Abstract
Neuronal outgrowth occurs via coordinated remodeling of the cytoskeleton involving both actin and microtubules. Microtubule stabilization drives the extending neurite, yet little is known of the molecular mechanisms whereby extracellular cues regulate microtubule dynamics. Bone morphogenetic proteins (BMPs) play an important role in neuronal differentiation and morphogenesis, and BMP7 in particular induces the formation of dendrites. Here, we show that BMP7 induces stabilization of microtubules in both a MAP2-dependent neuronal cell culture model and in dendrites of primary cortical neurons. BMP7 rapidly activates c-Jun N-terminal kinases (JNKs), known regulators of microtubule dynamics, and we show that JNKs associate with the carboxy terminus of the BMP receptor, BMPRII. Activation and binding of JNKs to BMPRII is required for BMP7-induced microtubule stabilization and for BMP7-mediated dendrite formation in primary cortical neurons. These data indicate that BMPRII acts as a scaffold to localize and coordinate cytoskeletal remodeling and thereby provides an efficient means for extracellular cues, such as BMPs, to control neuronal dendritogenesis.
Neurons are highly polarized cells comprised of a single long axon, which transmits signals, and multiple shorter dendrites, which are specialized to receive signals. This polarized property of neurons allows for the establishment of appropriate connections within the central nervous system and ensures unidirectional signal propagation (15, 52). Dendrite formation is critical for normal mammalian brain function, including cognition and memory formation, and dendritic abnormalities closely correlate with mental retardation and underlie the pathology of a number of central nervous system disorders, including Down's, Rett, and Fragile X syndromes and lissencephalies (28, 30). Although the importance of dendrite morphology is easily appreciated, the signaling mechanisms by which dendrites develop have only recently begun to be identified (2, 4, 5).
Neurite outgrowth occurs as a result of coordinated remodeling of the neuronal cytoskeleton involving actin and microtubules (25). In general, neurite outgrowth occurs as a result of local actin instability in the growth cone and coordinated microtubule stabilization that allows microtubules to protrude their dynamic ends more distally (52). Extracellular growth factors have the ability to regulate the cytoskeleton by activating signaling pathways that change the activity, localization, and stability of cytoskeletal regulators (16). However, the molecular mechanisms by which extracellular factors modulate microtubule stability during dendrite formation remain unclear. c-Jun N-terminal kinase (JNK) is a member of the mitogen-activated protein kinase family with a well-documented role in the regulation of gene transcription, cell death, and survival (9, 50). In the nervous system, JNKs have essential functions in the brain during development, neurite formation, regeneration, and memory formation (27, 47). Moreover, accumulating evidence now suggests that JNK is directly involved in the regulation of the cytoskeleton, particularly in maintaining the stability of microtubules by controlling the phosphorylation of microtubule-associated proteins (MAPs). Indeed, JNK1−/− mice display a decrease in MAP1B and MAP2 phosphorylation and microtubule stability (8, 12) and, in cultured neurons, JNK-dependent phosphorylation of SCG10 and doublecortin (DCX) contributes to neurite formation (24, 44).
Bone morphogenetic proteins (BMPs) are members of the transforming growth factor β (TGF-β) superfamily of ligands that are crucial in numerous steps during the differentiation and morphogenesis of the central and peripheral nervous system in vertebrates (33, 40). BMP7 signaling in particular induces the formation of dendrites in cultured sympathetic, cerebral cortical, and hippocampal neurons (29, 39, 51). BMPs transduce signals by binding type I and type II Ser/Thr kinase receptors and regulate transcription through the intracellular signaling mediators, Smads (3, 22). The BMP type II receptor (BMPRII) in vertebrates and the Drosophila ortholog, Wit, are unique among the receptors, being comprised of a long carboxy-terminal extension that is dispensable for Smad signaling (1, 22, 36). Mice lacking BMPRII display embryonic lethality prior to gastrulation (7), and mutations in the carboxy-terminal tail region are associated with primary pulmonary hypertension, indicating a function in the vascular system (18, 19). In addition, a role for this receptor in neurons has been well established. BMPRII is highly expressed in brain-derived tissues (13) and is localized to the tips of cortical dendrites (32). The BMPRII carboxy-terminal tail is particularly important for neuronal activity and has been shown to be essential for BMP7-dependent dendrite outgrowth in cortical neurons (32), nerve growth cone steering of Xenopus spinal neurons (49), and for synaptic stability in Drosophila (21). LIM kinase 1 (LIMK1), which phosphorylates the actin depolymerizing factor (ADF)/cofilin, binds to a region of the BMPRII tail to mediate BMP7-induced remodeling of the actin cytoskeleton and dendrite formation (23, 32).
Neurite outgrowth requires remodeling of both actin and microtubules, but whether BMPs regulate microtubule network dynamics is unknown. We demonstrate here that BMP7 activates JNK and thereby induces microtubule stabilization in neuronal cells. Moreover, we show that JNK binds to the carboxy-terminal tail of BMPRII and that this binding is required both for microtubule stabilization and for BMP7-induced dendritogenesis in primary cortical neurons. Given that BMPRII also binds the actin regulator, LIMK1, these findings indicate that BMPRII-mediated scaffolding of diverse cytoskeletal regulators serves to localize both actin and microtubule remodeling complexes, thereby facilitating BMP-dependent dendrite formation.
MATERIALS AND METHODS
Dendritogenesis in primary cortical neurons.
Primary cortical neurons were isolated and infected with recombinant adenoviruses as previously described (32). For JNK inhibitor studies, cells were transfected with a green fluorescent protein (GFP)-expressing plasmid using Lipofectamine 2000 (Invitrogen) at 3 days in vitro (DIV). At 24 h posttransfection, neuronal cultures were treated with either a chemical JNK inhibitor, SP600125 (Calbiochem), at 20 μM or a cell-permeable peptide inhibitor of JNK, L-JNKI1 (Alexis Biochemicals), at 0.5 and 0.75 μM and cultured in the presence or absence of BMP7 for 48 h. The number of primary dendrites in GFP-expressing neurons that costained with Map2 (a+b) antibody, which recognizes the Map2 isoforms specifically expressed in dendrites, was quantitated by blinded counting of at least 20 neurons per treatment condition.
Immunofluorescence microscopy and quantitation.
Primary cortical neurons were fixed as previously described (32). Cells were analyzed with a Zeiss inverted microscope equipped with fluorescence optics. Images of neurons were taken with a charge-coupled device camera (Hamamatsu Photonic Systems) and analyzed with Velocity imaging software (Improvision). For nuclear Smad accumulation, infected neurons were stained with polyclonal phospho-Smad1,5,8 (Cell Signaling) antibodies. For BMP-dependent JNK activation, neurons were stained with polyclonal phospho-JNK1,2 (Biosource) antibodies, imaged with a Leica TCS Sp2 confocal microscope equipped with fluorescence optics and multi-photon lasers (Spectral Physics, California), and analyzed with Leica TCS confocal software. The levels of P-JNK and JNK at the tips of dendrites were determined using Velocity (Improvision) by measuring pixel intensities of images immunostained with anti-phospho-JNK1,2 (Biosource) and anti-JNK1,2 (BD Biosciences) antibodies. The pixel intensities were then divided by the median calculated from the values determined for all dendrites in cells cultured in the absence of BMP7. Dendritic tips were then divided into categories based on the fold difference over the value of the median. The percentage of dendrite tips in each category is plotted as a percentage of the total.
Constructs and vectors.
BMPRII and its mutant derivatives were subcloned into pCMV5 for mammalian cell work, into pGEX4T1 for bacterial expression, and into pAdTrack-CMV shuttle vector containing GFP (Q-Biogene) for adenoviral infections. All constructs were generated by PCR or were previously described (32). BMPRII and ALK2 were Renilla luciferase-tagged for LUMIER. Flag-JNK1, Flag-JNK2, Flag-JNK3, and Flag-LIMK1 were subcloned into pCMV5 from previously described vectors (32, 37). GFP-MAP2 was previously described (8).
Biochemical assays and antibodies.
Immunoprecipitation, immunoblotting and glutathione S-transferase (GST) pulldown assays were conducted as described previously (20, 31). Antibodies used were anti-JNK (Cell Signaling, catalog no. 9252), anti-phospho-JNK (Cell Signaling, catalog no. 9251), and anti-Actin (Sigma). Analysis of interactions between Renilla luciferase and Flag-tagged proteins was performed using manual LUMIER (6, 38, 41, 45).
MAP2-dependent protrusion formation in N1E115 cells.
N1E115 mouse neuroblastoma cells were transfected with GFP-MAP2 using Turbofect (Fermentas), treated with BMP7, and fixed with 4% paraformaldehyde in phosphate-buffered saline at 24 h after ligand addition. For JNK inhibitor studies, cells were pretreated with a chemical JNK inhibitor, SP600125 (Calbiochem), at 5 μM or a cell-permeable peptide inhibitor of JNK, L-JNKI1 (Alexis Biochemicals), at 2 and 5 μM for 30 min prior to BMP7 addition.
Microtubule stabilization assays in corticals.
Primary cortical neurons were incubated with nocodazole (Sigma) at 5 μM in the presence or absence of BMP7 for 1 h. The cells were then fixed with methanol at −20°C and stained with monoclonal anti-Ace-tubulin (Chemicon) and anti-Tyr-tubulin (Sigma) antibodies, which stain stable and dynamic MT, respectively. For quantification of BMP-dependent microtubule stabilization, the length of stable microtubules in dendrites as detected by anti-Ace-tubulin antibodies was expressed as a percentage of the total length of dendritic microtubules as detected by anti-Tyr-tubulin antibodies. For cortical neurons infected with BMPRII adenoviral constructs coexpressing GFP, cells were fixed with 4% paraformaldehyde in phosphate-buffered saline prior to incubation with −20°C methanol and then stained with anti-Ace-tubulin (Sigma) antibodies. The length of stable microtubules (Ace MT) in dendrites was expressed as a percentage of total dendrite length (GFP staining).
RESULTS
BMP7 enhances microtubule stability in dendrites of primary cortical neurons.
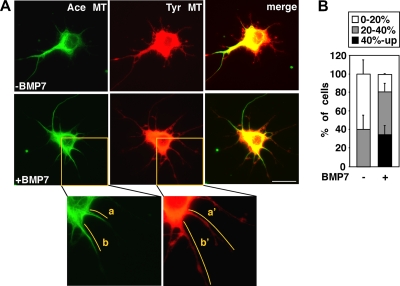
Neurite outgrowth requires coordinated remodeling of the actin and microtubule cytoskeleton (25). BMP signaling induces dendrite formation in primary neurons, and we showed previously that BMP7 modulates the actin cytoskeleton (32). Since microtubules are key architectural components of dendrites and are important for neurite formation (15), we sought to determine whether BMP signaling might also regulate the microtubule cytoskeleton. Dendritic microtubules of neuronal cells are intrinsically stable; thus, we incubated cortical cultures with the microtubule destabilizing agent, nocodazole, in the presence or absence of BMP7 for 1 h and then measured the extent of stabilized microtubules. Microtubules were visualized by immunostaining with antibodies to acetylated tubulin (Ace MT) to detect the stabilized microtubules and with antibodies to tyrosinated tubulin (Tyr MT) to detect total length of microtubules (Fig. 1A). The percentage of stable microtubules in dendrites was then determined by expressing the total length of stable microtubules as a fraction of total microtubule length in all dendritic protrusions. In nocodazole-treated cells coincubated with BMP7, an increase in microtubule stabilization in cortical dendrites was observed, with more than 35% of cells displaying a high degree of stabilized microtubules (40% and up) compared to 0% of cells treated only with nocodazole (Fig. 1B). No change in the total length of microtubules (Tyr MT) in cells coincubated with BMP7 was observed (data not shown). Thus, these results show that BMP7 signaling can rapidly promote microtubule stabilization in dendritic protrusions of cortical neurons.
FIG. 1.
BMP7 affects microtubule stability in dendrites of primary cortical neurons. (A) Primary cortical neurons were incubated with nocodazole (5 μM) in the presence or absence of BMP7 for 1 h. The microtubules were visualized by immunofluorescence microscopy using antibodies to acetylated (Ace MT) and tyrosinated (Tyr MT) microtubules. As seen in the inset images, the length of stable microtubules in dendrites (a and b) was expressed as a percentage of total MT length (a′ and b′). Scale bar, 20 μm. (B) The percentage of cells in each category based on stable MT content (40%-up, 20 to 40% or 0 to 20%) is plotted as a percentage of total. Shown are the means ± the standard errors of the mean (SEM) of three independent experiments with at least 20 cells analyzed per condition (P < 0.05 [Student t test for the 40% and up category]).
BMP7 promotes MAP2-dependent protrusion formation in N1E115 cells.
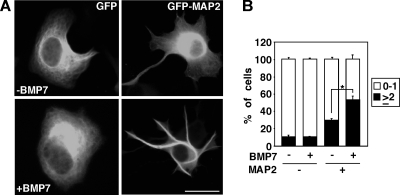
N1E115 cells are mouse neuroblastoma cells that are sensitive to BMPs, as measured by induction of Smad1 phosphorylation (data not shown), but do not form neurites in response to BMP7. MAPs bind to and regulate microtubule dynamics (8, 12, 24, 44) and overexpression of MAP2 induces neurite-like extensions in cells that otherwise do not form any processes (11, 43). Thus, we next examined the effect of BMP7 on MAP2-dependent protrusion formation in N1E115 cells. Consistent with previous reports (11, 43), cells expressing GFP-MAP2 display an increase in protrusion formation (Fig. 2A), and these protrusions are comprised of stabilized microtubules (see Fig. S1 in the supplemental material). Upon BMP7 stimulation, the number of protrusions/cell increased, and quantitation of the number of GFP-MAP2-positive processes revealed that the number of protrusions was enhanced with 54% of BMP7-treated cells with two or more protrusions versus 30% in controls (Fig. 2B). These results indicate that BMP7 can induce microtubule stabilization that results in MAP2-dependent protrusion formation and suggests the possibility that BMP7 may signal to MAP2 to mediate this effect.
FIG. 2.
BMP7 induces MAP2-dependent protrusion formation in N1E115 cells. (A) N1E115 cells transfected with GFP or MAP2-GFP were incubated in the presence or absence of BMP7 for 24 h. Scale bar, 50 μm. (B) The percentage of cells in each category based on the number of protrusions formed (0 to 1 and ≥2 protrusions) is plotted as a percentage of the total. Shown are the means ± the SEM of three independent experiments with at least 20 MAP2-negative and 70 MAP2-positive cells analyzed per condition (P < 0.001 [Student t test]).
BMP7-induced JNK activation is required for MAP2-dependent protrusion formation in N1E115 cells.
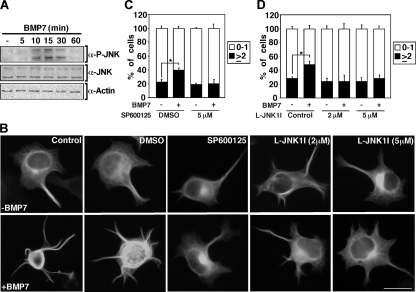
Phosphorylation of MAPs plays a key role in regulating microtubule dynamics (8, 12, 24, 44) and JNKs are cytoskeletal regulators that control the stability of microtubules by regulating the phosphorylation of MAPs (8, 12, 24, 44). Previous reports have demonstrated that the induction of neurite-like extensions in MAP2 overexpression models is enhanced upon JNK activation (8). Thus, we next sought to determine whether the activity of JNKs is regulated by BMP7 in N1E115 cells. Consistent with this possibility, immunoblotting of total cell lysates revealed that treatment of cells with BMP7 increased endogenous JNK phosphorylation as early as 10 min after ligand addition, and this remained elevated for up to 30 min (Fig. 3A). Given that BMP7 signaling activates JNK, we next determined whether this activation is required for BMP7-induced MAP2-dependent protrusion formation. For this, we used a chemical JNK inhibitor, SP600125, and a cell-permeable peptide comprised of the JNK binding domain of JIP-1, L-JNKI1 (10). This analysis revealed that the JNK inhibitors abolished BMP7-induced MAP2-dependent protrusion formation (Fig. 3B to D). Collectively, these results indicate that BMP7 can rapidly activate JNK and that JNK activation is required for BMP7 induced MAP2-dependent protrusion formation in N1E115 cells.
FIG. 3.
BMP7-induced JNK activation is required for MAP2-dependent protrusion formation in N1E115 cells. (A) BMP7 induces endogenous JNK activation in N1E115 cells. The levels of phosphorylated JNK were determined in N1E115 cell lysates treated with BMP7 at various times and visualized by immunoblotting with anti-phospho-JNK1,2 antibody. Total levels of JNK and actin were determined by immunoblotting. (B to D) JNK inhibition blocks BMP7 induced MAP2-dependent protrusion formation in N1E115 cells. N1E115 cells transfected with MAP2-GFP were treated with JNK inhibitors, SP600125 at 5 μM in dimethyl sulfoxide (DMSO) (C) or L-JNK1I at 2 and 5 μM (D), incubated in the presence or absence of BMP7 for 24 h, and analyzed as shown in Fig. 2B. Shown are the means ± the SEM of two (C) and three (D) independent experiments with at least 50 cells analyzed per condition (P < 0.05 [Student t test for both panels C and D]). Scale bar, 50 μm.
BMP7 induces JNK activation in primary cortical neurons.
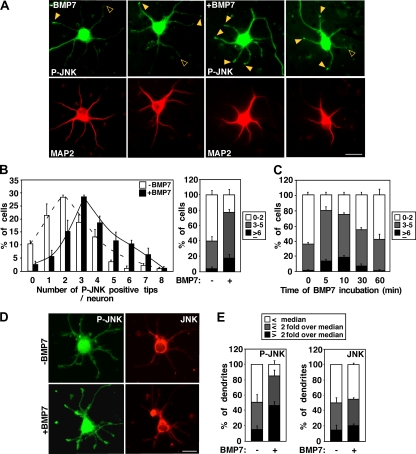
BMP7 induces dendrite formation in primary neurons (29, 39, 51) and can activate JNKs in N1E115 cells; thus, we next investigated whether JNKs have a role in BMP-dependent dendritogenesis. For this, we first determined whether BMP7 treatment alters JNK phosphorylation and/or subcellular distribution in embryonic cortical neurons by immunofluorescence microscopy. The cells were counterstained with a Map2 (a+b) antibody, which recognizes Map2 isoforms specifically expressed in dendrites. In control cells, phospho-JNK staining was occasionally observed in the tips of dendrites (Fig. 4A). However, in cells treated with BMP7 for 5 min, an increase in phospho-JNK staining at dendrite tips was observed. Quantitation of Map2 (a+b)-positive dendrites revealed that BMP7-treated cells have an increased number of phospho-JNK-positive tips. Specifically, 77% of BMP7-treated cells contained three to eight phospho-JNK-positive tips compared to 40% of untreated cells (Fig. 4B, right). Time course analysis demonstrated that BMP-dependent JNK activation peaked at 10 min after ligand addition (Fig. 4C). Thus, JNK is subject to rapid activation by BMP7 in primary cortical neurons and activated JNK can be detected in the tips of neurites of primary neurons. We also examined BMP-induced JNK activation in dendritic tips of cells that were costained with antibodies to detect total JNK. The levels of P-JNK and JNK at the tips of dendrites were quantitated from immunostained images (Fig. 4D), and the fold change over the median in control cultures was determined. This analysis revealed that levels of P-JNK at dendritic tips increased, with over 45% of BMP7-treated cells having a P-JNK intensity 2-fold higher than the median versus 15% in controls (Fig. 4E). In contrast, the levels of total JNK were unchanged (Fig. 4E). These results are consistent with a model in which BMP7 induces activation of localized JNK rather than promoting JNK recruitment to dendrite tips.
FIG. 4.
BMP7-induced JNK activation in primary cortical neurons is required for dendrite formation. (A) BMP7 activates endogenous JNK at the tips of dendrites. Primary cortical neurons were incubated with BMP7 for 5 min (A and B) or for 5, 10, 30, and 60 min (C), and the localization of phosphorylated JNK was visualized by immunofluorescence microscopy using phospho-JNK1,2 primary antibody and Alexa Fluor 488-conjugated secondary antibody. Representative P-JNK positive and negative tips are indicated with solid and open arrowheads, respectively. Map2 (a+b) (to mark dendrites) was detected using Map2 primary antibody and Alexa Fluor 546 secondary antibody. Scale bar, 20 μm. (B and C) The number of phospho-JNK-positive neurites in each MAP2-positive neuron was determined and is plotted as a percentage of total. Shown are the means ± the SEM of three independent experiments with 40 to 50 neurons analyzed per condition (P < 0.05 and < 0.01 [Student t test] for the ≥6 category for panels B, right, and C, 0 to 5 min, respectively). (D and E) BMP7 enhances P-JNK but not total JNK levels at the tips of dendrites. Primary cortical neurons were incubated with BMP7 for 10 min and phosphorylated, and total JNK was visualized by immunofluorescence microscopy using anti-phospho-JNK1,2 and anti-JNK1,2 antibodies. The levels of P-JNK and JNK at the tips of dendrites were quantitated by measuring pixel intensities of immunostained images, and the fold change over the median of all dendrites analyzed from the cultures maintained in the absence of BMP7 was determined. Dendritic tips were divided into categories based on the fold differences over the value of the median: ≤ the median, ≤2-fold over median, and >2-fold over median. The percentage of dendrite tips in each category is plotted as a percentage of total. Shown are the means ± the SEM of two independent experiments with at least 20 cells analyzed (P < 0.05 [Student t test] for the >2-fold over the median category in P-JNK). Scale bar, 20 μm.
BMP7-induced JNK activation in primary cortical neurons is required for dendrite formation.
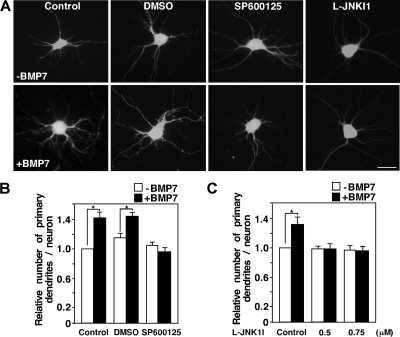
To determine whether JNK was required for BMP7-dependent dendrite formation, we examined the effects of JNK inhibitors, SP600125 and L-JNK1I, in primary mouse cortical neurons. For this, cells were transfected with a GFP encoding adenoviral vector to facilitate the visualization of dendrites (Fig. 5A) and dendrite formation determined in GFP-positive cells counterstained with Map2 (a+b) antibody (data not shown). In cultures stimulated with BMP7, we observed a statistically significant (P < 0.0001) increase of 30 to 40% in the number of dendrites formed compared to control cells, a finding similar to our previous observations (32). However, in cells incubated with the JNK inhibitors, BMP-dependent dendrite formation was completely abolished (Fig. 5B and C). Overall, these results indicate that BMP7 rapidly activates JNK and that JNK activity is required for BMP-dependent dendrite formation. Given that JNK activation is required for BMP-induced protrusion formation in a MAP2-dependent model of microtubule stabilization, altogether our results suggest that JNKs play a key role in mediating BMP7-induced microtubule stabilization in established cell lines and in primary neurons.
FIG. 5.
Inhibition of JNK blocks BMP-dependent dendrite formation. (A) Primary cortical neurons expressing GFP were incubated with the JNK inhibitors, SP600125 at 20 μM in dimethyl sulfoxide (DMSO) or L-JNK1I, at 0.5 and 0.75 μM in the presence or absence of BMP7 for 48 h after 4 DIV. The number of dendrites per neuron in GFP-positive cells (A) that costained with the dendrite-specific Map2 (a+b) antibody (not shown) was determined. (B and C) Fold changes in dendrite numbers relative to the unstimulated GFP empty control are shown with 30 to 40 neurons analyzed per condition. Shown are the means ± the SEM of three (B) and two (C) independent experiments (P < 0.0001 and P < 0.05 [Student t test] for panels B and C, respectively). Scale bar, 20 μm.
JNK associates with the tail of BMP type II receptor, BMPRII, via two JNK binding regions, JBR-A and JBR-B.
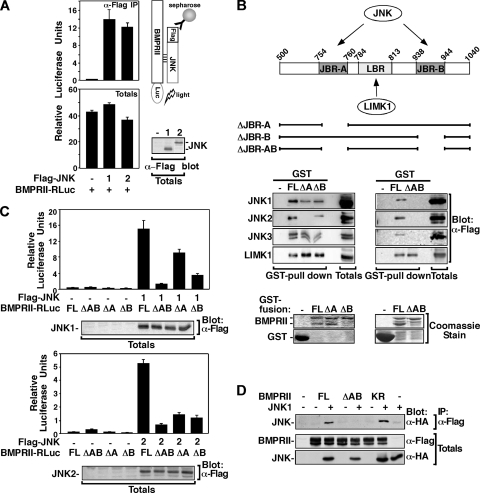
To better understand how JNKs might mediate BMP effects, we sought to determine where in the BMP pathway JNKs might act. BMPs initiate signaling by binding and inducing formation of type I and type II Ser/Thr kinase receptors (3, 22). The BMP type II receptor, BMPRII, has a 512-amino-acid carboxy-terminal tail downstream of the kinase domain, and we previously showed that this tail is required for BMP7-induced dendrite formation (32). Using a yeast-two-hybrid screen with JNK as bait, we identified the tail of BMPRII as an interacting partner (data not shown), suggesting that JNK binding to BMP receptors may be linked to the requirement for JNK in BMP-induced dendritogenesis. To examine this possibility, we first determined whether JNK physically interacts with BMPRII in mammalian cells using the LUMIER method (6, 38, 41, 45). Lysates from COS-1 cells transfected with Flag-JNK and BMPRII-Renilla luciferase (BMPRII-RLuc) were subjected to anti-Flag immunoprecipitation, and associated BMPRII-RLuc was detected by measuring luciferase activity. An interaction between BMPRII and all three JNK isoforms, JNK1, JNK2, and JNK3 was observed (Fig. 6A and B and data not shown).
FIG. 6.
JNK, associates with the tail of BMP type II receptor, BMPRII via two JNK binding regions, JBR-A and JBR-B. (A) Cell lysates from COS-1 cells transfected with Flag-JNK1,2 and BMPRII-Renilla luciferase (BMPRII-RLuc) were subjected to anti-Flag immunoprecipitation, and associated BMPRII-RLuc was detected by measuring the luciferase activity. Total BMPRII-RLuc levels were measured from total cell lysates. (B) A schematic representation of GST fusion constructs of the BMPRII tail is shown. Cell lysates from COS-1 cells transiently transfected with Flag-JNK1,2,3 or LIMK1-Flag were incubated with bacterially expressed GST fusion proteins encoding various BMPRII deletions. Interactions were visualized by anti-Flag immunoblotting. Levels of GST fusion proteins were confirmed by Coomassie blue staining (bottom). (C) Cell lysates from COS-1 cells transfected with Flag-JNK1 (top) or Flag-JNK2 (bottom) and BMPRII-RLuc full length (FL), ΔJBR-AB, ΔJBR-A, and ΔJBR-B, were subjected to anti-Flag immunoprecipitation, and associated BMPRII-RLuc was detected by measuring luciferase activity. The data were corrected to total BMPRII-RLuc levels measured from total cell lysates. The total levels of Flag-JNK were visualized by anti-Flag immunoblotting. (D) Cell lysates from COS-1 cells transfected with HA-JNK1 and BMPRII-Flag full length (FL), ΔAB, or kinase dead (KR) were subjected to anti-Flag immunoprecipitation, and associated JNK1 was detected by anti-HA immunoblotting.
Analysis of the tail for consensus JNK binding sites (9) identified two putative JNK binding regions (JBR) spanning amino acids 754 to 760 and amino acids 938 to 944, encoding RPTSLPL and RPNSLDL, which we termed JBR-A and JBR-B, respectively (Fig. 6B, top). Of note, these pairs of consensus JNK binding sites are conserved in diverse species, including flies (see Fig. S2 in the supplemental material). To determine whether these regions mediate JNK binding to BMPRII, the ability of JNK expressed in COS-1 cells to interact with bacterially expressed BMPRII tail was examined. Deletion of either JBR-A or JBR-B decreased BMPRII interaction with all JNK isoforms tested (Fig. 6B, left), whereas deletion of both JNK binding regions, JBR-AB, completely abolished the interaction between JNK and BMPRII (Fig. 6B, right). In contrast, the interaction of LIMK1, a known BMPRII-tail partner (23, 32), was retained in BMPRII mutants lacking JBR-A, JBR-B, or both (Fig. 5B). JBR-A was located within the region we previously defined as the LIMK1 binding domain (amino acids 751 to 813) (32); however, with further mapping experiments, we refined the LIMK1 binding region (LBR) to amino acids 784 to 813 (Fig. 6B and see Fig. S3A in the supplemental material).
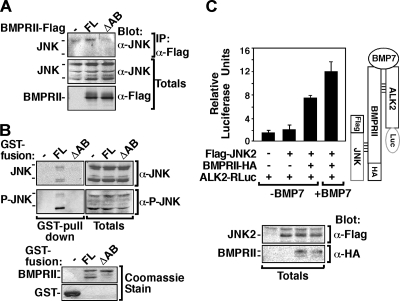
The interaction of JNK with JBR deletion mutants of BMPRII in mammalian cells was also examined in COS-1 cells using the LUMIER method. As expected, we observed a decrease in JNK interaction with BMPRII lacking either JNK binding region JBR-A or JBR-B, and a loss of interaction with the BMPRII mutant lacking both JNK binding regions, JBR-AB (Fig. 6C). Similar results were observed by immunoprecipitation and immunoblotting with HA-tagged JNK1 and Flag-tagged BMPRII (Fig. 6D). Specifically, JNK was immunoprecipitated from COS-1 cells with full-length BMPRII but not with ΔJBR-AB. Of note, the kinase-deficient mutant of BMPRII (BMPRII KR) interacted with JNK, as well as the wild-type receptor (Fig. 6D). Immunoprecipitation of endogenous JNK from COS-1 cells also showed JNK association with full-length BMPRII but not with ΔJBR-AB (Fig. 7A). Moreover, the association of endogenous JNK with the JNK binding regions within the BMPRII tail was also confirmed in the neuroblastoma-derived N1E115 cells by GST pulldown assay (Fig. 7B). Immunoblotting with anti-phospho-JNK antibodies revealed that phosphorylated, and thus activated, JNK was capable of associating with BMPRII (Fig. 7B). Overall, these results demonstrate the presence of a cluster of kinase binding sites in the BMPRII tail, including one for LIMK1, a regulator of the actin cytoskeleton, and two for JNK located on either side of the LIMK1 binding region.
FIG. 7.
JNK binds to the BMP receptor complex via BMPRII. (A) Lysates from COS-1 cells transiently transfected with BMPRII-Flag full length (FL) or BMPRII ΔJBR-AB were subjected to anti-Flag immunoprecipitation, and endogenous JNK was visualized by immunoblotting with anti-JNK1,2 antibody. The levels of total JNK and BMPRII protein levels were detected by immunoblotting. (B) N1E115 cell lysates were incubated with bacterially expressed GST fusion proteins encoding GST alone, BMPRII full length (FL), or BMPRII ΔJBR-AB, and the association of endogenous phosphorylated or total JNK was detected by immunoblotting. Levels of GST fusion proteins were confirmed by Coomassie blue staining (bottom). (C) JNK interacts with the BMP receptor complex. Cell lysates from COS-1 cells transfected with Flag-JNK2, BMPRII-HA, and ALK2-RLuc, were subjected to anti-Flag immunoprecipitation, and associated ALK2-RLuc was detected by measuring the luciferase activity. The data were corrected to total ALK2-RLuc levels measured from total cell lysates. Total expression of Flag- and HA-tagged proteins was determined by immunoblotting as indicated.
Since BMP ligands signal by inducing formation of a heteromeric receptor complex comprised of type I and type II Ser/Thr kinase receptors, we next examined whether JNK can interact with this complex. Lysates from COS-1 cells transfected with Flag-JNK2, BMPRII-HA, and the BMP type I receptor, ALK2 tagged with Renilla luciferase (ALK2-RLuc), were subjected to anti-Flag JNK2 immunoprecipitation, and associated ALK2-RLuc was detected by measuring luciferase activity. This analysis revealed that JNK does not interact with ALK2 when ALK2 is expressed alone (Fig. 7C). However, in cells coexpressing the BMP type II receptor, BMPRII, which forms a complex with ALK2, an association between JNK and ALK2 was observed. Moreover, this interaction was enhanced by BMP7 treatment (Fig. 7C). These results indicate that JNK binds to the carboxy-terminal tail of BMPRII and can remain bound in the context of a BMP7-induced type I and type II containing heteromeric receptor complex.
BMP7-induced dendrite formation and enhancement of microtubule stability is dependent on JNK binding to BMPRII.
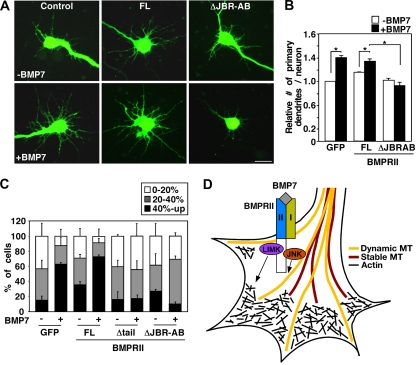
To examine whether the binding of JNK to BMPRII is specifically required for BMP-stimulated dendritogenesis, primary cortical neurons were infected with adenoviruses encoding BMPRII, together with a GFP marker, and dendrite formation was determined in GFP-positive cells (Fig. 8A) counterstained with Map2 (a+b) (data not shown). As previously observed, BMP7 induced an increase of 40% (P < 0.0001) in the number of primary dendrites. Overexpression of wild-type BMPRII slightly increased the basal number of primary dendrites, and this was enhanced upon BMP7 stimulation to a level similar to that in controls. In contrast, expression of the JNK-binding mutant of BMPRII (ΔJBR-AB) completely abolished the BMP7-induced increase in dendrite formation (Fig. 8B). This effect is not due to disruption of Smad1 activation as BMP7-induced Smad1 nuclear accumulation was retained in cortical neurons overexpressing the JNK-binding mutant of BMPRII (ΔJBR-AB) (see Fig. S4 in the supplemental material) and is consistent with previous work showing that the BMPRII tail is dispensable for Smad1-dependent transcriptional responses (34). Thus, BMPRII ΔJBR-AB acts in a dominant-negative manner to block dendrite formation, thereby indicating that the association of JNK with the tail of BMPRII is required for dendritogenesis.
FIG. 8.
JNK binding to BMPRII is required for BMP-induced dendritogenesis and microtubule stabilization. (A and B) Deletion of JNK binding regions, JBR-AB, of BMPRII blocks BMP-dependent dendrite formation. Primary cortical neurons were infected with adenoviruses encoding GFP empty vector, BMPRII full length (FL), or BMPRII ΔJBR-AB and treated with BMP7 for 48 h after 4 DIV. The number of dendrites per neuron in GFP-expressing neuronal cells (A) that costained with the dendrite-specific Map2 (a+b) antibody (data not shown) was quantified. The fold changes in dendrite numbers relative to the unstimulated GFP empty control are shown (B), with 30 to 40 neurons analyzed per condition. Shown are the means + the SEM of three independent experiments (P < 0.0001 [Student t test] for points infected with GFP, FL). Scale bar, 20 μm. (C) Deletion of JBR-AB on BMPRII blocks BMP7-induced microtubule stabilization in dendrites of cortical neurons. Primary cortical neurons were infected with the indicated adenoviral constructs and incubated with nocodazole (5 μM) in the presence or absence of BMP7 for 1 h. The length of stable microtubules in dendrites (Ace MT) was expressed as a percentage of total dendrite length (GFP staining) and analyzed as shown in Fig. 1B. Shown are the means ± the SEM for two independent experiments with at least 20 cells analyzed per condition (P < 0.05 [Student t test] for the category of 40% and up in points infected with GFP, BRII). (D) Model of BMP7-induced dendrite formation in neurons. BMPRII serves as a scaffold that binds cytoskeletal regulators such as JNK and LIMK1 and thus is poised in neurite tips to mediate rapid remodeling of the actin and microtubule networks in response to BMP7.
We next determined whether binding of JNK to the tail region of BMPRII is required for BMP7-induced microtubule stabilization. For this, cortical neurons were infected with adenoviral constructs containing full-length BMPRII (BRII FL), and two dominant-negative constructs, a BMPRII mutant lacking the entire tail region (BRII Δtail) and BMPRII lacking only the two JNK binding regions (BRII ΔJBR-AB). The extent of microtubule stabilization in dendrites in nocodazole-treated cells coincubated with or without BMP7 was then determined. The length of stable microtubules detected by anti-Ace-tubulin antibody was expressed as a percentage of total microtubule length as detected by GFP staining. In nocodazole-treated GFP control cells, BMP7 increased microtubule stabilization (Fig. 8C) and a similar effect was observed in BMPRII-expressing cells. However, in cells overexpressing BMPRII that lacks the entire tail region (BRII Δtail), or the JNK binding regions alone (BRII ΔJBR-AB), there was a loss of the BMP7-induced microtubule stabilization in dendritic protrusions (Fig. 8C). Thus, BMP7 signaling regulates microtubule stabilization in dendritic protrusions of cortical neurons, and the binding of JNK to BMPRII tail is required for this effect.
Taken together, our results show that BMP7 activates JNK in the tips of dendrites, that BMP signaling regulates microtubule stabilization, and that the binding of JNK to the BMPRII carboxy-terminal tail is required for BMP-induced microtubule stabilization and dendritogenesis in cortical neurons. Since BMPRII also binds LIMK, we propose a model in which BMPRII-mediated scaffolding of cytoskeletal regulators, including JNKs and LIMK, facilitate rapid rearrangement of the cytoskeletal network to promote dendritogenesis in response to extracellular BMP cues (Fig. 8D).
DISCUSSION
BMP signaling controls many crucial steps in the development of the vertebrate central nervous system by acting at different stages and in various regions to regulate cell fate, proliferation and differentiation (33, 40). The BMP type II receptor, BMPRII, in vertebrates as well as the Drosophila ortholog, Wit, are highly expressed in neuronal cells and play key roles in neuronal morphogenesis (1, 21, 32, 36, 49). Previous work has shown that an extrinsic growth factor, BMP7, signaling through BMPRII modulates the actin cytoskeleton to promote dendrite formation in neurons via direct binding of LIMK1 to the cytoplasmic tail of BMPRII (32). Here, we demonstrate that BMP7 also regulates another architectural component of dendrites, the microtubule network. We show that BMP7 activates JNK to regulate microtubule stabilization in primary cortical neurons and, using a MAP2-dependent model of cytoskeletal remodeling, that BMP7-induced JNK activation is required for protrusion formation in a neuronally derived cell line. Moreover, we show that activation of the microtubule regulator, JNK, and the binding of JNK to BMPRII is required both for microtubule stabilization and for BMP7-induced dendritogenesis. We conclude that the interaction of JNK with BMPRII is essential for BMP7-dependent stabilization of microtubules, an activity that is essential for driving dendritogenesis.
Microtubules are key architectural components of dendrites and are important for neurite formation (15). Although many cytoskeletal regulators have been identified, the molecular mechanisms by which extracellular factors modulate microtubule stability are not well understood. Here, we show that BMP7 promotes microtubule stabilization in neurons. Although not previously reported in vertebrates, a role for BMP signaling in the regulation of the microtubule cytoskeleton has been described in Drosophila. Specifically, Thickveins (Tkv), a type I receptor for the BMP-like ligand, DPP, was shown to contribute to the maintenance of axonal and neuromuscular junction microtubules (48), as well as apical microtubule arrays in the wing imaginal disc (26). Although the downstream effectors were not elucidated, these data, coupled with our results, suggest a broadly conserved role for BMP-like signaling pathways in microtubule stabilization. Dynamic assembly and disassembly of microtubules is controlled by a variety of MAPs, and MAPs, such as MAP2, MAP1B, DCX, and SCG10, are subject to phosphorylation that controls their ability to bind and stabilize microtubules (8, 12, 24, 44). Although not much is known about how this phosphorylation is regulated, JNK has been shown to be essential for MAP phosphorylation and neuronal microtubule dynamics (8, 12, 24, 44). Indeed, we used a MAP2-dependent model of cytoskeletal remodeling to demonstrate that BMP7-induced JNK activation induces protrusion formation and microtubule stabilization. Although this indicates that MAP2 is a likely downstream effector of the BMP/JNK pathway, other JNK targets such as DCX that regulate microtubule stability may also be involved.
Dynamic microtubules explore the growth cone and search for sites of stabilization during neuronal outgrowth (15), but how this localized microtubule stabilization is regulated is not clearly understood. Our initial attempts to detect BMP7-dependent JNK activation by immunoblotting of total cell lysates from primary cortical neurons were unsuccessful. However, BMP7-dependent JNK activation was readily detected in the tips of primary cortical neurons when analyzed by immunofluorescence microscopy, while no increase in total JNK levels in tips was detected. The simplest interpretation of these data leads to a model of compartment specific activation of JNK by BMP7. Consistent with this, a requirement for JNK activation in the cytosol and not in the nucleus has been shown to be required for appropriate dendritic length and branching in cultured neurons (14). Our biochemical analysis demonstrated that JNK interacts with BMPRII, independent of BMPRII kinase activity, indicating that prior receptor complex activation is not required for JNK binding. In addition, we showed that JNK remains bound to the BMPRII/ALK2 receptor complex after activation by BMP7. Moreover, deletion of the JNK binding regions in the tail of BMPRII, an approach that leaves endogenous JNK unperturbed, abrogated BMP7-induced microtubule stabilization and dendrite outgrowth, clearly indicating that specific binding of JNK is essential for dendritogenesis. Given that BMPRII is localized at the tips of neurites in cortical neurons (32), these results together suggest a model in which BMPRII functions as a scaffold that binds JNK and that anchoring of JNK to the cytoplasmic tail of BMPRII provides a means to spatially restrict BMP7-dependent JNK activation (Fig. 8D). We speculate that this allows for a confined regulation of MAP phosphorylation that results in localized microtubule stabilization that can serve to promote neurite outgrowth. The molecular pathway whereby BMP7 activates JNK and whether BMPRII-binding independent paths to JNK activation may exist remain to be determined. Interestingly, high levels of the JNK scaffold, JIP (17), and activated JNK (42) have been reported in axons. Since microtubule stabilization is particularly important for axon formation (25, 52, 53), it would be interesting to determine whether BMPs, via regulation of JNK activity, also contribute to microtubule stability in axonal processes. Studies of this as well as the potential relevance of BMP/JNK pathway in non-neuronal cells are intriguing areas for future investigations.
It has become increasingly clear that dynamic reorganization of both the actin and the microtubule cytoskeleton requires spatial and temporal control and, even more importantly, that functional coordination of these two networks is essential during neuronal morphogenesis (25, 52). Indeed, a key role for scaffolding proteins that function to modulate both the microtubule and the actin cytoskeleton to regulate neurite outgrowth and dentritogenesis has emerged (46). LIMK1 associates with the BMPRII tail and allows for local actin cytoskeletal remodeling by modulating the activity of ADF/cofilin (32). Here, we demonstrated that the microtubule cytoskeletal regulator, JNK, also associates with the tail of the BMPRII receptor in a region that is in close proximity to the LIMK binding site. Interestingly, the dynein-associated protein Tctex-1, a regulator of the cytoskeleton that is involved neurite outgrowth, has been reported to bind to BMPRII (35). Since BMPRII is localized to the tip of neurites in primary neurons (32), it is tempting to speculate that by binding multiple actin and microtubule cytoskeleton regulators and components, such as LIMK1, JNK, and Tctex-1, BMPRII might act as a scaffold to both spatially restrict and coordinate remodeling of the neuronal actin and microtubule cytoskeleton during neurite outgrowth. Since BMPRII transduces BMP signals, our results indicate that this scaffolding function provides an efficient means for extracellular cues, such as BMPs, to control the timing of neurite outgrowth and dendritogenesis.
Supplementary Material
Acknowledgments
This study was supported by grant 178082 to L.A. from the Canadian Institutes of Health Research. L.A. holds a Canada Research Chair. R.J.D. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Published ahead of print on 22 February 2010.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Aberle, H., A. P. Haghighi, R. D. Fetter, B. D. McCabe, T. R. Magalhaes, and C. S. Goodman. 2002. wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron 33:545-558. [DOI] [PubMed] [Google Scholar]
- 2.Arimura, N., and K. Kaibuchi. 2007. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat. Rev. Neurosci. 8:194-205. [DOI] [PubMed] [Google Scholar]
- 3.Attisano, L., and J. L. Wrana. 2002. Signal transduction by the TGF-beta superfamily. Science 296:1646-1647. [DOI] [PubMed] [Google Scholar]
- 4.Barnes, A. P., and F. Polleux. 2009. Establishment of axon-dendrite polarity in developing neurons. Annu. Rev. Neurosci. 32:347-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes, A. P., D. Solecki, and F. Polleux. 2008. New insights into the molecular mechanisms specifying neuronal polarity in vivo. Curr. Opin. Neurobiol. 18:44-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrios-Rodiles, M., K. R. Brown, B. Ozdamar, R. Bose, Z. Liu, R. S. Donovan, F. Shinjo, Y. Liu, J. Dembowy, I. W. Taylor, V. Luga, N. Przulj, M. Robinson, H. Suzuki, Y. Hayashizaki, I. Jurisica, and J. L. Wrana. 2005. High-throughput mapping of a dynamic signaling network in mammalian cells. Science 307:1621-1625. [DOI] [PubMed] [Google Scholar]
- 7.Beppu, H., M. Kawabata, T. Hamamoto, A. Chytil, O. Minowa, T. Noda, and K. Miyazono. 2000. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev. Biol. 221:249-258. [DOI] [PubMed] [Google Scholar]
- 8.Bjorkblom, B., N. Ostman, V. Hongisto, V. Komarovski, J. J. Filen, T. A. Nyman, T. Kallunki, M. J. Courtney, and E. T. Coffey. 2005. Constitutively active cytoplasmic c-Jun N-terminal kinase 1 is a dominant regulator of dendritic architecture: role of microtubule-associated protein 2 as an effector. J. Neurosci. 25:6350-6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogoyevitch, M. A., and B. Kobe. 2006. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol. Mol. Biol. Rev. 70:1061-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borsello, T., P. G. Clarke, L. Hirt, A. Vercelli, M. Repici, D. F. Schorderet, J. Bogousslavsky, and C. Bonny. 2003. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat. Med. 9:1180-1186. [DOI] [PubMed] [Google Scholar]
- 11.Boucher, M., D. Belanger, C. Beaulieu, and N. Leclerc. 1999. Tau-mediated process outgrowth is differentially altered by the expression of MAP2b and MAP2c in Sf9 cells. Cell Motil. Cytoskel. 42:257-273. [DOI] [PubMed] [Google Scholar]
- 12.Chang, L., Y. Jones, M. H. Ellisman, L. S. Goldstein, and M. Karin. 2003. JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Dev. Cell 4:521-533. [DOI] [PubMed] [Google Scholar]
- 13.Charytoniuk, D. A., E. Traiffort, E. Pinard, O. Issertial, J. Seylaz, and M. Ruat. 2000. Distribution of bone morphogenetic protein and bone morphogenetic protein receptor transcripts in the rodent nervous system and upregulation of bone morphogenetic protein receptor type II in hippocampal dentate gyrus in a rat model of global cerebral ischemia. Neuroscience 100:33-43. [DOI] [PubMed] [Google Scholar]
- 14.Coffey, E. T., V. Hongisto, M. Dickens, R. J. Davis, and M. J. Courtney. 2000. Dual roles for c-Jun N-terminal kinase in developmental and stress responses in cerebellar granule neurons. J. Neurosci. 20:7602-7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conde, C., and A. Caceres. 2009. Microtubule assembly, organization and dynamics in axons and dendrites. Nat. Rev. Neurosci. 10:319-332. [DOI] [PubMed] [Google Scholar]
- 16.Dajas-Bailador, F., E. V. Jones, and A. J. Whitmarsh. 2008. The JIP1 scaffold protein regulates axonal development in cortical neurons. Curr. Biol. 18:221-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.da Silva, J. S., and C. G. Dotti. 2002. Breaking the neuronal sphere: regulation of the actin cytoskeleton in neuritogenesis. Nat. Rev. Neurosci. 3:694-704. [DOI] [PubMed] [Google Scholar]
- 18.Davies, R. J., and N. W. Morrell. 2008. Molecular mechanisms of pulmonary arterial hypertension: role of mutations in the bone morphogenetic protein type II receptor. Chest 134:1271-1277. [DOI] [PubMed] [Google Scholar]
- 19.De Caestecker, M., and B. Meyrick. 2001. Bone morphogenetic proteins, genetics and the pathophysiology of primary pulmonary hypertension. Respir. Res. 2:193-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Guglielmo, G. M., C. Le Roy, A. F. Goodfellow, and J. L. Wrana. 2003. Distinct endocytic pathways regulate TGF-beta receptor signaling and turnover. Nat. Cell Biol. 5:410-421. [DOI] [PubMed] [Google Scholar]
- 21.Eaton, B. A., and G. W. Davis. 2005. LIM Kinase1 controls synaptic stability downstream of the type II BMP receptor. Neuron 47:695-708. [DOI] [PubMed] [Google Scholar]
- 22.Feng, X. H., and R. Derynck. 2005. Specificity and versatility in TGF-beta signaling through Smads. Annu. Rev. Cell Dev. Biol. 21:659-693. [DOI] [PubMed] [Google Scholar]
- 23.Foletta, V. C., M. A. Lim, J. Soosairajah, A. P. Kelly, E. G. Stanley, M. Shannon, W. He, S. Das, J. Massague, and O. Bernard. 2003. Direct signaling by the BMP type II receptor via the cytoskeletal regulator LIMK1. J. Cell Biol. 162:1089-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gdalyahu, A., I. Ghosh, T. Levy, T. Sapir, S. Sapoznik, Y. Fishler, D. Azoulai, and O. Reiner. 2004. DCX, a new mediator of the JNK pathway. EMBO J. 23:823-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Georges, P. C., N. M. Hadzimichalis, E. S. Sweet, and B. L. Firestein. 2008. The yin-yang of dendrite morphology: unity of actin and microtubules. Mol. Neurobiol. 38:270-284. [DOI] [PubMed] [Google Scholar]
- 26.Gibson, M. C., and N. Perrimon. 2005. Extrusion and death of DPP/BMP-compromised epithelial cells in the developing Drosophila wing. Science 307:1785-1789. [DOI] [PubMed] [Google Scholar]
- 27.Haeusgen, W., R. Boehm, Y. Zhao, T. Herdegen, and V. Waetzig. 2009. Specific activities of individual c-Jun N-terminal kinases in the brain. Neuroscience 161:951-959. [DOI] [PubMed] [Google Scholar]
- 28.Huttenlocher, P. R. 1991. Dendritic and synaptic pathology in mental retardation. Pediatr. Neurol. 7:79-85. [DOI] [PubMed] [Google Scholar]
- 29.Jan, Y. N., and L. Y. Jan. 2001. Dendrites. Genes Dev. 15:2627-2641. [DOI] [PubMed] [Google Scholar]
- 30.Kaufmann, W. E., and H. W. Moser. 2000. Dendritic anomalies in disorders associated with mental retardation. Cereb. Cortex 10:981-991. [DOI] [PubMed] [Google Scholar]
- 31.Labbe, E., A. Letamendia, and L. Attisano. 2000. Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-beta and Wnt pathways. Proc. Natl. Acad. Sci. U. S. A. 97:8358-8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee-Hoeflich, S. T., C. G. Causing, M. Podkowa, X. Zhao, J. L. Wrana, and L. Attisano. 2004. Activation of LIMK1 by binding to the BMP receptor, BMPRII, regulates BMP-dependent dendritogenesis. EMBO J. 23:4792-4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu, A., and L. A. Niswander. 2005. Bone morphogenetic protein signaling and vertebrate nervous system development. Nat. Rev. Neurosci. 6:945-954. [DOI] [PubMed] [Google Scholar]
- 34.Liu, F., F. Ventura, J. Doody, and J. Massague. 1995. Human type II receptor for bone morphogenic proteins (BMPs): extension of the two-kinase receptor model to the BMPs. Mol. Cell. Biol. 15:3479-3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Machado, R. D., N. Rudarakanchana, C. Atkinson, J. A. Flanagan, R. Harrison, N. W. Morrell, and R. C. Trembath. 2003. Functional interaction between BMPR-II and Tctex-1, a light chain of dynein, is isoform-specific and disrupted by mutations underlying primary pulmonary hypertension. Hum. Mol. Genet. 12:3277-3286. [DOI] [PubMed] [Google Scholar]
- 36.Marques, G., H. Bao, T. E. Haerry, M. J. Shimell, P. Duchek, B. Zhang, and M. B. O'Connor. 2002. The Drosophila BMP type II receptor Wishful Thinking regulates neuromuscular synapse morphology and function. Neuron 33:529-543. [DOI] [PubMed] [Google Scholar]
- 37.McDonald, P. H., C. W. Chow, W. E. Miller, S. A. Laporte, M. E. Field, F. T. Lin, R. J. Davis, and R. J. Lefkowitz. 2000. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science 290:1574-1577. [DOI] [PubMed] [Google Scholar]
- 38.Miller, B. W., G. Lau, C. Grouios, E. Mollica, M. Barrios-Rodiles, Y. Liu, A. Datti, Q. Morris, J. L. Wrana, and L. Attisano. 2009. Application of an integrated physical and functional screening approach to identify inhibitors of the Wnt pathway. Mol. Syst. Biol. 5:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller, F. D., and D. R. Kaplan. 2003. Signaling mechanisms underlying dendrite formation. Curr. Opin. Neurobiol. 13:391-398. [DOI] [PubMed] [Google Scholar]
- 40.Munoz-Sanjuan, I., and A. H. Brivanlou. 2002. Neural induction, the default model and embryonic stem cells. Nat. Rev. Neurosci. 3:271-280. [DOI] [PubMed] [Google Scholar]
- 41.Narimatsu, M., R. Bose, M. Pye, L. Zhang, B. Miller, P. Ching, R. Sakuma, V. Luga, L. Roncari, L. Attisano, and J. L. Wrana. 2009. Regulation of planar cell polarity by Smurf ubiquitin ligases. Cell 137:295-307. [DOI] [PubMed] [Google Scholar]
- 42.Oliva, A. A., Jr., C. M. Atkins, L. Copenagle, and G. A. Banker. 2006. Activated c-Jun N-terminal kinase is required for axon formation. J. Neurosci. 26:9462-9470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanchez, C., M. Perez, and J. Avila. 2000. GSK3beta-mediated phosphorylation of the microtubule-associated protein 2C (MAP2C) prevents microtubule bundling. Eur. J. Cell Biol. 79:252-260. [DOI] [PubMed] [Google Scholar]
- 44.Tararuk, T., N. Ostman, W. Li, B. Bjorkblom, A. Padzik, J. Zdrojewska, V. Hongisto, T. Herdegen, W. Konopka, M. J. Courtney, and E. T. Coffey. 2006. JNK1 phosphorylation of SCG10 determines microtubule dynamics and axodendritic length. J. Cell Biol. 173:265-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Varelas, X., R. Sakuma, P. Samavarchi-Tehrani, R. Peerani, B. M. Rao, J. Dembowy, M. B. Yaffe, P. W. Zandstra, and J. L. Wrana. 2008. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat. Cell Biol. 10:837-848. [DOI] [PubMed] [Google Scholar]
- 46.Vessey, J. P., and D. Karra. 2007. More than just synaptic building blocks: scaffolding proteins of the post-synaptic density regulate dendritic patterning. J. Neurochem. 102:324-332. [DOI] [PubMed] [Google Scholar]
- 47.Waetzig, V., Y. Zhao, and T. Herdegen. 2006. The bright side of JNKs-Multitalented mediators in neuronal sprouting, brain development and nerve fiber regeneration. Prog. Neurobiol. 80:84-97. [DOI] [PubMed] [Google Scholar]
- 48.Wang, X., W. R. Shaw, H. T. Tsang, E. Reid, and C. J. O'Kane. 2007. Drosophila spichthyin inhibits BMP signaling and regulates synaptic growth and axonal microtubules. Nat. Neurosci. 10:177-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wen, Z., L. Han, J. R. Bamburg, S. Shim, G. L. Ming, and J. Q. Zheng. 2007. BMP gradients steer nerve growth cones by a balancing act of LIM kinase and Slingshot phosphatase on ADF/cofilin. J. Cell Biol. 178:107-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weston, C. R., and R. J. Davis. 2007. The JNK signal transduction pathway. Curr. Opin. Cell Biol. 19:142-149. [DOI] [PubMed] [Google Scholar]
- 51.Whitford, K. L., P. Dijkhuizen, F. Polleux, and A. Ghosh. 2002. Molecular control of cortical dendrite development. Annu. Rev. Neurosci. 25:127-149. [DOI] [PubMed] [Google Scholar]
- 52.Witte, H., and F. Bradke. 2008. The role of the cytoskeleton during neuronal polarization. Curr. Opin. Neurobiol. 18:479-487. [DOI] [PubMed] [Google Scholar]
- 53.Witte, H., D. Neukirchen, and F. Bradke. 2008. Microtubule stabilization specifies initial neuronal polarization. J. Cell Biol. 180:619-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.