Abstract
The transcription factor CCAAT/enhancer-binding protein α (C/EBPα) coordinates proliferation arrest and the differentiation of myeloid progenitors, adipocytes, hepatocytes, keratinocytes, and cells of the lung and placenta. C/EBPα transactivates lineage-specific differentiation genes and inhibits proliferation by repressing E2F-regulated genes. The myeloproliferative C/EBPα BRM2 mutant serves as a paradigm for recurrent human C-terminal bZIP C/EBPα mutations that are involved in acute myeloid leukemogenesis. BRM2 fails to repress E2F and to induce adipogenesis and granulopoiesis. The data presented here show that, independently of pocket proteins, C/EBPα interacts with the dimerization partner (DP) of E2F and that C/EBPα-E2F/DP interaction prevents both binding of C/EBPα to its cognate sites on DNA and transactivation of C/EBP target genes. The BRM2 mutant, in addition, exhibits enhanced interaction with E2F-DP and reduced affinity toward DNA and yet retains transactivation potential and differentiation competence that becomes exposed when E2F/DP levels are low. Our data suggest a tripartite balance between C/EBPα, E2F/DP, and pocket proteins in the control of proliferation, differentiation, and tumorigenesis.
The CCAAT/enhancer-binding protein α (C/EBPα) belongs to a family of bZIP (basic region leucine zipper) transcription factors that are involved in cell cycle arrest and induction of lineage-specific differentiation genes in several cell types, as shown during hepatic, adipogenic, granulocytic, skin, lung, and placenta development (2, 12, 19, 26, 29, 43, 57). C/EBPα knockout mice die perinatally from hypoglycemia due to defective expression of liver-specific enzymes required for glucose homeostasis (56). Furthermore, C/EBPα-deficient mice lack white adipose tissue and granulocytes of the eosinophil and neutrophil lineages (56, 59). The C/EBPα gene may mutate to produce oncogenic protein forms that are defective for cell cycle inhibition and that no longer promote cell differentiation (19, 22, 34, 37).
Several lines of evidence suggest an intricate relationship between C/EBPα and early gene 2 factor (E2F) gene products. The E2F-dimerization partner (DP) family of transcription factors regulate key genes of cell cycle progression, apoptosis, and DNA damage (17, 33, 41, 44). Formation of E2F-DP heterodimeric complexes is required for induction of E2F-regulated genes (1, 15, 25), while association with pocket proteins (the retinoblastoma family, retinoblastoma protein [pRB], p107, and p130) inhibits the transcriptional activity of E2F and thus restricts cell cycle progression and tumor development (35). Proliferation arrest by C/EBPα involves repression of E2F target genes (50). The murine C-terminal basic region mutant 2 (BRM2) of C/EBPα is unable to repress E2F transcription and induces a myeloproliferative disorder in the mouse (42, 43). BRM2 is of particular interest, since it resembles recurrent C/EBPα mutations isolated from human acute myeloid leukemia (AML) (34, 37, 42). E2F interacts with the bZIP domain of C/EBPα, and yet the N-terminal transactivation domain of C/EBPα is also required for the suppression of E2F genes (9, 18). Curiously, mice expressing the transactivation-deficient N-terminal truncated C/EBPα isoform p30 that is unable to repress E2F develop AML (3, 22), suggesting that the transcriptional activity of C/EBPα is important for its tumor-suppressing function. Failure to abrogate E2F-mediated proliferation may therefore only partially explain the BRM2 phenotype, since the transactivation domain of BRM2 remains intact and the transcriptional capacity of BRM2 may persist (9, 21). Along these lines, it has been shown that C/EBPα-mediated proliferation arrest and differentiation capacity can be separated from each other by highly malignant E7 papilloma viral oncoproteins, independently of pRB (32). BRM2 knockin mice display defects in proliferation and in differentiation of adipocytes and neutrophils (43), suggesting that altered interaction with E2F is involved in regulating both, cell cycle progression, and differentiation. Moreover, a fraction of adult BRM2 mice recover granulopoiesis (42), suggesting that functionality of BRM2 may be restored by readjustment of the balance between E2F and C/EBPα.
The data presented here identify DP as a novel C/EBPα interacting protein and E2F-DP complexes as inhibitors of the transcriptional activity of C/EBPα. Both “activator” and “repressor” E2Fs (E2F1, E2F3, E2F4, and E2F5) in conjunction with DP, but independently of pocket proteins, may suppress transactivation of C/EBPα by interfering with its binding to DNA. DNA binding, transactivation, and differentiation potential of the BRM2 mutant was restored upon knockdown of either E2F or DP proteins. These data suggest an intricate interdependence between transcriptional inactive C/EBPα bound to E2F-DP and transcriptional active C/EBPα bound to DNA. Our data suggest that the ratio between E2F-DP complexes and C/EBPα critically determines precursor cell expansion and C/EBPα-mediated differentiation and suggests a therapeutic opportunity in readjustment of this balance.
MATERIALS AND METHODS
Plasmids.
pCMV-HA-hDP2, pCMV-HA-hDP1, pBabe-ER-E2F1 wild type (WT) and E132 (33), pE2Fx6-TATA-LUC reporter, and the pRB-binding-defective point mutant E2F1 Y411C (15) were provided by Kristian Helin. The pcDNA3 based amino-terminal hemagglutinin (HA)-tagged E2F constructs were obtained from Stefan Gaubatz. The coding regions of E2F1 and E2F4 contained in the BamHI-EcoRI fragments of these constructs were introduced into the pGEX4T2 BamHI-EcoRI site to generate glutathione S-transferase (GST) fusion proteins. All DP1 and DP2 GST fusion proteins were obtained by introducing a PCR product containing a BamHI and a NotI site, respectively, 5′ and 3′ of the coding region. Alternatively, a PCR product with BamHI-BamHI site was used to generate pCMV-HA-DP2Δ83. The cyclin A binding-deficient pcDNA1-E2F1Δ24 construct was provided by Liang Zhu (24). A BamHI fragment containing the E2F1 DNA-binding-deficient mutant E132 was cloned into pcDNA3 and pGEX4T1. The pBabePuro-based retroviral C/EBPα basic region point mutants (BRM2, I294A and R297A; BRM3, D301A and K304A; and BRM5, Y285A) were obtained from Claus Nerlov (43). For transient transfection, EcoRI-BamHI fragments of these mutants were fused to a carboxy-terminal triple FLAG, contained in a pcDNA3 plasmid. For bacterial expression purposes, DNA encoding the bZIP domain of C/EBPα (both WT and BRM2 mutant), comprising the basic region and the leucine zipper, were fused to an N-terminal His7-tagged protein, contained within the pQLinkH vector (47). The C/EBP-responsive −82 cMGF-luciferase reporter has been described previously (51). Small hairpin RNAs (shRNAs) were expressed in psiRNA (Invivogen), harboring a zeocin selection marker fused to green fluorescent protein (GFP). shRNA oligonucleotides against DP1, E2F1, E2F3, or E2F4 were designed by using the InvivoGen's siRNA Wizard program and subjected to BLAST searching to exclude homology to any additional known sequences. Double-stranded DNA oligonucleotides were ligated to the BbsI site of the psiRNA construct. As a control, a nonspecific shRNA was used. The sequences targeted by shRNAs were as follows: control, 5′-GTC CAT CGA ACT CAG TAG CT-3′; DP1, 5′-GCA GCA TCT CCA ATG ACA AAT-3′; E2F1, 5′-GCC AAG AAG TCC AAG AAT CAT-3′; E2F3, 5′-GCT CAC CAA GAA GTT CAT TCA-3′; and E2F4, 5′-CGA GAG TGA AGG TGT CTG T-3′.
Cell culture and immunoblotting.
293T, 3T3-L1, Phoenix-E ecotropic retroviral packaging cells, mouse embryonic fibroblasts (MEFs) generated from the C/EBPα knockout strain (56), and pRB−/− p107−/− p130−/− MEFs (10) were grown in Dulbecco modified Eagle medium (DMEM) plus GlutaMAX, supplemented with 10% fetal bovine serum (FBS; Gibco). Prior and during adipogenesis DMEM was replaced by alpha-MEM medium plus GlutaMAX. For detection of endogenous proteins, cells were lysed with RIPA buffer (1% NP-40, 0.1% sodium dodecyl sulfate [SDS], 50 mM Tris-HCl [pH 7.5], 50 mM NaCl, and protease inhibitors) while transiently transfected cells were lysed with Triton buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1% Triton, and protease inhibitors). Immunoblot analyses were conducted with the following Santa Cruz antibodies: rabbit anti-C/EBPα (14AA), mouse anti-E2F1 (KH95), rabbit anti-E2F1 (C-20), rabbit anti-E2F3 (N-20), rabbit anti-E2F4 (C-20), rabbit anti-DP1 (K-20), rabbit anti-DP2 (C-20), and mouse anti-α-tubulin (B-7), as well as goat anti-GST (Amersham), mouse anti-HA 12CA5 (Roche), mouse anti-FLAG M2 (Sigma), and mouse anti-DP1 Ab-6 (NeoMarkers). Antigen-antibody complexes were detected either by chemiluminescence (ECL System; Amersham) using secondary antibodies conjugated to horseradish peroxidase or by the Odyssey infrared imaging system, using secondary antibodies conjugated to IRDye (Biomol). The latter allowed quantification of signals using the Odyssey software.
Transfection, immunoprecipitation, and reporter assays.
Coimmunoprecipitation assays were performed with anti-FLAG-M2-agarose (Sigma) according to the manufacturer's specifications. Immunoprecipitated samples and input control (one-fifth of input lysate) were resolved by immunoblotting. For reporter assays, 293T cells and MEFs were transfected with Metafectene (Biontex) and TransIT-LT1 (Mirus), respectively, according to the manufacturer's specifications. Cells were lysed at 48 h posttransfection, and the reporter activities were determined in a Berthold Lumat LB9501. The luciferase values were normalized to protein levels, and protein expression was controlled by immunoblotting.
Fluorescence-activated cell sorting.
Cells were treated with trypsin and resuspended in phosphate-buffered saline (PBS), and GFP-positive cells were sorted by using FACSVantage SE.
RNA isolation and cDNA synthesis.
Total RNA of cells was isolated using the TriPure isolation reagent (Roche) and cDNA was prepared by using random primers and SuperScriptII reverse transcriptase (Invitrogen) according to the manufacturer's specifications. Real-time reverse transcription-PCR was performed on an ABI Prism 7000 (Applied Biosystems) using SYBR green PCR master mix 7000 (Applied Biosystems) according to the manufacturer's instructions. The relative RNA expression levels were calculated by using the comparative threshold cycle (CT) method, and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) expression values were used to normalize the analyzed RNA levels. The sequences of the primer pairs can be obtained upon request.
Chromatin immunoprecipitation (ChIP) analysis.
Cells were cross-linked with 1% formaldehyde for 20 min. After the addition of glycine to a final concentration of 250 mM, the cells were washed twice with ice-cold PBS and swelled in hypotonic buffer (10 mM Tris-HCl [pH 8], 10 mM NaCl, 0.2% NP-40, and protease inhibitors). Nuclei were resuspended in lysis buffer (50 mM Tris-HCl [pH 8], 10 mM EDTA, 1% SDS, and protease inhibitors) and incubated 20 min on ice prior to sonication with Bioruptor (Diagenode) to an average DNA length of 500 bp. Cell debris were removed by centrifugation at 10,000 × g for 20 min. At this point 10% of the input was kept as a control, whereas the rest of the supernatant was diluted with 2 volumes of dilution buffer (1% Triton X-100, 150 mM NaCl, 20 mM Tris-HCl [pH 8], 2 mM EDTA, and protease inhibitors) and incubated overnight with 5 μg of rabbit anti-C/EBPα (14AA; Santa Cruz) or 5 μg of rabbit IgG (Sigma). Immunoprecipitates were collected with protein G-Dynabeads (Invitrogen). Beads were washed four times with Wash I buffer (20 mM Tris-HCl [pH 8], 2 mM EDTA, 50 mM NaCl, 1% Triton X-100, 0.1% SDS, and protease inhibitors), twice with Wash II buffer (10 mM Tris-HCl [pH 8], 1 mM EDTA, 250 mM LiCl, 1% NP-40, 1% deoxycholic acid, and protease inhibitors), and twice with TE buffer (10 mM Tris-HCl [pH 8], 1 mM EDTA, and protease inhibitors). Protein-DNA complexes were eluted with 100 μl of elution buffer (1% SDS and 50 mM NaHCO3). NaCl was added to a final concentration of 200 mM, and cross-linking was reversed by incubation at 67°C overnight. After 30 min RNase A treatment, DNA was purified by using QIAquick PCR purification kit (Qiagen) and quantified with ABI Prism 7000 (Applied Biosystems) using SYBR green PCR master mix 7000 (Applied Biosystems) according to the manufacturer's instructions. ChIP DNA levels were calculated by using the comparative CT method, normalized to input, and expressed as anti-C/EBPα versus IgG. The primers used in the present study have been previously described: peroxisome proliferator-activated receptor gamma 2 (PPARγ2) promoter (52), PPARγ2 distal (2.8 kb upstream of ATG) (39), and apolipoprotein 2 (AP2) promoter (52).
GST and His pulldown assays.
GST and His fusion proteins were expressed in Escherichia coli BL21(DE3) and prepared according to standard procedures. For in vitro binding assays, in vitro-translated 35S-labeled proteins (Promega TNT kit) were incubated with equal amounts of affinity-purified GST fusion proteins coupled to glutathione-Sepharose. GST protein served as a negative control. The beads were washed three times with NP-40 buffer (0.4% NP-40, 50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA) and once with Tris-buffered saline. The proteins were resolved by SDS-PAGE and visualized by autoradiography. GST fusion proteins were identified by Coomassie blue staining to verify that equal amounts were present in all reactions. Alternatively, GST proteins were incubated with an equal volume of lysates of 293T cells transfected with C/EBPα expression plasmids. Bound proteins were detected by immunoblotting. GST fusion proteins were identified by Ponceau S staining. His-tagged proteins were bound to Ni beads (Qiagen) in a buffer (pH 8) containing 50 mM Tris-HCl (pH 8.0), 300 mM NaCl, 0.3% NP-40, and 20 mM imidazole. This buffer was also used for washing the beads.
Retroviral infection.
Retroviral plasmids were transfected into Phoenix E by using the CaPO4 method. Culture supernatant were recollected 48 h after transfection, passed through 0.45-μm-pore-size polyvinylidene difluoride filters (Millipore), supplemented with 5 μg of Polybrene/ml, and used to infect subconfluent layers of C/EBPα−/− MEFs or 3T3-L1. After 10 h of infection, the cells were selected for 3 days in the presence of 2 μg of puromycin (InvivoGen)/ml.
Adipogenesis and shRNA.
3T3-L1 or C/EBPα−/− MEFs were differentiated with alpha-MEM medium, supplemented with 10% FBS, 0.5 mM IBMX [3-isobutyl-1-methylxanthine]), 10 μg of insulin/ml, and 1 μM dexamethasone (DEX) for 2 days. From day 3 onward, cells were cultured in alpha-MEM containing only 10% FBS and 10 μg of insulin/ml. The medium was refreshed every second day. After 8 days, cellular morphology was documented by using bright-field microscopy, and the cells were lysed with RIPA buffer. For shRNA experiments, 2 × 105 C/EBPα−/− MEFs were seeded in 24-well containing alpha-MEM medium supplemented with 10% FBS and were transfected 12 h later with 50 ng of psiRNA constructs, when indicated in combination with E2F/DP expression constructs. Drug treatment for induction of adipogenesis was started 24 h posttransfection. Eight days after start of the treatment, cells were washed twice in PBS and fixed 10 min with Roti-Histofix 4% (Roth). GFP expression contained in the psiRNA construct permitted the recognition of transfected cells under UV light using an AxioVert 100 (Zeiss) inverted microscope. Adipocytes were determined by cell morphology, since phase-contrast microscopy allowed a clear recognition of accumulated lipid droplets within the cells. GFP-expressing cells were counted as either nonadipocytes or adipocytes (at least 400 cells per duplicate). Finally, cells were stained with Oil-Red-O and analyzed by bright-field microscopy.
Electrophoretic mobility shift assays.
Bandshift and competition analyses with oligonucleotides were performed as described previously (51). The C/EBP probes were derived from the cMGF promoter (51). The E2F probes were derived from the dihydrofolate reductase (DHFR) promoter (48). The relative intensity of signals arising from shifted radiolabeled probes was determined by quantification of signals from scanned autoradiography films using the Odyssey software.
Protein expression and purification.
His-bZIP-domain of C/EBPα was overexpressed at 20°C in E. coli Rosetta (DE3). The purification procedure comprises an affinity chromatography on a 5-ml HisTrap FF crude column (GE Healthcare), charged with Ni2+, and a size exclusion chromatography on a Superdex 75 prep grade column (26 by 60 cm; GE Healthcare). The His7 tag was cleaved with tobacco etch virus protease prior to the gel filtration step.
ITC.
Isothermal titration calorimetry (ITC) measurements (58) were performed in 20 mM HEPES (pH 7.5) and 0.15 M KCl at 25°C using a VP-ITC microcalorimeter (MicroCal, LLC, Northampton, IL). In an experiment 5 μl of DNA solution (200 or 250 μM) was injected into the sample cell containing 20 μM protein solution (monomeric WT and BRM2 variant of C/EBPα bZIP domain). The DNA sequences used were as follows: forward, 5′-GTC AGT CAG ATT GCG CAA TAT CGG TCA G-3′; and reverse, 5′-C TGA CCG ATA TTG CGC AAT CTG ACT GAC-3′ (31). A total of 50 injections were performed with a spacing of 240 s and a reference power of 18 μcal/s. Binding isotherms were plotted and analyzed using Origin Software (MicroCal, LLC).
Statistical analysis.
Statistical differences between indicated values were determined by one-way analysis of variance with Dunnett post-test. A P value of <0.05 was considered statistically significant.
RESULTS
E2F-DP complexes repress transcriptional activity by C/EBPα.
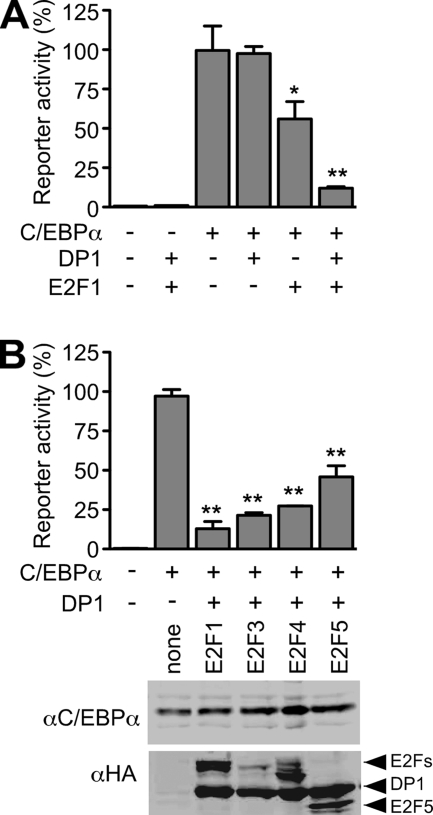
Several studies have shown that interaction between C/EBPα and E2F1 results in repression of E2F target genes (9, 18, 43, 50). Whether and how E2F affects gene activation by C/EBPα has not yet been addressed. The expression of E2F1 or DP1 (or DP2) weakly suppressed C/EBPα, whereas the coexpression of E2F1/DP1 (or E2F1/DP2) strongly repressed the transcriptional activity of C/EBPα (Fig. 1A and data not shown). On the basis of homology and function, E2F proteins can be subgrouped into family members primarily involved in gene activation (“activator” E2F1-E2F3) or in gene repression (“repressor” E2F4 and E2F5). Figure 1B shows that in conjunction with DP all E2F proteins examined suppressed transactivation by C/EBPα.
FIG. 1.
E2F-DP represses the transcriptional activity of C/EBPα. (A) Activation of a C/EBP responsive reporter (100 ng) by a C/EBPα expression vector (100 ng) in the absence or presence of DP1 (100 ng), E2F1 (100 ng), or both expression vectors. The E2F1-DP1 complex represses C/EBPα activity, also when transfected at 50 ng each (data not shown). (B) Several members of the E2F family repress the transcriptional activity of C/EBPα. 293T cells were transfected as indicated (upper panel) with C/EBP-responsive gene reporter, C/EBPα, HA-DP1, and HA-tagged E2F expression constructs (100 ng each). Protein expression was quantified by immunoblotting (lower panel). All gene reporter assays were carried out in duplicate and are plotted as the means ± the standard error of the mean (SEM). *, P < 0.05; **, P < 0.01 (versus C/EBPα alone, which was set to 100). The data are representative of at least three independent experiments.
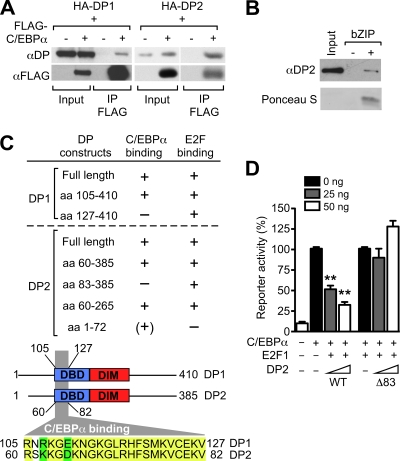
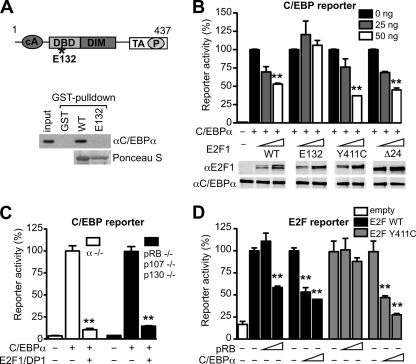
Coimmunoprecipitation and in vitro binding studies showed that both DP1 and DP2 bind to C/EBPα and interact with the bZIP domain of C/EBPα (Fig. 2A and B and data not shown). The structural requirements for repression of C/EBPα activity by DP and by E2F1 were therefore examined. First, amino- and carboxy-terminal DP deletions were tested for interaction with C/EBPα. As shown in Fig. 2C, C/EBPα binding depends on conserved N-terminal regions contained in both DPs (DP1, amino acids [aa] 105 to 127; DP2, aa 60 to 82) that partially overlap with the DNA-binding domain (DBD) but not with the E2F-DP dimerization domain (DIM). Our data showed that a C/EBPα-binding-deficient DP2 mutant lacking 83 N-terminal amino acids (equivalent to mutant aa 83 to 385 of Fig. 2A, referred from now on as Δ83) fails to cooperate with E2F1 in repressing the transcriptional activity of C/EBPα (Fig. 2D). Interaction assays and gene reporter studies showed that mutations within the E2F1 DBD (E2F1 E132) also abrogate binding to C/EBPα (Fig. 3A) and fail to repress C/EBPα activity (Fig. 3B). These results are in agreement with previous findings, showing that the E2F1 E132 mutant failed to repress C/EBPα-induced adipogenesis (43). Altogether, these data indicate that the physical interaction between E2F-DP and C/EBPα are required to suppress the transcriptional activity of C/EBPα.
FIG. 2.
Repression of C/EBPα by DP requires interaction of both proteins. (A) Lysates of 293T cells transfected with FLAG-C/EBPα and either HA-DP1 or HA-DP2 expression vectors were immunoprecipitated with anti-FLAG (IP), separated by SDS-PAGE, and analyzed by immunoblotting. DP1 and DP2 proteins were detected with anti-DP1 and anti-DP2 sera, respectively. (B) DP2 interacts with the bZIP domain of C/EBPα. His-bZIP immobilized on beads was incubated with GST-DP2. As a negative control, beads were incubated with GST-DP2 in the absence of His-bZIP proteins. Bound GST-DP2 was detected by immunoblotting and pulldown of His-bZIP was controlled by Ponceau S staining. (C [from top to bottom]) GST fusion deletion mutants of DP1 and DP2 were tested for their ability to interact with C/EBPα (data not shown). The results are summarized in the table. Brackets represent reduced affinity. A schematic representation of the domains within DP1 and DP2 is presented (DBD, DNA-binding domain; DIM, dimerization domain; gray box, sequence required for C/EBPα binding). Alignment of the amino acid sequences of the DP1 and DP2 motifs involved in C/EBPα binding. Yellow, identity; green, similarity. (D) The C/EBPα-binding-deficient DP2 mutant Δ83 fails to repress C/EBPα. 293T cells were transfected with a C/EBP-responsive gene reporter, C/EBPα (100 ng), and HA-E2F1 (50 ng) and with either full-length (WT) or N-terminal truncated (Δ83) DP2 expression constructs, as indicated. Protein expression was controlled by immunoblotting (data not shown). **, P < 0.01 versus C/EBPα alone, which was set to 100.
FIG. 3.
Cross-repression of C/EBPα and E2F1 is independent of pocket proteins. (A) At the top is a schematic representation of E2F1. cA, cyclin A-binding domain; DBD, DNA-binding domain; DIM, dimerization domain; TA, transactivation domain; P, pocket protein interaction domain; asterisk, approximate location of the E132 mutations. Indicated below are the results of GST-pulldown assays performed to assess the binding of C/EBPα (expressed in 293T cells) to either E2F1 WT or E2F1 E132 (GST fusion proteins). Bound proteins were detected by immunoblotting, and pulldown of GST proteins was controlled by Ponceau S staining. (B) An intact DNA-binding domain of E2F1 is required to repress the transcriptional activity of C/EBPα. Different E2F1 mutants were tested for their ability to inhibit C/EBPα-mediated transcription: E132 (DNA binding deficient), Y411C (pRB binding deficient), and Δ24 (cyclin A binding deficient). 293T cells were transfected with a C/EBP responsive gene reporter, C/EBPα (100 ng), and with E2F1 mutant expression constructs, as indicated (E2F1 is always in combination with 50 ng of HA-DP1). The dose-dependent effects of E2F1 mutants were tested. Protein expression was controlled by immunoblotting, and the lanes were run on the same gel, but the image was divided into different panels for the shake of simplicity (lower panels). **, P < 0.01 versus C/EBPα alone, which was set to 100. (C) E2F1-mediated repression of C/EBPα is independent of pocket proteins. C/EBPα−/− MEFs or pRB−/− p107−/− p130−/− MEFs were transfected with C/EBP-responsive gene reporter, C/EBPα, HA-DP1, and HA-E2F1 (all at 100 ng). **, P < 0.01 versus C/EBPα alone, which was set to 100. (D) C/EBPα-mediated repression of E2F is independent of pocket proteins. pRB−/− p107−/− p130−/− MEFs were transfected with E2F-responsive gene reporter (100 ng) and with HA-DP1 (50 ng), in combination with 50 ng of either E2F1 WT or E2F1 Y411C expression constructs. Increasing amounts of pRB or C/EBPα (triangle: 100 or 200 ng) were also transfected as indicated to assess their repression on E2F1. As a control, cells were transfected with empty vector. **, P < 0.01 versus E2F1-DP1 alone, which was set to 100. Reporter assays were performed in duplicate, plotted as the means ± the SEM. The data are representative of at least three independent experiments.
The connection between pRB- and C/EBPα-mediated proliferation arrest and gene repression remains under debate (16, 18, 48). pRB belongs to the family of pocket proteins, including p107 and p130, that regulate the cell cycle and cell differentiation by inhibition of E2F (7). As shown in Fig. 3B, E2F1 mutants that are defective for pRB (Y411C) or for cyclin A-CDK2 binding (Δ24) still repress the transcriptional activity of C/EBPα. MEFs that lack all three pocket proteins (triple-knockout [TKO] cells) (10) were used to further examine the suppression of C/EBPα by E2F-DP. As shown in Fig. 3C, E2F-DP suppressed C/EBPα-mediated transcription also in the absence of pocket proteins. Next, we explored whether repression of E2F by C/EBPα may depend on pRB. Figure 3D shows that transcriptional activity of E2F1-DP1 was repressed by either pRB or by C/EBPα. Moreover, the pRB-binding-deficient E2F1 mutant (Y411C) that escapes pRB repression (15) was still repressed by C/EBPα. Altogether, these data suggest that in addition to the well-studied repression of E2F by pRB, the repression of E2F by C/EBPα or the repression of C/EBPα by E2F may also occur independently of pRB.
E2F-DP complexes dominantly repress the myeloproliferative BRM2 C/EBPα mutant.
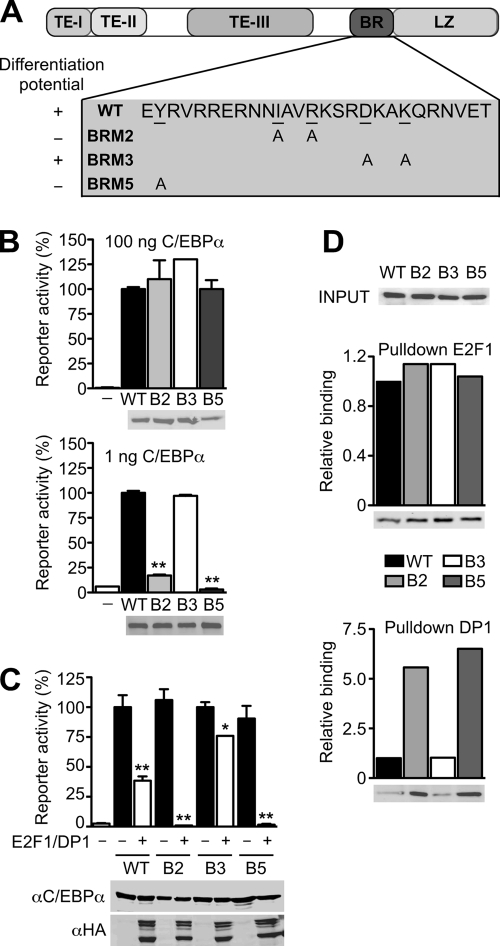
It is thought that basic region mutants of C/EBPα (Fig. 4A), such as BRM2 and BRM5, fail to abrogate cell proliferation due to defective repression of E2F-regulated S-phase genes (43). Both mutants evoked a myeloproliferative phenotype (42) and also failed to support adipogenic and granulocytic differentiation (43). Although these studies showed that the BRM mutants are deficient for inhibition of proliferation, their transactivation potential remained equivocal (9, 21, 30). Therefore, we compared the transactivation potential of the BRM mutants depicted in Fig. 4A with that of WT C/EBPα and BRM3, a mutant that functions similarly as WT (43). WT C/EBPα and BRM mutants were tested at different concentrations in gene reporter assays. As shown in Fig. 4B, all C/EBPα proteins displayed similar transactivation potential at a high C/EBPα concentration (100 ng of expression plasmid, which is within the dosage range frequently used for reporter assays). At a low concentration (1 ng of C/EBPα expression plasmid, which is close to the endogenous level of C/EBPα [data not shown]), however, transactivation by BRM2 and BRM5 was severely diminished compared to the WT or BRM3. This was not due to decreased protein stability or concentration-dependent expression effects, since both BRM2 and BRM5 proteins were expressed at similar amounts compared to the WT or BRM3 (see the expression controls under the graphs in Fig. 4B). Next, we examined the possibility that E2F-DP becomes rate-limiting at a high C/EBPα concentration. As shown in Fig. 4C, BRM2 and BRM5 activities were strongly repressed by E2F-DP compared to WT C/EBPα or BRM3. Binding experiments showed that DP1 and DP2 displayed much stronger interactions with BRM2 and BRM5, compared to WT or BRM3, whereas binding between WT or C/EBPα mutants and E2F1 or E2F4 was indistinguishable (Fig. 4D and data not shown).
FIG. 4.
Repression of C/EBPα BRM2 by E2F complexes. (A) Schematic representation of C/EBPα and the basic region mutants (BRMs). TE-I to TE-III, transactivation domains; BR, basic region; LZ, leucine zipper. BRM2 (B2) and BRM5 (B5) are unable to induce adipogenesis and granulopoiesis, while BRM3 (B3) shows a phenotype similar to that of WT. (B) Differences in the transcriptional activation between BRMs and WT are concentration dependent. 293T cells were transfected with a C/EBP-responsive gene reporter and with either 100 ng (upper panel) or 1 ng (lower panel) of C/EBPα BRMs or WT, as indicated. Bar graphs represent relative luciferase reporter activity. **, P < 0.01 versus WT C/EBPα, which was set to 100. C/EBPα expression was controlled by anti-C/EBPα immunoblotting (below graphs). (C) Repression of BRMs by E2F1-DP1. Transcriptional activation of WT, BRM2, BRM3, or BRM5 (100 ng) in the presence or absence of HA-E2F1/HA-DP1 (50 ng each) was determined in 293T cells. *, P < 0.05; **, P < 0.01 (versus C/EBPα alone, which was set to 100). Protein expression was analyzed by immunoblotting (lower panel). Luciferase reporter assays were performed in duplicate and plotted as means ± the SEM. The data are representative of at least three independent experiments. (D) Increased binding of BRM2 and BRM5 to DPs. Binding of BRMs or WT C/EBPα expressed in 293T cells (input shown in upper panel) to E2F1 or DP1 (GST fusion proteins) was examined by GST pulldown assay. Bound proteins were quantified by anti-C/EBPα immunoblotting. Bar graphs represent the quantification of the immunoblotting located below. The results are representative of at least three independent experiments.
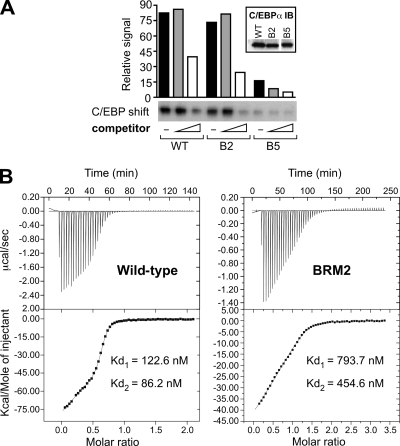
Structural and binding analysis of C/EBPα had suggested a crucial role for Tyr285 (mutated in BRM5) in C/EBPα-DNA complexes (30). The crystal structure of C/EBPα may also suggest that Arg297 (mutated in BRM2) interacts with the DNA backbone. In vitro-translated BRM2, BRM5 or WT C/EBPα proteins were subjected to gel retardation analysis using a radiolabeled C/EBP DNA binding site and the same unlabeled DNA probe as a competitor. As shown in Fig. 5A, BRM5 displayed defective DNA binding, whereas BRM2 retained DNA binding capacity. Determination of the DNA binding constants of WT C/EBPα and BRM2 by ITC revealed biphasic binding to C/EBP sites for both WT and BRM2, with 5- to 7-fold reduced binding affinity for BRM2 (Fig. 5B).
FIG. 5.
Differential binding of C/EBPα mutants to DNA. (A) In vitro-translated FLAG-C/EBPα WT, BRM2 (B2), or BRM5 (B5) were incubated with radiolabeled probes containing a palindromic C/EBP binding site of the cMGF promoter (51). Competition assays were performed with increasing amounts of cold C/EBP probe (triangle: competitor). Binding to radiolabeled probes was examined by gel shift assay and detected by autoradiography. Bar graphs represent the signal quantification of the autoradiogram. The expression of C/EBPα mutants was controlled with anti-C/EBPα serum (inset). The lanes were run on the same gel but were noncontiguous. IB, immunoblot. (B) Reduced DNA binding affinity of BRM2. ITC data for C/EBP DNA site binding to the bZIP-domain of C/EBPα are presented. The panels show the sequential heat pulses and integrated heat data, fitted to a two-site binding model, for the WT protein (left) and for the BRM2 variant (right), respectively. Dissociation constant (Kd) values derived from these measurements (lower panels) correspond to 1/K (K, association constant).
E2F-DP inhibits the interaction between C/EBPα and its DNA binding sites.
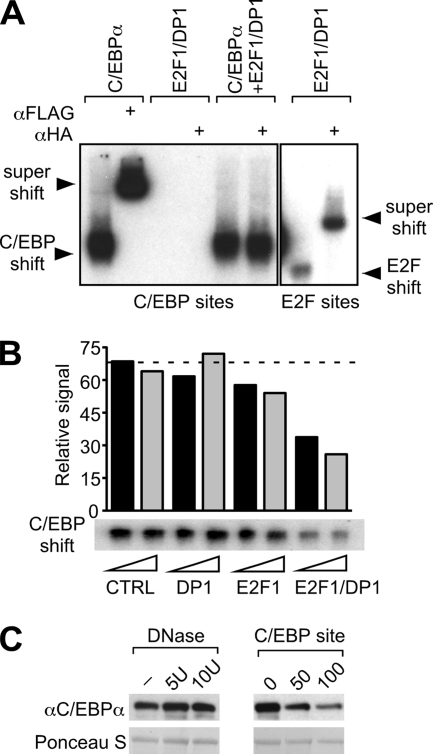
E2F may repress the transcriptional activity of C/EBPα either when bound to DNA or by abrogating the binding of C/EBPα to DNA. To distinguish between these possibilities, DNA binding site interaction analysis was performed by gel shift assay. As shown in Fig. 6A, the E2F1-DP1 complex did not bind to the C/EBP probe, neither in the absence nor in the presence of C/EBPα (left panel). Control shifts and antibody super shifts confirmed that the C/EBP site was bound by C/EBPα and that E2F1-DP1 complexes associated with E2F sites only (Fig. 6A, right panel). Thus, E2F-DP complexes did not detectably associate with C/EBP DNA-binding sites nor with C/EBPα proteins bound to DNA.
FIG. 6.
E2F-DP disrupts C/EBPα binding to its consensus DNA sites. (A) Electrophoretic mobility shift assay using radiolabeled probes containing either a C/EBP-binding site (left panel) or an E2F consensus binding site (right panel). Nuclear extracts of 293T cells, transfected with FLAG-C/EBPα and/or HA-E2F1/DP1 expression constructs, were incubated with probes and with anti-FLAG or anti-HA antibodies, as indicated. Binding and supershift are shown on this representative autoradiogram. (B) DNA binding of C/EBPα in the presence of purified E2F1 and DP1 proteins. GST-DP1 or GST-E2F1 fusion proteins were bound to GST beads and detached from the GST tag by thrombin digestion, resulting in the elution of DP1 or E2F1 proteins. In vitro-translated FLAG-C/EBPα was preincubated with increasing amounts (triangle) of DP1, E2F1, E2F1-DP1, or control (CTRL, GST alone) eluate. When DP1 or E2F1 eluate was added separately, it was combined with control eluate to maintain equal protein amounts. Binding of C/EBPα to a radiolabeled C/EBP probe was examined by gel shift assay and detected by autoradiography. The bar graph represents the signal quantification of the autoradiogram. The dashed line is set up at the CTRL level. (C) DNA competes with interaction between C/EBPα and E2F1. Binding of C/EBPα expressed in 293T cells (input) to E2F1 (GST fusion proteins) was examined in a GST pulldown assay under conditions of DNA depletion (DNase treatment) or addition of DNA (C/EBP-binding site). In the left panel, E2F1-C/EBPα binding reactions were incubated in the presence of 5, 10, or 10 U of heat-inactivated DNase (−). In the right panel, 0, 50, or 100 ng of C/EBP probe was added to the C/EBPα-E2F1 binding reactions. Bound proteins were detected and quantified by immunoblotting and pulldown of GST proteins controlled by Ponceau S staining.
Next, we investigated whether E2F-DP complexes interfere with the binding of C/EBPα to DNA. As shown in Fig. 6B, addition of E2F1-DP1 complex diminished the association of C/EBPα with DNA, while individual addition of DP1 or E2F1 did only barely affect C/EBPα binding to DNA. To examine whether contaminating DNA might have affected interaction between C/EBPα and E2F proteins, binding assay samples were treated with DNase (Fig. 6C, left panel). The removal of residual DNA by DNase treatment slightly increased the interaction between C/EBPα and E2F1, and the addition of the C/EBP DNA binding site (as used in Fig. 6A and B) reduced the interaction between C/EBPα and E2F1 (right panel). These data suggest that binding of C/EBPα to the E2F1 or to C/EBP DNA binding sites is competitive and exclusive.
E2F-DP knockdown unlocks the differentiation potential of BRM2.
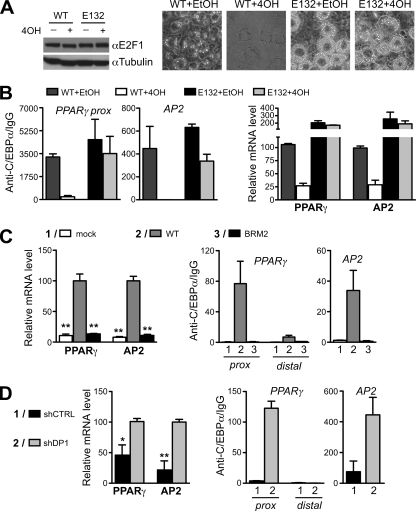
A physiological function of E2F-mediated disruption of C/EBPα-DNA complexes was addressed using the preadipogenic 3T3L1 cell line that may undergo C/EBPα-dependent differentiation into fat cells (27). 3T3L1 cells were transduced with a conditional estrogen receptor E2F1 fusion construct (ER-E2F1) that may inhibit adipogenic differentiation upon addition of tamoxifen (4OH) (43). As a negative control, the DNA- and C/EBPα-binding-deficient E2F1 mutant E132 was used, which does not repress adipogenesis (43). 3T3L1 expressing ER-E2F1 WT or E132 were hormonally stimulated to differentiate into adipocytes either in the presence or absence of 4OH (Fig. 7A). A strong inhibition of adipogenesis was observed with the ER-E2F1 WT compared to ER-E132 after 4OH treatment. Some inhibition was also discernible with WT ER-E2F1 in the absence of 4OH, suggesting some leakiness of the ER-fusion proteins to the nucleus. ChIP assay revealed that the association of C/EBPα with adipocytic genes, including apolipoprotein 2 (AP2) and peroxisome proliferator-activated receptor γ2 (PPARγ2), was strongly diminished after activation of WT E2F1 but not of E132 (Fig. 7B, left panel). Dissociation of C/EBPα from adipocytic genes coincided with decreased levels of adipogenic gene transcripts (Fig. 7B, right panel). The data suggested that the interaction between E2F-DP and C/EBPα prevented the binding of C/EBPα to promoters of differentiation genes.
FIG. 7.
E2F-DP competes with binding of C/EBPα to the promoters of target genes. (A) E2F1 WT but not the E132 mutant represses adipogenesis. 3T3L1 cells were transduced with constructs expressing ER-E2F1 (WT or E132), a 4OH-responsive E2F1 fusion protein (33). Cells were treated with adipogenic stimulation cocktail (insulin, IBMX, and DEX), in combination with either 4OH (1 μM) or ethanol (EtOH; solvent only; −). E2F1 expression in these cell lines was controlled by immunoblotting (left). Right panels show representative photographs of cells after 8 days of treatment. (B) Cells treated as in panel A were harvested after 48 h for ChIP or mRNA expression analysis. On the left, chromatin samples were immunoprecipitated with antibodies directed against C/EBPα or IgG and analyzed by quantitative PCR, using primers flanking C/EBP-binding sites in the proximal promoters of the PPARγ2 (PPARγ prox) and AP2 genes. ChIP quantification data are expressed as anti-C/EBPα versus IgG and normalized to input. On the right, bar graphs represent AP2 and PPARγ2 transcript levels normalized to GAPDH. (C) BRM2 fails to bind to adipocytic gene promoters. ChIP and mRNA expression analyses of C/EBPα−/− MEFs, transduced with either control vector (mock), WT, or BRM2 C/EBPα. ChIP analysis was performed as in panel B. As a negative control, a region 2.8 kb upstream of the PPARγ2 ATG (PPARγ distal) was amplified. **, P < 0.01 versus WT C/EBPα, which was set to 100. (D) Knockdown of DP1 permits binding of BRM2 to endogenous gene promoters and restores transcription of adipocytic genes. Transcript expression and ChIP analyses were performed with cells transduced with BRM2 and stably expressing shRNA directed against DP1 (shDP1) or control (shCTRL). All ChIP and mRNA expression analyses were performed with cells harvested after 48 h treatment with adipogenic stimulation cocktail. Values represent mean of triplicates ± the SEM. *, P < 0.05; **, P < 0.01 (versus shDP1, which was set to 100).
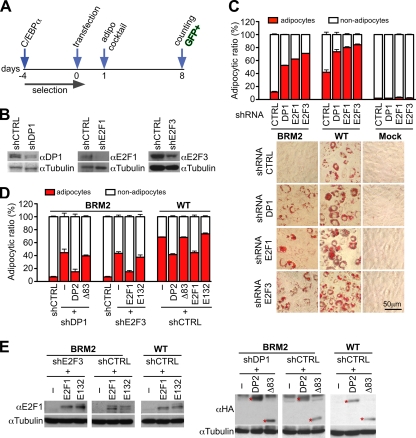
MEFs from C/EBPα-deficient mice (αKO MEFs) do not undergo adipocytic differentiation upon hormonal stimulation (56). The induction of adipocytic genes and differentiation into adipocytes was restored by retroviral expression of WT C/EBPα but not by the expression of BRM2 in αKO MEFs (Fig. 7C, left panel, and Fig. 8C). In accordance with this, WT C/EBPα but not BRM2 associated with promoters of adipocytic genes (Fig. 7C, right panel). Importantly, knockdown of DP1 with shRNA constructs, however, restored the binding of BRM2 to adipogenic target genes and induced their activation (Fig. 7D). Adipogenic differentiation by BRM2 was also restored by knocking down either E2F1, E2F3, E2F4, or DP1 proteins (Fig. 8B and C and data not shown) and was overruled by reconstitution of E2F3 or DP1 knockdowns with E2F1 or DP2, respectively, whereas C/EBPα-binding-deficient mutants E2F1 E132 or DP2 Δ83 did not inhibit the function of BRM2 (Fig. 8D and E). Consistently, the adipogenic activity of WT C/EBPα was enhanced by the E2F-DP knockdown constructs and inhibited by WT E2F1 or DP2 but not by the mutants E2F1 E132 or DP2 Δ83, lending further support to a mechanism of E2F-C/EBP cross-inhibition. No adipogenic differentiation was observed with shRNAs in the αKO MEFs, excluding off-target effects of the shRNA constructs (Fig. 8C). These data show that C/EBPα-mediated adipogenesis is intrinsically repressed by E2F-DP complexes, that BRM2 is more susceptible to E2F-DP-mediated suppression, and that the differentiation potential of BRM2 can be recovered despite its attenuated DNA binding capacity.
FIG. 8.
Knockdown of E2F-DP restores BRM2-mediated adipogenesis. (A) Schematic representation of the experimental strategy: adipogenesis of C/EBPα-deficient MEFs, transduced with either control vector (mock), WT C/EBPα, or BRM2 and transfected with shRNA constructs (expressing GFP) directed against DP1, E2F1, E2F3, or control shRNA and where indicated with E2F or DP expression constructs. On day 1, adipogenic stimulation cocktail (insulin, IBMX, and DEX) was added. (B) Reduction of shRNA targeted endogenous proteins was confirmed by immunoblotting analysis of GFP-positive sorted cells on day 3. (C) After 8 days of insulin-IBMX-DEX treatment, GFP-positive cells were quantified as adipocytes or nonadipocytes (bar graphs, upper panel) and stained with Oil-Red-O (photographs, lower panel). Duplicates of at least 400 cells were counted and values were plotted as the means ± the SEM. The data are representative of two independent experiments. (D) C/EBPα-deficient MEFs transduced with BRM2 were transfected with shRNA E2F3 constructs in combination with E2F1 WT (E2F1) or E2F1 E132 mutant (E132) expression constructs. Alternatively, cells were transfected with DP1 shRNA and with HA-tagged expression constructs coding for either DP2 full-length (DP2) or for DP2 N-terminal deletion mutant (Δ83). Knockdown was specific for each family member, thus, shDP1 did not target ectopically expressed DP2 and shE2F3 did not target ectopically expressed E2F1, allowing reconstitution of knockdown (data now shown). WT C/EBPα reconstituted MEFs were transfected with E2F1 or DP2 expression constructs in combination with a GFP expression construct. GFP-positive cells were analyzed as described in panel C. (E) Immunoblot analyses of lysates from experiments shown in Fig. 8D. Asterisks mark bands corresponding to exogenous DP2 proteins.
DISCUSSION
Cross-regulation of the transcription factors C/EBPα and E2F.
C/EBPα-mediated gene regulation entails the activation of cell lineage-specific terminal differentiation genes and the repression of E2F-regulated S-phase genes (43, 50); thus, C/EBPα coordinates both proliferation arrest and differentiation. The C/EBPα mutant BRM2, similar to mutations found in human AML, fails to suppress E2F-regulated S-phase genes or to support adipogenic and granulocytic differentiation (43) and evokes a myeloproliferative disease in mice (42). BRM2 therefore represents a paradigm to examine C/EBPα-regulated proliferation and differentiation processes. Although failure to repress E2F had been documented, it remained to be explored why BRM2 fails to induce differentiation, since it retains an intact transactivation domain and may also bind to cis-regulatory sites (9, 21, 30).
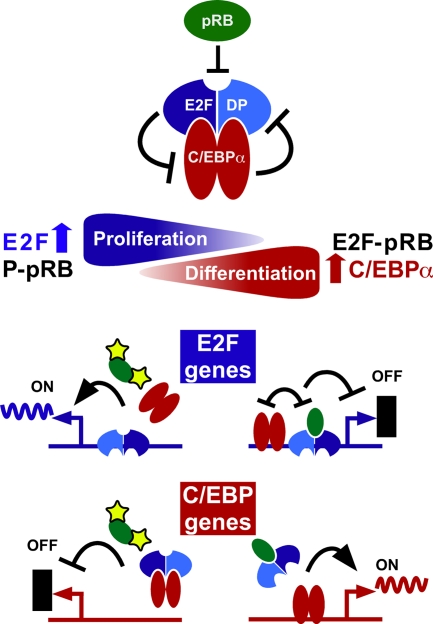
We show here that C/EBPα interacts with DP, the essential dimerization partner of E2F, and that the BRM2 mutant displays enhanced E2F-DP interaction. The data presented show that E2F-DP counteracts induction of differentiation by C/EBPα or by BRM2 and that diminishing E2F-DP complexes restores differentiation by BRM2. This conclusion is also supported by published data, showing that (i) E2F1 downregulation occurs during adipogenesis (11), (ii) forced upregulation of E2F1 inhibits adipogenesis, and (iii) an E2F1 mutant that is unable to repress transcriptional activity of C/EBPα also fails to repress adipogenesis (43). Taken together, our data uncover a novel regulatory function of E2F-DP and suggest reciprocal regulation of the transcriptional activities of C/EBPα and E2F-DP as an important step in proliferation and differentiation control, as summarized in the model shown in Fig. 9.
FIG. 9.
The C/EBPα-E2F-pRB network. Model of how E2F-DP and C/EBPα may function as proliferation-differentiation switches by repressing each other's activity. Proliferation involves the induction of E2F genes and disruption of C/EBPα-mediated transactivation of differentiation genes by E2F-DP. Repression is reversed during differentiation and C/EBP-target genes are activated when E2F-DP complexes become limiting. pRB is a regulator that balances the transcriptional activity of E2F-DP versus C/EBPα. Proliferating cells have high levels of phosphorylated pRB (phosphorylation represented by a yellow star) that cannot bind E2F. Cell maturation toward terminal differentiation is accompanied by an increase in the levels of the E2F-binding hypophosphorylated pRB form, progressively removing more and more E2F away from E2F-C/EBPα complexes. This model may explain the positive effect that pRB has on C/EBPα-mediated transactivation.
DP is required for induction of E2F-regulated genes (1, 15, 25). Although many proteins have been identified that interact with E2F, DP binding partners are rare. We show that C/EBPα is a binding partner of DP1 or DP2 and identify a sequence within DP that is required for interaction with C/EBPα but not for interaction with E2F. Although DP and E2F may also bind to C/EBPα independently from each other, efficient repression of the transcriptional activity of C/EBPα requires their combination, strongly suggesting E2F-DP complexes as suppressors of C/EBPα functions.
E2F-DP complexes repress C/EBPα-mediated transactivation by inhibition of binding of C/EBPα to DNA. The BRM2 mutations apparently display two effects, decreasing the binding to DNA, and augmenting binding to DP, suggesting that a balancing mechanism may exist that switches C/EBPα from a DNA-bound transcriptional active to a DP-bound transcriptional inactive state. BRM2 was found to be more susceptibility to inactivation, and yet it retains transactivation and differentiation potential. These data also provide an explanation as to why some studies observed, while others did not, a transcriptional activity of BRM2 (9, 21) and why BRM2 mice may recover granulopoietic potential (42). Different circumstances may affect the equilibrium between BRM2 and E2F-DP proteins, including conformational changes within C/EBPα or posttranslational modifications of C/EBPα.
Other studies proposed that protein-protein interaction between C/EBPα and E2F at E2F cis-regulatory sites are important for E2F repression (50). This model was supported by a study showing that BRM2 failed to interact with E2F4 and to repress E2F-regulated genes (9). However, another study did not confirm differences in binding affinities between BRM2 or WT C/EBPα and E2F1 (21). In agreement with the latter study, our data suggest that BRM2 displays even increased binding to DP. This may seem counterintuitive at first, if one assumes that C/EBPα represses transcription of E2F target genes by binding via DNA-bound E2F complexes only. A recent report, however, has pointed out that repression of E2F by C/EBPβ may require cryptic C/EBP sites in the proximity of consensus E2F sites, such as those contained in the DHFR promoter (16, 48). Our data confirmed that mutation of the cryptic C/EBP site in the DHFR promoter abolishes binding of C/EBPα proteins but does not affect binding of E2F-DP complexes (data not shown). Thus, the data presented here support the notion that C/EBPα represses transcription of E2F-regulated genes through cryptic cis-regulatory C/EBP binding sites (48). This interpretation may also help to explain why the ability of C/EBPα to repress E2F depends on the DNA-binding function of C/EBPα and why BRM2 and BRM5 mutants, both with compromised DNA binding, fail to repress E2F genes.
Pocket protein-independent repression mechanisms of E2F-DP complexes.
It is widely accepted that E2F proteins function as repressors by association with pocket proteins. Our data show that E2Fs may repress C/EBPα-target genes independently of pocket proteins and that C/EBPα may repress E2Fs independently of pRB. Thus, pocket proteins are not strictly required for cross-transcriptional repression of E2F and C/EBPα. In agreement with this interpretation are results showing that C/EBPα represses E2F-mediated transcription in Saos cells that are deficient for both pRB and p53 (18). These findings are of major interest, since they suggest pocket protein-independent repressive functions of E2F complexes (23, 33). Although repression of E2F by pRB does not require the presence of cryptic C/EBP binding elements, the antiproliferative function of C/EBPα appears to depend on the presence of pocket proteins (48). This raises the possibility that the collaborative action of C/EBPα and pocket proteins during proliferation arrest may not depend on repression of E2F but may affect another key cell cycle regulator. How exactly the interaction between E2F and C/EBPα at compound sites such as at the DHFR promoter converts both transactivators into repressors still needs to be explored. In any case, our data suggest a mechanism of E2F-DP-mediated disruption of binding of C/EBPα to its differentiation genes that may account for the block of cell differentiation.
The fact that under experimental conditions transcriptional repression of E2F or C/EBPα occurs in the absence of pocket proteins does not, however, exclude a role for pocket proteins in affecting the balance of transcriptional activities between C/EBPα and E2F. In fact, pRB is a good candidate to regulate this balance since pRB displays opposing functions on E2F and on C/EBPα: while pRB represses E2F function, it activates the transcription of C/EBPα genes (8). Both C/EBPα and pRB are required for adipogenesis (5), whereas E2F4 counteracts adipogenesis (11). However, no correlation is observed between pRB-C/EBPα interaction and adipogenesis (43). These data may be explained by our model that predicts activation of C/EBPα-regulated differentiation genes by pRB-mediated neutralization of E2F and block of differentiation by dissociation of pRB, increasing the availability of E2Fs (Fig. 9). When pRB is hypophosphorylated it associates with E2F, reducing the amount of available E2F that may bind to and inhibit C/EBPα. Proliferating cells contain high levels of pRB in phosphorylated, E2F-nonbinding form (4). As cells progress toward terminal differentiation the level of phosphorylated pRB decreases (49), resulting in enhanced pRB-E2F complex formation and release of C/EBPα from E2F-C/EBPα complexes. This would result in concomitant elevation of free C/EBPα proteins that support differentiation, e.g., toward the adipocytic lineage. Our data also show that the negative effect of E2F on adipogenesis depends on C/EBPα, since the knockdown of E2F proteins in the absence of C/EBPα does not restore adipogenesis. Thus, we favor a scenario wherein pRB is a regulator of available E2F-DP complexes that in turn titrates C/EBPα activities in proliferation and differentiation control.
Interestingly, C/EBPα and C/EBPβ appear to be functionally distinct, as Sebastian and coworkers have shown that C/EBPβ fails to repress E2F in an equivalent TKO cell line (46, 48). It is known that despite their extended homology and partial redundancy (2, 6, 20), C/EBPα and C/EBPβ may also have antagonistic functions, e.g., in the case of skin tumorigenesis, where the lack of C/EBPα promotes tumorigenesis (28), whereas the lack of C/EBPβ protects against chemical-induced skin carcinogenesis (60). Curiously, pRB deficiency also protects from tumorigenesis in a murine skin carcinogenesis model (45). These results may point to a general difference in pocket protein requirements of C/EBPα- versus C/EBPβ-mediated E2F functions. Alternatively, the different TKO cell lines used in the Johnson lab studies and in our study may have acquired additional mutations that obscure distinct aspects of C/EBP biology. Nevertheless, how pocket proteins modify the functional interactions between E2F and C/EBP family members requires further investigations.
Pathological relevance of the C/EBPα-E2F proliferation-differentiation switch.
In addition to the adipogenic phenotype, BRM2 mice display impaired myelopoiesis and serve as a model for C-terminal AML-C/EBPα mutations (42). We show that knockdown of E2F restores the adipogenic potential of BRM2 and one could therefore envisage that interfering with the interaction between E2F-DP and C/EBPα would also restore granulocytic differentiation and revert the tumorigenic phenotype.
The data presented here suggest that E2F represses the transactivation of C/EBPα target genes by disrupting the binding of C/EBPα to its cis-regulatory sites. Interestingly, disturbed DNA binding of C/EBPα is frequently observed in AML patients that carry C-terminal point mutations or small insertions in the basic region of C/EBPα (13, 14, 37). This often occurs in conjunction with N-terminal frameshift mutations that cause expression of the truncated p30 C/EBPα isoform that is defective for E2F repression and for transactivation (18, 22, 37). Modeling human leukemogenesis in the mouse has shown that the combinations of these two types of mutations accelerate the malignancy of AML-type leukemias compared to homozygous mutant mice (3). Abrogation of C/EBPα functions has also been reported in AML-ETO and BCR-ABL leukemias (36, 38), and ectopic expression of C/EBPα induces the differentiation of such leukemic cells (53, 54). Moreover, the loss of C/EBPα expression in the epidermis increases susceptibility to Ras-induced skin tumorigenesis (28), similarly to the overexpression of E2F1 or DP1 transgenes in the mouse (40, 55). To further delineate the contribution of failure to activate terminal differentiation genes in tumorigenesis, it would be interesting to identify a DP mutant that retains E2F coregulation but is defective for C/EBPα interaction. Pharmacological interference of C/EBPα-E2F-DP interaction and/or restoration of DNA-binding functions of C/EBPα might also provide a therapeutic opportunity in various proliferative diseases.
Acknowledgments
Katrin Zaragoza was partially supported by a fellowship of the MDC-HU International Ph.D. Program. The Protein Sample Production Facility at the Max Delbrueck Center is funded by the Helmholtz Association of German Research Centres.
We are grateful to Elisabeth Kowenz-Leutz, Hein te Riele, Kristian Helin, Claus Nerlov, Stefan Gaubatz, and Liang Zhu for kindly providing cells and plasmids. We thank Hans-Peter Rahn for fluorescence-activated cell sorting; Jeske Smink for critical comments on the manuscript; and Janett Tischer, Silke Kurths, and Ingrid Berger for excellent technical assistance.
Footnotes
Published ahead of print on 22 February 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Bandara, L. R., V. M. Buck, M. Zamanian, L. H. Johnston, and N. B. La Thangue. 1993. Functional synergy between DP-1 and E2F-1 in the cell cycle-regulating transcription factor DRTF1/E2F. EMBO J. 12:4317-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Begay, V., J. Smink, and A. Leutz. 2004. Essential requirement of CCAAT/enhancer binding proteins in embryogenesis. Mol. Cell. Biol. 24:9744-9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bereshchenko, O., E. Mancini, S. Moore, D. Bilbao, R. Mansson, S. Luc, A. Grover, S. E. Jacobsen, D. Bryder, and C. Nerlov. 2009. Hematopoietic stem cell expansion precedes the generation of committed myeloid leukemia-initiating cells in C/EBPα mutant AML. Cancer Cell 16:390-400. [DOI] [PubMed] [Google Scholar]
- 4.Bracken, A. P., M. Ciro, A. Cocito, and K. Helin. 2004. E2F target genes: unraveling the biology. Trends Biochem. Sci. 29:409-417. [DOI] [PubMed] [Google Scholar]
- 5.Chen, P. L., D. J. Riley, Y. Chen, and W. H. Lee. 1996. Retinoblastoma protein positively regulates terminal adipocyte differentiation through direct interaction with C/EBPs. Genes Dev. 10:2794-2804. [DOI] [PubMed] [Google Scholar]
- 6.Chen, S. S., J. F. Chen, P. F. Johnson, V. Muppala, and Y. H. Lee. 2000. C/EBPβ, when expressed from the C/EBPα gene locus, can functionally replace C/EBPα in liver but not in adipose tissue. Mol. Cell. Biol. 20:7292-7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Classon, M., and N. Dyson. 2001. p107 and p130: versatile proteins with interesting pockets. Exp. Cell Res. 264:135-147. [DOI] [PubMed] [Google Scholar]
- 8.Classon, M., B. K. Kennedy, R. Mulloy, and E. Harlow. 2000. Opposing roles of pRB and p107 in adipocyte differentiation. Proc. Natl. Acad. Sci. U. S. A. 97:10826-10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Alo, F., L. M. Johansen, E. A. Nelson, H. S. Radomska, E. K. Evans, P. Zhang, C. Nerlov, and D. G. Tenen. 2003. The amino terminal and E2F interaction domains are critical for C/EBPα-mediated induction of granulopoietic development of hematopoietic cells. Blood 102:3163-3171. [DOI] [PubMed] [Google Scholar]
- 10.Dannenberg, J. H., A. van Rossum, L. Schuijff, and H. te Riele. 2000. Ablation of the retinoblastoma gene family deregulates G1 control causing immortalization and increased cell turnover under growth-restricting conditions. Genes Dev. 14:3051-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fajas, L., R. L. Landsberg, Y. Huss-Garcia, C. Sardet, J. A. Lees, and J. Auwerx. 2002. E2Fs regulate adipocyte differentiation. Dev. Cell 3:39-49. [DOI] [PubMed] [Google Scholar]
- 12.Flodby, P., C. Barlow, H. Kylefjord, L. Ahrlund-Richter, and K. G. Xanthopoulos. 1996. Increased hepatic cell proliferation and lung abnormalities in mice deficient in CCAAT/enhancer binding protein alpha. J. Biol. Chem. 271:24753-24760. [DOI] [PubMed] [Google Scholar]
- 13.Frohling, S., and H. Dohner. 2004. Disruption of C/EBPα function in acute myeloid leukemia. N. Engl. J. Med. 351:2370-2372. [DOI] [PubMed] [Google Scholar]
- 14.Gombart, A. F., W. K. Hofmann, S. Kawano, S. Takeuchi, U. Krug, S. H. Kwok, R. J. Larsen, H. Asou, C. W. Miller, D. Hoelzer, and H. P. Koeffler. 2002. Mutations in the gene encoding the transcription factor CCAAT/enhancer binding protein alpha in myelodysplastic syndromes and acute myeloid leukemias. Blood 99:1332-1340. [DOI] [PubMed] [Google Scholar]
- 15.Helin, K., C. L. Wu, A. R. Fattaey, J. A. Lees, B. D. Dynlacht, C. Ngwu, and E. Harlow. 1993. Heterodimerization of the transcription factors E2F-1 and DP-1 leads to cooperative transactivation. Genes Dev. 7:1850-1861. [DOI] [PubMed] [Google Scholar]
- 16.Iakova, P., S. S. Awad, and N. A. Timchenko. 2003. Aging reduces proliferative capacities of liver by switching pathways of C/EBPα growth arrest. Cell 113:495-506. [DOI] [PubMed] [Google Scholar]
- 17.Irwin, M., M. C. Marin, A. C. Phillips, R. S. Seelan, D. I. Smith, W. Liu, E. R. Flores, K. Y. Tsai, T. Jacks, K. H. Vousden, and W. G. Kaelin, Jr. 2000. Role for the p53 homologue p73 in E2F-1-induced apoptosis. Nature 407:645-648. [DOI] [PubMed] [Google Scholar]
- 18.Johansen, L. M., A. Iwama, T. A. Lodie, K. Sasaki, D. W. Felsher, T. R. Golub, and D. G. Tenen. 2001. c-Myc is a critical target for C/EBPα in granulopoiesis. Mol. Cell. Biol. 21:3789-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson, P. F. 2005. Molecular stop signs: regulation of cell-cycle arrest by C/EBP transcription factors. J. Cell Sci. 118:2545-2555. [DOI] [PubMed] [Google Scholar]
- 20.Jones, L. C., M. L. Lin, S. S. Chen, U. Krug, W. K. Hofmann, S. Lee, Y. H. Lee, and H. P. Koeffler. 2002. Expression of C/EBPβ from the C/EBPα gene locus is sufficient for normal hematopoiesis in vivo. Blood 99:2032-2036. [DOI] [PubMed] [Google Scholar]
- 21.Keeshan, K., G. Santilli, F. Corradini, D. Perrotti, and B. Calabretta. 2003. Transcription activation function of C/EBPα is required for induction of granulocytic differentiation. Blood 102:1267-1275. [DOI] [PubMed] [Google Scholar]
- 22.Kirstetter, P., M. B. Schuster, O. Bereshchenko, S. Moore, H. Dvinge, E. Kurz, K. Theilgaard-Monch, R. Mansson, T. A. Pedersen, T. Pabst, E. Schrock, B. T. Porse, S. E. Jacobsen, P. Bertone, D. G. Tenen, and C. Nerlov. 2008. Modeling of C/EBPα mutant acute myeloid leukemia reveals a common expression signature of committed myeloid leukemia-initiating cells. Cancer Cell 13:299-310. [DOI] [PubMed] [Google Scholar]
- 23.Koziczak, M., W. Krek, and Y. Nagamine. 2000. Pocket protein-independent repression of urokinase-type plasminogen activator and plasminogen activator inhibitor 1 gene expression by E2F1. Mol. Cell. Biol. 20:2014-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krek, W., M. E. Ewen, S. Shirodkar, Z. Arany, W. G. Kaelin, Jr., and D. M. Livingston. 1994. Negative regulation of the growth-promoting transcription factor E2F-1 by a stably bound cyclin A-dependent protein kinase. Cell 78:161-172. [DOI] [PubMed] [Google Scholar]
- 25.Krek, W., D. M. Livingston, and S. Shirodkar. 1993. Binding to DNA and the retinoblastoma gene product promoted by complex formation of different E2F family members. Science 262:1557-1560. [DOI] [PubMed] [Google Scholar]
- 26.Li, F., E. Rosenberg, C. I. Smith, K. Notarfrancesco, S. R. Reisher, H. Shuman, and S. I. Feinstein. 1995. Correlation of expression of transcription factor C/EBPα and surfactant protein genes in lung cells. Am. J. Physiol. 269:L241-L247. [DOI] [PubMed] [Google Scholar]
- 27.Lin, F. T., and M. D. Lane. 1992. Antisense CCAAT/enhancer-binding protein RNA suppresses coordinate gene expression and triglyceride accumulation during differentiation of 3T3-L1 preadipocytes. Genes Dev. 6:533-544. [DOI] [PubMed] [Google Scholar]
- 28.Loomis, K. D., S. Zhu, K. Yoon, P. F. Johnson, and R. C. Smart. 2007. Genetic ablation of CCAAT/enhancer binding protein alpha in epidermis reveals its role in suppression of epithelial tumorigenesis. Cancer Res. 67:6768-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martis, P. C., J. A. Whitsett, Y. Xu, A. K. Perl, H. Wan, and M. Ikegami. 2006. C/EBPα is required for lung maturation at birth. Development 133:1155-1164. [DOI] [PubMed] [Google Scholar]
- 30.Miller, M., J. D. Shuman, T. Sebastian, Z. Dauter, and P. F. Johnson. 2003. Structural basis for DNA recognition by the basic region leucine zipper transcription factor CCAAT/enhancer-binding protein alpha. J. Biol. Chem. 278:15178-15184. [DOI] [PubMed] [Google Scholar]
- 31.Moll, J. R., A. Acharya, J. Gal, A. A. Mir, and C. Vinson. 2002. Magnesium is required for specific DNA binding of the CREB B-ZIP domain. Nucleic Acids Res. 30:1240-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller, C., M. Alunni-Fabbroni, E. Kowenz-Leutz, X. Mo, M. Tommasino, and A. Leutz. 1999. Separation of C/EBPα-mediated proliferation arrest and differentiation pathways. Proc. Natl. Acad. Sci. U. S. A. 96:7276-7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller, H., A. P. Bracken, R. Vernell, M. C. Moroni, F. Christians, E. Grassilli, E. Prosperini, E. Vigo, J. D. Oliner, and K. Helin. 2001. E2Fs regulate the expression of genes involved in differentiation, development, proliferation, and apoptosis. Genes Dev. 15:267-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nerlov, C. 2004. C/EBPα mutations in acute myeloid leukaemias. Nat. Rev. Cancer 4:394-400. [DOI] [PubMed] [Google Scholar]
- 35.Nevins, J. R. 2001. The Rb/E2F pathway and cancer. Hum. Mol. Genet. 10:699-703. [DOI] [PubMed] [Google Scholar]
- 36.Pabst, T., B. U. Mueller, N. Harakawa, C. Schoch, T. Haferlach, G. Behre, W. Hiddemann, D. E. Zhang, and D. G. Tenen. 2001. AML1-ETO downregulates the granulocytic differentiation factor C/EBPα in t(8;21) myeloid leukemia. Nat. Med. 7:444-451. [DOI] [PubMed] [Google Scholar]
- 37.Pabst, T., B. U. Mueller, P. Zhang, H. S. Radomska, S. Narravula, S. Schnittger, G. Behre, W. Hiddemann, and D. G. Tenen. 2001. Dominant-negative mutations of CEBPA, encoding CCAAT/enhancer binding protein-alpha (C/EBPα), in acute myeloid leukemia. Nat. Genet. 27:263-270. [DOI] [PubMed] [Google Scholar]
- 38.Perrotti, D., V. Cesi, R. Trotta, C. Guerzoni, G. Santilli, K. Campbell, A. Iervolino, F. Condorelli, C. Gambacorti-Passerini, M. A. Caligiuri, and B. Calabretta. 2002. BCR-ABL suppresses C/EBPα expression through inhibitory action of hnRNP E2. Nat. Genet. 30:48-58. [DOI] [PubMed] [Google Scholar]
- 39.Picard, F., M. Kurtev, N. Chung, A. Topark-Ngarm, T. Senawong, R. Machado De Oliveira, M. Leid, M. W. McBurney, and L. Guarente. 2004. Sirt1 promotes fat mobilization in white adipocytes by repressing PPARγ. Nature 429:771-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pierce, A. M., I. B. Gimenez-Conti, R. Schneider-Broussard, L. A. Martinez, C. J. Conti, and D. G. Johnson. 1998. Increased E2F1 activity induces skin tumors in mice heterozygous and nullizygous for p53. Proc. Natl. Acad. Sci. U. S. A. 95:8858-8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polager, S., and D. Ginsberg. 2003. E2F mediates sustained G2 arrest and down-regulation of Stathmin and AIM-1 expression in response to genotoxic stress. J. Biol. Chem. 278:1443-1449. [DOI] [PubMed] [Google Scholar]
- 42.Porse, B. T., D. Bryder, K. Theilgaard-Monch, M. S. Hasemann, K. Anderson, I. Damgaard, S. E. Jacobsen, and C. Nerlov. 2005. Loss of C/EBPα cell cycle control increases myeloid progenitor proliferation and transforms the neutrophil granulocyte lineage. J. Exp. Med. 202:85-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porse, B. T., T. A. Pedersen, X. Xu, B. Lindberg, U. M. Wewer, L. Friis-Hansen, and C. Nerlov. 2001. E2F repression by C/EBPα is required for adipogenesis and granulopoiesis in vivo. Cell 107:247-258. [DOI] [PubMed] [Google Scholar]
- 44.Ren, B., H. Cam, Y. Takahashi, T. Volkert, J. Terragni, R. A. Young, and B. D. Dynlacht. 2002. E2F integrates cell cycle progression with DNA repair, replication, and G2/M checkpoints. Genes Dev. 16:245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruiz, S., M. Santos, M. F. Lara, C. Segrelles, C. Ballestin, and J. M. Paramio. 2005. Unexpected roles for pRb in mouse skin carcinogenesis. Cancer Res. 65:9678-9686. [DOI] [PubMed] [Google Scholar]
- 46.Sage, J., G. J. Mulligan, L. D. Attardi, A. Miller, S. Chen, B. Williams, E. Theodorou, and T. Jacks. 2000. Targeted disruption of the three Rb-related genes leads to loss of G1 control and immortalization. Genes Dev. 14:3037-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheich, C., D. Kummel, D. Soumailakakis, U. Heinemann, and K. Bussow. 2007. Vectors for coexpression of an unrestricted number of proteins. Nucleic Acids Res. 35:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sebastian, T., R. Malik, S. Thomas, J. Sage, and P. F. Johnson. 2005. C/EBPβ cooperates with RB:E2F to implement Ras(V12)-induced cellular senescence. EMBO J. 24:3301-3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shao, D., and M. A. Lazar. 1997. Peroxisome proliferator activated receptor gamma, CCAAT/enhancer-binding protein alpha, and cell cycle status regulate the commitment to adipocyte differentiation. J. Biol. Chem. 272:21473-21478. [DOI] [PubMed] [Google Scholar]
- 50.Slomiany, B. A., K. L. D'Arigo, M. M. Kelly, and D. T. Kurtz. 2000. C/EBPα inhibits cell growth via direct repression of E2F-DP-mediated transcription. Mol. Cell. Biol. 20:5986-5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sterneck, E., C. Muller, S. Katz, and A. Leutz. 1992. Autocrine growth induced by kinase type oncogenes in myeloid cells requires AP-1 and NF-M, a myeloid specific, C/EBP-like factor. EMBO J. 11:115-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang, Q. Q., J. W. Zhang, and M. D. Lane. 2004. Sequential gene promoter interactions by C/EBPβ, C/EBPα, and PPARγ during adipogenesis. Biochem. Biophys. Res. Commun. 318:213-218. [DOI] [PubMed] [Google Scholar]
- 53.Trivedi, A. K., P. Pal, G. Behre, and S. M. Singh. 2008. Multiple ways of C/EBPα inhibition in myeloid leukaemia. Eur. J. Cancer 44:1516-1523. [DOI] [PubMed] [Google Scholar]
- 54.Truong, B. T., Y. J. Lee, T. A. Lodie, D. J. Park, D. Perrotti, N. Watanabe, H. P. Koeffler, H. Nakajima, D. G. Tenen, and S. C. Kogan. 2003. CCAAT/Enhancer binding proteins repress the leukemic phenotype of acute myeloid leukemia. Blood 101:1141-1148. [DOI] [PubMed] [Google Scholar]
- 55.Wang, D., J. Russell, H. Xu, and D. G. Johnson. 2001. Deregulated expression of DP1 induces epidermal proliferation and enhances skin carcinogenesis. Mol. Carcinog. 31:90-100. [DOI] [PubMed] [Google Scholar]
- 56.Wang, N. D., M. J. Finegold, A. Bradley, C. N. Ou, S. V. Abdelsayed, M. D. Wilde, L. R. Taylor, D. R. Wilson, and G. J. Darlington. 1995. Impaired energy homeostasis in C/EBPα knockout mice. Science 269:1108-1112. [DOI] [PubMed] [Google Scholar]
- 57.Wang, X., H. A. Pasolli, T. Williams, and E. Fuchs. 2008. AP-2 factors act in concert with Notch to orchestrate terminal differentiation in skin epidermis. J. Cell Biol. 183:37-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiseman, T., S. Williston, J. F. Brandts, and L. N. Lin. 1989. Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Anal. Biochem. 179:131-137. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, D. E., P. Zhang, N. D. Wang, C. J. Hetherington, G. J. Darlington, and D. G. Tenen. 1997. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein alpha-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 94:569-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu, S., K. Yoon, E. Sterneck, P. F. Johnson, and R. C. Smart. 2002. CCAAT/enhancer binding protein-beta is a mediator of keratinocyte survival and skin tumorigenesis involving oncogenic Ras signaling. Proc. Natl. Acad. Sci. U. S. A. 99:207-212. [DOI] [PMC free article] [PubMed] [Google Scholar]