Abstract
Enterotoxigenic Bacteroides fragilis (ETBF) produces an approximately 20-kDa heat-labile enterotoxin (BFT) that plays an essential role in mucosal inflammation. Although spontaneous disappearance of ETBF infection is common, little information is available on regulated expression of antibacterial factors in response to BFT stimulation. This study investigates the role of BFT in human β-defensin 2 (hBD-2) induction from intestinal epithelial cells. Stimulation of HT-29 and Caco-2 intestinal epithelial cell lines with BFT resulted in the induction of hBD-2. Activation of a reporter gene for hBD-2 was dependent on the presence of NF-κB binding sites. In contrast, suppression of AP-1 did not affect hBD-2 expression in BFT-stimulated cells. Inhibition of p38 mitogen-activated protein kinase (MAPK) using SB203580 and small interfering RNA (siRNA) transfection resulted in a significant reduction in BFT-induced IκB kinase (IKK)/NF-κB activation and hBD-2 expression. Our results suggest that a pathway including p38 MAPK, IKK, and NF-κB activation is required for hBD-2 induction in intestinal epithelial cells exposed to BFT, and may be involved in the host defense following infection with ETBF.
Enterotoxigenic Bacteroides fragilis (ETBF) strains have been identified during an investigation of diarrheal illness in animals and young children (27, 28, 36, 47). Recently, ETBF infection has been reported to be associated with inflammatory bowel diseases (IBD) (2, 32, 37) and colorectal cancer (39, 46). B. fragilis enterotoxin (BFT), an approximately 20-kDa heat-labile metalloprotease, is regarded as a virulence factor for the diseases. Although ETBF strains are proposed to be enteric pathogens, all human studies of ETBF infection have demonstrated that between ∼4% and 30% of infected individuals asymptomatically colonize with ETBF and that infection is self-limited (33, 36). These reports suggest that antibacterial factors induced by ETBF infection may be upregulated in the infected area of the intestine and regulate enteric inflammation.
To prevent intestinal infection, the luminal flora and pathogens are controlled by epithelially derived antimicrobial peptides that are constitutively expressed or inducible (29). For example, defensins, cathelicidin LL-37, lysozyme, phospholipase A, and proteins with bactericidal properties such as ubiquicidin, ribosomal proteins, and eosinophilic proteins have been reported (4, 11, 29, 38).
Defensins can act as endogenous antimicrobials by membrane permeabilization, activation of cell wall lytic enzymes, and disruption of membrane-bound multienzyme complexes and intracellular events (10, 29, 34). Human defensins are cationic, amphipathic peptides of 3.5 to ∼6 kDa that are characterized by three intramolecular disulfide bonds. They are subdivided into α- and β-defensins. Four different neutrophil α-defensins have been reported: human neutrophil protein 1 (HNP-1) to HNP-4. In addition, two human α-defensins (HD-5 and HD-6) are constitutively expressed in Paneth cells of the small intestine (6). The epithelial human β-defensin (hBD-1) is constitutive in the intestinal tract, whereas epithelial hBD-2 is induced by cytokines or in response to inflammatory stimuli such as bacterial infection (29, 35, 42). Recently, human α-defensins have been reported to inhibit the activity of Clostridium difficile toxin B (9). In light of these reports, it is possible that defensins may affect inflammatory responses due to ETBF-derived BFT and contribute to host defense. However, little is known about the regulated expression of β-defensins in response to BFT stimulation.
Activation of mitogen-activated protein kinase (MAPK), nuclear factor κB (NF-κB), or activator protein 1 (AP-1) is known to be important for the induction of hBD-2 in several cell lines (24, 30, 35, 40, 41, 43, 44). Although these signaling molecules are reported to be upregulated in intestinal epithelial cells exposed to BFT (12, 14, 19, 20, 21, 23, 45), there is no evidence of BFT-induced MAPK and NF-κB activation leading to hBD-2 expression. In the studies reported here, we investigated the regulation of hBD-2 induction in response to BFT stimulation and found that stimulation with BFT enhances hBD-2 expression in intestinal epithelial cells through activation of the MAPK/IκB kinase (IKK)/NF-κB pathway.
MATERIALS AND METHODS
Reagents.
Lipopolysaccharide (LPS)-free fetal bovine serum (FBS), antibiotics, l-glutamine, and Trizol were obtained from GIBCO BRL (Gaithersburg, MD). Dulbecco's modified Eagle's medium (DMEM) was purchased by Sigma Chemical Co. (St. Louis, MO). Antibodies against IKK-α, IKK-β, phospho-IKK-α/β, pan-extracellular signal-regulated kinase 1/2 (pan-ERK1/2 [p44/p42]), phospho-ERK1/2, pan-Jun N-terminal kinase (pan-JNK [p54/p46]), phospho-JNK, pan-p38, phospho-p38, and actin were acquired from Cell Signaling Technology, Inc. (Beverly, MA). Goat anti-rabbit and anti-mouse secondary antibodies conjugated to horseradish peroxidase were purchased from Transduction Laboratories (Lexington, KY). Antibodies against p50, p52, p65, c-Rel, and RelB were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). PD98059, SB203580, SP600125, and Bay 11-7085 were acquired from Calbiochem (La Jolla, CA).
Purification of BFT and cell culture conditions.
BFT was purified from the culture supernatants of a highly toxigenic strain of ETBF as described previously (12, 14, 19-21, 23). The purity of the BFT preparations was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Typical preparations of BFT contained 0.5 to 1.2 mg of protein per milliliter as measured by the bicinchoninic acid (BCA) protein assay. The buffers used in the purification were prepared using LPS-free water (Baxter Healthcare Corp., Deerfield, IL). The activity of LPS in BFT solutions (1 mg/ml) was less than 1 endotoxin unit/ml (quantitative chromogenic Limulus amebocyte lysate; BioWhittaker, Walkersville, MD). BFT was frozen in aliquots at −80°C immediately after purification. The human colon adenocarcinoma cell line HT-29 (ATCC HTB 38) and Caco-2 human ileocecal epithelial cell line (ATCC HTB 37) were grown in DMEM with 10% FBS and 2 mM glutamine. Cells were seeded at 0.5 × 106 to 2 × 106 cells per well onto six-well plates and allowed to attach overnight. After 12 h of serum starvation, cells were incubated with BFT for the indicated period.
Quantitative RT-PCR and ELISA.
Cells were treated with BFT, after which total cellular RNA was extracted using Trizol. Reverse transcription (RT)-PCR amplification was performed as described previously (23). The primers and expected PCR product sizes were as follows (1, 30): hBD-1, 5′-CTCTGTCAGCTCAGCCTC-3′ (sense) and 5′-CTTGCAGCACTTGGCCTTCCC-3′ (antisense), 272 bp; hBD-2, 5′-CCAGCCATCAGCCATGAGGGT-3′ (sense) and 5′-GGAGCCCTTTCTGAATCCGCA-3′ (antisense), 254 bp; and human β-actin, 5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′ (sense) and 5′-CTAGAAGCATTGCGGTGGACGATGGAGGG-3′ (antisense), 661 bp. To quantify mRNA molecules, standard RNAs for hBD-1 and hBD-2 were generated by an in vitro transcription using T7 RNA polymerase, as described previously (7, 16). Standard RNA for human β-actin was kindly provided by Martin F. Kagnoff of the University of California, San Diego. The sizes of PCR products generated from standard RNAs for hBD-1, hBD-2, and human β-actin are 368 bp, 371 bp, 319 bp, and 520 bp, respectively. mRNA levels of 5 × 103 mRNA molecules/μg of total RNA were considered positive; although lower levels could be detected and quantified, they were considered unlikely to be biologically meaningful, as they would reflect, on average, less than one mRNA transcript/20 cells (13, 22).
The amounts of hBD-2 in culture supernatants were measured by a commercially available enzyme-linked immunosorbent assay (ELISA; Phoenix Pharmaceuticals, Belmont, CA) according to the manufacturer's instructions. One experiment was performed in triplicate wells. This experiment was repeated more than 3 times. The detection limit of the ELISA kit was 10 pg/ml.
EMSA.
Cells were harvested and nuclear extracts prepared as described previously (21). Concentrations of protein in the extracts were determined by the Bradford assay (Bio-Rad, Hercules, CA). The electrophoretic mobility shift assay (EMSA) was performed according to the manufacturer's instructions (Promega, Madison, WI). In brief, 5 μg of nuclear extract was incubated for 30 min at room temperature with a γ-32P-labeled oligonucleotide probe (5′-AGTTGAGGGGACTTTCCCAGGC-3′ for the NF-κB binding site; 5′-CGCTTGATGACTCAGCCGGAA-3′ for the AP-1 binding site). After incubation, both bound DNA and free DNA were resolved on 5% polyacrylamide gels, as described previously (14, 21). A supershift assay was used to identify the specific members of the NF-κB family activated by BFT stimulation. EMSA was performed as described above, except that rabbit antibodies (1 μg/reaction) against NF-κB proteins p50, p52, p65, c-Rel, and RelB were added during the binding reaction period (21).
Plasmids, transfection, and luciferase assays.
To analyze hBD-2 promoter activity, wild-type (hBD-2-2338-luc) and mutant plasmids (NF-κB-mutant-1+2+3-luc, AP-1-mutant-luc, and AP-1+NF-κB-mutant-luc) were kindly donated by Jürgen Harder, Clinical Research Unit, Department of Dermatology, University Hospital Kiel, Kiel, Germany (44). The reporter plasmid containing AP-1-luciferase was purchased from BD Sciences (Franklin Lakes, NJ) (14). p2x NF-κB-, pβ-actin- and pRSV-β-galactosidase transcriptional reporters were kindly provided by Martin F. Kagnoff of the University of California, San Diego (8). Cells in six-well dishes were transfected with 1.5 μg of plasmid DNA using FuGENE 6 transfection reagent (Roche, Mannheim, Germany). The transfected cells were incubated for 24 h at 37°C in a 5% CO2 incubator and were then treated with BFT for the indicated time. Luciferase activity was determined in accordance with the manufacturer's instructions (Tropix, Inc., Bedford, MA). Light release was quantitated for 10 s using a luminometer (MicroLumat Plus, Berthold GmbH & Co. KG, Bad Wildbad, Germany), as previously described (25).
TAM-67 is a dominant-negative c-Jun superrepressor that lacks the transactivation domain of c-Jun and is a potent inhibitor of AP-1-mediated transactivation (3, 26). TAM-67 dimerizes with c-Jun or c-Fos family members and binds DNA, resulting in the inhibition of wild-type c-Jun and c-Fos function. The TAM-67 used in the present study was a gift from Andreas von Knethen of the University of Erlangen, Erlangen, Germany. Small interfering RNAs (siRNAs) against IKK-β, the NF-κB p65 subunit, and p38 were designed as described previously (17, 25). The siRNAs were synthesized by Qiagen (Valencia, CA). The negative (nonsilencing) control siRNA was also purchased from Qiagen. Transfection of dominant-negative superrepressor or siRNA into cells was performed as described previously (14). Cells were cultured in 6-well plates to 50 to 80% confluence, and the cells were then transfected with the dominant-negative superrepressor, siRNA, or nonsilencing siRNA using FuGENE 6 (Roche) as a transfection reagent. Briefly, 1 μg of siRNA or 1.5 μg plasmid DNA was diluted in serum-free medium to produce a final volume of 100 μl, to which 3 μl of FuGENE 6 was added, and the mixture was incubated for 15 min at room temperature. The transfection mixture was added to the respective wells, each containing 300 μl of medium (10% FBS content). Transfected cells were incubated for 48 h prior to the assay.
A retroviral system containing a mammalian expression vector encoding a hemagglutinin (HA) epitope-tagged mutant IκBα (IκBα-AA) with substitutions of serine for alanine at positions 32 and 36 was used to block NF-κB activation as described previously (15).
Immunoblots.
Cells were washed with ice-cold PBS and lysed in 0.5 ml/well lysis buffer (150 mM NaCl, 20 mM Tris pH 7.5, 0.1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride [PMSF], and 10 μg/ml aprotinin). Fifteen to 50 μg of protein per lane was size fractionated on a 6% polyacrylamide minigel (Mini-Protein II; Bio-Rad) and electrophoretically transferred to a nitrocellulose membrane (0.1-μm pore size). The immunoreactive proteins to which the primary antibodies had bound were visualized using goat anti-rabbit or anti-mouse secondary antibodies conjugated to horseradish peroxidase, followed by enhanced chemiluminescence (ECL system; Amersham Life Science, Buckinghamshire, England) and exposure to X-ray film.
In vitro kinase assay.
An HTScan IKK-β kinase assay kit was purchased from Cell Signaling Technology. This kit contains the GST-IKK-β kinase protein, a biotinylated peptide substrate, and a phosphoserine antibody for detection of the phosphorylated form of the substrate peptide. TransAM NF-κB and TransAM AP-1 ELISA kits were obtained from Active Motif (Carlsbad, CA). Each assay was performed according to the manufacturer's instructions (12, 18, 25).
Statistical analyses.
Data are presented as the mean ± standard deviation (SD) for quantitative RT-PCR and the mean ± standard error of the mean (SEM) for ELISA, luciferase assays, and kinase assays. Wilcoxon's rank sum test was used for statistical analysis. P values of <0.05 were considered statistically significant.
RESULTS
BFT induces hBD-2 expression in intestinal epithelial cells.
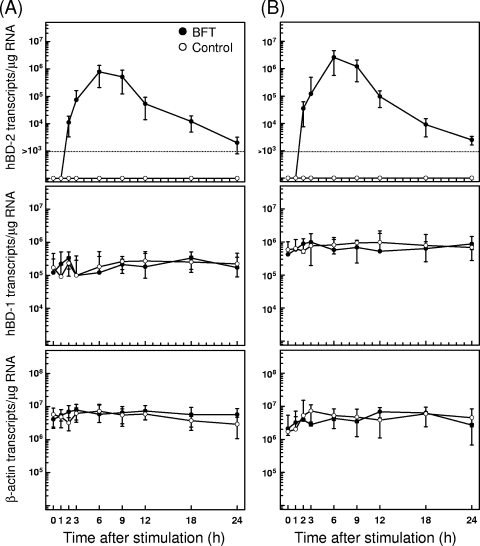
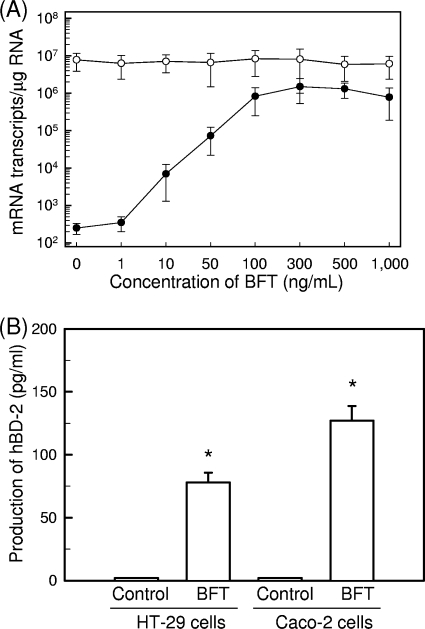
Stimulation with BFT increased the expression of hBD-2 mRNA as assessed by quantitative RT-PCR. Increased hBD-2 mRNA expression in HT-29 cells was first noted at 2 h after stimulation, peaked at 6 h poststimulation, and decreased to baseline thereafter (Fig. 1A). Similar increases in hBD-2 transcripts were observed following BFT stimulation of one additional human intestinal epithelial cell line, Caco-2 (Fig. 1B). In contrast to hBD-2 expression, expression of hBD-1 mRNA was constitutive and not affected by BFT in either cell line. hBD-1 transcript levels in each cell line ranged from ∼105 to ∼106 transcripts/μg cellular RNA. The magnitude of hBD-2 mRNA expression was dependent on the concentration of stimulated BFT (Fig. 2A). To confirm that the expressed hBD-2 transcripts are linked to protein synthesis, we measured the production of the hBD-2 protein in culture supernatants. As shown in Fig. 2B, stimulation of HT-29 and Caco-2 cells with BFT resulted in the increased hBD-2 release.
FIG. 1.
Time course of hBD mRNA expression in HT-29 and Caco-2 cells after treatment with BFT. HT-29 (A) and Caco-2 cells (B) were treated with BFT (300 ng/ml) for the indicated periods. Levels of hBD-1, hBD-2, and β-actin mRNA were analyzed by quantitative RT-PCR using each standard RNA. The values are expressed as means ± SD of five different experiments.
FIG. 2.
Expression of hBD-2 transcripts and proteins in intestinal epithelial cells stimulated with BFT. (A) HT-29 cells were treated with the indicated concentrations of BFT for 6 h. mRNA expression of hBD-2 (closed circles) and β-actin (open circles) was analyzed by quantitative RT-PCR using each standard RNA. The values are expressed as means ± SD of three different experiments. (B) HT-29 and Caco-2 cells were treated with BFT (300 ng/ml) for 24 h. The concentration of hBD-2 protein in culture supernatants was determined by ELISA (mean ± SEM; n = 5). *, P < 0.05 versus untreated controls.
Binding of NF-κB to the hBD-2 promoter is required for the induction of the hBD-2 gene in BFT-stimulated intestinal epithelial cells.
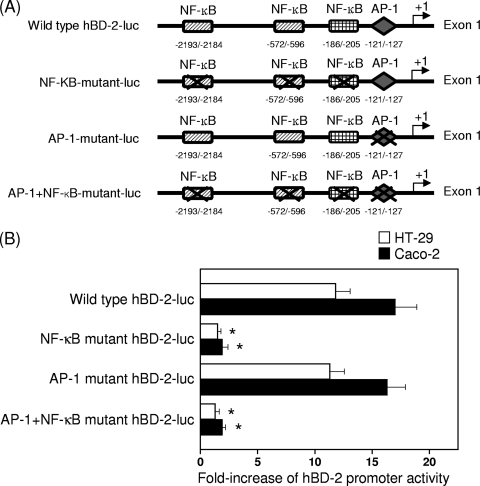
Since promoters for hBD-2 gene induction contain binding sites for NF-κB and AP-1, we asked whether hBD-2 induction might correlate with NF-κB and AP-1 binding sites in HT-29 and Caco-2 cells stimulated with BFT. In this experiment, we used four different hBD-2 promoter constructs that were kindly donated by Jürgen Harder, Clinical Research Unit, Department of Dermatology, University Hospital Kiel, Kiel, Germany (Fig. 3A) (44). BFT stimulation led to a significant increase in luciferase transcription from the hBD-2 promoter. In this system, mutation of all three NF-κB sites significantly decreased BFT-induced hBD-2 promoter activation. However, mutation of the AP-1 binding site did not result in any significant changes in hBD-2 promoter activation (Fig. 3B).
FIG. 3.
Activation of the hBD-2 promoter in HT-29 and Caco-2 cells after stimulation with BFT. (A) The hBD-2 promoter constructs. Nucleotide positions are marked relative to the hBD-2 transcription start. Three NF-κB sites and one AP-1 site in the hBD-2 promoter (bp −2338 to −1) linked to the luciferase gene were mutated in different combinations. (Adapted from reference 44.) (B) HT-29 or Caco-2 cells were transfected with the wild-type (−2338-luc) or several mutated hBD-2-promoter-luciferase plasmids for 24 h. After transfection, cells were stimulated with BFT (300 ng/ml) for another 6 h. Data are expressed as mean fold induction ± SEM of luciferase activity relative to unstimulated controls (n = 5). The mean fold induction of β-actin reporter gene activity relative to that of unstimulated controls remained relatively constant throughout each experiment.
AP-1 is not involved in the induction of the hBD-2 gene in BFT-stimulated cells.
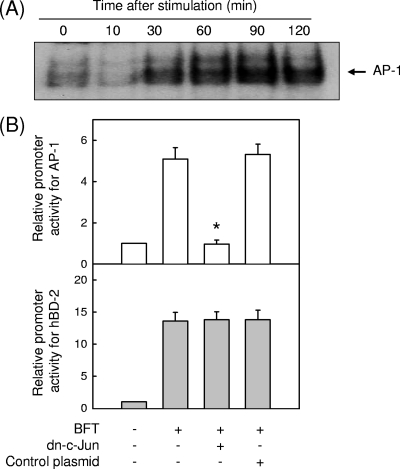
Because we found that mutation of the AP-1 binding site in a reporter plasmid did not affect hBD promoter activity (Fig. 3B), we reevaluated whether AP-1 signaling could not regulate the induction of hBD-2 in BFT-stimulated intestinal epithelial cells. In a preliminary study, stimulation of Caco-2 cells with BFT did not increase AP-1 signals (data not shown); however, BFT increased the DNA binding activity of AP-1 in HT-29 cells (Fig. 4A). Transfection with a dominant-negative c-Jun superrepressor was used for suppression of AP-1 activity in HT-29 cells. As shown in Fig. 4B, transfection with the c-Jun superrepressor did not reduce hBD-2 promoter activation induced by BFT stimulation, although the c-Jun superrepressor almost completely suppressed AP-1 activity in HT-29 cells stimulated with BFT. Consistent with this, the levels (mean ± SD transcripts/μg of total RNA) of hBD-2 mRNA induced by BFT did not change when the AP-1 signal was blocked: unstimulated control, <1.0 × 103; BFT, (8.9 ± 6.1) × 105; BFT plus dominant-negative c-Jun, (7.7 ± 5.8) × 105; and BFT plus control plasmid, (10.5 ± 5.3) × 105 (n = 3). These results indicate that AP-1 is not involved in the induction of hBD-2 in intestinal epithelial cells stimulated with BFT.
FIG. 4.
Relationship between AP-1 signaling and hBD-2 expression in BFT-stimulated HT-29 cells (A) HT-29 cells were stimulated with BFT (300 ng/ml) for the indicated periods of time. AP-1 activity was assessed by EMSA. The results are representative of three repeated experiments. (B) HT-29 cells were transfected with the pAP-1- or wild-type hBD-2-luciferase transcriptional reporter, together with the dominant-negative c-Jun superrepressor, as indicated. Then, 48 h later, the cells were stimulated with BFT (300 ng/ml) for another 1 h (AP-1) or 6 h (hBD-2), after which luciferase assays were performed. Data are expressed as mean fold induction in luciferase activity relative to unstimulated controls ± SEM (n = 5). The mean fold induction of the β-actin reporter gene relative to unstimulated controls remained relatively constant throughout each experiment. *, P < 0.05 compared with BFT alone.
Suppression of NF-κB activity attenuates hBD-2 expression in HT-29 cells stimulated with BFT.
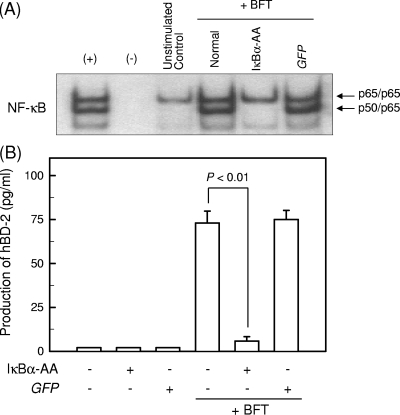
We next reevaluated whether BFT-induced NF-κB activation is associated with hBD-2 expression. For these experiments, HT-29 cells were transfected with retrovirus containing dominant-negative IκBα (retrovirus-IκBα-AA). The transfected cells were then stimulated with BFT for 1 h, and the ability of NF-κB to bind to DNA was assessed by EMSA. Transfection with retrovirus-IκBα-AA completely blocked NF-κB binding in BFT-stimulated HT-29 cells; however, the control retrovirus containing a green fluorescent protein (GFP) plasmid (retrovirus-GFP) did not reduce NF-κB binding (Fig. 5A). The cells transfected with retrovirus-IκBα-AA were stimulated with BFT for 24 h, and the level of hBD-2 production was determined by ELISA. Transfection with retrovirus-IκBα-AA resulted in significant inhibition of hBD-2 secretion (Fig. 5B).
FIG. 5.
Inhibition of NF-κB suppresses hBD-2 mRNA expression in HT-29 cells stimulated with BFT. (A) HT-29 cells were transfected with either retrovirus containing IκBα-superrepressor (IκBα-AA) or control virus (GFP). At 48 h after transfection, the cells were stimulated with BFT (300 ng/ml) for 1 h. NF-κB binding activity was assayed by EMSA. + represents the positive control in which HT-29 cells were treated with tumor necrosis factor alpha (TNF-α) (20 ng/ml); − represents the negative control. The results are representative of three repeated experiments. (B) Cells were treated with BFT (300 ng/ml) for 24 h. The concentration of hBD-2 protein in culture supernatants was determined by ELISA (mean ± SEM; n = 5).
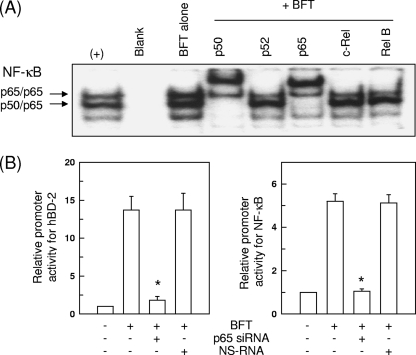
Since activation of p65/p50 heterodimeric NF-κB in response to BFT stimulation was observed (Fig. 6A), we performed another experiment using p65 siRNA to suppress NF-κB. In this experiment, blocking of NF-κB with p65 siRNA significantly decreased BFT-induced expression of hBD-2 (Fig. 6B). However, the nonsilencing control siRNA (NS-RNA) had no significant effect. These results demonstrate a direct connection between NF-κB-dependent signaling and hBD-2 induction.
FIG. 6.
Effects of NF-κB p65 siRNA transfection on hBD-2 induction in HT-29 cells stimulated with BFT. (A) HT-29 cells were stimulated with BFT (300 ng/ml) for 1 h. Supershift EMSA using nuclear extracts was performed using antibodies to p50, p52, p65, c-Rel, and RelB. The result shows that antibodies to p65 and p50 shifted the NF-κB signal complex significantly. In contrast, anti-p52, anti-c-Rel, or anti-RelB antibodies did not shift the NF-κB signal complex. The results are representative of three repeated experiments. (B) HT-29 cells were transfected with NF-κB p65-specific silencing siRNA (siRNA) or nonsilencing control siRNA (NS RNA) for 48 h, after which the transfected cells were cotransfected with wild-type hBD-2-luciferase or p2x NF-κB-luciferase transcriptional reporter. After 24 h, the transfected cells were stimulated with BFT (300 ng/ml) for another 1 h (NF-κB) or 6 h (hBD-2). Data are expressed as mean fold induction ± SEM of luciferase activity relative to unstimulated controls (n = 5). The mean fold induction of the β-actin reporter gene relative to unstimulated controls remained relatively constant throughout each experiment. *, P < 0.05 compared with BFT alone.
MAPK is associated with hBD-2 induction in BFT-stimulated HT-29 cells.
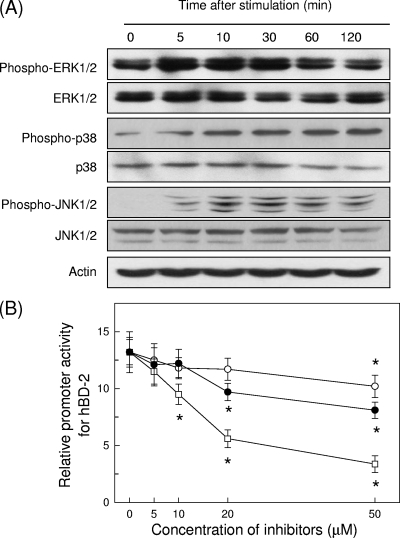
BFT strongly activated the phosphorylation of ERK1/2, p38, and JNK in HT-29 cells. Activation of all three MAPK signaling molecules was first noted 5 min after stimulation (Fig. 7A). To evaluate the relationship between MAPK activation and hBD-2 induction in BFT-stimulated HT-29 cells, we examined the effect of the following kinase inhibitors: PD98059, an inhibitor of MEK1/2, a MAPK that phosphorylates ERK1/2; pyridinyl imidazole SB203580, which specifically inhibits p38; and SP600125, which inhibits JNK. As shown in Fig. 7B, pretreatment of HT-29 cells with PD98059 (≥50 μM), SB203580 (≥10 μM), or SP600125 (≥20 μM) for 30 min significantly inhibited the BFT-induced hBD-2 gene activation. However, SB203580 showed a greater inhibitory effect on hBD-2 gene activation than SP600125. These results suggest that BFT-induced p38 may be more essential for the induction of hBD-2 than ERK or JNK.
FIG. 7.
BFT activates MAPKs in HT-29 cells. (A) HT-29 cells were stimulated with BFT (300 ng/ml) for the indicated periods of time. ERK1/2, p38, and JNK activities were measured by immunoblot analysis. Results are representative of five independent experiments. (B) HT-29 cells were transfected with the wild-type hBD-2-luciferase transcriptional reporter for 24 h. The transfected cells were preincubated with PD98059 (open circles), SB203580 (open squares), or SP600125 (filled circles) for 30 min and then stimulated with BFT (300 ng/ml) for another 6 h. Data are expressed as the mean fold induction in luciferase activity relative to unstimulated controls ± SEM (n = 5). *, P < 0.05 compared with BFT alone.
Suppression of p38 MAPK inhibits IKK and NF-κB signals leading to hBD-2 induction in BFT-stimulated HT-29 cells.
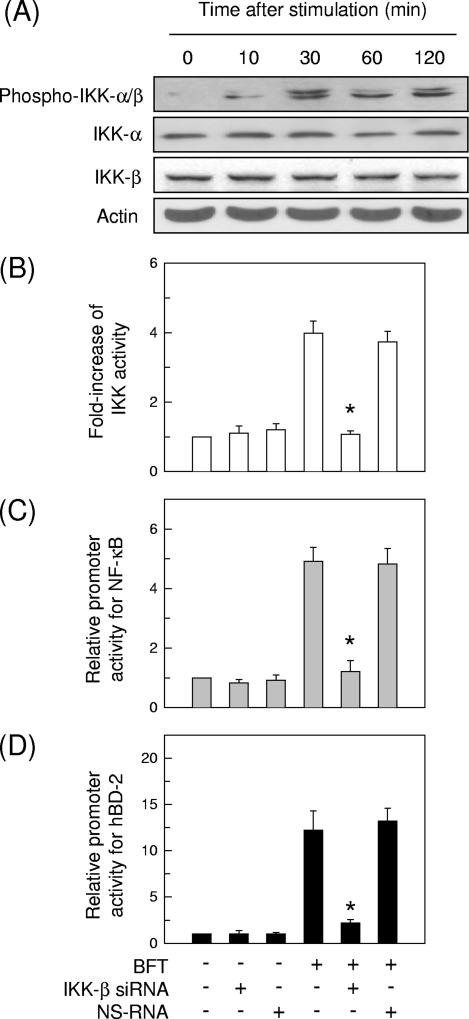
Since p38 MAPK signal appears to be more essential for the induction of hBD-2 in intestinal epithelial cells stimulated with BFT, we asked whether p38 might be associated with hBD-2 induction via the NF-κB signaling pathway. Stimulation of HT-29 cells with BFT increased levels of phosphorylated IKK-α/β, which were first observed 10 min after stimulation and reached a maximum after 30 min (Fig. 8A). Since we demonstrated previously that IKK-β is involved in IκBα phosphorylation and NF-κB activation in BFT-stimulated cells (12), siRNA against IKK-β was transfected into HT-29 cells to suppress IKK activity (Fig. 8B). IKK-β siRNA significantly reduced both NF-κB activation and hBD-2 induction in BFT-stimulated HT-29 cells (Fig. 8C and D).
FIG. 8.
Suppression of IKK activity by siRNA in HT-29 intestinal epithelial cells stimulated with BFT. (A) HT-29 cells were stimulated with BFT (300 ng/ml) for the indicated periods. Phosphorylation and protein expression of IKK-α, IKK-β, and actin were assessed by immunoblot analysis. The results are representative of three repeated experiments. (B) HT-29 cells were transfected with siRNA against IKK-β for 48 h. The transfected cells were stimulated with BFT (300 ng/ml) for 1 h. IKK kinase activity was measured using the HTScan IKK-β kinase assay kit. Data are expressed as mean fold induction ± SEM of kinase activity relative to untreated controls (n = 5). (C and D) The siRNA-transfected cells were then cotransfected with p2x NF-κB- or wild-type hBD-2-luciferase reporter for another 24 h. BFT (300 ng/ml) was added to cotransfected cells for 1 h (NF-κB) (C) or 6 h (hBD-2) (D). Data are expressed as mean fold induction ± SEM of luciferase activity relative to untreated controls (n = 5). The mean fold induction of the β-actin reporter gene relative to untreated controls remained relatively constant throughout each experiment. Asterisks indicate values of BFT plus siRNA that are significantly different from those of BFT alone (P < 0.05).
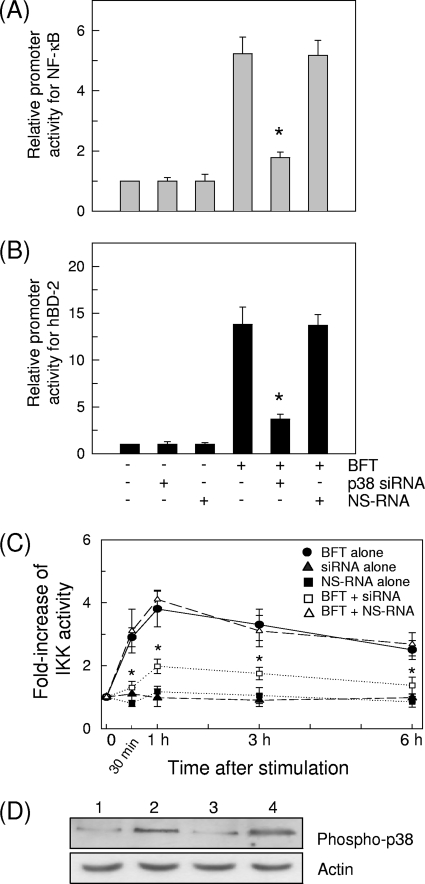
To investigate the role of MAPK signaling in IKK/NF-κB activation and hBD-2 induction by BFT, we transfected siRNA against p38 into HT-29 cells. As shown in Fig. 9A and B, transfection with p38 siRNA significantly decreased the NF-κB activation and hBD-2 induction following BFT stimulation. Concurrently, p38 siRNA significantly inhibited IKK activity in BFT-stimulated HT-29 cells (Fig. 9C). In this system, phosphorylation of p38 was almost completely suppressed in HT-29 cells transfected with p38 siRNA (Fig. 9D). These results suggest that the exposure of intestinal epithelial cells with BFT can activate a signaling cascade involving p38 MAPK/IKK/NF-κB, leading to induction of hBD-2.
FIG. 9.
Transfection with siRNA against p38 inhibits the activation of IKK and NF-κB and the expression of hBD-2 in HT-29 cells stimulated with BFT. (A and B) The siRNA-transfected cells were cotransfected with p2x NF-κB- or wild-type hBD-2-luciferase reporter for another 24 h. BFT (300 ng/ml) was added to cotransfected cells for 1 h (NF-κB) (A) or 6 h (hBD-2) (B). Data are expressed as mean fold induction ± SEM of luciferase activity relative to untreated controls (n = 5). The mean fold induction of the β-actin reporter gene relative to untreated controls remained relatively constant throughout each experiment. Asterisks indicate values of BFT plus siRNA that are significantly different from those of BFT alone (P < 0.05). (C) BFT (300 ng/ml) was added to the siRNA-transfected HT-29 cells for the indicated period. IKK kinase activity was measured using an HTScan IKK-β kinase assay kit. Data are expressed as mean fold induction ± SEM of kinase activity relative to untreated controls (n = 5). Asterisks indicate values of BFT plus siRNA that are significantly different from those of BFT alone (P < 0.05). (D) The siRNA-transfected cells were combined with BFT (300 ng/ml) for 30 min. Cell lysates were analyzed by immunoblotting with the indicated antibodies. Results shown are representative of three independent experiments. Lanes: 1, unstimulated control in nontransfected cells; 2, BFT in nontransfected cells; 3, BFT in p38 siRNA-transfected cells; 4, BFT in NS-RNA-transfected cells.
DISCUSSION
In the present study, we demonstrated that one of the early responses to BFT stimulation in human intestinal epithelial cells was the induction of hBD-2 in a dose- and time-dependent manner. BFT-induced hBD-2 expression could be regulated by a MAPK-, IKK-, and NF-κB-dependent signaling pathway.
The promoter region of the hBD-2 gene contains binding sites for both transcription factors NF-κB and AP-1. Thus, the induction of hBD-2 by cytokines or various bacteria such as Salmonella spp., pathogenic Escherichia coli, and Helicobacter pylori requires the activation of NF-κB and/or AP-1 and their binding to the promoter region (30, 40, 41, 43, 44). Since BFT stimulation activates NF-κB and AP-1 signaling in intestinal epithelial cells (14, 23), it is possible that BFT-mediated induction of hBD-2 may be associated with the binding of NF-κB and/or AP-1 to the hBD-2 promoter. The present study showed that mutation of NF-κB sites significantly reduced BFT-mediated hBD-2 promoter activation. The importance of NF-κB in the signal transduction pathway leading to hBD-2 gene induction by BFT was confirmed by the observation that the transfection of HT-29 cells with retrovirus-IκBα-AA or p65 siRNA significantly downregulated the hBD-2 induction by BFT stimulation.
In contrast, a reporter with an AP-1 binding site mutation did not show any significant change in hBD-2 promoter activation in BFT-stimulated cells. The lack of involvement of AP-1 in BFT-induced hBD-2 expression was further confirmed by transfecting HT-29 cells with a dominant-negative c-Jun superrepressor. Considering that AP-1 activation is linked to expression of chemokine interleukin-8 (IL-8) and monocyte chemoattractant protein 1 (MCP-1) in intestinal epithelial cells exposed to BFT and may be involved in the development of enteritis (14), AP-1 activation seems to be primarily involved in the BFT-induced mucosal inflammation rather than hBD-2 induction.
Our findings are different from those of a study in which hBD-2 induction in human gingival epithelial cells infected with the periodontal bacterium Fusobacterium nucleatum was not blocked by NF-κB inhibitors but was mainly regulated by AP-1 (24). Both NF-κB and AP-1 are required for full hBD-2 promoter activation upon stimulation of keratinocytes with Pseudomonas aeruginosa (44) and of Caco-2 intestinal epithelial cells with pathogenic E. coli (43). Therefore, NF-κB-dependent and AP-1-independent hBD-2 induction seems to be unique to BFT-stimulated intestinal epithelial cells.
MAPK activation is known to be an important event underlying hBD-2 induction. Although BFT activates MAPK and NF-κB signaling in intestinal epithelial cells (12, 14, 19, 20, 21, 23, 45), the relationship between NF-κB and MAPK signaling in intestinal epithelial cells stimulated with BFT is unclear. To gain insight into BFT-induced signaling pathways involved in hBD-2 induction, we attempted to determine whether NF-κB and MAPK signaling might cooperate to induce hBD-2 expression in intestinal epithelial cells. In the present study, the p38 inhibitor SB203580 showed a greater inhibitory effect on hBD-2 induction than the ERK inhibitor PD98059 or the JNK inhibitor SP600125. These results suggest that p38 MAPK is more essential for hBD-2 induction in BFT-stimulated intestinal epithelial cells than ERK or JNK. Since several reports have demonstrated that p38 MAPK may be associated with IKK-dependent NF-κB activation (5, 25, 31), we assessed the effects of p38 activation on IKK and NF-κB signaling in BFT-stimulated cells. Our study showed that suppression of p38 activity in BFT-stimulated HT-29 cells significantly reduced phospho-IKK-α/β activity, NF-κB activity, and hBD-2 expression, suggesting that the activated p38 molecule may act upstream of IKK and NF-κB in BFT-induced hBD-2 expression.
In summary, we have demonstrated that exposure of intestinal epithelial cells to BFT results in the activation of a signaling cascade involving p38 MAPK, IKK, NF-κB, and subsequent hBD-2 induction in intestinal epithelial cells. Based on these findings, we propose that the induction of hBD-2 seems to enhance the innate epithelial defense against ETBF infection. If dysregulation of this cascade occurs, it may lead to insufficient expression of hBD-2 in intestine, thereby increasing the chances of ETBF-associated diseases such as colitis, IBD, and colon tumorigenesis.
Acknowledgments
We thank Jürgen Harder for providing hBD-2-luciferase plasmids, Martin F. Kagnoff for providing pβ-actin- and pRSV-β-galactosidase-luciferase plasmids and standard β-actin RNA, Andreas von Knethen for providing the dominant-negative c-Jun plasmid, and Han Jin Lee for expert technical help.
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the government of Korea (MEST) (MRC program no. 2009-0091463) and by the Basic Science Research Program through the NRF funded by the Ministry of Education, Science and Technology (R11-2008-044-01004-0).
None of the authors of this study has any financial or commercial conflicts of interest.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 15 March 2010.
REFERENCES
- 1.Bals, R., X. Wang, R. L. Meegalla, S. Wattler, D. J. Weiner, M. C. Nehls, and J. M. Wilson. 1999. Mouse beta-defensin 3 is an inducible antimicrobial peptide expressed in the epithelia of multiple organs. Infect. Immun. 67:3542-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basset, C., J. Holton, A. Bazeos, D. Vaira, and S. Bloom. 2004. Are Helicobacter species and enterotoxigenic Bacteroides fragilis involved in inflammatory bowel disease? Dig. Dis. Sci. 49:1425-1432. [DOI] [PubMed] [Google Scholar]
- 3.Brown, P. H., R. Alani, L. H. Preis, E. Szabo, and M. J. Birrer. 1993. Suppression of oncogene-induced transformation by a deletion mutant of c-jun. Oncogene 8:877-886. [PubMed] [Google Scholar]
- 4.Cash, H. L., C. V. Whitham, C. L. Behrendt, and L. V. Hooper. 2006. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313:1126-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chio, C. C., Y. H. Chang, Y. W. Hsu, K. H. Chi, and W. W. Lin. 2004. PKA-dependent activation of PKC, p38 MAPK and IKK in macrophage: implication in the induction of inducible nitric oxide synthase and interleukin-6 by dibutyryl cAMP. Cell. Signal. 16:565-575. [DOI] [PubMed] [Google Scholar]
- 6.De Smet, K., and R. Contreras. 2005. Human antimicrobial peptides: defensins, cathelicidins and histatins. Biotechnol. Lett. 27:1337-1347. [DOI] [PubMed] [Google Scholar]
- 7.Eckmann, L., J. Fierer, and M. F. Kagnoff. 1996. Genetically resistant (Ityr) and susceptible (Itys) congenic mouse strains show similar cytokine responses following infection with Salmonella dublin. J. Immunol. 156:2894-2900. [PubMed] [Google Scholar]
- 8.Elewaut, D., J. A. DiDonato, J. M. Kim, F. Truong, L. Eckmann, and M. F. Kagnoff. 1999. NF-kappa B is a central regulator of the intestinal epithelial cell innate immune response induced by infection with enteroinvasive bacteria. J. Immunol. 163:1457-1466. [PubMed] [Google Scholar]
- 9.Giesemann, T., G. Guttenberg, and K. Aktories. 2008. Human alpha-defensins inhibit Clostridium difficile toxin B. Gastroenterology 134:2049-2058. [DOI] [PubMed] [Google Scholar]
- 10.Hale, J. D., and R. E. Hancock. 2007. Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Expert Rev. Anti Infect. Ther. 5:951-959. [DOI] [PubMed] [Google Scholar]
- 11.Howell, S. J., D. Wilk, S. P. Yadav, and C. L. Bevins. 2003. Antimicrobial polypeptides of the human colonic epithelium. Peptides 24:1763-1770. [DOI] [PubMed] [Google Scholar]
- 12.Kim, J. M., D. H. Lee, J. S. Kim, J. Y. Lee, H. G. Park, Y. J. Kim, Y. K. Oh, H. C. Jung, and S. Kim. 2009. 5,7-Dihydroxy-3,4,6-trimethoxyflavone inhibits the inflammatory effects induced by Bacteroides fragilis enterotoxin via dissociating the complex of heat shock protein 90 and IκBα and IκB kinase-γ in intestinal epithelial cell culture. Clin. Exp. Immunol. 155:541-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim, J. M., H. C. Jung, D. J. Jin, K. I. Im, I. S. Song, and C. Y. Kim. 1997. Cytokine genes are downregulated when attachment of Entamoeba histolytica to HT-29 colon epithelial cells is prevented. Scand. J. Immunol. 45:613-617. [DOI] [PubMed] [Google Scholar]
- 14.Kim, J. M., H. Y. Jung, J. Y. Lee, J. Youn, C. H. Lee, and K. H. Kim. 2005. Mitogen-activated protein kinase and activator protein-1 dependent signals are essential for Bacteroides fragilis enterotoxin-induced enteritis. Eur. J. Immunol. 35:2648-2657. [DOI] [PubMed] [Google Scholar]
- 15.Kim, J. M., J. S. Kim, H. C. Jung, Y. K. Oh, H. Y. Chung, C. H. Lee, and I. S. Song. 2003. Helicobacter pylori infection activates NF-kappaB signaling pathway to induce iNOS and protect human gastric epithelial cells from apoptosis. Am. J. Physiol. Gastrointest. Liver Physiol. 285:G1171-G1180. [DOI] [PubMed] [Google Scholar]
- 16.Kim, J. M., J. S. Kim, J. Y. Lee, Y. J. Kim, H. J. Youn, I. Y. Kim, Y. J. Chee, Y. K. Oh, N. Kim, H. C. Jung, and I. S. Song. 2007. Vacuolating cytotoxin in Helicobacter pylori water-soluble proteins upregulates chemokine expression in human eosinophils via Ca2+ influx, mitochondrial reactive oxygen intermediates, and NF-κB activation. Infect. Immun. 75:3373-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim, J. M., J. S. Kim, Y. J. Kim, Y. K. Oh, I. Y. Kim, Y. J. Chee, J. S. Han, and H. C. Jung. 2008. Conjugated linoleic acids produced by Lactobacillus dissociates IKK-gamma and Hsp90 complex in Helicobacter pylori-infected gastric epithelial cells. Lab. Invest. 88:541-552. [DOI] [PubMed] [Google Scholar]
- 18.Kim, J. M., J. W. Kang, M. Y. Cha, D. Yoo, N. Kim, I. K. Kim, J. Ku, S. Kim, H. S. Ma, H. C. Jung, I. S. Song, and J. S. Kim. 1 March 2010, posting date. Novel guggulsterone derivative GG-52 inhibits NF-κB signaling in intestinal epithelial cells and attenuates acute murine colitis. Lab. Invest. [Epub ahead of print.] [DOI] [PubMed]
- 19.Kim, J. M., J. Y. Lee, and Y. J. Kim. 2008. Inhibition of apoptosis in Bacteroides fragilis enterotoxin-stimulated intestinal epithelial cells through the induction of c-IAP-2. Eur. J. Immunol. 38:2190-2199. [DOI] [PubMed] [Google Scholar]
- 20.Kim, J. M., J. Y. Lee, Y. M. Yoon, Y. K. Oh, J. S. Kang, Y. J. Kim, and K. H. Kim. 2006. Bacteroides fragilis enterotoxin induces cyclooxygenase-2 and fluid secretion in intestinal epithelial cells through NF-κB activation. Eur. J. Immunol. 36:2446-2456. [DOI] [PubMed] [Google Scholar]
- 21.Kim, J. M., S. J. Cho, Y. K. Oh, H. Y. Jung, Y. J. Kim, and N. Kim. 2002. Nuclear factor-kappa B activation pathway in intestinal epithelial cells is a major regulator of chemokine gene expression and neutrophil migration induced by Bacteroides fragilis enterotoxin. Clin. Exp. Immunol. 130:59-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, J. M., Y. J. Kim, and Y. J. Cho. 2000. Synergy of Bacteroides fragilis and Escherichia coli in the induction of KC gene expression in mouse peritoneal tissues. Scand. J. Infect. Dis. 32:643-649. [DOI] [PubMed] [Google Scholar]
- 23.Kim, J. M., Y. K. Oh, Y. J. Kim, H. B. Oh, and Y. J. Cho. 2001. Polarized secretion of CXC chemokines by human intestinal epithelial cells in response to Bacteroides fragilis enterotoxin: NF-kappaB plays a major role in the regulation of IL-8 expression. Clin. Exp. Immunol. 123:421-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krisanaprakornkit, S., J. R. Kimball, and B. A. Dale. 2002. Regulation of human beta-defensin-2 in gingival epithelial cells: the involvement of mitogen-activated protein kinase pathways, but not the NF-kappaB transcription factor family. J. Immunol. 168:316-324. [DOI] [PubMed] [Google Scholar]
- 25.Lee, J. Y., H. Kim, M. Y. Cha, H. G. Park, Y. J. Kim, I. Y. Kim, and J. M. Kim. 2009. Clostridium difficile toxin A promotes dendritic cell maturation and chemokine CXCL2 expression through p38, IKK, and the NF-kappaB signaling pathway. J. Mol. Med. 87:169-180. [DOI] [PubMed] [Google Scholar]
- 26.Lee, J. Y., H. R. Park, Y. K. Oh, Y. J. Kim, J. Youn, J. S. Han, and J. M. Kim. 2007. Effects of transcription factor activator protein-1 on interleukin-8 expression and enteritis in response to Clostridium difficile toxin A. J. Mol. Med. 85:1393-1404. [DOI] [PubMed] [Google Scholar]
- 27.Myers, L. L., and D. S. Shoop. 1987. Association of enterotoxigenic Bacteroides fragilis with diarrheal disease in young pigs. Am. J. Vet. Res. 48:774-775. [PubMed] [Google Scholar]
- 28.Niyogi, S. K., P. Dutta, U. Mitra, and D. K. Pal. 1997. Association of enterotoxigenic Bacteroides fragilis with childhood diarrhoea. Indian J. Med. Res. 105:167-169. [PubMed] [Google Scholar]
- 29.Nuding, S., L. T. Zabel, C. Enders, E. Porter, K. Fellermann, J. Wehkamp, H. A. Mueller, and E. F. Stange. 2009. Antibacterial activity of human defensins on anaerobic intestinal bacterial species: a major role of HBD-3. Microbes Infect. 11:384-393. [DOI] [PubMed] [Google Scholar]
- 30.O'Neil, D. A., E. M. Porter, D. Elewaut, G. M. Anderson, L. Eckmann, T. Ganz, and M. F. Kagnoff. 1999. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J. Immunol. 163:6718-6724. [PubMed] [Google Scholar]
- 31.Park, K. J., R. B. Gaynor, and Y. T. Kwak. 2003. Heat shock protein 27 association with the IκB kinase complex regulates tumor necrosis factor alpha-induced NF-κB activation. J. Biol. Chem. 278:35272-35278. [DOI] [PubMed] [Google Scholar]
- 32.Prindiville, T. P., R. A. Sheikh, S. H. Cohen, Y. J. Tang, M. C. Cantrell, and J. Silva, Jr. 2000. Bacteroides fragilis enterotoxin gene sequences in patients with inflammatory bowel disease. Emerg. Infect. Dis. 6:171-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhee, K. J., S. Wu, X. Wu, D. L. Huso, B. Karim, A. A. Franco, S. Rabizadeh, J. E. Golub, L. E. Mathews, J. Shin, R. B. Sartor, D. Golenbock, A. R. Hamad, C. M. Gan, F. Housseau, and C. L. Sears. 2009. Induction of persistent colitis by a human commensal, enterotoxigenic Bacteroides fragilis, in wild-type C57BL/6 mice. Infect. Immun. 77:1708-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sahl, H. G., U. Pag, S. Bonness, S. Wagner, N. Antcheva, and A. Toss. 2005. Mammalian defensins: structures and mechanism of antibiotic activity. J. Leukoc. Biol. 77:466-475. [DOI] [PubMed] [Google Scholar]
- 35.Schneider, J. J., A. Unholzer, M. Schaller, M. Schäfer-Korting, and H. C. Korting. 2005. Human defensins. J. Mol. Med. 83:587-595. [DOI] [PubMed] [Google Scholar]
- 36.Sears, C. L. 2009. Enterotoxigenic Bacteroides fragilis: a rogue among symbiotes. Clin. Microbiol. Rev. 22:349-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sears, C. L., S. Islam, A. Saha, M. Arjumand, N. H. Alam, A. S. Faruque, M. A. Salam, J. Shin, D. Hecht, A. Weintraub, R. B. Sack, and F. Qadri. 2008. Association of enterotoxigenic Bacteroides fragilis infection with inflammatory diarrhea. Clin. Infect. Dis. 47:797-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tollin, M., P. Bergman, T. Svenberg, H. Jornvall, G. H. Gudmundsson, and B. Agerberth. 2003. Antimicrobial peptides in the first line defence of human colon mucosa. Peptides 24:523-530. [DOI] [PubMed] [Google Scholar]
- 39.Toprak, N. U., A. Yagci, B. M. Gulluoglu, M. L. Akin, P. Demirkalem, T. Celenk, and G. A. Soyletir. 2006. Possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin. Microbiol. Infect. 12:782-786. [DOI] [PubMed] [Google Scholar]
- 40.Wada, A., K. Ogushi, T. Kimura, H. Hojo, N. Mori, S. Suzuki, A. Kumatori, M. Se, Y. Nakahara, M. Nakamura, J. Moss, and T. Hirayama. 2001. Helicobacter pylori-mediated transcriptional regulation of the human beta-defensin 2 gene requires NF-kappaB. Cell. Microbiol. 3:115-123. [DOI] [PubMed] [Google Scholar]
- 41.Wada, A., N. Mori, K. Oishi, H. Hojo, Y. Nakahara, Y. Hamanaka, M. Nagashima, I. Sekine, K. Ogushi, T. Niidome, T. Nagatake, J. Moss, and T. Hirayama. 1999. Induction of human beta-defensin-2 mRNA expression by Helicobacter pylori in human gastric cell line MKN45 cells on cag pathogenicity island. Biochem. Biophys. Res. Commun. 263:770-774. [DOI] [PubMed] [Google Scholar]
- 42.Wehkamp, J., J. Schauber, and E. F. Stange. 2007. Defensins and cathelicidins in gastrointestinal infections. Curr. Opin. Gastroenterol. 23:32-38. [DOI] [PubMed] [Google Scholar]
- 43.Wehkamp, J., J. Harder, K. Wehkamp, B. Wehkamp-von Meissner, M. Schlee, C. Enders, U. Sonnenborn, S. Nuding, S. Bengmark, K. Fellermann, J. M. Schröder, and E. F. Stange. 2004. NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect. Immun. 72:5750-5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wehkamp, K., L. Schwichtenberg, J. M. Schröder, and J. Harder. 2006. Pseudomonas aeruginosa- and IL-1β-mediated induction of human beta-defensin-2 in keratinocytes is controlled by NF-kappaB and AP-1. J. Invest. Dermatol. 126:121-127. [DOI] [PubMed] [Google Scholar]
- 45.Wu, S., J. Powell, N. Mathioudakis, S. Kane, E. Fernandez, and C. L. Sears. 2004. Bacteroides fragilis enterotoxin induces intestinal epithelial cell secretion of interleukin-8 through mitogen-activated protein kinases and a tyrosine kinase-regulated nuclear factor-κB pathway. Infect. Immun. 72:5832-5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu, S., K. J. Rhee, E. Albesiano, S. Rabizadeh, X. Wu, H. R. Yen, D. L. Huso, F. L. Brancati, E. Wick, F. McAllister, F. Housseau, D. M. Pardoll, and C. L. Sears. 2009. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 15:1016-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang, G., B. Svenungsson, A. Karnell, and A. Weintraub. 1999. Prevalence of enterotoxigenic Bacteroides fragilis in adult patients with diarrhea and healthy controls. Clin. Infect. Dis. 29:590-594. [DOI] [PubMed] [Google Scholar]