Abstract
Bacillus cereus EntFM displays an NlpC/P60 domain, characteristic of cell wall peptidases. The protein is involved in bacterial shape, motility, adhesion to epithelial cells, biofilm formation, vacuolization of macrophages, and virulence. These data provide new information on this, so far, poorly studied toxin and suggest that this protein is a cell wall peptidase, which we propose to rename CwpFM.
Bacillus cereus is a Gram-positive spore-forming bacterium responsible for two types of food-associated toxi-infections: an emetic and a diarrheal syndrome (44). Rare but severe opportunistic infections have been attributed to B. cereus (4, 12, 34). The pleiotropic regulator PlcR controls the expression of several B. cereus secreted factors, such as the nonhemolytic enterotoxin (Nhe), the hemolysin BL (Hbl), and the cytotoxin K (CytK) (1, 19). Some of these factors are prevalent in diarrheal strains (21) and might play a role during B. cereus gastroenteritis and opportunistic infections, although no direct link has been demonstrated. Deletion of plcR reduces, but does not abolish, the virulence of the bacterium in various infection models (11, 41), suggesting that other factors are required for pathogenicity. It has consistently been shown that flagella are involved in virulence-related properties (32, 49): they confer motility, adhesion to epithelial cells, and virulence (9, 18, 39). Other PlcR-independent factors, like InhA1 (20, 40), HlyII (3; S. Tran, E. Guillemet, C. Clybouw, A. Moris, M. Gohar, D. Lereclus, and N. Ramarao, submitted for publication), and IlsA (14), have been shown to play a role in B. cereus pathogenicity. Additionally, a protein, first isolated from the B. cereus FM1 strain, was named enterotoxin FM (EntFM) because it was suspected to cause at high doses fluid accumulation in rabbit and mouse ligated intestinal loop tests (5, 8, 42). However, very few studies have been performed on this protein, and its specific role during B. cereus virulence has not been reported. The entFM gene is located on the chromosome and appears to be common to Bacillus thuringiensis and B. cereus strains. Prevalence studies revealed that entFM is detected in most outbreak-associated strains (25, 36).
To induce a potent infection, B. cereus has to colonize and persist in the host gut. Previous studies have shown that B. cereus adheres to epithelial cells (2, 35, 39) and forms biofilms at both solid-liquid and liquid-air interfaces (26, 48). A biofilm is a multicellular bacterial community composed of microorganisms attached to a surface and embedded in an exopolymeric matrix. Bacteria inside the biofilm are protected from the host immune system and are less susceptible to antimicrobial agents (37), thus explaining why chronic infections involving biofilms are so difficult to treat (13, 22).
In this study, we show that the B. cereus EntFM is related to cell wall peptidases (Cwps), and we therefore propose to rename this protein CwpFM. CwpFM is involved in bacterial motility and shape, in B. cereus adhesion to epithelial cells, and in biofilm formation. Moreover, CwpFM induces vacuolization of macrophages. All these phenotypic traits might explain the role of CwpFM during virulence in our insect model.
Sequence analysis of the B. cereus EntFM protein.
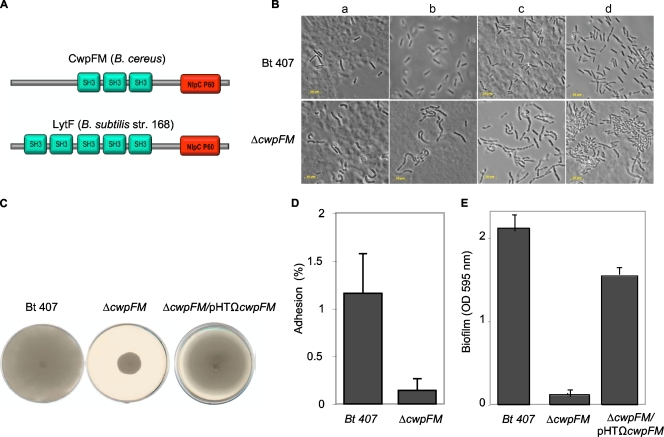
BLAST analysis of the entFM gene of the reference strain B. cereus ATCC 14579 (http://blast.ncbi.nlm.nih.gov) revealed that this gene is present as a single copy in all members of the B. cereus group and only in this group (data not shown). This gene is annotated either as coding for an enterotoxin or as coding for a putative cell wall peptidase of the NlpC/P60 protein family (identity of 99% within the B. cereus group). Moreover, the EntFM protein secondary structure (http://pfam.sanger.ac.uk/) indicates the existence of four predicted domains: three protein-protein interaction SH3 domains and an NlpC/P60 family domain (Fig. 1A). This domain organization is very similar to that of Bacillus subtilis CwlE (lytF) (Fig. 1A), a cell wall peptidase responsible for the normal rod-shaped morphology of the bacterium and deletion of which results in a long filament-like phenotype (17, 33). The NlpC/P60 proteins define a family of cell wall peptidases that can modify the cell wall (46). Many of theses proteins display, in addition to their catalytic site, a domain necessary for the interaction with peptides, carbohydrates, and lipids of the cell wall, such as SH3, LysM, and the peptidoglycan binding domain. Bacterial cell wall peptidases are involved in various processes, such as peptidoglycan modification during growth, cell wall turnover, separation of daughter cells during cell division, motility, bacterial adhesion and invasiveness, and biofilm formation, and therefore contribute directly to bacterial pathogenicity (10, 23, 28, 38, 43, 45, 47). EntFM was first named an enterotoxin, but BLAST and secondary structure analyses revealed similarity to cell wall peptidases of the NlpC/P60 family. For these reasons, and the hereby-described phenotypes, we propose to rename this protein CwpFM.
FIG. 1.
(A) Domain organization of B. cereus CwpFM and B. subtilis LytF cell wall peptidases. Green boxes represent SH3 cell wall binding domains. Red boxes define the NlpC/P60 peptidase domain. (B) Bacterial morphology, as observed with phase-contrast microscopy. Bacterial morphology of the B. thuringiensis 407 and the cwpFM mutant strains was analyzed by phase-contrast light microscopy (Leica). Magnification, ×100. Cultures were sampled at different growth phases: the beginning of the exponential phase (OD600, 0.3) (a) or the beginning (OD600, 3) (b), middle (OD600, 7) (c), or end (OD600, 9) (d) of the stationary phase. Bar, 10 μm. (C) Motility assay. Bacteria were inoculated on 0.3% agar plates. Motility of the wild-type B. thuringiensis 407, the cwpFM mutant, and the complemented strain cwpFM/pHTΩcwpFM was assessed after 24 h of incubation at 37°C. Images are representative of two independent experiments with three replicates. (D) Quantitative analysis of bacterial adhesion to HeLa cells. HeLa cells were incubated with wild-type B. thuringiensis 407 and the cwpFM mutant strain, at a density of 10 bacteria per cell, for 20 min. Cells were washed to eliminate nonadherent bacteria, and the bacteria associated with cells were quantified by dilution plating on LB agar plates. Adhesion was calculated as the ratio of adherent bacteria to the total number of bacteria used for inoculation. Results are shown as means of two independent experiments, each including three replicates. (E) Biofilm formation by B. cereus at the air-liquid interface. The B. thuringiensis 407 strain, the cwpFM mutant, and the complemented strain cwpFM/pHTΩcwpFM were incubated in the wells of a PVC microtiter plate for 48 h at 30°C. Biofilm formation was quantified using crystal violet. Bars represent OD595 values, and results are the mean of three independent experiments with 16 replicates.
Six other potential cell wall endopeptidases exist in the genome of several B. cereus sequenced strains (27). They, however, belong to the M23/M37 family and show no significant homologies with CwpFM. To elucidate the biological function of CwpFM and its potential role in bacterial shape, motility, adhesion, biofilm formation, and virulence, a cwpFM-deficient B. cereus strain was constructed and a recombinant B. cereus CwpFM protein was produced.
Phenotypical analysis.
The B. thuringiensis 407 Cry− strain was used as a model for B. cereus. This sequenced strain (http://www.ncbi.nlm.nih.gov/nuccore/NZ_ACMZ00000000) was originally described as a B. thuringiensis strain, but cured of its plasmid it is acrystalliferous and shows high phylogenic similarity with the B. cereus reference strain ATCC 14579 (29). The cwpFM gene was disrupted in the B. thuringiensis 407 strain through simple homologous recombination by using a 231-bp internal region of the cwpFM gene generated from the B. thuringiensis 407 chromosome by PCR using the primer pair cwpFM-1 (5′-CCCAAGCTTGACTTCGTAACCACTGGTGGC-3′) and cwpFM-2 (5′-CGCGGATCCGCTTACGTATCCAGTTCCACC-3′). This DNA fragment was inserted into the thermosensitive vector pRN5101, and the resulting plasmid was introduced into B. thuringiensis 407 by electroporation as described previously (30, 39). The cwpFM mutant stability as measured by replica plating was 100% over 30 generations. The cwpFM mutant strain growth curve was indistinguishable from that of the wild-type strain in LB medium (data not shown). However, morphological observation by light microscopy (Fig. 1B) showed that bacteria of the cwpFM mutant strain were shorter and larger than the wild-type strain and displayed a “peanut shape” throughout bacterial growth. Moreover, the mutant bacteria seemed to agglutinate and to form filaments, suggesting a modification of surface properties and a deficiency in septum separation.
To analyze if the deletion of the cwpFM gene affected bacterial motility, the wild-type strain, the cwpFM mutant, and the complemented strain, cwpFM/pHTΩcwpFM, obtained by insertion in the cwpFM mutant of the pHT304-Kan plasmid carrying the functional cwpFM gene of the B. thuringiensis 407 strain (sequenced verified), were spotted onto 0.3% agar plates. Motility was analyzed after 24 h of growth at 37°C (Fig. 1C). For the cwpFM mutant, capacity to spread was affected as the diameter of the migration zone decreased by about 73.5% (±0.9%) compared to the wild-type strain. In contrast, the complemented strain was as motile as the wild-type strain.
Adhesion of bacteria to eukaryotic cells is often the first key event during infection of susceptible hosts. To determine whether CwpFM contributes to adhesion to epithelial cells, the wild-type and cwpFM mutant strains were investigated for their abilities to adhere to epithelial HeLa cells. Adhesion was recorded as the ratio between adherent bacteria and total inoculated bacteria as previously described (7). Adhesion of the cwpFM mutant was reduced 10-fold compared to the wild-type strain (P < 0.012) (Fig. 1D).
To determine whether CwpFM is involved in biofilm formation, the wild-type and mutant strains were grown in polyvinylchloride (PVC) microtiter plates for 48 h at 30°C as described in reference 6. The results (Fig. 1E) indicated that the cwpFM mutant was severely compromised in its ability to form biofilm. Biofilm formation by the mutant (optical density [OD], 0.181 ± 0.052 [mean ± standard deviation]) was over 10-fold less than with the wild-type strain (OD, 2.232 ± 0.254) (P < 9 × 10−6). The complementation of the cwpFM mutant by a functional cwpFM gene partially restored its ability to form biofilm. CwpFM is involved in adhesion and motility, and this might be linked to its role during biofilm formation (24, 48).
EntFM induces vacuolization of macrophages.
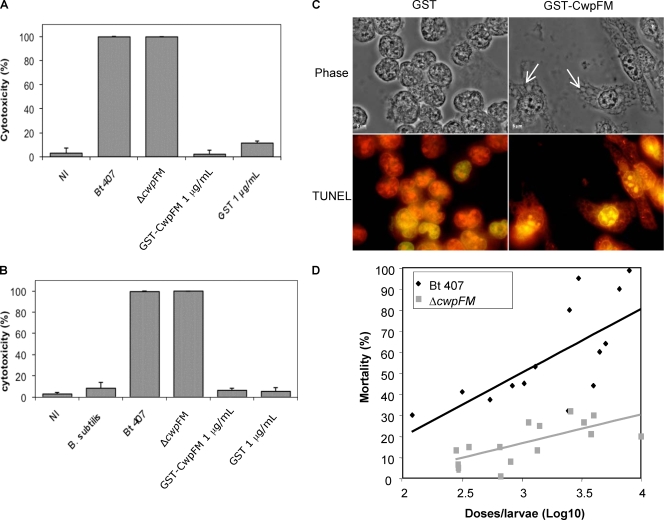
It has been previously reported that B. cereus induces cytotoxicity against various eukaryotic cells, such as epithelial cells or macrophages (31, 39-41; Tran et al., submitted for publication). The B. cereus cytotoxic effect toward epithelial cells is PlcR dependent (39). However, a plcR-deficient strain induced strong vacuolization in insect hemocytes (41). This implies that PlcR-independent factors are also responsible for cytotoxicity toward immune cells. A putative role of CwpFM, regulation of which is independent of PlcR (19), in cytotoxicity was assessed by incubating purified CwpFM with epithelial HeLa cells (Fig. 2A) and with J774 macrophage cells (Fig. 2B). Cell viability was assessed by the trypan blue dye exclusion method as previously described, and cytotoxicity was determined as the percentage of cells that were permeable to the blue dye, compared to all cells (39). Purified CwpFM protein was obtained as a recombinant glutathione S-transferase (GST)-CwpFM protein produced in Escherichia coli strain M15(pREP4) (Qiagen) harboring the plasmid pGEX6P1-GST-CwpFM by using a bulk GST purification module (GE Healthcare) according to the manufacturer's instructions. After 2 h, purified CwpFM induced no toxicity on HeLa or J774 cells. Moreover, the cwpFM mutant was as cytotoxic (100%) as the wild-type strain (100%), showing that CwpFM is not involved in B. cereus toxicity toward either epithelial cells or macrophages. However, cell morphology observation by light microscopy showed that CwpFM induced lengthening and strong vacuolization of macrophages (Fig. 2C, top right panel). No vacuolization was observed when cells were incubated with GST alone (top left panel) or with untreated cells (data not shown). Despite CwpFM-induced vacuolization of macrophages, we did not observe macrophage death, either by necrosis (trypan blue staining [Fig. 2B]) or by apoptosis (Fig. 2C, bottom panel). For determination of apoptosis, cells were stained using the DeadEnd fluorometric terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) system as directed by the manufacturer (Promega) and observed under a fluorescence microscope with filters at 633 nm and 488 nm (red, all cells; green, apoptotic cells).
FIG. 2.
(A and B) Evaluation of cytotoxicity using trypan blue dye. HeLa (A) or macrophage (B) cells (1 × 105) were incubated with the culture supernatant of B. thuringiensis 407, the cwpFM mutant, or B. subtilis, or with purified GST-CwpFM (1 μg/ml). Control cells were either noninfected (NI) or incubated with purified GST (1 μg/ml). After 2 h of incubation, cell mortality was evaluated by trypan blue dye exclusion. (C) CwpFM induces vacuolization of macrophages. Macrophages (106 cells/well) were incubated with 1 μg/ml of purified GST-CwpFM or GST. After treatment, cells were fixed with 4% paraformaldehyde for 25 min at 4°C. Cell morphology was observed under a phase-contrast microscope (100× objective; Leica) (top panels). Arrows indicate cell vacuoles. Alternatively, cells were stained using the DeadEnd fluorometric TUNEL kit and observed under a fluorescence microscope (bottom panels). All cells are labeled in red, and potential apoptotic cells are stained green. Images are representative of at least three independent experiments. (D) CwpFM virulence against G. mellonella larvae. Various concentrations of wild-type and cwpFM strains were inoculated into the hemocoel of three groups of 20 G. mellonella larvae. Mortality was recorded after 24 h at 37°C, and the LD50 was determined using the probit method.
CwpFM is involved in virulence.
To determine whether CwpFM contributes to B. cereus virulence, we tested the ability of the wild-type strain and the cwpFM mutant to kill larvae of the insect model Galleria mellonella. Groups of 20 G. mellonella larvae were infected by injection of different doses of bacterial cultures (6.5 × 102 to 104 CFU/larva) into the hemocoel as described previously (15, 20). Mortality was assessed after 24 h of incubation. The 50% lethal dose (LD50) was calculated using probit analysis (16) (Fig. 2D). Results showed that bacterial virulence in insects was strongly reduced in the cwpFM mutant (LD50, 69,000 CFU/larva, as estimated by the probit software) compared to the wild-type strain (LD50, 1,600 CFU/larva), revealing a clear role of CwpFM in B. cereus virulence.
Altogether, these findings suggest that CwpFM might be a cell wall peptidase involved in the bacterial cell wall dynamic that could explain the mutant bacterial shape and its impairments in motility, adhesion to eukaryotic cells, and biofilm formation. These different effects of CwpFM could explain its involvement in bacterial virulence in the insect model and indicate that CwpFM has properties which may facilitate the infectious process at multiple steps.
Acknowledgments
We thank Julien Brillard for the generous gift of the pHT304-Kan plasmid. We thank Alain Lereec for excellent technical assistance.
This work was supported by grant ANR-05-PNRA-013.
We declare that we have no conflict of interest.
Footnotes
Published ahead of print on 12 March 2010.
REFERENCES
- 1.Agaisse, H., M. Gominet, O. A. Økstad, A. B. Kolstø, and D. Lereclus. 1999. PlcR is a pleiotropic regulator of extracellular virulence factor gene expression in Bacillus thuringiensis. Mol. Microbiol. 32:1043-1053. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, A., P. E. Granum, and U. Ronner. 1998. The adhesion of Bacillus cereus spores to epithelial cells might be an additional virulence mechanism. Int. J. Food Microbiol. 39:93-99. [DOI] [PubMed] [Google Scholar]
- 3.Andreeva, Z., V. Nesterenko, I. Yurkov, Z. I. Budarina, E. Sineva, and A. S. Solonin. 2006. Purification and cytotoxic properties of Bacillus cereus hemolysin II. Protein Express. Purif. 47:186-193. [DOI] [PubMed] [Google Scholar]
- 4.Arnaout, M., R. Tamburro, S. Bodner, J. Sandlund, G. Rivera, and C. Pui. 1999. Bacillus cereus causing fulminant sepsis and hemolysis in two patients with acute leukemia. J. Pediatr. Hematol. Oncol. 21:431-435. [DOI] [PubMed] [Google Scholar]
- 5.Asano, S., Y. Nukumizu, H. Bando, T. Iizuka, and T. Yamamoto. 1997. Cloning of novel enterotoxin genes from Bacillus cereus and Bacillus thuringiensis. Appl. Environ. Microbiol. 63:1054-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auger, S., E. Krin, S. Aymerich, and M. Gohar. 2006. Autoinducer 2 affects biofilm formation by Bacillus cereus. Appl. Environ. Microbiol. 72:937-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auger, S., N. Ramarao, C. Faille, A. Fouet, S. Aymerich, and M. Gohar. 2009. Biofilms formation and cell surface properties among pathogenic and nonpathogenic strains of the Bacillus cereus group. Appl. Environ. Microbiol. 75:6616-6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boonchai, N., S. Asano, H. Bando, and C. Wiwat. 2008. Study on cytotoxicity and nucleotide sequences of enterotoxin FM of Bacillus cereus isolated from various food sources. J. Med. Assoc. Thai. 91:1425-1432. [PubMed] [Google Scholar]
- 9.Bouillaut, L., N. Ramarao, C. Buisson, N. Gilois, M. Gohar, D. Lereclus, and C. Nilesen-LeRoux. 2005. FlhA influences Bacillus thuringiensis PlcR-regulated gene transcription, protein production, and virulence. Appl. Environ. Microbiol. 71:8903-8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calabrese, D., and K. W. Nickerson. 1980. A comparison of protein crystal subunit sizes in Bacillus thuringiensis. Can. J. Microbiol. 26:1006-1010. [DOI] [PubMed] [Google Scholar]
- 11.Callegan, M. C., S. T. Kane, D. C. Cochran, M. S. Gilmore, M. Gominet, and D. Lereclus. 2003. Relationship of PlcR-regulated factors to Bacillus endophthalmitis virulence. Infect. Immun. 71:3116-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callegan, M. C., S. T. Kane, D. C. Cochran, B. Novosad, M. S. Gilmore, M. Gominet, and D. Lereclus. 2005. Bacillus endophthalmitis: roles of bacterial toxins and motility during infection. Invest. Ophthalmol. Vis Sci. 46:3233-3238. [DOI] [PubMed] [Google Scholar]
- 13.Costerton, J., P. Stewart, and E. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 14.Fedhila, S., N. Daou, D. Lereclus, and C. Nielsen-LeRoux. 2006. Identification of Bacillus cereus internalin and other candidate virulence genes specifically induced during oral infection in insects. Mol. Microbiol. 62:339-355. [DOI] [PubMed] [Google Scholar]
- 15.Fedhila, S., P. Nel, and D. Lereclus. 2002. The InhA2 metalloprotease of Bacillus thuringiensis strain 407 is required for pathogenicity in insects infected via the oral route. J. Bacteriol. 184:3296-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finney, D. J. 1971. Probit analysis. Cambridge University Press, London, England.
- 17.Fukushima, T., A. Afkham, S. Kurosawa, T. Tanabe, H. Yamamoto, and J. Sekiguchi. 2006. A new D,L-endopeptidase gene product, YojL (renamed CwlS), plays a role in cell separation with LytE and LytF in Bacillus subtilis. J. Bacteriol. 188:5541-5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghelardi, E., F. Celandroni, S. Salvetti, D. J. Beecher, M. Gominet, D. Lereclus, A. C. Wong, and S. Senesi. 2002. Requirement of flhA for swarming differentiation, flagellin export, and secretion of virulence-associated proteins in Bacillus thuringiensis. J. Bacteriol. 184:6424-6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gohar, M., K. Faegri, S. Perchat, S. Ravnum, O. A. Økstad, M. Gominet, A. B. Kolstø, and D. Lereclus. 2008. The PlcR virulence regulon of Bacillus cereus. PLoS One 3:e2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guillemet, E., C. Cadot, S. Tran, M. H. Guinebretière, D. Lereclus, and N. Ramarao. 2010. The InhA metalloproteases of B. cereus contribute concomitantly to virulence. J. Bacteriol. 192:286-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guinebretière, M. H., V. Broussolle, and C. Nguyen-The. 2002. Enterotoxigenic profiles of food-poisoning and food-borne Bacillus cereus strains. J. Clin. Microbiol. 40:3053-3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall-Stoodley, L., and P. Stoodley. 2009. Evolving concepts in biofim infections. Cell. Microbiol. 11:1034-1043. [DOI] [PubMed] [Google Scholar]
- 23.Hirst, R., B. Gosai, A. Rutman, C. Guerin, P. Nicotera, P. Andrew, and C. O'Callaghan. 2008. Streptococcus pneumoniae deficient in pneumolysin or autolysin has reduced virulence in meningitis. J. Infect. Dis. 1997:744-751. [DOI] [PubMed] [Google Scholar]
- 24.Houry, A., R. Briandet, S. Aymerich, and M. Gohar. 2010. Motility and flagellum involvement in Bacillus cereus biofilm formation. Microbiology 156:1009-1018. [DOI] [PubMed]
- 25.Hsieh, Y., S. Sheu, Y. Chen, and H. Tsen. 1999. Enterotoxigenic profiles and polymerase chain reaction detection of Bacillus cereus group cells and B. cereus strains from foods and food-borne ourbreaks. J. Appl. Microbiol. 87:481-490. [DOI] [PubMed] [Google Scholar]
- 26.Hsueh, Y., E. Somers, D. Lereclus, and A. C. Wong. 2006. Biofilm formation by Bacillus cereus is influenced by PlcR, a pleiotropic regulator. Appl. Environ. Microbiol. 72:5089-5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivanova, N., A. Sorokin, I. Anderson, N. Galleron, B. Candelon, V. Kapatral, A. Bhattacharyya, G. Reznik, N. Mikhailova, A. Lapidus, L. Chu, M. Mazur, E. Goltsman, N. Larsen, M. D'Souza, T. Walunas, Y. Grechkin, G. Pusch, R. Haselkorn, M. Fonstein, S. D. Ehrlich, R. Overbeek, and N. Kyrpides. 2003. Genome sequence of Bacillus cereus and comparative analysis with Bacillus anthracis. Nature 423:87-91. [DOI] [PubMed] [Google Scholar]
- 28.Kajimura, J., T. Fujlwara, S. Yamada, Y. Suzawa, T. Nishida, Oyamada, I. Y. Hayashi, J. Yamagishi, H. Komatsuzawa, and M. Sugai. 2005. Identification and molecular characterization of an N-acetylmuramyl-L-alanine amidase Sle1 involved in cell separation of Staphylococcus aureus. Mol. Microbiol. 58:1087-1101. [DOI] [PubMed] [Google Scholar]
- 29.Lereclus, D., O. Arantes, J. Chaufaux, and M.-M. Lecadet. 1989. Transformation and expression of a cloned ∂-endotoxin gene in Bacillus thuringiensis. FEMS Microbiol. Lett. 60:211-218. [DOI] [PubMed] [Google Scholar]
- 30.Lereclus, D., M. Vallade, J. Chaufaux, O. Arantes, and S. Rambaud. 1992. Expansion of insecticidal host range of Bacillus thuringiensis by in vivo genetic recombination. Biotechnology 10:418-421. [DOI] [PubMed] [Google Scholar]
- 31.Lindbäck, T., O. A. Økstad, A. L. Rishovd, and A. B. Kolstø. 1999. Insertional inactivation of hblC encoding the L2 component of Bacillus cereus ATCC 14579 haemolysin BL strongly reduces enterotoxigenic activity, but not the haemolytic activity against human erythrocytes. Microbiology 145:3139-3146. [DOI] [PubMed] [Google Scholar]
- 32.Lövgren, A., M.-Y. Zhang, A. Engström, and R. Landén. 1993. Identification of two expressed flagellin genes in the insect pathogen Bacillus thuringiensis subsp. alesti. J. Gen. Microbiol. 139:21-30. [DOI] [PubMed] [Google Scholar]
- 33.Margot, P., M. Wahlen, A. Gholamhoseinian, P. Piggot, and D. Karamata. 1998. The lytE gene of Bacillus subtilis 168 encodes a cell wall hydrolase. J. Bacteriol. 180:749-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller, J. M., J. G. Hair, M. Hebert, L. Hebert, F. J. Roberts, and R. S. Weyant. 1997. Fulminating bacteremia and pneumonia due to Bacillus cereus. J. Clin. Microbiol. 35:504-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minnaard, J., V. Lievin-Le Moal, M. H. Coconnier, A. L. Servin, and P. F. Pérez. 2004. Disassembly of F-actin cytoskeleton after interaction of Bacillus cereus with fully differentiated human intestinal Caco-2 cells. Infect. Immun. 72:3106-3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ngamwongsatit, P., W. Buasri, P. Puianariyanon, C. Pulsrikarn, M. Ohba, A. Assavanig, and W. Panbangreb. 2008. Broad distribution of enterotoxin genes (hblCDA, nheABC, cytK, and entFM) among Bacillus thuringiensis and Bacillus cereus as shown by novel primers. Int. J. Food Microbiol. 121:352-356. [DOI] [PubMed] [Google Scholar]
- 37.Pan, Y., F. J. Breidt, and S. Kathariou. 2006. Resistance of Listeria monocytogenes biofilms to sanitizing agents in a simulated food processing environment. Appl. Environ. Microbiol. 72:7711-7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin, Z. O., Y. L. Yang, Y. Zhu, T. Tolker-Nielsen, S. Molin, and D. Qu. 2007. Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermis. Microbiology 153:2083-2092. [DOI] [PubMed] [Google Scholar]
- 39.Ramarao, N., and D. Lereclus. 2006. Adhesion and cytotoxicity of Bacillus cereus and Bacillus thuringiensis to epithelial cells are FlhA and PlcR dependent, respectively. Microbes Infect. 8:1483-1491. [DOI] [PubMed] [Google Scholar]
- 40.Ramarao, N., and D. Lereclus. 2005. The InhA1 metalloprotease allows spores of the B. cereus group to escape macrophages. Cell. Microbiol. 7:1357-1364. [DOI] [PubMed] [Google Scholar]
- 41.Salamitou, S., F. Ramisse, M. Brehélin, D. Bourguet, N. Gilois, M. Gominet, E. Hernandez, and D. Lereclus. 2000. The plcR regulon is involved in the opportunistic properties of Bacillus thuringiensis and Bacillus cereus in mice and insects. Microbiology 146:2825-2832. [DOI] [PubMed] [Google Scholar]
- 42.Shinagawa, K., J. Sugiyama, T. Terada, N. Matsusaka, and S. Sugii. 1991. Improved methods for purification of an enterotoxin produced by Bacillus cereus. FEMS Microbiol. Lett. 80:1-6. [DOI] [PubMed] [Google Scholar]
- 43.Smith, T. J., S. Blackman, and S. Foster. 2000. Autolysins of Bacillus subtilis: multiple enzymes with multiple functions. Microbiology 146:249-262. [DOI] [PubMed] [Google Scholar]
- 44.Stenfors Arnesen, L., A. Fagerlund, and P. Granum. 2008. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 32:579-606. [DOI] [PubMed] [Google Scholar]
- 45.VanDyke, D. J., J. Wu, S. M. Logan, J. F. Kelly, S. Mizuno, S. I. Alzawa, and K. F. Jarrell. 2 April 2009, posting date. Identification of genes involved in the assembly and attachment of a novel flagellinN-linked tetrasaccharide important for motility in the archaeon Methanococcus maripaludis. Mol. Microbiol. [Epub ahead of print.] doi: 10.1111/j.1365-2958.2009.06671.x. [DOI] [PubMed]
- 46.Vollmer, W., B. Joris, P. Charlier, and S. Foster. 2008. Bacterial peptidoglycan (murein) hydrolases. FEMS Microbiol. Rev. 32:259-286. [DOI] [PubMed] [Google Scholar]
- 47.Wang, L., and M. Lin. 2008. A novel cell-wall anchored peptidoglycan hydrolase (autolysin), IspC, essential for Listeria monocytogenes virulence: genetic and proteomic analysis. Microbiology 154:1900-1913. [DOI] [PubMed] [Google Scholar]
- 48.Wijman, J., P. de Leeuw, R. Moezelaar, M. Zwietering, and T. Abee. 2007. Air-liquid interface biofilms of Bacillus cereus: formation, sporulation and dispersion. Appl. Environ. Microbiol. 73:1481-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang, M.-Y., A. Lövgren, M. G. Low, and R. Landén. 1993. Characterization of an avirulent pleiotropic mutant of the insect pathogen Bacillus thuringiensis: reduced expression of flagellin and phospholipases. Infect. Immun. 61:4947-4954. [DOI] [PMC free article] [PubMed] [Google Scholar]