Abstract
The current interest in malaria elimination has led to a renewed interest in drugs that can be used for mass administration to minimize malaria transmission. Primaquine (PQ) is the only generally available drug with a strong activity against mature Plasmodium falciparum gametocytes, the parasite stage responsible for transmission. Despite concerns about PQ-induced hemolysis in glucose-6-phosphate dehydrogenase (G6PD)-deficient individuals, a single dose of PQ may be safe and efficacious in clearing gametocytes that persist after conventional treatment. As part of a mass drug intervention, we determined the hemolytic effect of sulfadoxine-pyrimethamine (SP) plus artesunate (AS) plus a single dose of primaquine (PQ; 0.75 mg/kg of body weight) in children aged 1 to 12 years. Children were randomized to receive SP+AS+PQ or placebo; those with a hemoglobin (Hb) level below 8 g/dl were excluded from receiving PQ and received SP+AS. The Hb concentration was significantly reduced 7 days after SP+AS+PQ treatment but not after placebo or SP+AS treatment. This reduction in Hb was most pronounced in G6PD-deficient (G6PD A−) individuals (−2.5 g/dl; 95% confidence interval [95% CI], −1.2 to −3.8 g/dl) but was also observed in heterozygotes (G6PD A) (−1.6 g/dl; 95% CI, −0.9 to −2.2 g/dl) and individuals with the wild-type genotype (G6PD B) (−0.5 g/dl; 95% CI, −0.4 to −0.6 g/dl). Moderate anemia (Hb level of <8 g/dl) was observed in 40% (6/15 individuals) of the G6PD A−, 11.1% (3/27 individuals) of the G6PD A, and 4.5% (18/399 individuals) of the G6PD B individuals; one case of severe anemia (Hb level of <5 g/dl) was observed. PQ may cause moderate anemia when coadministered with artemisinins, and excluding individuals based on G6PD status alone may not be sufficient to prevent PQ-induced hemolysis.
Primaquine (PQ) is receiving renewed attention in light of its potential role in malaria elimination strategies (22, 45, 49). Malaria elimination is back on the agenda after recent reports of substantial reductions in Plasmodium falciparum malaria transmission intensity throughout the African continent (5, 8, 14, 31). Wide-scale implementation of artemisinin-based combination therapy (ACT) has had a beneficial effect on the burden of malaria in several areas (5, 8), through its high cure rates (1, 9, 39, 41) and activity against sexual-stage parasites that ensure malaria transmission (9, 39, 41). However, the effect of ACT against sexual-stage parasites is probably largely restricted to immature gametocytes (33), and mature infectious gametocytes may persist for several weeks after ACT treatment (9, 37). As a consequence, ACT reduces but does not prevent posttreatment malaria transmission (9, 39, 41). The addition of a more efficacious gametocytocidal drug may be of value in situations where antimalarial drugs are employed for control programs that specifically aim to reduce or eliminate malaria transmission and where the force of transmission is sufficiently great that ACT in combination with vector control measures will not lead to elimination.
PQ is an 8-aminoquinolone that is widely used for the radical treatment of the liver hypnozoites of Plasmodium vivax parasites (26, 42). It also actively clears mature P. falciparum gametocytes (12, 37, 44). PQ has been suggested to be of added value to ACT in clearing P. falciparum gametocytes (37, 44, 45, 49) and was previously recommended as a useful addition to schizontocidal drugs to reduce malaria transmission (47, 49). A recently published mathematical model suggests that interventions with an antimalarial drug combination containing PQ may result in the interruption of malaria transmission (25). PQ may therefore also be a valuable component of mass drug interventions that aim to eliminate malaria. One major concern that complicates wide-scale use of PQ is its potential hemolytic side effect (37, 42, 45, 47). This hemolytic effect may be most pronounced in individuals receiving PQ in high doses (3, 42) or over several days (3, 17), and clinically important hemolysis is generally thought to be restricted to individuals who are glucose-6-phosphate dehydrogenase (G6PD) deficient (17, 42, 45, 47), which is a common genetic variant in areas where malaria is endemic (35). However, even a single dose of PQ (0.75 mg/kg of body weight; 45 mg for adults) may result in a transient reduction in hemoglobin levels (37), or possibly in severe anemia (32, 34). If clinically significant hemolysis with single-dose PQ is restricted to G6PD-deficient individuals and near-patient phenotypic G6PD testing becomes feasible (40), it will potentially be possible to test and exclude G6PD-deficient individuals in mass drug interventions. If mass drug interventions combine ACT with PQ, it is important to acknowledge the possibility that artemisinins could potentiate the hemolytic effects of PQ. This may have been the basis for the significant hemolysis seen with the (now-withdrawn) chlorproguanil-dapsone (CD)-plus-artesunate (AS) combination (21). Here we describe a study undertaken as part of a large-scale mass drug intervention with sulfadoxine-pyrimethamine plus artesunate followed by a single dose of PQ (SP+AS+PQ). Since hemolysis is the major practical limitation of PQ, we studied the impact of the drug combination on hemoglobin concentrations in relation to G6PD deficiency and other factors that may be associated with drug-induced hemolysis.
MATERIALS AND METHODS
This study was conducted in February and March 2008 as part of a large mass drug administration study in the area of Lower Moshi, northern Tanzania (latitude, 3°61′ to 3°68′S; longitude, 37°32′ to 37°38′E). Malaria transmission in this area is low, with an estimated entomologic inoculation rate (EIR) of 3.4 (95% confidence interval [95% CI], 0.7 to 9.9) infectious bites per person per year in 2004 (30). Transmission in the area is largely restricted to the long rainy season (May to July) and an unpredictable short rainy season (October to November). In a cross-sectional survey that preceded the mass drug administration, a microscopic P. falciparum parasite prevalence of 0.62% (95% CI, 0.13 to 1.8%) was detected.
Intervention.
In the mass drug intervention, 16 geographically defined clusters of households were randomized at a ratio of 1:1 to receive either placebo (once daily over 3 days) or a gametocytocidal drug combination that consisted of (i) sulfadoxine-pyrimethamine (25 mg sulfadoxine plus 1.25 mg/kg as a single dose on the first day; Fansidar, Roche, Switzerland), (ii) artesunate (AS) (4 mg/kg/day for 3 days; Arsumax, Sanofi, France), and (iii) primaquine (0.75 mg/kg as a single dose on the third day of treatment, in conjunction with the last dose of AS; Tiofarma BV, Netherlands) (37). Children of less than 1 year of age were excluded from the intervention. A total of 2,437 individuals received placebo (951 of whom were <12 years of age), and 2,164 received gametocytocidal drugs (926 of whom <12 years of age). PQ was produced as 7.5- and 15-mg tablets following the regulations of the European Pharmacopeia and were tested for content and stability. Treatment was given regardless of the presence of parasites, and treatment was observed directly by field workers. If vomiting occurred within 20 min, a replacement dose was administered. In case of repeated vomiting, the child was excluded. Dosing was based on age because weighing individuals was considered unfeasible under field conditions, where rapid administration of drugs relied on small teams visiting individual households. Treatment teams consisted of one clinically qualified person (clinician, nurse, or health officer) and one or two fieldworkers. The weight-age relationship was determined based on data from two cross-sectional studies that were conducted in 2005 and included a total of 2,752 inhabitants (38). Drugs were administered as described in Table 1. Individual written consent was obtained from all literate adults; an independent literate adult witness gave consent for illiterate adults. Parents/caretakers gave consent for their children. Ethical approval was given by the ethical board of the Tanzanian National Institute of Medical Research (NIMR/HQ/R.8a/Vol. IX/491) and the Tanzanian Food and Drug Authority (CE.57/180/01/8). The protocol for the mass drug administration study was registered online at Clinical Trials.gov (http://clinicaltrials.gov/ct2/show/NCT00509015).
TABLE 1.
Dosing scheme for intervention with sulfadoxime-pyrimethamine plus artesunate plus primaquinea
| Age (yr) | Weight (kg) (IQR)b | Gametocytocidal drug combination |
||
|---|---|---|---|---|
| Sulfadoxine (mg)/ pyrimethamine (mg) | Artesunate (mg) | Primaquine (mg) | ||
| 1-2 | 11.0 (9.5-11.6) | 250/12.5 | 25 | 7.5 |
| 3-4 | 12.9 (11.5-14.5) | 500/25 | 50 | 15 |
| 5-7 | 16.7 (14.8-19.0) | 500/25 | 75 | 15 |
| 8-11 | 23.0 (20.5-27.1) | 750/37.5 | 100 | 22.5 |
All individuals receiving placebo were given a dose of 1 tablet. Sulfadoxine-pyrimethamine and artesunate were administered at the nearest half tablet, and primaquine was administered as a combination of 7.5-mg and 15-mg tablets.
IQR, interquartile range (25th to 75th percentiles). The weights of different age groups were determined using previously published cross-sectional surveys of 2,752 inhabitants of the study population.
Safety.
The hemoglobin (Hb) concentration was determined by use of a HemoCue photometer (Angelholm, Sweden) for all children of <12 years of age randomized to receive SP+AS+PQ and for a random sample of children randomized to receive placebo. The HemoCue 201+ system has an internal electronic self test which automatically verifies the calibration of the instrument each time the analyzer is switched on and every second hour thereafter. The imprecision in Hb measurements was previously determined to be 0.75%, and the correlation coefficient with the ICSH international reference method was >0.99 (4). Individuals with an Hb level below 5 g/dl were excluded from the intervention and transported to the nearby Kilimanjaro Christian Medical Centre (KCMC) for blood transfusion and adequate clinical care. Those with an Hb level below 8 g/dl were excluded from PQ treatment, received only SP+AS, and were given hematinic drugs (ferrous sulfate and folic acid). This meant that of the 926 children of <12 years of age who were initially considered eligible for SP+AS+PQ, 37 were excluded from PQ treatment because of an Hb level of <8 g/dl and instead received SP+AS.
Sampling and follow-up.
The hematolytic effect of the drug combination was evaluated in a selection of children under 12 years of age who provided an additional day 7 finger-prick sample for Hb measurement and a filter paper DNA sample. The selection for this follow-up sampling was done prior to treatment and at the household level. Selected households were visited by a study team with a list of all individuals who had received treatment. All treated children were asked to donate a finger-prick blood sample, regardless of symptoms suggestive of anemia. Day 7 after initiation of treatment was chosen because hemolysis may last for ∼7 days after PQ treatment (23), and we previously observed that the reduction in hemoglobin concentration was most pronounced on day 7 (37). Because of the transient nature of PQ-induced reductions in hemoglobin concentration (37), individuals were not routinely followed up afterwards but were seen during the evaluation phase of the mass drug intervention, which continued for 5 months postintervention.
Sample size considerations.
The sample size for hemoglobin measurements was based on an estimated prevalence of G6PD A− individuals of 4% and of G6PD A individuals of 10% in the study area (36) and an expected proportion of 50% of the individuals with the G6PD A− genotype and 5% of the individuals with the G6PD B genotype experiencing hemolysis after treatment, defined as a reduction in Hb level of at least 20% compared to baseline values (37). Based on these assumptions, enrolling 500 individuals who were treated with SP+AS+PQ would result in an estimated 20 individuals with the G6PD A− genotype, 50 with the G6PD A genotype, and 430 with the G6PD B wild-type genotype, giving sufficient power to detect the expected difference between the G6PD A− and G6PD B groups (Zα = 1.96; Zβ = −0.84). To allow comparison with the placebo group, where no hemolysis was expected, we aimed at including 100 placebo-treated individuals. No formal randomization was done to include children in this ancillary study. A team of field workers visited the largest feasible number of randomly selected households for day 7 sampling without prior selection of individuals.
DNA extraction and genotyping.
DNA was extracted from filter paper by use of a Chelex 100-based method. G6PD deficiency was determined by screening human DNA for single nucleotide polymorphisms in the G6PD gene (G202A and A376G) by a simple high-throughput method using PCR, sequence-specific oligonucleotide probes (SSOPs), and enzyme-linked immunosorbent assay (ELISA)-based technology (20). Primers described by Mombo et al. (28) were used to amplify a 919-bp fragment of the G6PD gene covering the mutation site at codon 68. Individuals with no G202A mutation were classified as having the G6PD B genotype, heterozygotes for the G202A mutation were classified as having the G6PD A genotype, and homozygotes or hemizygotes (males) for the G202A mutation were classified as having the G6PD A− genotype. The prevalence of α+-thalassemia was determined by detection of the African α-globin deletion, α3.7, by PCR as described by Liu et al. (27). Glutathione S-transferase-μ (GSTM1) polymorphisms were determined by a multiplex PCR using primers described by Bröckmoller et al. (11). Beta-globin primers were included in the reaction mixture as an internal positive control. A 650-bp PCR product was expected for GSTM1, and the absence of this fragment indicated 2 null alleles (GSTM1-null). Sickle cell status was not determined in this study because of a low prevalence of this hemoglobinopathy in the study area. The mean prevalence of the HbAS genotype in the Kilimanjaro region was previously estimated to be 2.1% (21/990 individuals), and that of the HbSS genotype was estimated to be 0% (19; A. Manjurano et al., unpublished data). A pilot study in the study area confirmed this low prevalence (M. Daou et al., unpublished data).
Statistical analyses.
Data were double entered and imported into STATA 10.0 (Statacorp, TX) for statistical analysis. Hemoglobin concentrations were normally distributed, and values before and after treatment were compared by a paired-sample t test; values between treatment groups were compared by analysis of variance (ANOVA). The change in hemoglobin concentration after treatment was expressed as a percentage of the enrolment value. To determine a possible influence of relative overdosing, a variable was created that indicated whether children were in the lowest year of their age category (i.e., being a 1-year-old in the 1- to 2-year-old age group, a 3-year-old in the 3- to 4-year-old age group, etc.). Multivariate linear regression models were used to determine whether relative overdosing was independently associated with a change in Hb level. Factors with a P value of <0.1 in univariate models were included in a multivariate model and retained if the P value was <0.05 in the multivariate model. Multivariate regression models were also used to adjust the association between G6PD status and the change in Hb level for relative overdosing.
RESULTS
Baseline screening revealed six children with severe anemia (0.5% [6/1,106 children]) and 73 children with moderate anemia (7.1% [78/1,106 children]). Fourteen children vomited after receiving SP+AS+PQ, compared to 5 in the placebo arm and 0 in the SP+AS arm. Vomiting did not lead to exclusion from the study; no repeated vomiting occurred. A total of 734 children from 375 households were selected and asked to donate a second blood sample. Of these, 3.7% (22/587 children) refused in the SP+AS+PQ arm, 8.0% in the SP+AS arm (2/25 children), and 4.9% (6/122 children) in the placebo arm. As a result, combined day 0 and day 7 Hb measurements were available for 565 children who received SP+AS+PQ, 116 children who received placebo, and 23 children who received SP+AS. Baseline values and changes in Hb concentration after the intervention are presented in Table 2. In the placebo group, there was no change in Hb concentration 7 days after the intervention (mean difference, −0.05 g/dl [95% CI, −0.28 to 0.18 g/dl]; P = 0.65), and none of the children developed moderate or severe anemia in the week after administration of the placebo. In the group of individuals treated with SP+AS+PQ, there was a statistically significant reduction in Hb concentration on day 7 relative to that on day 0 (mean difference, −0.58 g/dl [95% CI, −0.46 to −0.71 g/dl]; P < 0.0001). In this group of children, all of whom had a baseline Hb concentration of ≥8 g/dl, one 5-year-old child developed severe anemia, with an Hb level of 4.8 g/dl compared with a baseline of 8.3 g/dl. This child was given hematinic drugs and recovered without the need for additional treatment (Hb concentration, 7.8 g/dl 2 weeks after initiation of the intervention and 12.3 g/dl 3 months after intervention). Children who developed moderate anemia were given hematinic drugs and were observed in the follow-up period, which continued for 5 months after the intervention. In a small group (n = 23) of individuals who were excluded from PQ treatment because their baseline Hb concentration was below 8 g/dl, we observed a statistically significant increase in Hb concentration between enrolment and day 7 after the initiation of treatment (mean difference, +0.62 g/dl [95% CI, +0.10 to +1.13 g/dl]; P = 0.02).
TABLE 2.
Baseline values and changes in Hb concentration in children who received placebo, SP+AS+PQ, or SP+AS
| Parameter | Placebo group | SP+AS+PQ group | SP+AS group |
|---|---|---|---|
| n | 116 | 564 | 23 |
| Age (yr) (median [IQR]) | 4 (2-6.5) | 5 (3-8) | 3 (2-4) |
| Gender (% male [no. of males/total no. of individuals]) | 47.4 (55/116) | 54.8 (309/564) | 56.5 (13/23) |
| Hb concn at enrolment (g/dl) (median [IQR]) | 11.3 (9.9-12.3) | 11.7 (10.4-12.8) | 6.7 (6.2-7.3) |
| Mean difference in Hb concn on day 7 vs that on day 0 (g/dl) (95% CI) | −0.05 (−0.28-+0.18) | −0.58 (−0.46-−0.71)a | +0.62 (+0.10-+1.13)b |
P < 0.0001.
P = 0.02.
Factors related to drug-induced hemolysis.
DNA samples were available for 562 children. The frequencies of G6PD deficiency were 8.4% (G6PD A heterozygotes; 95% CI, 6.1 to 10.7%) and 3.9% (G6PD A− homo-/hemizygotes; 95% CI, 2.3 to 5.5%). The prevalences of α+-thalassemia were 25.2% (αα/α− heterozygotes; 95% CI, 21.2 to 29.2%) and 2.4% (α−/α− homozygotes; 95% CI, 1.0 to 3.8%). The prevalence of the GSTM1 null genotype was 29.1% (95% CI, 25.2 to 32.9%). The change in Hb concentration in the intervention group was related to G6PD deficiency status (P = 0.0001), although a statistically significant reduction in Hb concentration was observed for all genotypes (Fig. 1). The mean change in Hb concentration compared to baseline values was −0.5 g/dl (95% CI, −0.4 to −0.6 g/dl) for children with the wild-type G6PD B genotype. The reduction in Hb concentration was significantly more pronounced in heterozygotes (G6PD A) (mean change, −1.6 g/dl [95% CI, −0.9 to −2.2 g/dl] compared to G6PD B group; P < 0.001) and in homozygote deficient children (G6PD A−) (mean change, −2.5 g/dl [95% CI, −1.2 to −3.8 g/dl] compared to G6PD B group; P < 0.001). Hb concentrations before and after treatment in individuals who developed moderate or severe anemia are shown in Fig. 2. Although the categorization has the disadvantage of classifying some minimal reductions in Hb as “development of moderate anemia” (Fig. 2, first observation), it illustrates reductions with potential clinical relevance. While all children had an Hb level of >8 g/dl prior to the intervention, 4.5% (18/399 children) of the G6PD B children became moderately anemic (Hb < 8 g/dl) after the intervention. For G6PD A and G6PD A− children, this proportion was 11.1% (3/27 children) and 40.0% (6/15 children), respectively. Compared to children with the G6PD B genotype, the relative risk of becoming moderately anemic after the intervention was 2.46 (95% CI, 0.77 to 7.84) for G6PD A children and 8.87 (95% CI, 4.12 to 19.09) for G6PD A− children. Despite the lower occurrence of moderate anemia in children with the G6PD A or G6PD B genotype, some children in each category experienced a considerable reduction in Hb concentration. The absolute number of children who became moderately anemic after the intervention was largest for children with the G6PD B genotype (Fig. 2). The previously mentioned child who developed severe anemia after the intervention had the G6PD A genotype. Hemolysis was not related to α+-thalassemia (P = 0.40) (Fig. 3A) or the GSTM1 polymorphism (P = 0.22) (Fig. 3B) and was not more common in G6PD B males than in G6PD B females (P = 0.23). In the placebo group, there was no relationship between a change in Hb concentration and any of the genetic polymorphisms studied (data not shown).
FIG. 1.
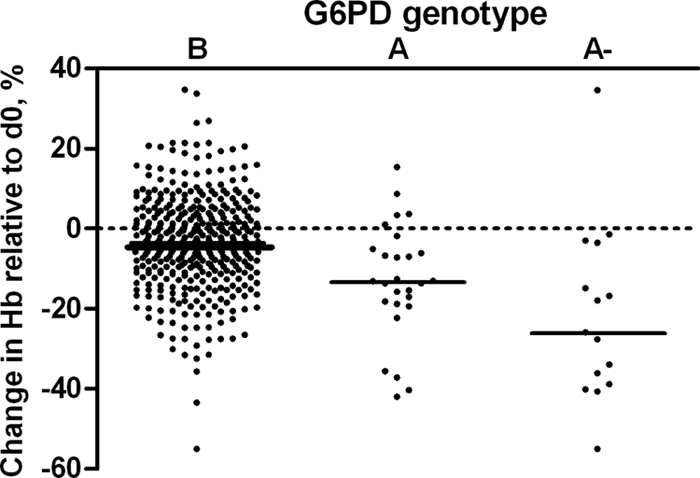
Hb concentration on day 7 after treatment with SP+AS+PQ, shown as a percentage of the baseline value in relation to G6PD genotype. There was a significant reduction in Hb concentration in G6PD B (P < 0.0001), G6PD A (P < 0.0001), and G6PD A− (P = 0.001) individuals on day 7 after treatment compared to that on day 0 (d0).
FIG. 2.
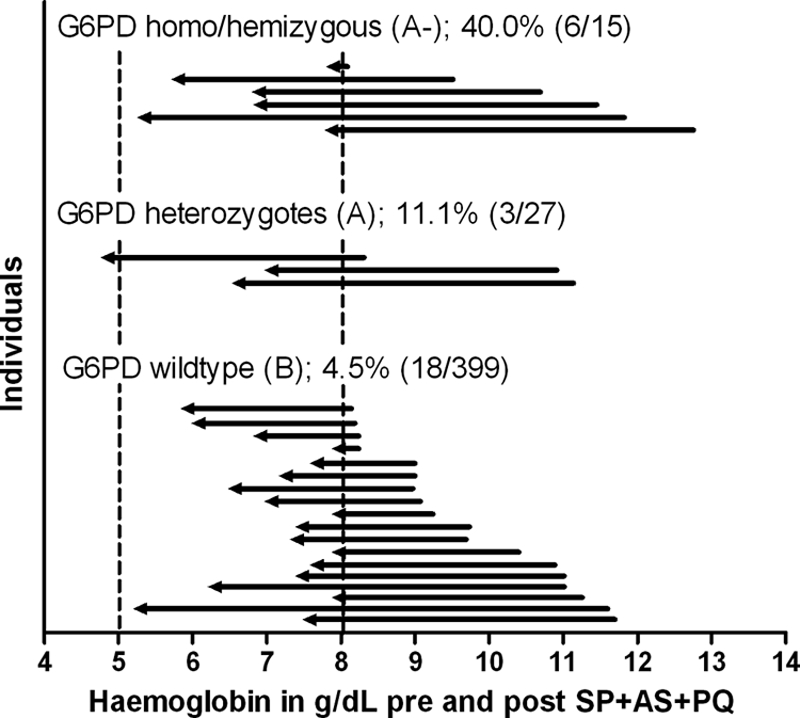
Hb concentrations before and after SP+AS+PQ treatment for those individuals who became moderately anemic (Hb level of <8 g/dl) after the intervention. On the x axis is the hemoglobin concentration in g/dl; on the y axis are individual observations for G6PD homo-/hemizygous individuals (6 of 15 individuals treated [40%] became moderately anemic), heterozygotes (3 of 27 individuals [11.1%]), and wild-type individuals (18 of 399 individuals [4.5%]). Arrows represent individual baseline (right) and postintervention (left) hemoglobin measurements. Dashed lines represent the values below which individuals are considered to have severe (5 g/dl) or moderate (8 g/dl) anemia.
FIG. 3.
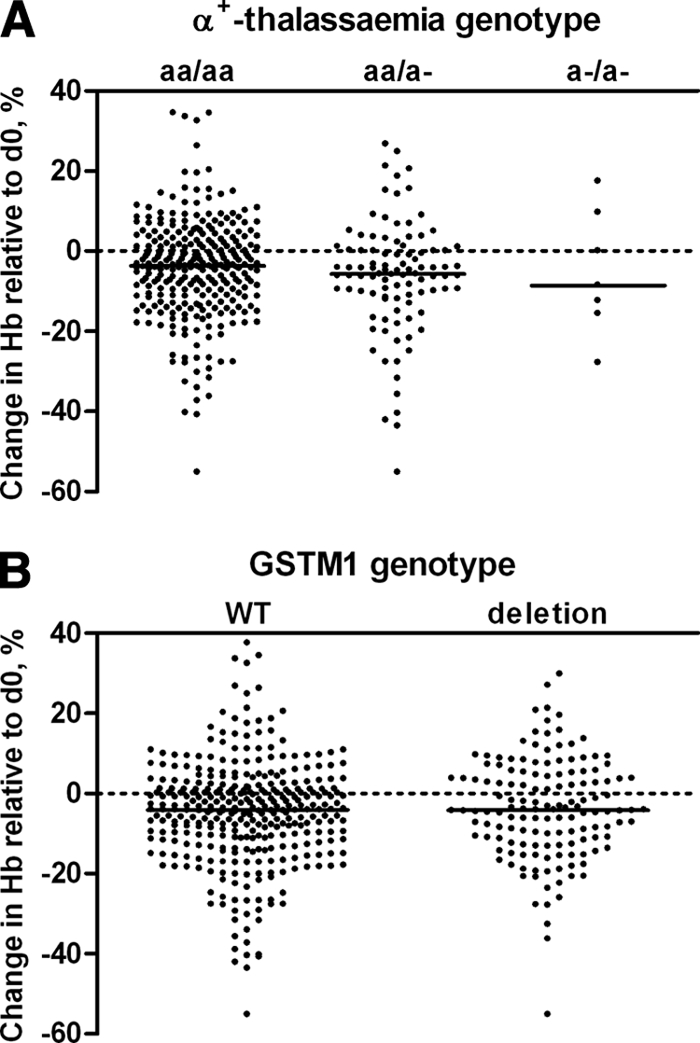
Hb concentration on day 7 after treatment with SP+AS+PQ, shown as a percentage of the baseline value in relation to α+-thalassemia genotype (A) or GSTM1 genotype (B). The reduction in Hb concentration was not related to α+-thalassemia genotype (P = 0.23) or GSTM1 genotype (P = 0.50).
Dosing based on age group creates the risk of giving children in the lower part of their age group a relatively high dose of drugs. This was examined by determining whether being in the lowest year of the age group was associated with a reduction in Hb concentration relative to that at enrolment. In children below 5 years of age, those who were in the lowest year of their age group had significant reductions in Hb levels that were independent of G6PD genotype or other genetic polymorphisms (Table 3). For older age groups, this increased risk of overdosing was not observed (P = 0.93), while the effect of G6PD deficiency was still apparent (Table 3).
TABLE 3.
Impact of G6PD status and being in the lower part of an age group on relative Hb level in children
| Patient group and genotype or portion of age group | Proportion (no. of children/total no. in group) | Coefficient β (95% CI) | P value |
|---|---|---|---|
| Children under 5 years of age | |||
| G6PD genotype | |||
| Wild type (B) | 87.7 (222/253) | Reference | |
| Heterozygote (A) | 7.9 (20/253) | −6.90 (−15.37-1.58) | 0.11 |
| Homozygote (A−) | 4.3 (11/253) | −14.64 (−24.84-−4.43) | 0.005 |
| Portion of age group | |||
| Not in lowest year of age group | 53.8 (136/253) | Reference | |
| In lowest year of age group | 46.2 (117/253) | −4.41 (−8.58-−0.23) | 0.04 |
| Children aged 5 to 11 years | |||
| G6PD genotype | |||
| Wild type (B) | 91.1 (234/257) | Reference | |
| Heterozygote (A) | 6.2 (16/257) | −12.22 (−17.81-−6.64) | <0.001 |
| Homozygote (A−) | 2.7 (7/257) | −21.40 (−29.45-−13.35) | <0.001 |
DISCUSSION
In this study, we observed a statistically significant reduction in Hb concentration in children after administration of a gametocytocidal drug combination containing PQ and an artemisinin. The observed hemolysis was strongly related to G6PD deficiency but was not restricted to individuals with the G6PD A− or A genotype. In the study area, with a moderate prevalence of the African-variant G6PD deficiency, the majority of children who developed moderate anemia were wild type at the G6PD A− allele. More than five percent of the children became moderately anemic after administration of the PQ combination. This is potentially important for mass drug administration both as part of attempts to eliminate falciparum malaria and in areas where vivax malaria is predominant, where coadministration of ACTs with PQ is increasingly likely (18).
This study provides evidence for reductions in Hb concentrations after a single dose of PQ, at least in the context of coadministration with SP+AS. Because the prevalence of the G6PD A− genotype was low in the current study population (∼4%) (36), because hemolysis in individuals with African-variant G6PD deficiency is generally milder than that in individuals with the Mediterranean variant (6), and because we excluded all children with moderate anemia, we did not expect any clinically relevant hemolysis in the current study. In contrast to our expectations, we observed considerable and statistically significant reductions in Hb concentrations, and >5% of the children became moderately anemic after the intervention with SP+AS+PQ. This reduction in Hb appeared transient (37), and none of the children needed hospitalization or experienced clinical consequences from the intervention in terms of clinical symptoms of anemia or life-threatening anemia. The asymptomatic nature of hemolysis is in line with previous studies where eight adult African-Americans with the G6PD A− variant developed mild, asymptomatic hemolysis (decrease in Hb of 0.5 to 2.5 g/dl) after weekly administration of 45 mg PQ for 8 weeks (10) and where 8 to 18% of the red cells of G6PD-deficient Thai adults were affected after a single dose of 45 mg PQ (15). This study was not designed to determine the hemolytic effect of PQ compared to antimalarial treatment without PQ. A reduction in Hb as a consequence of SP+AS treatment can therefore not be excluded (29). However, in line with our previous observations (37), we observed no hemolysis, but an increase in Hb concentration, in the small group of children who received only SP+AS. While this observation could be due partly to regression to the mean in individuals who had low baseline values (Hb < 8 g/dl), the increase was considerable and we found no indications for drug-induced hemolysis in either G6PD wild-type or three G6PD heterozygote individuals in this treatment arm. We therefore consider it plausible that the observed hemolysis was PQ induced. The combined treatment of PQ with artemisinins may have resulted in a more pronounced hemolytic effect of PQ, as was suggested for chlorproguanil-dapsone (CD). CD has a hemolytic effect when administered alone (2), and this effect may be more pronounced when CD is coadministered with AS (21), although another study suggested that the safety profile of CD was not influenced by coadministration with different doses of AS (46). Based on the current data, we therefore cannot exclude a potentiating effect of AS on the hematolytic effect of PQ; this would have required a study arm without AS.
The severity of hemolysis was highly variable between individuals and strongly related with G6PD genotype, being most pronounced in homo-/hemizygous (G6PD A−) individuals, less pronounced in heterozygotes (G6PD A), and least pronounced in individuals with the wild-type genotype (G6PD B). Even in G6PD B individuals, we observed a statistically significant reduction in Hb concentration after SP+AS+PQ treatment. Three percent of G6PD B children experienced a reduction in Hb of ≥3 g/dl (12/399 children), and 4.5% became moderately anemic. We hypothesized that other genetic factors would explain part of this hemolysis, but we observed no association with α+-thalassemia or the examined polymorphism in GSTM1, which is involved in protection against oxidative stress and has been implicated in various types of anemia (16). Relative overdosing was related to the degree of hemolysis in children below 5 years of age. This indicates that future studies should base treatment dose on weight rather than age and highlights a shortcoming of our approach. However, this apparent overdosing could not explain the hemolysis we observed in the G6PD B group. Our findings therefore suggest that other (genetic) factors play a role in PQ-induced hemolysis or that some G6PD-deficient individuals were not detected by our PCR-based screening. Direct measurement of enzyme activity, i.e., the NADPH production capacity of G6PD, can be used for diagnosis of all mutations associated with G6PD deficiency, while PCR determines only one mutation per set of primers. At present, 140 mutations are known in the corresponding DNA (7), and we screened only for one of the most prevalent mutations, G6PD A−. Although the G6PD A− genotype accounts for 90% of G6PD deficiency in Africa (13), it is possible that G6PD deficiency due to mutations other than G6PD A− explains part of the observed hemolysis. A recent study in Uganda reported that 30% of males who were enzymatically G6PD deficient were wild type for the G6PD A− allele (24). Although we found no evidence for an excess in G6PD A− allele-unrelated hemolysis in males, this finding indicates shortcomings of the PCR-based method to determine G6PD deficiency. Our study had several other shortcomings. Parasite carriage in the children was not determined prior to the intervention. A cross-sectional study of a selection of the population indicated a preintervention parasite prevalence of 0.6% by microscopy (S. A. Shekalaghe et al., unpublished data), making malaria-induced hemolysis a very unlikely explanation for hemolysis after the intervention. In addition, children were not routinely followed up after day 7 postintervention, and as a result, we have no detailed hematological data beyond this time point for all children, although we previously reported complete hematological recovery between day 7 and day 14 (37). The current data are not conclusive and are insufficient to lead to public health policy changes. The value of PQ in malaria transmission-reducing strategies depends on the individual risks in relation to the individual and community benefits, which both depend on the transmission setting. Substantial community benefits in terms of a reduction in the burden of malaria may justify side effects even if the immediate individual benefit is limited. In the current study, the immediate individual benefits of drug administration were negligible: only 0.6% of the individuals enrolled in the mass drug intervention were parasitemic. The community effects will be described in detail elsewhere (Shekalaghe et al., unpublished data); the current study describes solely the potential risk for the individual.
Together with a recently published study (37), our data suggest that PQ at the current dosing schedule may not be optimal for wide-scale implementation in combination with artemisinins, even in areas with a relatively low prevalence of G6PD deficiency. PCR-based screening for G6PD deficiency would not have been sufficient to prevent hemolysis in our study population, where ∼5% of the G6PD B children became moderately anemic after PQ. The importance of hematolytic side effects of antimalarial drugs was recently shown for CD, which was withdrawn from the market after hematological adverse effects in trial participants (48). Because of the potential role of PQ in combination with artemisinins in malaria elimination efforts (22, 25, 45, 49), we consider it important to conduct dose-finding studies to define a dosage of PQ that has gametocytocidal properties that are similar to those of PQ at 0.75 mg/kg (37) but is sufficiently low to prevent hemolytic side effects. Alternatively, gametocytocidal drugs with lower hematotoxicity, including the 8-aminoquinoline bulaquine (43), could be considered for malaria transmission-reducing interventions.
Acknowledgments
We thank the community and village elders of Lower Moshi for their participation in the study. We acknowledge the assistance of Paul Mrema, Charles Lukwaro, and Tumaini Azra. We also thank the National Institute for Medical Research (NIMR) for their support during the study.
This study was supported by NWO-WOTRO (W07.05.203.00) through APRIORI. Seif Shekalaghe was supported by NWO-WOTRO (WIZ93-465) through PRIOR, Teun Bousema was supported by a Rubicon fellowship of the Netherlands Organization for Scientific Research (NWO; Rubicon 825.08.025), and Chris Drakeley was supported by a grant from the Wellcome Trust (078925).
This study was conducted by PRIOR and the Joint Malaria Programme, a collaboration between the National Institute for Medical Research in Tanzania, Kilimanjaro Christian Medical Centre, London School of Hygiene and Tropical Medicine, and the University of Copenhagen.
Footnotes
Published ahead of print on 1 March 2010.
REFERENCES
- 1.Adjuik, M., A. Babiker, P. Garner, P. Olliaro, W. Taylor, and N. White. 2004. Artesunate combinations for treatment of malaria: meta-analysis. Lancet 363:9-17. [DOI] [PubMed] [Google Scholar]
- 2.Alloueche, A., W. Bailey, S. Barton, J. Bwika, P. Chimpeni, C. O. Falade, F. A. Fehintola, J. Horton, S. Jaffar, T. Kanyok, P. G. Kremsner, J. G. Kublin, T. Lang, M. A. Missinou, C. Mkandala, A. M. Oduola, Z. Premji, L. Robertson, A. Sowunmi, S. A. Ward, and P. A. Winstanley. 2004. Comparison of chlorproguanil-dapsone with sulfadoxine-pyrimethamine for the treatment of uncomplicated falciparum malaria in young African children: double-blind randomised controlled trial. Lancet 363:1843-1848. [DOI] [PubMed] [Google Scholar]
- 3.Alving, A. S., C. F. Johnson, A. R. Tarlov, G. J. Brewer, R. W. Kellermeyer, and P. E. Carson. 1960. Mitigation of the haemolytic effect of primaquine and enhancement of its action against exoerythrocytic forms of the Chesson strain of Plasmodium vivax by intermittent regimens of drug administration: a preliminary report. Bull. World Health Organ. 22:621-631. [PMC free article] [PubMed] [Google Scholar]
- 4.Back, S. E., C. G. M. Magnusson, L. K. Norlund, H. H. von Schenk, M. E. Menschik, and P. E. S. Lindberg. 2004. Multiple-site analytic evaluation of a new portable analyzer, HemoCue Hb 201+, for point-of-care testing. Point Care 3:60-65. [Google Scholar]
- 5.Barnes, K. I., D. N. Durrheim, F. Little, A. Jackson, U. Mehta, E. Allen, S. S. Dlamini, J. Tsoka, B. Bredenkamp, D. J. Mthembu, N. J. White, and B. L. Sharp. 2005. Effect of artemether-lumefantrine policy and improved vector control on malaria burden in KwaZulu-Natal, South Africa. PLoS Med. 2:e330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beutler, E., and S. Duparc. 2007. Glucose-6-phosphate dehydrogenase deficiency and antimalarial drug development. Am. J. Trop. Med. Hyg. 77:779-789. [PubMed] [Google Scholar]
- 7.Beutler, E., and T. J. Vulliamy. 2002. Hematologically important mutations: glucose-6-phosphate dehydrogenase. Blood Cells Mol. Dis. 28:93-103. [DOI] [PubMed] [Google Scholar]
- 8.Bhattarai, A., A. S. Ali, S. P. Kachur, A. Martensson, A. K. Abbas, R. Khatib, A. W. Al Mafazy, M. Ramsan, G. Rotllant, J. F. Gerstenmaier, F. Molteni, S. Abdulla, S. M. Montgomery, A. Kaneko, and A. Bjorkman. 2007. Impact of artemisinin-based combination therapy and insecticide-treated nets on malaria burden in Zanzibar. PLoS Med. 4:e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bousema, J. T., P. Schneider, L. C. Gouagna, C. J. Drakeley, A. Tostmann, R. Houben, J. I. Githure, R. Ord, C. J. Sutherland, S. A. Omar, and R. W. Sauerwein. 2006. Moderate effect of artemisinin-based combination therapy on transmission of Plasmodium falciparum. J. Infect. Dis. 193:1151-1159. [DOI] [PubMed] [Google Scholar]
- 10.Brewer, G. J., and C. J. Zarafonetis. 1967. The haemolytic effect of various regimens of primaquine with chloroquine in American Negroes with G6PD deficiency and the lack of an effect of various antimalarial suppressive agents on erythrocyte metabolism. Bull. World Health Organ. 36:303-308. [PMC free article] [PubMed] [Google Scholar]
- 11.Brockmoller, J., R. Kerb, N. Drakoulis, M. Nitz, and I. Roots. 1993. Genotype and phenotype of glutathione S-transferase class mu isoenzymes mu and psi in lung cancer patients and controls. Cancer Res. 53:1004-1011. [PubMed] [Google Scholar]
- 12.Butcher, G. A. 1997. Antimalarial drugs and the mosquito transmission of Plasmodium. Int. J. Parasitol. 27:975-987. [DOI] [PubMed] [Google Scholar]
- 13.Cappellini, M. D., and G. Fiorelli. 2008. Glucose-6-phosphate dehydrogenase deficiency. Lancet 371:64-74. [DOI] [PubMed] [Google Scholar]
- 14.Ceesay, S. J., C. Casals-Pascual, J. Erskine, S. E. Anya, N. O. Duah, A. J. Fulford, S. S. Sesay, I. Abubakar, S. Dunyo, O. Sey, A. Palmer, M. Fofana, T. Corrah, K. A. Bojang, H. C. Whittle, B. M. Greenwood, and D. J. Conway. 2008. Changes in malaria indices between 1999 and 2007 in The Gambia: a retrospective analysis. Lancet 372:1545-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charoenlarp, P., S. Areekul, T. Harinasuta, and P. Sirivorasarn. 1972. The haemolytic effect of a single dose of 45 mg of primaquine in G-6-PD deficient Thais. J. Med. Assoc. Thai. 55:631-638. [PubMed] [Google Scholar]
- 16.Chiang, W. L., Y. S. Hsieh, S. F. Yang, T. A. Lu, and S. C. Chu. 2007. Differential expression of glutathione-S-transferase isoenzymes in various types of anemia in Taiwan. Clin. Chim. Acta 375:110-114. [DOI] [PubMed] [Google Scholar]
- 17.Clyde, D. F. 1981. Clinical problems associated with the use of primaquine as a tissue schizontocidal and gametocytocidal drug. Bull. World Health Organ. 59:391-395. [PMC free article] [PubMed] [Google Scholar]
- 18.Dao, N. V., B. T. Cuong, N. D. Ngoa, T. T. Thuyle, N. D. The, D. N. Duy, B. Dai, N. X. Thanh, M. Chavchich, K. H. Rieckmann, and M. D. Edstein. 2007. Vivax malaria: preliminary observations following a shorter course of treatment with artesunate plus primaquine. Trans. R. Soc. Trop. Med. Hyg. 101:534-539. [DOI] [PubMed] [Google Scholar]
- 19.Enevold, A., M. Alifrangis, J. J. Sanchez, I. Carneiro, C. Roper, C. Borsting, J. Lusingu, L. S. Vestergaard, M. M. Lemnge, N. Morling, E. Riley, and C. J. Drakeley. 2007. Associations between alpha+-thalassemia and Plasmodium falciparum malarial infection in northeastern Tanzania. J. Infect. Dis. 196:451-459. [DOI] [PubMed] [Google Scholar]
- 20.Enevold, A., L. S. Vestergaard, J. Lusingu, C. J. Drakeley, M. M. Lemnge, T. G. Theander, I. C. Bygbjerg, and M. Alifrangis. 2005. Rapid screening for glucose-6-phosphate dehydrogenase deficiency and haemoglobin polymorphisms in Africa by a simple high-throughput SSOP-ELISA method. Malar. J. 4:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fanello, C. I., C. Karema, P. Avellino, G. Bancone, A. Uwimana, S. J. Lee, U. d'Alessandro, and D. Modiano. 2008. High risk of severe anaemia after chlorproguanil-dapsone+artesunate antimalarial treatment in patients with G6PD (A−) deficiency. PLoS One 3:e4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenwood, B. M. 2008. Control to elimination: implications for malaria research. Trends Parasitol. 24:449-454. [DOI] [PubMed] [Google Scholar]
- 23.Hill, D. R., J. K. Baird, M. E. Parise, L. S. Lewis, E. T. Ryan, and A. J. Magill. 2006. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis I. Am. J. Trop. Med. Hyg. 75:402-415. [PubMed] [Google Scholar]
- 24.Johnson, M. K., T. D. Clark, D. Njama-Meya, P. J. Rosenthal, and S. Parikh. 2009. Impact of the method of G6PD deficiency assessment on genetic association studies of malaria susceptibility. PLoS One 4:e7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawpoolsri, S., E. Y. Klein, P. Singhasivanon, S. Yimsamran, N. Thanyavanich, W. Maneeboonyang, L. L. Hungerford, J. H. Maguire, and D. L. Smith. 2009. Optimally timing primaquine treatment to reduce Plasmodium falciparum transmission in low endemicity Thai-Myanmar border populations. Malar. J. 8:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leslie, T., I. Mayan, N. Mohammed, P. Erasmus, J. Kolaczinski, C. J. Whitty, and M. Rowland. 2008. A randomised trial of an eight-week, once weekly primaquine regimen to prevent relapse of plasmodium vivax in Northwest Frontier Province, Pakistan. PLoS One 3:e2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, Y. T., J. M. Old, K. Miles, C. A. Fisher, D. J. Weatherall, and J. B. Clegg. 2000. Rapid detection of alpha-thalassaemia deletions and alpha-globin gene triplication by multiplex polymerase chain reactions. Br. J. Haematol. 108:295-299. [DOI] [PubMed] [Google Scholar]
- 28.Mombo, L. E., F. Ntoumi, C. Bisseye, S. Ossari, C. Y. Lu, R. L. Nagel, and R. Krishnamoorthy. 2003. Human genetic polymorphisms and asymptomatic Plasmodium falciparum malaria in Gabonese schoolchildren. Am. J. Trop. Med. Hyg. 68:186-190. [PubMed] [Google Scholar]
- 29.Obonyo, C. O., W. Taylor, H. Ekvall, A. Kaneko, F. Ter Kuile, P. Olliaro, A. Bjorkman, and A. J. Oloo. 2007. Effect of artesunate plus sulfadoxine-pyrimethamine on haematological recovery and anaemia, in Kenyan children with uncomplicated, Plasmodium falciparum malaria. Ann. Trop. Med. Parasitol. 101:281-295. [DOI] [PubMed] [Google Scholar]
- 30.Oesterholt, M. J., J. T. Bousema, O. K. Mwerinde, C. Harris, P. Lushino, A. Masokoto, H. Mwerinde, F. W. Mosha, and C. J. Drakeley. 2006. Spatial and temporal variation in malaria transmission in a low endemicity area in northern Tanzania. Malar. J. 5:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Meara, W. P., P. Bejon, T. W. Mwangi, E. A. Okiro, N. Peshu, R. W. Snow, C. R. Newton, and K. Marsh. 2008. Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet 372:1555-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pannacciulli, I., E. Salvidio, A. Tizianello, and G. Parravidino. 1969. Hemolytic effects of standard single dosages of primaquine and chloroquine on G-6-PD-deficient Caucasians. J. Lab. Clin. Med. 74:653-661. [PubMed] [Google Scholar]
- 33.Pukrittayakamee, S., K. Chotivanich, A. Chantra, R. Clemens, S. Looareesuwan, and N. J. White. 2004. Activities of artesunate and primaquine against asexual- and sexual-stage parasites in falciparum malaria. Antimicrob. Agents Chemother. 48:1329-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reeve, P. A., H. Toaliu, A. Kaneko, J. J. Hall, and M. Ganczakowski. 1992. Acute intravascular haemolysis in Vanuatu following a single dose of primaquine in individuals with glucose-6-phosphate dehydrogenase deficiency. J. Trop. Med. Hyg. 95:349-351. [PubMed] [Google Scholar]
- 35.Ruwende, C., S. C. Khoo, R. W. Snow, S. N. Yates, D. Kwiatkowski, S. Gupta, P. Warn, C. E. Allsopp, S. C. Gilbert, and N. Peschu. 1995. Natural selection of hemi- and heterozygotes for G6PD deficiency in Africa by resistance to severe malaria. Nature 376:246-249. [DOI] [PubMed] [Google Scholar]
- 36.Shekalaghe, S., M. Alifrangis, C. Mwanziva, A. Enevold, S. Mwakalinga, H. Mkali, R. Kavishe, A. Manjurano, R. Sauerwein, C. Drakeley, and T. Bousema. 2009. Low density parasitaemia, red blood cell polymorphisms and Plasmodium falciparum specific immune responses in a low endemic area in northern Tanzania. BMC Infect. Dis. 9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shekalaghe, S., C. Drakeley, R. Gosling, A. Ndaro, M. van Meegeren, A. Enevold, M. Alifrangis, F. Mosha, R. Sauerwein, and T. Bousema. 2007. Primaquine clears submicroscopic Plasmodium falciparum gametocytes that persist after treatment with sulphadoxine-pyrimethamine and artesunate. PLoS One 2:e1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shekalaghe, S. A., J. T. Bousema, K. K. Kunei, P. Lushino, A. Masokoto, L. R. Wolters, S. Mwakalinga, F. W. Mosha, R. W. Sauerwein, and C. J. Drakeley. 2007. Submicroscopic Plasmodium falciparum gametocyte carriage is common in an area of low and seasonal transmission in Tanzania. Trop. Med. Int. Health 12:547-553. [DOI] [PubMed] [Google Scholar]
- 39.Sutherland, C. J., R. Ord, S. Dunyo, M. Jawara, C. J. Drakeley, N. Alexander, R. Coleman, M. Pinder, G. Walraven, and G. A. Targett. 2005. Reduction of malaria transmission to anopheles mosquitoes with a six-dose regimen of co-artemether. PLoS Med. 2:e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tantular, I. S., and F. Kawamoto. 2003. An improved, simple screening method for detection of glucose-6-phosphate dehydrogenase deficiency. Trop. Med. Int. Health 8:569-574. [DOI] [PubMed] [Google Scholar]
- 41.Targett, G., C. Drakeley, M. Jawara, L. von Seidlein, R. Coleman, J. Deen, M. Pinder, T. Doherty, C. Sutherland, G. Walraven, and P. Milligan. 2001. Artesunate reduces but does not prevent posttreatment transmission of Plasmodium falciparum to Anopheles gambiae. J. Infect. Dis. 183:1254-1259. [DOI] [PubMed] [Google Scholar]
- 42.Taylor, W. R., and N. J. White. 2004. Antimalarial drug toxicity: a review. Drug Saf. 27:25-61. [DOI] [PubMed] [Google Scholar]
- 43.Tekwani, B. L., and L. A. Walker. 2006. 8-Aminoquinolines: future role as antiprotozoal drugs. Curr. Opin. Infect. Dis. 19:623-631. [DOI] [PubMed] [Google Scholar]
- 44.Weerasinghe, K. L., G. Galappaththy, W. P. Fernando, D. R. Wickremasinghe, H. M. Faizal, and A. R. Wickremasinghe. 2002. A safety and efficacy trial of artesunate, sulphadoxine-pyrimethamine and primaquine in P falciparum malaria. Ceylon Med. J. 47:83-85. [DOI] [PubMed] [Google Scholar]
- 45.White, N. J. 2008. The role of anti-malarial drugs in eliminating malaria. Malar. J. 7(Suppl. 1):S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wootton, D. G., H. Opara, G. A. Biagini, M. K. Kanjala, S. Duparc, P. L. Kirby, M. Woessner, C. Neate, M. Nyirenda, H. Blencowe, Q. Dube-Mbeye, T. Kanyok, S. Ward, M. Molyneux, S. Dunyo, and P. A. Winstanley. 2008. Open-label comparative clinical study of chlorproguanil-dapsone fixed dose combination (Lapdap) alone or with three different doses of artesunate for uncomplicated Plasmodium falciparum malaria. PLoS One 3:e1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization. 2006. Guidelines for the treatment of malaria. WHO/HTM/MAL/2006.1108. World Health Organization, Geneva, Switzerland.
- 48.World Health Organization. 2008. Antimalarial chlorproguanil-dapsone (LapDap™) withdrawn following demonstration of posttreatment haemolytic anaemia in G6PD deficient patients in a phase III trial of chlorproguanil-dapsone-artesunate (Dacart™) versus artemether-lumefantrine (Coartem®) and confirmation of findings in a comparative trial of LapDap™ versus Dacart™. Information exchange system alert no. 117. QSM/MC/IEA.117. World Health Organization, Geneva, Switzerland.
- 49.World Health Organization. 2008. Global malaria control and elimination: report of a technical review. World Health Organization, Geneva, Switzerland.


