C. Truyens, O. Detournay, and Y. Carlier. 2004. Systemic and placental productions of tumor necrosis factor contribute to induce fetal mortality in mice acutely infected with Trypanosoma cruzi. Exp. Parasitol. 107:58-64. [DOI] [PubMed] [Google Scholar]
- 29.Mor, G. 2008. Inflammation and pregnancy: the role of toll-like receptors in trophoblast-immune interaction. Ann. N. Y. Acad. Sci. 1127:121-128. [DOI] [PubMed] [Google Scholar]
- 30.Ohtsuka, Y., J. Lee, D. S. Stamm, and I. R. Sanderson. 2001. MIP-2 secreted by epithelial cells increases neutrophil and lymphocyte recruitment in the mouse intestine. Gut 49:526-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orndorff, P. E., T. S. Hamrick, I. W. Smoak, and E. A. Havell. 2006. Host and bacterial factors in listeriosis pathogenesis. Vet. Microbiol. 114:1-15. [DOI] [PubMed] [Google Scholar]
- 32.Ozer, O., F. B. Cebesoy, I. Sari, and V. Davutoglu. 2009. A case of Salmonella typhi endocarditis in pregnancy. Am. J. Med. Sci. 337:210-211. [DOI] [PubMed] [Google Scholar]
- 33.Pejcic-Karapetrovic, B., K. Gurnani, M. S. Russell, B. B. Finlay, S. Sad, and L. Krishnan. 2007. Pregnancy impairs the innate immune resistance to Salmonella typhimurium leading to rapid fatal infection. J. Immunol. 179:6088-6096. [DOI] [PubMed] [Google Scholar]
- 34.Pinkus, G. S., and J. L. Pinkus. 1991. Myeloperoxidase: a specific marker for myeloid cells in paraffin sections. Mod. Pathol. 4:733-741. [PubMed] [Google Scholar]
- 35.Raupach, B., and S. H. Kaufmann. 2001. Bacterial virulence, proinflammatory cytokines and host immunity: how to choose the appropriate Salmonella vaccine strain? Microbes Infect. 3:1261-1269. [DOI] [PubMed] [Google Scholar]
- 36.Raupach, B., N. Kurth, K. Pfeffer, and S. H. Kaufmann. 2003. Salmonella typhimurium strains carrying independent mutations display similar virulence phenotypes yet are controlled by distinct host defense mechanisms. J. Immunol. 170:6133-6140. [DOI] [PubMed] [Google Scholar]
- 37.Ravindran, R., and S. J. McSorley. 2005. Tracking the dynamics of T-cell activation in response to Salmonella infection. Immunology 114:450-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Redline, R. W. 2004. Placental inflammation. Semin. Neonatol. 9:265-274. [DOI] [PubMed] [Google Scholar]
- 39.Rogerson, S. J., L. Hviid, P. E. Duffy, R. F. Leke, and D. W. Taylor. 2007. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect. Dis. 7:105-117. [DOI] [PubMed] [Google Scholar]
- 40.Rose-John, S., J. Scheller, G. Elson, and S. A. Jones. 2006. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J. Leukoc. Biol. 80:227-236. [DOI] [PubMed] [Google Scholar]
- 41.Sad, S., R. Dudani, K. Gurnani, M. Russell, H. van Faassen, B. Finlay, and L. Krishnan. 2008. Pathogen proliferation governs the magnitude but compromises the function of CD8 T cells. J. Immunol. 180:5853-5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saji, F., Y. Samejima, S. Kamiura, K. Sawai, K. Shimoya, and T. Kimura. 2000. Cytokine production in chorioamnionitis. J. Reprod. Immunol. 47:185-196. [DOI] [PubMed] [Google Scholar]
- 43.Scapini, P., J. A. Lapinet-Vera, S. Gasperini, F. Calzetti, F. Bazzoni, and M. A. Cassatella. 2000. The neutrophil as a cellular source of chemokines. Immunol. Rev. 177:195-203. [DOI] [PubMed] [Google Scholar]
- 44.Schloesser, R. L., V. Schaefer, and A. H. Groll. 2004. Fatal transplacental infection with non-typhoidal Salmonella. Scand. J. Infect. Dis. 36:773-774. [DOI] [PubMed] [Google Scholar]
- 45.Sebkova, A., D. Karasova, M. Crhanova, E. Budinska, and I. Rychlik. 2008. aro mutations in Salmonella enterica cause defects in cell wall and outer membrane integrity. J. Bacteriol. 190:3155-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stallmach, T., G. Hebisch, H. Joller, P. Kolditz, and M. Engelmann. 1995. Expression pattern of cytokines in the different compartments of the feto-maternal unit under various conditions. Reprod. Fertil. Dev. 7:1573-1580. [DOI] [PubMed] [Google Scholar]
- 47.Wick, M. J. 2004. Living in the danger zone: innate immunity to Salmonella. Curr. Opin. Microbiol. 7:51-57. [DOI] [PubMed] [Google Scholar]