Abstract
This study assessed the spectrum of activity of torezolid (TR-700), the active moiety of torezolid phosphate (TR-701), and proposes tentative MIC and disk diffusion breakpoints as well as quality control ranges. The in vitro susceptibilities of 1,096 bacterial isolates, representing 23 different species or phenotypic groups, were determined for torezolid, linezolid, cefotaxime, and levofloxacin using Clinical and Laboratory Standards Institute (CLSI) broth microdilution MICs, minimum bactericidal concentrations (MBCs), agar dilution, and disk diffusion testing methods. Torezolid was very active against the majority of Gram-positive strains, including methicillin-susceptible and -resistant Staphylococcus aureus (MIC50 = 0.25 μg/ml, MIC90 ≤ 0.5 μg/ml), coagulase-negative staphylococci (CNS; MIC50 = 0.25 μg/ml, MIC90 ≤ 0.5 μg/ml), enterococci (MIC50 and MIC90 ≤ 0.5 μg/ml), and streptococci (MIC50 and MIC90 ≤ 0.25 μg/ml). Based upon MIC90s, torezolid was 4-fold more active than linezolid against S. aureus, coagulase-negative staphylococci, and the enterococci and 8-fold more active than linezolid against the streptococci. With the use of tentative MIC breakpoints of ≤2 μg/ml for susceptibility, torezolid disk diffusion zone diameter breakpoints are proposed using a 20-μg disk. In addition, MIC quality control ranges of torezolid were determined for three CLSI-recognized standard ATCC reference strains.
Torezolid phosphate (TR-701, DA-7218) is an oxazolidinone prodrug which is currently under clinical development. It is a novel oral oxazolidinone which displays good activity against important Gram-positive pathogens, particularly methicillin-resistant Staphylococcus aureus (MRSA) and some linezolid-resistant staphylococci (11). Torezolid (TR-700) is the active moiety of torezolid phosphate (TR-701). In plasma, the prodrug torezolid phosphate (TR-701) is rapidly converted into active torezolid (13). Torezolid has been shown to be 4- to 8-fold more active than linezolid against Gram-positive isolates collected from South Korea (3, 8), as well as from the United States and Europe (10). Preliminary reports have shown that torezolid was 4-fold more active than linezolid against the staphylococci and enterococci and 8- to >128-fold more active than cefotaxime and levofloxacin against staphylococci, enterococci, and streptococci (1). Torezolid is in phase 3 clinical trials for treatment of hospital- and community-acquired infections, including complicated skin and skin structure infections and community-associated pneumonia.
The present study was designed to (i) assess the in vitro antibacterial activity of torezolid and compare its activity with that of linezolid, cefotaxime, and levofloxacin against a broad range of bacterial pathogens for which torezolid might be considered for therapy; (ii) determine the appropriate disk mass for disk diffusion antimicrobial susceptibility testing; (iii) determine preliminary torezolid disk diffusion interpretive criteria for these microorganisms; (iv) determine the correlation of torezolid agar dilution MICs with broth microdilution MICs versus approximately 100 strains of each of three target species; and (v) propose MIC quality control ranges for 3 different aerobic quality control strains.
(This study was presented in part at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 2008 [1].)
MATERIALS AND METHODS
Bacteria tested.
A total of 1,096 recent clinical bacterial isolates representing over 23 species or phenotypic groups were selected as representative pathogens that cause infections for which torezolid might be considered for therapy. These included 361 streptococci, 203 enterococci, 234 S. aureus strains, 104 coagulase-negative staphylococci, 99 Haemophilus influenzae strains, 50 Moraxella catarrhalis strains, 12 Corynebacterium jeikeium strains, and 33 Listeria monocytogenes strains. The majority of these strains (72.9%) were recent (<3 years) clinical isolates at the time of testing. The remainder of the strains (27.1%) were specifically selected in order to provide a challenge set of phenotypic resistance patterns. All isolates were from within the United States.
Antimicrobial susceptibility testing.
Torezolid (TR-700) standardized powder was provided by Trius Pharmaceuticals, Inc. (lot DP-70-1465/wt). Linezolid (lot 1000891018) was obtained from Pfizer, Inc. Cefotaxime (lot 036K1623), oxacillin (lot 085K1923), levofloxacin (lot 1333515), and penicillin (lot 095K0625) were purchased from Sigma. All aerobic microorganisms were tested by the disk diffusion method using the following disks: 30-μg cefotaxime BDMS (Becton Dickinson Microbiology Systems) (lot 7176383), 30-μg linezolid BDMS (lot 8028004), 5-μg levofloxacin BDMS (lot 7285689), 30-μg cefoxitin BDMS (lot 7277165), and 2-μg, 5-μg, 10-μg, and 20-μg torezolid disks prepared by the Clinical Microbiology Institute (CMI).
Broth microdilution and agar dilution tests were performed according to the latest CLSI document, M7-A7, 2006 (5). Disk diffusion tests were performed according to the CLSI document M2-A9, 2006 (7). MIC trays were produced at CMI using cation-adjusted Mueller-Hinton broth (CAMHB; Difco lot 7306781). The medium was supplemented with lysed horse blood (Hemostat lot H05287) for testing the streptococci or made up as Haemophilus test medium (HTM) for testing Haemophilus influenzae. Disk diffusion plates were purchased from commercial suppliers. Agar dilution plates were prepared at CMI using Difco dehydrated Mueller-Hinton agar medium (lot 5011641) supplemented as needed with 5% sheep blood (Hema Resources lot 0414-100140-03) or made up as HTM agar. Disk diffusion zone diameters for torezolid and linezolid versus all staphylococci were read using transmitted light as recommended by the CLSI. Zone diameters for all other genera were read using reflected light as specified by the CLSI.
MIC versus zone diameter scattergrams were prepared for each of the major groups of microorganisms. MIC “microbiological cutoff breakpoints” were selected using the method described by Turnidge and Paterson (12). This method requires the construction of histograms and estimation of the upper end of the wild-type distribution and thus the wild-type cutoff values, also known as microbiological cutoff or breakpoints. Using an error minimization approach (2, 6, 9, 12), disk diffusion interpretive criteria are proposed. The zone diameter breakpoints proposed were designed to minimize the interpretive discrepancies between the two types of susceptibility testing methods. The tentative MIC breakpoints were those proposed by the sponsor based upon a conservative interpretation of previous in vivo studies. Pharmacokinetic/pharmacodynamic (PK/PD) studies are in progress.
Agar dilution versus microbroth dilution.
In order to determine if there are differences between agar dilution and broth microdilution techniques, 315 strains were tested in parallel by the two methods. This phase of testing included 104 strains of Staphylococcus aureus, 106 strains of Streptococcus pneumoniae, 53 strains of Enterococcus faecalis, and 52 strains of Enterococcus faecium.
Quality control studies.
For the quality control portion of the study, bacteria were tested by the broth microdilution method as described by the CLSI (5). An eight-laboratory study was undertaken in order to propose MIC quality control ranges for torezolid against three standard quality control bacteria. The testing laboratories included both hospital and commercial microbiology laboratories in the United States. The eight participants included D. Bade, Microbial Research, Inc., Fort Collins, CO; S. Brown, Clinical Microbiology Institute, Wilsonville, OR; J. Daly, Primary Children's Medical Center, Salt Lake City, UT; G. Hall, Cleveland Clinic Foundation, Cleveland, OH; D. Hardy, University of Rochester Medical Center, Rochester, NY; J. Hindler, University of California Los Angeles, Los Angeles, CA; C. Knapp, Trek Diagnostic Systems, Cleveland, OH; and R. Rennie, University of Alberta Hospital, Edmonton, Alberta, Canada. This study closely followed the protocol described by the CLSI (6) with the exception that eight testing facilities were used rather than the required seven. The quality control organisms were those recommended by the CLSI and included S. aureus ATCC 29213, S. pneumoniae ATCC 49619, and E. faecalis ATCC 29212. Internal quality control results for the control drug, linezolid, were within published ranges (4) for all tests. There were no instances where the results for the control were outside the ranges recommended by the CLSI. This study involved replicate tests of torezolid diluted from 8 to 0.004 μg/ml in three lots of Mueller-Hinton broth. This exercise generated 240 MICs with each appropriate quality control strain.
RESULTS AND DISCUSSION
In vitro activity.
The antimicrobial activities of torezolid against all isolates are summarized in Table 1. This table demonstrates the modal MIC, the MIC range, the MIC50, and MIC90. Data are presented comparing broth microdilution MICs of torezolid against all comparator drugs. Bactericidal data are presented for torezolid and linezolid only.
TABLE 1.
Susceptibilities of aerobic bacteria to torezolid and comparator drugsa
| Species | n | Drug | Type of value | Concn (μg/ml) |
|||
|---|---|---|---|---|---|---|---|
| Mode | Range | 50%b | 90%c | ||||
| All Staphylococcus spp. combined | 338 | Torezolid | MIC | 0.25 | 0.12-16 | 0.25 | 0.5 |
| 112 | Torezolid | MBC | 16 | 0.5->32 | 8 | 32 | |
| 104 | Torezolid agar | MIC | 0.5 | 0.25-0.5 | 0.5 | 0.5 | |
| 338 | Cefotaxime | MIC | 2 | 0.03->64 | 4 | >64 | |
| 338 | Levofloxacin | MIC | 0.25 | 0.06->16 | 0.5 | >16 | |
| 338 | Linezolid | MIC | 2 | 0.5->8 | 2 | 2 | |
| 112 | Linezolid | MBC | >8 | 2->8 | >8 | >8 | |
| All S. aureus strains combined | 234 | Torezolid | MIC | 0.25 | 0.12-16 | 0.5 | 0.5 |
| 82 | Torezolid | MBC | 16 | 0.5->32 | 4 | 16 | |
| 104 | Torezolid agar | MIC | 0.5 | 0.25-0.5 | 0.5 | 0.5 | |
| 234 | Cefotaxime | MIC | 2 | 0.03->64 | 8 | >64 | |
| 234 | Levofloxacin | MIC | 0.25 | 0.12->16 | 4 | >16 | |
| 234 | Linezolid | MIC | 2 | 1->8 | 2 | 2 | |
| 82 | Linezolid | MBC | 16 | 2->8 | 8 | >8 | |
| S. aureus, methicillin susceptible | 105 | Torezolid | MIC | 0.25 | 0.25-8 | 0.25 | 0.5 |
| 25 | Torezolid | MBC | 16 | 1-32 | 16 | 32 | |
| 52 | Torezolid agar | MIC | 0.25 | 0.25-0.5 | 0.25 | 0.5 | |
| 105 | Cefotaxime | MIC | 2 | 0.03-4 | 2 | 2 | |
| 105 | Levofloxacin | MIC | 0.25 | 0.12->16 | 0.25 | 4 | |
| 105 | Linezolid | MIC | 2 | 1->8 | 2 | 2 | |
| 25 | Linezolid | MBC | >8 | 4->8 | >8 | >8 | |
| S. aureus, methicillin resistant | 129 | Torezolid | MIC | 0.5 | 0.12-16 | 0.5 | 1 |
| 57 | Torezolid | MBC | 1 | 0.5->32 | 2 | 16 | |
| 52 | Torezolid agar | MIC | 0.5 | 0.25-0.5 | 0.5 | 0.5 | |
| 129 | Cefotaxime | MIC | 8 | 2->64 | 16 | >64 | |
| 129 | Levofloxacin | MIC | 4 | 0.12->16 | 8 | >16 | |
| 129 | Linezolid | MIC | 2 | 1->8 | 2 | 4 | |
| 57 | Linezolid | MBC | 8 | 2->8 | 8 | >8 | |
| S. aureus, linezolid resistant | 13 | Torezolid | MIC | 4 | 0.25-16 | 4 | 8 |
| 2 | Torezolid | MBC | None | 16->32 | 16 | >32 | |
| 13 | Cefotaxime | MIC | >64 | 2->64 | >64 | >64 | |
| 13 | Levofloxacin | MIC | >16 | 0.25->16 | >16 | >16 | |
| 13 | Linezolid | MIC | >8 | 2->8 | >8 | >8 | |
| 2 | Linezolid | MBC | >8 | >8 | >8 | >8 | |
| S. aureus, vancomycin nonsusceptible | 32 | Torezolid | MIC | 0.25 | 0.12-1 | 0.25 | 1 |
| 4 | Torezolid | MBC | 0.5 | 0.5-1 | 0.5 | 1 | |
| 32 | Cefotaxime | MIC | >64 | 2->64 | >64 | >64 | |
| 32 | Levofloxacin | MIC | >16 | 4->16 | 16 | >16 | |
| 32 | Linezolid | MIC | 2 | 1-4 | 2 | 4 | |
| 4 | Linezolid | MBC | 2 | 2-4 | 2 | 4 | |
| All coagulase-negative staphylococci combined (54 S. epidermidis, 14 S. haemolytica, 10 S. hominis, 7 S. lugdunensis, 13 S. saprophyticus, 6 CNS-no other speciation) | 104 | Torezolid | MIC | 0.25 | 0.12-1 | 0.25 | 0.5 |
| 32 | Torezolid | MBC | 16 | 2->32 | 16 | 32 | |
| 104 | Cefotaxime | MIC | 0.5 | 0.03->64 | 2 | >64 | |
| 104 | Levofloxacin | MIC | 0.25 | 0.06->16 | 0.5 | >16 | |
| 104 | Linezolid | MIC | 1 | 0.5-8 | 1 | 2 | |
| 32 | Linezolid | MBC | >8 | 2->8 | >8 | >8 | |
| All methicillin-resistant, coagulase-negative staphylococci combined | 58 | Torezolid | MIC | 0.12 | 0.12-1 | 0.25 | 0.5 |
| 21 | Torezolid | MBC | 16 | 2->32 | 16 | 32 | |
| 58 | Cefotaxime | MIC | 4 | 0.5->64 | 8 | >64 | |
| 58 | Levofloxacin | MIC | 8 | 0.12->16 | 8 | >16 | |
| 58 | Linezolid | MIC | 1 | 0.5-8 | 1 | 4 | |
| 21 | Linezolid | MBC | >8 | 8->8 | >8 | >8 | |
| All methicillin-susceptible, coagulase-negative staphylococci combined | 46 | Torezolid | MIC | 0.25 | 0.12-1 | 0.25 | 0.5 |
| 11 | Torezolid | MBC | 16 | 2->32 | 16 | 32 | |
| 46 | Cefotaxime | MIC | 0.5 | 0.03-4 | 0.5 | 2 | |
| 46 | Levofloxacin | MIC | 0.25 | 0.06-16 | 0.25 | 0.5 | |
| 46 | Linezolid | MIC | 1 | 0.5-4 | 1 | 2 | |
| 11 | Linezolid | MBC | >8 | 2->8 | >8 | >8 | |
| All enterococci combined | 203 | Torezolid | MIC | 0.5 | 0.25-2 | 0.5 | 0.5 |
| 70 | Torezolid | MBC | 32 | 1->32 | 32 | 32 | |
| 105 | Torezolid agar | MIC | 0.5 | 0.25-1 | 0.5 | 0.5 | |
| 203 | Cefotaxime | MIC | >64 | 0.25->64 | >64 | >64 | |
| 203 | Levofloxacin | MIC | >16 | 0.5->16 | >16 | >16 | |
| 203 | Linezolid | MIC | 2 | 1->8 | 2 | 2 | |
| 70 | Linezolid | MBC | >8 | 4->8 | >8 | >8 | |
| E. faecalis, vancomycin resistant | 45 | Torezolid | MIC | 0.5 | 0.25-1 | 0.5 | 0.5 |
| 20 | Torezolid | MBC | 32 | 16->32 | 32 | >32 | |
| 28 | Torezolid agar | MIC | 0.5 | 0.5 | 0.5 | 0.5 | |
| 45 | Cefotaxime | MIC | >64 | 0.25->64 | >64 | >64 | |
| 45 | Levofloxacin | MIC | >16 | 0.5->16 | >16 | >16 | |
| 45 | Linezolid | MIC | 2 | 1-4 | 2 | 2 | |
| 20 | Linezolid | MBC | >8 | >8 | >8 | >8 | |
| E. faecalis, vancomycin susceptible | 54 | Torezolid | MIC | 0.5 | 0.25-1 | 0.5 | 0.5 |
| 15 | Torezolid | MBC | 32 | 16-32 | 32 | 32 | |
| 25 | Torezolid agar | MIC | 0.5 | 0.5 | 0.5 | 0.5 | |
| 54 | Cefotaxime | MIC | >64 | 0.25->64 | >64 | >64 | |
| 54 | Levofloxacin | MIC | 1 | 1->16 | 1 | >16 | |
| 54 | Linezolid | MIC | 2 | 1-4 | 2 | 2 | |
| 15 | Linezolid | MBC | >8 | >8 | >8 | >8 | |
| E. faecium, vancomycin resistant | 52 | Torezolid | MIC | 0.5 | 0.25-2 | 0.5 | 0.5 |
| 20 | Torezolid | MBC | 32 | 1-32 | 32 | 32 | |
| 27 | Torezolid agar | MIC | 0.5 | 0.25-1 | 0.5 | 0.5 | |
| 52 | Cefotaxime | MIC | >64 | >64 | >64 | >64 | |
| 52 | Levofloxacin | MIC | >16 | 1->16 | >16 | >16 | |
| 52 | Linezolid | MIC | 2 | 1->8 | 2 | 4 | |
| 20 | Linezolid | MBC | >8 | 4->8 | >8 | >8 | |
| E. faecium, vancomycin susceptible | 52 | Torezolid | MIC | 0.5 | 0.25-1 | 0.5 | 0.5 |
| 15 | Torezolid | MBC | 32 | 16-32 | 32 | 32 | |
| 25 | Torezolid agar | MIC | 0.5 | 0.25-0.5 | 0.5 | 0.5 | |
| 52 | Cefotaxime | MIC | >64 | 0.5->64 | >64 | >64 | |
| 52 | Levofloxacin | MIC | >16 | 0.5->16 | 4 | >16 | |
| 52 | Linezolid | MIC | 2 | 2-4 | 2 | 2 | |
| 15 | Linezolid | MBC | >8 | >8 | >8 | >8 | |
| All streptococcal species combined | 361 | Torezolid | MIC | 0.25 | 0.03-0.5 | 0.25 | 0.25 |
| 53 | Torezolid | MBC | 1 | 0.5-32 | 1 | 16 | |
| 106 | Torezolid agar | MIC | 0.25 | 0.12-0.5 | 0.25 | 0.5 | |
| 361 | Cefotaxime | MIC | 0.015 | 0.015-8 | 0.03 | 1 | |
| 361 | Levofloxacin | MIC | 1 | 0.25-4 | 1 | 1 | |
| 361 | Linezolid | MIC | 1 | 0.12-4 | 1 | 2 | |
| 53 | Linezolid | MBC | >8 | 2->8 | 8 | >8 | |
| All Streptococcus pneumoniae strains combined | 133 | Torezolid | MIC | 0.25 | 0.03-0.5 | 0.25 | 0.25 |
| 33 | Torezolid | MBC | 1 | 0.5-16 | 1 | 2 | |
| 106 | Torezolid agar | MIC | 0.25 | 0.12-0.5 | 0.25 | 0.5 | |
| 133 | Cefotaxime | MIC | 0.015 | 0.015-8 | 0.12 | 2 | |
| 133 | Levofloxacin | MIC | 1 | 0.25-4 | 1 | 1 | |
| 133 | Linezolid | MIC | 1 | 0.12-4 | 1 | 2 | |
| 33 | Linezolid | MBC | 4 | 2->8 | 4 | 8 | |
| S. pneumoniae, penicillin susceptible | 53 | Torezolid | MIC | 0.25 | 0.03-0.5 | 0.25 | 0.25 |
| 12 | Torezolid | MBC | 1 | 0.5-8 | 1 | 4 | |
| 26 | Torezolid agar | MIC | 0.25 | 0.12-0.5 | 0.25 | 0.5 | |
| 53 | Cefotaxime | MIC | 0.015 | 0.015-0.25 | 0.015 | 0.03 | |
| 53 | Levofloxacin | MIC | 1 | 0.25-4 | 1 | 1 | |
| 53 | Linezolid | MIC | 1 | 0.12-2 | 1 | 2 | |
| 12 | Linezolid | MBC | 2 | 2->8 | 4 | 8 | |
| S. pneumoniae, penicillin intermediate | 26 | Torezolid | MIC | 0.25 | 0.12-0.5 | 0.25 | 0.5 |
| 10 | Torezolid | MBC | 1 | 0.5-16 | 1 | 1 | |
| 26 | Torezolid agar | MIC | 0.25 | 0.12-0.5 | 0.25 | 0.5 | |
| 26 | Cefotaxime | MIC | 0.12 | 0.03-1 | 0.12 | 0.5 | |
| 26 | Levofloxacin | MIC | 1 | 0.5-1 | 1 | 1 | |
| 26 | Linezolid | MIC | 1 | 0.5-4 | 1 | 2 | |
| 10 | Linezolid | MBC | 2 | 2->8 | 4 | 8 | |
| S. pneumoniae, penicillin resistant | 54 | Torezolid | MIC | 0.25 | 0.12-0.5 | 0.25 | 0.25 |
| 11 | Torezolid | MBC | 1 | 1-2 | 1 | 2 | |
| 54 | Torezolid agar | MIC | 0.25 | 0.25-0.5 | 0.25 | 0.5 | |
| 54 | Cefotaxime | MIC | 1 | 0.5-8 | 1 | 8 | |
| 54 | Levofloxacin | MIC | 1 | 0.5-2 | 1 | 1 | |
| 54 | Linezolid | MIC | 1 | 0.5-2 | 1 | 2 | |
| 11 | Linezolid | MBC | 4 | 4-8 | 4 | 8 | |
| All β-hemolytic streptococcal strains combined (101 S. agalactiae, 101 S. pyogenes) | 202 | Torezolid | MIC | 0.25 | 0.12-0.5 | 0.25 | 0.25 |
| 22 | Torezolid | MBC | 16 | 8-32 | 16 | 32 | |
| 202 | Cefotaxime | MIC | 0.015 | 0.015-0.06 | 0.03 | 0.06 | |
| 202 | Levofloxacin | MIC | 0.5 | 0.25-2 | 0.5 | 1 | |
| 202 | Linezolid | MIC | 1 | 1-4 | 1 | 2 | |
| 22 | Linezolid | MBC | >8 | >8 | >8 | >8 | |
| S. viridans group | 30 | Torezolid | MIC | 0.25 | 0.06-0.5 | 0.25 | 0.25 |
| 30 | Cefotaxime | MIC | 0.12 | 0.015-2 | 0.12 | 0.5 | |
| 30 | Levofloxacin | MIC | 1 | 0.25-2 | 1 | 2 | |
| 30 | Linezolid | MIC | 2 | 0.5-2 | 2 | 2 | |
| C. jeikeium | 12 | Torezolid | MIC | 0.25 | 0.25-0.5 | 0.25 | 0.5 |
| 12 | Cefotaxime | MIC | 32 | 8-32 | 32 | 32 | |
| 12 | Levofloxacin | MIC | >16 | 16-> 16 | >16 | >16 | |
| 12 | Linezolid | MIC | 1 | 0.5-1 | 1 | 1 | |
| L. monocytogenes | 33 | Torezolid | MIC | 0.25 | 0.25-0.5 | 0.25 | 0.25 |
| 33 | Cefotaxime | MIC | 32 | 2-32 | 32 | 32 | |
| 33 | Levofloxacin | MIC | 1 | 1-2 | 1 | 1 | |
| 33 | Linezolid | MIC | 2 | 2-2 | 2 | 2 | |
| M. catarrhalis | 50 | Torezolid | MIC | 4 | 2-4 | 4 | 4 |
| 50 | Cefotaxime | MIC | 0.06 | 0.03-2 | 0.250 | 1 | |
| 50 | Levofloxacin | MIC | 0.06 | 0.03-0.06 | 0.06 | 0.06 | |
| 50 | Linezolid | MIC | 8 | 8-16 | 8 | 8 | |
| All H. influenzae strains combined | 99 | Torezolid | MIC | 8 | 2-32 | 8 | 16 |
| 32 | Torezolid | MBC | >32 | 8->32 | >32 | >32 | |
| 99 | Cefotaxime | MIC | 0.008 | 0.008-2 | 0.015 | 0.5 | |
| 99 | Levofloxacin | MIC | 0.12 | 0.12 | 0.12 | 0.12 | |
| 99 | Linezolid | MIC | >8 | 4->8 | >8 | >8 | |
| 25 | Linezolid | MBC | >8 | >8 | >8 | >8 | |
| H. influenzae, β-lactamase negative | 32 | Torezolid | MIC | 8 | 4-32 | 8 | 16 |
| 11 | Torezolid | MBC | >32 | 16->32 | >32 | >32 | |
| 32 | Cefotaxime | MIC | 0.008 | 0.008-0.03 | 0.008 | 0.015 | |
| 32 | Levofloxacin | MIC | 0.12 | 0.12 | 0.12 | 0.12 | |
| 32 | Linezolid | MIC | >8 | 4->8 | >8 | >8 | |
| 10 | Linezolid | MBC | >8 | >8 | >8 | >8 | |
| H. influenzae, β-lactamase positive | 42 | Torezolid | MIC | 8 | 4-32 | 8 | 32 |
| 10 | Torezolid | MBC | >32 | 32->32 | >32 | >32 | |
| 42 | Cefotaxime | MIC | 0.015 | 0.008-0.03 | 0.015 | 0.015 | |
| 42 | Levofloxacin | MIC | 0.12 | 0.12 | 0.12 | 0.12 | |
| 42 | Linezolid | MIC | >8 | 8->8 | >8 | >8 | |
| 5 | Linezolid | MBC | >8 | >8 | >8 | >8 | |
| H. influenzae, β-lactamase negative, ampicillin nonsusceptible | 25 | Torezolid | MIC | 8 | 2-16 | 8 | 16 |
| 11 | Torezolid | MBC | >32 | 8->32 | >32 | >32 | |
| 25 | Cefotaxime | MIC | 0.5 | 0.03-2 | 0.5 | 0.5 | |
| 25 | Levofloxacin | MIC | 0.12 | 0.12 | 0.12 | 0.12 | |
| 25 | Linezolid | MIC | >8 | 8->8 | >8 | >8 | |
| 10 | Linezolid | MBC | >8 | >8 | >8 | >8 | |
Abbreviations: MBC, minimum bactericidal concentration; CNS-Nos, coagulase-no other speciation.
MIC50 or MBC50.
MIC90 or MBC90.
Torezolid was very active against the majority of the strains of methicillin-susceptible and methicillin-resistant staphylococci, alpha- and beta-hemolytic streptococci, Corynebacterium jeikeium, and Listeria monocytogenes. The torezolid MIC50 for each of these groups was ≤0.25 μg/ml. The MIC90 for each of these groups was ≤1 μg/ml. Based upon the MIC90, torezolid was 2-fold more active than linezolid against C. jeikeium, 4-fold more active than linezolid against the staphylococci and enterococci, and 8-fold more active than linezolid against L. monocytogenes. Compared to cefotaxime and levofloxacin, torezolid was 8- to >128-fold more active against all of the groups mentioned above.
Torezolid exhibited only moderate activity against Moraxella catarrhalis and Haemophilus influenzae. Torezolid was 2-fold more active than linezolid against Moraxella catarrhalis and comparable in activity to linezolid against Haemophilus influenzae. Both torezolid and linezolid were significantly less active than cefotaxime and levofloxacin against these species.
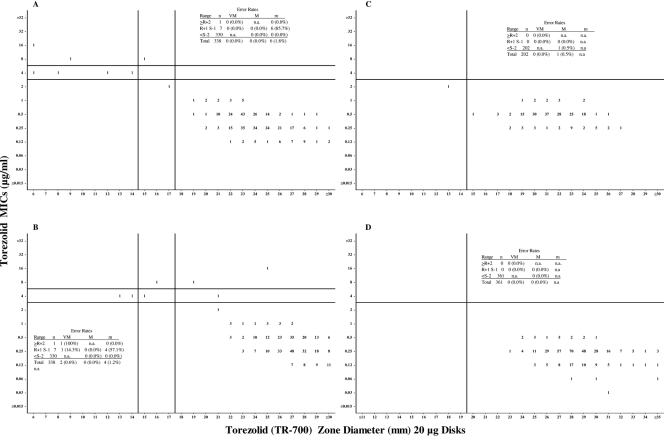
Scattergrams showing the distribution of MICs plotted against the corresponding zone diameters can be found in Fig. 1A to D. MIC breakpoints of ≤2 μg/ml for susceptible, 4 μg/ml for intermediate, and ≥8 μg/ml for resistant were used for the staphylococci. A susceptible-only breakpoint of ≤2 μg/ml for the streptococci and enterococci is proposed. Susceptible-only breakpoints are proposed whenever there is an absence or rare occurrence of resistant strains (4). It is fully recognized that the “official” MIC breakpoints will be based upon a variety of parameters such as PK/PD analysis, animal models, Monte Carlo simulations, and ultimately the clinical response of human patients (12). Using more conservative MIC breakpoints of ≤1 μg/ml for susceptible, 2 μg/ml for intermediate, and ≥4 μg/ml for resistant would have no impact upon the disk zone diameter breakpoints proposed here. CLSI-approved breakpoints were used for the comparator drugs when available. Not all of the comparator drugs have been assigned breakpoints for all species.
FIG. 1.
Scattergrams of torezolid versus zone diameters (20-μg disks). (A) All staphylococci combined using transmitted light (n = 338). (B) All staphylococci combined using reflected light (n = 338). (C) Enterococcal species combined (n = 202). (D) All streptococci combined (n = 361). Horizontal lines represent proposed susceptible (lower line), susceptible-only, and resistant (upper line) MIC breakpoints; vertical lines represent proposed susceptible (right line), susceptible-only, and resistant (left line) zone diameter breakpoints. Abbreviations: n, number of strains tested; VM, very major errors; M, major errors; m, minor errors; n.a., not applicable; R, resistant; S, susceptible.
Disk diffusion breakpoints.
Based upon the “microbiological” MIC breakpoints listed above, disk diffusion breakpoints were proposed for each of the groups tested and each of the four disk masses under study. Scattergrams depicting the proposed MIC and disk diffusion breakpoints are presented in Fig. 1A to D along with the associated error rates. As mentioned earlier, torezolid versus staphylococcal zone diameters were read using both transmitted (preferred) and reflected (not recommended) light sources.
All four disk masses provided adequate separation of susceptible and the infrequently encountered resistant microorganisms (data not shown). The error rates for all disk masses were well within acceptable limits. There was only one very major error which occurred with all four disk masses when testing H. influenzae. Although a few very major errors were noted for the staphylococci when using reflected light, there were no very major errors at all when using transmitted light (data not shown). Since there were no substantial differences between the four disk masses, the 20-μg disks are recommended primarily because of the subjective “robustness” of the zones with sharper, clearer, and easier-to-measure endpoints. The proposed MIC and disk diffusion breakpoints are presented in Table 2.
TABLE 2.
Proposed MIC and disk diffusion breakpoints of torezolida
| Species | Breakpoints (S, I, R) |
|
|---|---|---|
| MIC (μg/ml) | Disk diffusion using a 20- or 10-μg disk | |
| Staphylococcus aureus | ≤2, 4, ≥8 | ≥18, 15-17, ≤14 mm |
| Coagulase-negative staphylococci | ≤2, 4, ≥8 | ≥18, 15-17, ≤14 mm |
| Enterococci, Streptococcus pneumoniae, and Streptococcus other than S. pneumoniae | ≤2 for susceptible with no intermediate or resistant categories | ≥15 mm for susceptible with no intermediate or resistant categories |
| Corynebacterium jeikeium | ≤2 for susceptible with no intermediate or resistant categories | No range recommended due to low no. of isolates tested |
| Listeria monocytogenes | ≤2 for susceptible with no intermediate or resistant categories | ≥15 mm for susceptible with no intermediate or resistant categories |
| Moraxella catarrhalis | No range recommended | No range recommended |
| Haemophilus influenzae | No range recommended | No range recommended |
S, susceptible; I, intermediate; R, resistant.
Broth microdilution versus agar dilution.
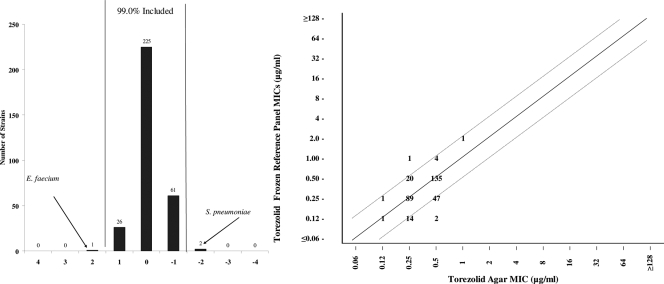
When torezolid broth microdilution MICs in frozen reference panels were compared to those of agar dilution against the 104 strains of S. aureus, 105 strains of enterococci, and 106 strains of S. pneumoniae, fully 99.0% of the results fell within ±1 log2 dilution (Fig. 2). Only 2 results for S. pneumoniae and 1 result for E. faecium were outside the normal range. These results were quite comparable to those of linezolid, where fully 100% of the values were within ±1 log2 dilution (data not shown).
FIG. 2.
Bar graph (left) and scattergram (right) showing torezolid broth microdilution MICs versus torezolid agar dilution MICs for S. aureus (n = 104), Enterococcus spp. (n = 105), and S. pneumoniae (n = 106). Vertical bars represent ±1 doubling dilution from complete agreement.
Quality control studies.
Quality control ranges for MIC testing were proposed on the basis of the modal MIC values observed plus or minus 1 log2 dilution. The proposed MIC ranges are presented in Table 3. These quality control ranges were accepted by the Antimicrobial Susceptibility Testing Subcommittee of the CLSI at their June 2008 meeting.
TABLE 3.
Torezolid MIC quality control ranges
| Quality control strain | No. of occurrences at the following MIC (μg/ml)a: |
% in rangeb | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.004 | 0.008 | 0.016 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | ||
| S. aureus ATCC 29213 | 52 | 149 | 37 | 2 | 99.2 | ||||||||
| S. pneumoniae ATCC 49619 | 1 | 14 | 201 | 24 | 99.6 | ||||||||
| E. faecalis ATCC 29212 | 54 | 179 | 7 | 100 | |||||||||
CLSI-recommended quality control ranges are shown in bold. The range of concentrations tested was 0.004 to 8 μg/ml.
Percentage of results which fall within the recommended range. The acceptable limit is ≥95%.
Discussion and conclusions.
Torezolid demonstrated excellent activity in vitro against the majority of Gram-positive strains tested, with particularly high activity against methicillin-susceptible and -resistant staphylococci, the enterococci, and all streptococci. The MIC50 and MIC90 for torezolid were ≤0.5 μg/ml for all key pathogens and for most resistant phenotypes. Torezolid was 4-fold more active than linezolid against the staphylococci and enterococci. In addition, torezolid was 8- to >128-fold more active against all of the groups tested compared to cefotaxime and levofloxacin. All staphylococcal and enterococcal isolates known to be intermediate or resistant to vancomycin were susceptible to torezolid. The inclusion of MRSA and vancomycin-nonsusceptible strains in torezolid's spectrum of activity sets this drug apart from the majority of antimicrobials in other classes. The disk diffusion test produced acceptable error rates against all strains of staphylococci tested. As with linezolid, the torezolid disk diffusion test should be read with transmitted light rather than by reflected light. Final breakpoint determinations will be based upon the “evaluation of pharmacokinetics, regression line analysis, overall discrepancy rates, and clinical verification of breakpoints by clinical and bacteriological response rates” as specified by the CLSI (6). Torezolid broth microdilution MICs compared very favorably to agar dilution MICs when tested against the staphylococci, enterococci, and streptococci. The proposed torezolid quality control ranges for MIC testing have been accepted by the CLSI.
Acknowledgments
Financial support for this project was provided by Trius Pharmaceuticals Inc., San Diego, CA.
Footnotes
Published ahead of print on 15 March 2010.
REFERENCES
- 1.Brown, S. D., and M. M. Traczewski. 2008. Relative potency of TR-700, the active moiety of prodrug TR-701, against selected bacterial pathogens and provisional disk test criteria, abstr. F1-2069. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 2.Brunden, M. N., G. E. Zurenko, and B. Kapik. 1992. Modification of the error-bounded classification scheme for use with two MIC breakpoints. Diagn. Microbiol. Infect. Dis. 15:135-140. [DOI] [PubMed] [Google Scholar]
- 3.Choi, S., T. Lee, W. Im, J. Rhee, and W. Kim. 2004. Comparative antibacterial activity of DA-70218, a new oxazolidinone, abstr F-1417. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 4.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement. M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Clinical and Laboratory Standards Institute. 2008. Development of in vitro susceptibility testing criteria and quality control parameters: approved standard, 3rd ed., M23-A3. Clinical and Laboratory Standards Institute, Wayne, PA.
- 7.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial disk susceptibility tests: approved standard M2-A9. Clinical and Laboratory Standards Institute, Wayne, PA.
- 8.Lee, K., J. H. Yum, D. Yong, Y. Chong, S. H. Choi, and J. Rhee. 2004. Comparative in vitro activity of DA-70157, a novel oxazolidinone, against recent clinical isolates of aerobic and anaerobic bacteria, abstr F-1419. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 9.Metzler, D. M., and R. M. DeHaan. 1974. Susceptibility tests of anaerobic bacteria statistical and clinical considerations. J. Infect. Dis. 130:588-594. [DOI] [PubMed] [Google Scholar]
- 10.Schaadt, R., D. Sweeney, D. Shinabarger, and G. Zurenko. 2007. The in vitro activity of TR-700: the active ingredient of the prodrug TR-701, a novel oxazolidinone antibacterial agent, abstr. F1-1687. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL. [DOI] [PMC free article] [PubMed]
- 11.Shaw, K. J., S. Poppe, R. Schaadt, V. Brown-Driver, J. Finn, C. M. Pillar, D. Shinabarger, and G. Zurenko. 2008. In vitro activity of TR-700, the antibacterial moiety of the prodrug TR-701, against linezolid-resistant strains. Antimicrob. Agents Chemother. 52:4442-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turnidge, J., and D. L. Paterson. 2007. Setting and revising antibacterial susceptibility breakpoints. Clin. Microbiol. Rev. 20:391-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vera-Cabrera, L., E. Gonzales, A. Rendon, J. Ocampo-Candiani, O. Welch, V. M. Velaques-Moreno, S. H. Choi, and C. Molina-Torres. 2006. In vitro activities of DA-7157 and DA-7218 against Mycobacterium tuberculosis and Nocardia brasiliensis. Antimicrob. Agents Chemother. 50:3170-3172. [DOI] [PMC free article] [PubMed] [Google Scholar]