Abstract
Antibiotic exposure exerts strong selective pressure and is an important modifiable risk factor for antibiotic resistance. We aimed to identify the role of various antibiotics as risk factors for the isolation of extended-spectrum-β-lactamase (ESBL)-producing Klebsiella spp. in hospitalized patients at a tertiary-care hospital. A parallel multivariable model was created to compare two groups of cases with either nosocomially acquired ESBL- or non-ESBL-producing Klebsiella spp. to a common control group of hospitalized patients (a case-case-control design). Seventy-eight ESBL cases, 358 non-ESBL cases, and 444 controls were analyzed. Significant factors associated with the isolation of Klebsiella spp. were an age of >65 years, transfer from a health care facility, an intensive care unit (ICU) stay, and the presence of a comorbid malignancy or lung, hepatic, or renal disease. A propensity score was generated from the above, and our ability to discriminate between Klebsiella cases and controls (area under the receiver-operating-characteristic [ROC] curve, 0.78) was good. The ESBL phenotype was tightly linked with fluoroquinolone resistance (95% versus 18%, P < 0.001). Factors associated with isolation of ESBL Klebsiella spp. in a multivariable analysis, adjusting for the propensity score, included exposure to β-lactam-β-lactamase inhibitor combinations (odds ratio [OR], 10.17; 95% confidence interval [CI], 1.19 to 86.92) and to fluoroquinolones (OR, 2.86; 95% CI, 1.37 to 5.97). Exposure to broad-spectrum cephalosporins was statistically associated with ESBL Klebsiella spp. only among the subgroup of patients not treated with fluoroquinolones. In our institution, where the ESBL-producing-Klebsiella phenotype is coselected with fluoroquinolone resistance, fluoroquinolone and β-lactam-β-lactamase inhibitor combinations, rather than cephalosporins, are the main risk factors for ESBL isolates. Formulary interventions to limit the spread of ESBL-producing isolates should be tailored to each setting.
The emergence of nosocomially as well as community-acquired extended-spectrum-β-lactamase (ESBL)-producing Klebsiella spp. has increased sharply in recent years (5, 7, 42). In the early 2000s, a high proportion of ESBL-producing Gram-negative bacilli was reported, particularly from countries in South America, Asia, and the Mediterranean basin, reaching 50% in many parts of the world (54). In the United States, during 2003, 20.6% of K. pneumoniae isolates from patients in the intensive care units (ICU) of hospitals participating in the National Nosocomial Infections Surveillance (NNIS) System were not susceptible to broad-spectrum cephalosporins, and the great majority of them were likely ESBL producers (9, 51). This proportion of resistant isolates represented a 47% increase in the proportion of resistant isolates compared to similar data collected between 1998 and 2002.
ESBL producers are often multidrug-resistant strains exhibiting coresistance to other classes of antibiotics, including trimethoprim-sulfamethoxazole, aminoglycosides, and the fluoroquinolones (12, 50). Thus, very few treatment options are available for empirical as well as for definite therapy (28, 37). Infections caused by ESBL producers have been associated with severe adverse clinical outcomes, leading to increased mortality, prolonged hospital stay, and increased costs (49). These adverse outcomes have been related, at least in part, to a delay in effective therapy (1, 20, 41, 53).
Various modes of ESBL dissemination, including clonal spread, plasmid spread, and β-lactamase gene transfer via integrons, have been described (10, 32). From the individual-patient perspective, all of these events represent acquisition of the resistant determinant from an extrinsic source. Both dissemination and mutational events are likely to occur under antibiotic selection pressure (4, 18). Indeed, untoward emergence of bacterial resistance by antibiotic agents, referred to as “collateral damage,” is now being appreciated (34).
Several studies have examined the association between antibiotic use and ESBL production either at the group level (“ecological studies”) or at the individual level (“risk factor analysis”). Most studies have implicated broad-spectrum cephalosporins as the major class associated with ESBL production (2, 23, 30, 31, 38, 43, 44), whereas results regarding the risk associated with β-lactam-β-lactamase inhibitor combinations vary (15, 19). In recent years, the use of fluoroquinolones has increased dramatically in U.S. hospitals (33). This may have affected the spread of ESBL-producing organisms. Moreover, most of the previously published risk factor studies used a case-control design with patients who have the resistant form of the organism versus those who have the susceptible form of the organism. This may have created selection bias, which may have generated distorted effect estimates of association between resistance and antibiotic utilization (14, 16, 17). Alternatively, the case-case-control study design contrasts two separate case-control analyses within a single study, which enables more accurate identification of risk factors for antimicrobial-resistant pathogens (21). Thus, for example, one is able to identify variables specifically predictive of ESBL-producing Klebsiella spp. without violating epidemiological principles of control group selection (14). In this study, we aimed to study the impact of antibiotic use on the isolation of ESBL-producing Klebsiella species isolates in an era of high fluoroquinolone use, by using the case-case-control study design.
MATERIALS AND METHODS
Study design, populations, and definitions.
A case-case-control study design was used. Two concurrent retrospective analyses were performed on patients admitted to the Beth Israel Deaconess Medical Center, a 640-bed tertiary-care hospital in Boston, MA, from 1999 to 2001. Patients were eligible for inclusion in this study if they were admitted to the hospital's medical or surgical service. No pediatric, obstetrics, gynecology, or psychiatry patients were included. Data were extracted from the hospital's computerized clinical, administrative, pharmacy, and laboratory data repositories by using data management software (Microsoft Access). The first group of cases was defined as patients from whom ESBL-producing Klebsiella spp. were isolated. The second group of cases was defined as patients from whom non-ESBL-producing Klebsiella spp. were isolated. Patients with Klebsiella spp. isolated <48 h after hospitalization or from a nonclinical surveillance culture (e.g., stool or nares) were excluded. To diminish the effect of potential confounding due to infection control interventions, each group was compared to a common control group of hospitalized patients without Klebsiella infection (with a length of stay of at least 2 days) during the same study period. Controls were randomly chosen by allocating a random number to all eligible controls. Each patient was included as a subject only once, at the time of the initial positive culture. Klebsiella species isolates were identified to the species level and MICs determined using the Vitek 2 system (bioMérieux, Inc., Durham, NC). ESBL production of Klebsiella spp. was confirmed by double disk diffusion according to CLSI guidelines.
Risk factors.
For all subjects, information on demographics, comorbidities, and antibiotic exposures were obtained from the hospital's computerized administrative, pharmacy, and laboratory databases. Variables analyzed as possible risk factors included age, gender, transfer from another health care facility (hospital, chronic-care facility), surgical procedure or intensive care unit stay prior to isolation of the Klebsiella spp., and number of days at risk (days of hospitalization prior to isolation of Klebsiella spp. for cases or days of hospitalization for controls). Comorbidities were acquired from the International Classification of Diseases diagnostic codes (ninth revision) (56). Antibiotics administered during the hospitalization but prior to the isolation of a positive Klebsiella species culture were documented and categorized by agent (penicillins, aminoglycosides, fluoroquinolones, vancomycin, metronidazole, β-lactam-β-lactamase inhibitor combinations, carbapenems, and narrow- and broad-spectrum cephalosporins). All risk factors were analyzed as dichotomous variables, except for number of days at risk, which remained a continuous variable.
Statistical analysis.
Parallel case-control analyses of ESBL-producing Klebsiella species cases versus controls and non-ESBL-producing Klebsiella species cases versus controls were performed to evaluate the relationship between each variable and the likelihood of isolating the organism. Risk factors established prior to the nosocomial isolation of Klebsiella spp. were examined by univariate analysis. All analyses were then adjusted for numbers of days the patients were at risk by including number of days at risk as a covariate in all analyses. Odds ratios (OR) and 95% confidence intervals (CI) were calculated for binomial variables. Individual risk factors were identified using logistic-regression models adjusted for numbers of days at risk. To assess the impact of exposure to individual antimicrobial agents on the isolation of Klebsiella spp. and limit confounding by nonantimicrobial risk factors, a propensity score was constructed using nonantimicrobial variables. In essence, the propensity score represents a composite risk incorporating the nonantimicrobial covariates for each subject and was used in subsequent models to adjust for potential confounding (8, 24, 48).
Those factors preliminarily associated with Klebsiella spp. by the logistic-regression models adjusted for number of days at risk (P ≤ 0.10) were included by stepwise selection in a multivariable logistic-regression model (where 0.05 was set as the limit for the acceptance or removal of new terms) to determine the relative contribution of each variable to the final propensity score. Next, with ESBL-producing and non-ESBL-producing Klebsiella spp. considered separately as outcomes in two different models, individual antibiotics were analyzed using logistic-regression models adjusted for nonantibiotic risk factors using the propensity score and for number of days at risk. Since several antibiotic agents are often used to treat an individual patient (either concomitantly or sequentially), antibiotics significantly associated with Klebsiella spp. (P ≤ 0.05) were then included in multivariable logistic-regression models adjusted for the propensity score, again with either ESBL- or non-ESBL-producing Klebsiella spp. as the outcome.
Analyses were performed using STATA version 7.0 (STATA Corp., College Station, TX) and SAS, version 8.1 (SAS Institute, Cary, NC). The study was approved by the Committee on Clinical Investigation of Beth Israel Deaconess Medical Center.
RESULTS
Patient characteristics.
During the 3 years studied, 426 Klebsiella species cases (78 ESBL- and 358 non-ESBL-producing isolates) were enrolled. Of the Klebsiella species isolates, 343 were K. pneumoniae, 85 were K. oxytoca, 1 was K. ozaenae, and 7 were not identified to the species level. Four hundred forty-four randomly selected patients without Klebsiella infection, admitted to a medical or surgical service during the same time period and hospitalized at least 2 days, were included as controls. The median age of cases with Klebsiella infection was 69 years, the median number of days at risk was 8 (mean, 13.9), and 40.1% of the patients were male. Of Klebsiella species cultures, 8.5% were from blood, 46.1% from urine, and 31.7% from respiratory samples. The majority of ESBL-producing Klebsiella species isolates were from respiratory samples (41.0%), whereas the majority of non-ESBL-producing Klebsiella spp. were from urine (49.2%). The ESBL phenotype was tightly linked with fluoroquinolone resistance; 95% of the ESBL-producing Klebsiella spp. were also fluoroquinolone resistant versus only 18% of the non-ESBL-producing isolates (P < 0.001). The median age of controls was 62 years, the median number of days at risk was 5 days (mean, 7.1 days), and 42.3% of the controls were male. The distribution of patients' demographics, clinical characteristics, and antibiotic covariates by cases and controls is presented in Table 1.
TABLE 1.
Distribution of demographics, clinical characteristics, and antibiotic exposures of case and control groups
| Variable | No. (%) of: |
||
|---|---|---|---|
| Cases |
Controls (n = 444) | ||
| ESBL (n = 78) | Non-ESBL (n = 358) | ||
| Demographics | |||
| Age of >65 yra | 39 (50.00) | 210 (58.66) | 205 (46.17) |
| Male | 35 (44.87) | 140 (39.11) | 188 (42.34) |
| Transferred from health care facilitya | 58 (74.36) | 252 (70.39) | 241 (54.28) |
| Surgerya | 39 (50.00) | 171 (47.77) | 165 (37.16) |
| ICU staya | 60 (76.92) | 193 (53.91) | 72 (16.22) |
| Comorbidities | |||
| Cardiovascular disease | 50 (64.10) | 257 (71.79) | 254 (57.21) |
| Respiratory diseasea | 53 (67.95) | 149 (41.62) | 94 (21.17) |
| Renal diseasea | 20 (25.64) | 58 (16.20) | 25 (5.63) |
| Hepatic diseasea | 12 (15.38) | 49 (13.69) | 30 (6.76) |
| Transplant | 3 (3.85) | 5 (1.40) | 10 (2.25) |
| Malignancya | 8 (10.26) | 63 (17.60) | 56 (12.61) |
| Diabetes mellitus | 18 (23.08) | 74 (20.67) | 104 (23.42) |
| Antibiotic exposure | |||
| Broad-spectrum cephalosporins | 34 (43.59) | 50 (13.97) | 38 (8.56) |
| Narrow-spectrum cephalosporins | 12 (15.38) | 69 (19.27) | 88 (19.82) |
| Penicillins | 38 (48.72) | 79 (22.07) | 58 (13.06) |
| β-Lactam-β-lactamase inhibitor combinations | 21 (26.92) | 28 (7.82) | 7 (1.58) |
| Piperacillin | 1 (1.28) | 3 (0.84) | 2 (0.45) |
| Fluoroquinolones | 52 (66.67) | 86 (24.02) | 115 (25.90) |
| Aminoglycosides | 21 (26.92) | 36 (10.06) | 24 (5.41) |
| Clindamycin | 10 (12.82) | 21 (5.87) | 13 (2.93) |
| Metronidazole | 45 (57.69) | 86 (24.02) | 75 (16.89) |
| Trimethoprim-sulfamethoxazole | 6 (7.69) | 11 (3.07) | 22 (4.95) |
| Carbapenems | 2 (2.56) | 5 (1.40) | 3 (0.68) |
Nonantibiotic covariates finally included in the propensity score model.
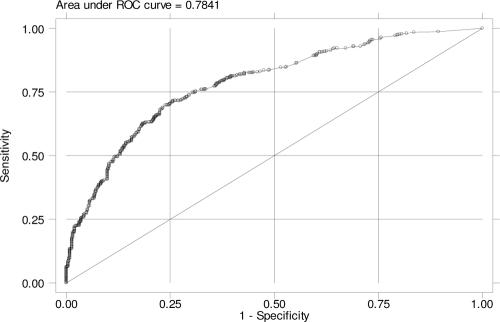
Using nonantibiotic variables, we constructed a propensity score predicting a patient's probability of being infected with Klebsiella spp. Of the nonantibiotic covariates ultimately included in the propensity-adjusted analysis, an age of >65 years, transfer from another health care facility, prior ICU stay, prior surgery, concomitant respiratory, renal, or hepatic disease, and concurrent malignancy were all associated (P < 0.05) with isolation of Klebsiella spp. (Table 2). The variable number of days at risk was included along with these covariates to generate the propensity score. Prior cardiovascular disease was removed from the final propensity-adjusted model, as it did not alter the effect estimates or improve the discriminatory power of the model whether or not it was included. The propensity score was generated from the above-named covariates with a good ability to discriminate between Klebsiella cases (both non-ESBL and ESBL producers) and controls (area under the receiver-operating-characteristic [ROC] curve, 0.78) (Fig. 1).
TABLE 2.
Time-at-risk-adjusted odds of nonantibiotic covariates for isolation of any Klebsiella species included in the propensity score model
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Age of >65 yr | 1.73 | 1.30-2.31 | 0.001 |
| Transfer from another health care facility | 2.21 | 1.22-4.01 | 0.009 |
| Surgery | 1.38 | 1.03-1.85 | <0.0001 |
| ICU stay | 11.74 | 6.35-21.69 | <0.0001 |
| Respiratory disease | 5.94 | 3.37-10.46 | <0.0001 |
| Renal disease | 4.63 | 2.25-9.54 | <0.0001 |
| Hepatic disease | 2.33 | 1.06-5.13 | 0.036 |
| Malignancy | 1.62 | 1.09-2.41 | 0.017 |
FIG. 1.
Propensity score-generated ROC curve.
Propensity score-adjusted analysis of antibiotic risk factors.
The results of propensity score-adjusted antibiotic risk factor analysis for the isolation of ESBL- and non-ESBL-producing Klebsiella spp. are presented in Table 3. Isolation of ESBL-producing Klebsiella spp. was associated with prior use of β-lactam-β-lactamase inhibitor combinations, penicillins, aminoglycosides, fluoroquinolones, and metronidazole. Of note, in this analysis, use of neither narrow- nor broad-spectrum cephalosporins was predictive of ESBL-producing Klebsiella species isolation, although a trend toward association (P = 0.07) was detected between the use of broad-spectrum cephalosporins and the isolation of ESBL-producing Klebsiella species. Bivariate analysis of non-ESBL-producing Klebsiella cases versus controls identified both fluoroquinolones and broad-spectrum cephalosporins as being protective against an outcome of isolation of non-ESBL-producing Klebsiella spp.
TABLE 3.
Propensity score-adjusted antibiotic risk factors for the isolation of ESBL- and non-ESBL-producing Klebsiella species from cases versus controls
| Variable (antibiotic exposure) | Bivariate result for the following comparisona: |
|||||
|---|---|---|---|---|---|---|
| ESBL vs control |
Non-ESBL vs control |
|||||
| OR | 95% CI | P | OR | 95% CI | P | |
| Broad-spectrum cephalosporins | 1.89 | 0.94-3.78 | 0.07 | 0.51 | 0.29-0.87 | 0.02 |
| Narrow-spectrum cephalosporins | 0.74 | 0.33-1.62 | 0.45 | 0.74 | 0.49-1.11 | 0.14 |
| Penicillins | 4.29 | 2.23-8.26 | <0.01 | 1.24 | 0.80-1.91 | 0.33 |
| β-Lactam-β-lactamase inhibitor combinations | 13.48 | 4.35-41.74 | <0.01 | 2.38 | 0.93-6.14 | 0.07 |
| Piperacillin | 2.94 | 0.20-44.13 | 0.44 | 1.33 | 0.17-10.66 | 0.79 |
| Fluoroquinolones | 2.52 | 1.37-4.64 | 0.01 | 0.37 | 0.25-0.56 | <0.01 |
| Aminoglycosides | 5.34 | 2.16-13.18 | <0.01 | 1.44 | 0.76-2.72 | 0.26 |
| Clindamycin | 2.30 | 0.75-7.03 | 0.15 | 1.64 | 0.73-3.69 | 0.23 |
| Metronidazole | 2.83 | 1.53-5.22 | <0.01 | 0.80 | 0.53-1.20 | 0.28 |
| Trimethoprim-sulfamethoxazole | 1.00 | 0.32-3.20 | 1.00 | 0.44 | 0.19-1.04 | 0.06 |
| Carbapenems | 0.46 | 0.07-3.28 | 0.44 | 0.32 | 0.07-1.42 | 0.13 |
Boldface indicates statistical significance.
Table 4 presents the results of the multivariable logistic-regression analysis of antibiotic risk factors for the outcome of ESBL-producing Klebsiella species isolation. When we adjusted for prediction variables, only exposure to β-lactam-β-lactamase inhibitor combinations and exposure to fluoroquinolone antibiotics were found to be independently associated (P < 0.05) with isolation of ESBL-Klebsiella spp. Additionally, we detected a significant effect modification between fluoroquinolones and broad-spectrum cephalosporins; i.e., the effect of broad-spectrum cephalosporins on the risk of isolation of ESBL-Klebsiella spp. differed between patients treated with fluoroquinolones and those who were not. Thus, a subanalysis was run to examine the effect of broad-spectrum cephalosporins on the isolation of ESBL-producing Klebsiella spp. after stratification for fluoroquinolone use. In this analysis, broad-spectrum cephalosporin use was found to be predictive of isolation of ESBL-producing Klebsiella spp. among the subgroup of 346 patients not exposed to fluoroquinolones (OR, 3.63; P = 0.025) and not among the subgroup of 167 fluoroquinolone-exposed patients (OR, 1.09; P = 0.45).
TABLE 4.
Multivariable model of antibiotic risk factors for the isolation of ESBL-producing Klebsiella spp. after adjustment for propensity score
| Variable (antibiotic exposure) | Isolation of ESBL-producing Klebsiella spp. |
||
|---|---|---|---|
| OR | 95% CI | Pa | |
| Penicillins | 1.50 | 0.65-3.52 | 0.33 |
| β-Lactam-β-lactamase inhibitor combinations | 10.17 | 1.19-86.92 | 0.03 |
| Fluoroquinolones | 2.86 | 1.37-5.97 | 0.01 |
| Aminoglycosides | 2.76 | 0.98-7.77 | 0.06 |
| Metronidazole | 1.74 | 0.85-3.57 | 0.13 |
Boldface indicates statistical significance.
DISCUSSION
Antibiotic resistance is an increasing clinical problem worldwide and a valid public health threat (42). ESBL-producing Enterobacteriaceae are prototypic examples of this threat, as these organisms are often multidrug resistant and only carbapenems are a proven effective therapy against them (37). Antibiotic pressure is a major determinant of the emergence and dissemination of antibiotic-resistant organisms (4). Moreover, it is among the few modifiable factors predisposing patients to infections with resistant organisms. Since antibiotic agents and classes may differ in their propensities to promote resistance (34), great interest exists in understanding the complex interaction between antibiotic use and the emergence and spread of resistance.
Data from ecological studies have been highly supportive of the association between cephalosporin use (particularly broad-spectrum cephalosporins) and ESBL resistance in Gram-negative bacilli (predominantly K. pneumoniae) (43). This association has been best displayed by interventional studies aimed at decreasing the use of cephalosporins, which were found to be effective in reducing the incidence of ESBL-producing K. pneumoniae (23, 26, 40, 44, 46). It has also been demonstrated that replacing cephalosporins with antibiotics containing β-lactamase inhibitors may help to reduce the occurrence of ESBL-producing organisms (26). While useful in providing cumulative data regarding antimicrobial use and resistance, ecological studies do not link individual exposure histories to individual outcome events. Therefore, elucidating causal relationship between antibiotic exposure and resistance based solely on aggregated data may yield distorted results, as has been demonstrated previously for Gram-negative bacilli (13). Moreover, most ecological studies regarding antibiotic resistance performed in the past did not use appropriate methodology, as has been discussed by the ORION group (52), and were subject to publication bias. We do not dismiss the evidence provided by previous ecological studies that the level of cephalosporins use is directly associated with the incidence of ESBL-producing Gram-negative bacilli. However, we think that these observations should be considered with caution.
The effect of antibiotic exposure on ESBL resistance has also been studied by several individual patient-level risk factor analyses, yielding diverse results. The use of ceftazidime (31), broad-spectrum cephalosporins and aminoglycosides (2), ciprofloxacin and/or trimethoprim-sulfamethoxazole (55), and cephalosporins, fluoroquinolones, and penicillins (11) was identified as a risk factor for isolation of ESBL-producing K. pneumoniae in different case-control studies. It is likely that differences in local epidemiology, e.g., nursing homes versus acute-care hospitals versus a community setting, explain part of the diversity of results. In addition, methodological differences, e.g., the definition of case patients and the selection of control groups, may lead to selection bias in some studies and contribute to the nonuniformity of the results, as has been discussed in detail by D. L. Paterson (35).
In order to avoid selection bias and to allow us to control for confounding elements, we used the case-case-control study design and compared in parallel two groups of case patients (i.e., those with ESBL- or non-ESBL-producing Klebsiella spp.) to a common control group of hospitalized patients without Klebsiella infection (21). This study design, as has been discussed previously, is considered to be an effective and more accurate tool to identify risk factors for resistant organisms than the standard case-control study design (35). To further control for confounding, we created a propensity score, which expressed well the individual composite risk of being a Klebsiella case (area under the ROC curve, 0.78), and included it in the multivariate analysis examining the relation between antibiotic exposure and isolation of ESBL- and non-ESBL-producing Klebsiella spp. After adjustment for the nonantimicrobial risk factors, the only antibiotics that were independently associated with the isolation of ESBL-producing Klebsiella spp. included β-lactam-β-lactamase inhibitor combinations and fluoroquinolones. Of note, exposure to broad-spectrum cephalosporins was associated with ESBL-producing Klebsiella spp. only among the subgroup of patients not treated with fluoroquinolones.
The association between fluoroquinolone resistance and ESBL-producing Escherichia coli and K. pneumoniae has been explored in previous studies (29). It is estimated that, worldwide, more than half of ESBL-producing E. coli and Klebsiella species isolates may be fluoroquinolone resistant (33, 55). Likewise, in a multicenter study of K. pneumoniae bacteremia, 60% of the ciprofloxacin-resistant isolates were also identified as ESBL producers (39). Indeed, among our study population, the ESBL phenotype was tightly linked with fluoroquinolone resistance (95% of the ESBL-producing Klebsiella spp. were also fluoroquinolone resistant versus only 18% of the non-ESBL-producing isolates [P < 0.001]).
Fluoroquinolone antimicrobials have a strong impact on the gastrointestinal flora; typical changes include a strong suppression of the Gram-negative facultative bacteria of the lower intestinal flora that is much more prominent and prolonged than that of other antibacterials (25, 45). Prior fluoroquinolone use indeed is a known risk factor for isolation of quinolone-resistant Gram-negative bacteria (27, 29).
The two aforementioned factors, i.e., ESBL and quinolone coresistance and the impact of fluoroquinolones on the gastrointestinal flora, provide a potential explanation for our findings that exposure to fluoroquinolones acts as an independent risk factor for isolation of ESBL-producing Klebsiella spp. but that exposure to cephalosporins acts as an independent risk factor only in the subgroup of patients not exposed to fluoroquinolones.
Another case-double-control study by Rodriguez-Bano et al. identified the use of fluoroquinolones as well as oxyimino-β-lactams as an independent risk factor for bloodstream infections caused by ESBL-producing E. coli (47). Since most non-ESBL-producing Klebsiella isolates are susceptible to cephalosporins and fluoroquinolones, infection during antibiotic treatment is unlikely with susceptible strains; thus, these agents are protective toward the outcome of non-ESBL-producing Klebsiella species isolation. Certainly, molecular data and clonality, which could shed more light on the mechanisms explaining the associations found, are missing.
The association between β-lactam-β-lactamase inhibitor combinations and ESBL-producing Klebsiella isolation in the present study is remarkable, particularly since in the multivariable analysis the effect estimate associated with β-lactam-β-lactamase inhibitor combinations was the greatest among all of the agents studied. β-Lactamase inhibitors currently in clinical use (clavulanate, sulbactam, and tazobactam) are Ambler class A enzyme inhibitors, yet ESBL-producing strains in most parts of the world show high rates of resistance to β-lactam-β-lactamase inhibitor combinations (3, 50). This is likely related to cocarriage of OXA enzymes (which are not inhibited by the Ambler class A enzyme inhibitors) on the ESBL plasmid, hyperproduction of the ESBL enzyme, or coresidence of non-ESBL enzymes, such as plasmid-encoded Ambler class C β-lactamases, potentially in combination with a porin mutation or, much less frequently, in combination with an inhibitor resistance mutation in the ESBL itself (e.g., the complex mutant TEM β-lactamases) (6, 36). Indeed, emergence of resistance and treatment failure during β-lactam-β-lactamase inhibitor combination treatment has been reported (57). Studies of the effects of these agents as risk factors for ESBL vary; some have described them as protective (19) while others have described them as a significant risk factor (15). This discrepancy may be related to geographical changes in ESBLs and cocarried resistance genes and differences in local usage patterns of specific inhibitor combinations, as well as to differences in study designs and choice of control groups. During this time period at the study institution, about half of the ESBL-producing Klebsiella isolates were susceptible to piperacillin-tazobactam, whereas none were susceptible to ampicillin-sulbactam (amoxicillin-clavulanate was not routinely tested). Due to data extraction limitations, we were not able to differentiate between specific inhibitor combinations.
Our study has several limitations. First, it is a retrospective study; thus, some of the data may be incompletely reported. We do not believe that this is a major problem, as the data included in the study are routinely recorded in the patient chart. Second, categorization of prior antibiotic use was based on drug classes rather than the specific agent. While agents belonging to the same class may have different effects on resistance (22), most studies assume a uniform class effect; therefore, comparison is possible. We suggest that future studies should focus on this issue of within-class differences, obviously necessitating much larger data sets. Third, the unequal sizes of the two case groups result in reduced statistical power to detect variables as significant risk factors for the ESBL cases compared to the non-ESBL cases.
In conclusion, exposure to fluoroquinolones or to β-lactam-β-lactamase inhibitor combinations during hospitalization is a risk factor for ESBL-producing Klebsiella infections, while broad-spectrum cephalosporins are predictive of isolation of ESBL-producing Klebsiella spp. among hospitalized patients not treated with fluoroquinolones. Knowledge of patients' prior antimicrobial exposures may be helpful in selecting an empirical treatment of nosocomial Klebsiella species infections. Formulary interventions to limit the spread of ESBLs may need to be tailored based on the local epidemiology and risk factors.
Footnotes
Published ahead of print on 8 March 2010.
REFERENCES
- 1.Anderson, D. J., J. J. Engemann, L. J. Harrell, Y. Carmeli, L. B. Reller, and K. S. Kaye. 2006. Predictors of mortality in patients with bloodstream infection due to ceftazidime-resistant Klebsiella pneumoniae. Antimicrob. Agents Chemother. 50:1715-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asensio, A., A. Oliver, P. Gonzalez-Diego, F. Baquero, J. C. Perez-Diaz, P. Ros, J. Cobo, M. Palacios, D. Lasheras, and R. Canton. 2000. Outbreak of a multiresistant Klebsiella pneumoniae strain in an intensive care unit: antibiotic use as risk factor for colonization and infection. Clin. Infect. Dis. 30:55-60. [DOI] [PubMed] [Google Scholar]
- 3.Babini, G. S., and D. M. Livermore. 2000. Antimicrobial resistance amongst Klebsiella spp. collected from intensive care units in Southern and Western Europe in 1997-1998. J. Antimicrob. Chemother. 45:183-189. [DOI] [PubMed] [Google Scholar]
- 4.Baquero, F., M. C. Negri, M. I. Morosini, and J. Blazquez. 1998. Antibiotic-selective environments. Clin. Infect. Dis. 27(Suppl. 1):S5-S11. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Ami, R., M. J. Schwaber, S. Navon-Venezia, D. Schwartz, M. Giladi, I. Chmelnitsky, A. Leavitt, and Y. Carmeli. 2006. Influx of extended-spectrum beta-lactamase-producing enterobacteriaceae into the hospital. Clin. Infect. Dis. 42:925-934. [DOI] [PubMed] [Google Scholar]
- 6.Canton, R., M. I. Morosini, O. M. de la Maza, and E. G. de la Pedrosa. 2008. IRT and CMT beta-lactamases and inhibitor resistance. Clin. Microbiol. Infect. 14(Suppl. 1):53-62. [DOI] [PubMed] [Google Scholar]
- 7.Canton, R., A. Novais, A. Valverde, E. Machado, L. Peixe, F. Baquero, and T. M. Coque. 2008. Prevalence and spread of extended-spectrum beta-lactamase-producing Enterobacteriaceae in Europe. Clin. Microbiol. Infect. 14(Suppl. 1):144-153. [DOI] [PubMed] [Google Scholar]
- 8.Carmeli, Y., G. M. Eliopoulos, and M. H. Samore. 2002. Antecedent treatment with different antibiotic agents as a risk factor for vancomycin-resistant Enterococcus. Emerg. Infect. Dis. 8:802-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention, National Nosocomial Surveillance System. 2004. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am. J. Infect. Control 32:470-485. [DOI] [PubMed] [Google Scholar]
- 10.Chmelnitsky, I., Y. Carmeli, A. Leavitt, M. J. Schwaber, and S. Navon-Venezia. 2005. CTX-M-2 and a new CTX-M-39 enzyme are the major extended-spectrum beta-lactamases in multiple Escherichia coli clones isolated in Tel Aviv, Israel. Antimicrob. Agents Chemother. 49:4745-4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colodner, R., W. Rock, B. Chazan, N. Keller, N. Guy, W. Sakran, and R. Raz. 2004. Risk factors for the development of extended-spectrum beta-lactamase-producing bacteria in nonhospitalized patients. Eur. J. Clin. Microbiol. Infect. Dis. 23:163-167. [DOI] [PubMed] [Google Scholar]
- 12.Colodner, R., Z. Samra, N. Keller, H. Sprecher, C. Block, N. Peled, T. Lazarovitch, R. Bardenstein, O. Schwartz-Harari, and Y. Carmeli. 2007. First national surveillance of susceptibility of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella spp. to antimicrobials in Israel. Diagn. Microbiol. Infect. Dis. 57:201-205. [DOI] [PubMed] [Google Scholar]
- 13.Harbarth, S., A. D. Harris, Y. Carmeli, and M. H. Samore. 2001. Parallel analysis of individual and aggregated data on antibiotic exposure and resistance in gram-negative bacilli. Clin. Infect. Dis. 33:1462-1468. [DOI] [PubMed] [Google Scholar]
- 14.Harris, A. D., T. B. Karchmer, Y. Carmeli, and M. H. Samore. 2001. Methodological principles of case-control studies that analyzed risk factors for antibiotic resistance: a systematic review. Clin. Infect. Dis. 32:1055-1061. [DOI] [PubMed] [Google Scholar]
- 15.Harris, A. D., J. C. McGregor, J. A. Johnson, S. M. Strauss, A. C. Moore, H. C. Standiford, J. N. Hebden, and J. G. Morris, Jr. 2007. Risk factors for colonization with extended-spectrum beta-lactamase-producing bacteria and intensive care unit admission. Emerg. Infect. Dis. 13:1144-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris, A. D., M. H. Samore, and Y. Carmeli. 2000. Control group selection is an important but neglected issue in studies of antibiotic resistance. Ann. Intern. Med. 133:159. [DOI] [PubMed] [Google Scholar]
- 17.Harris, A. D., M. H. Samore, M. Lipsitch, K. S. Kaye, E. Perencevich, and Y. Carmeli. 2002. Control-group selection importance in studies of antimicrobial resistance: examples applied to Pseudomonas aeruginosa, enterococci, and Escherichia coli. Clin. Infect. Dis. 34:1558-1563. [DOI] [PubMed] [Google Scholar]
- 18.Hoyen, C. K., N. J. Pultz, D. L. Paterson, D. C. Aron, and C. J. Donskey. 2003. Effect of parenteral antibiotic administration on establishment of intestinal colonization in mice by Klebsiella pneumoniae strains producing extended-spectrum beta-lactamases. Antimicrob. Agents Chemother. 47:3610-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang, Y., S. Zhuang, and M. Du. 2007. Risk factors of nosocomial infection with extended-spectrum beta-lactamase-producing bacteria in a neonatal intensive care unit in China. Infection 35:339-345. [DOI] [PubMed] [Google Scholar]
- 20.Hyle, E. P., A. D. Lipworth, T. E. Zaoutis, I. Nachamkin, W. B. Bilker, and E. Lautenbach. 2005. Impact of inadequate initial antimicrobial therapy on mortality in infections due to extended-spectrum beta-lactamase-producing enterobacteriaceae: variability by site of infection. Arch. Intern. Med. 165:1375-1380. [DOI] [PubMed] [Google Scholar]
- 21.Kaye, K. S., A. D. Harris, M. Samore, and Y. Carmeli. 2005. The case-case-control study design: addressing the limitations of risk factor studies for antimicrobial resistance. Infect. Control Hosp. Epidemiol. 26:346-351. [DOI] [PubMed] [Google Scholar]
- 22.Kaye, K. S., Z. A. Kanafani, A. E. Dodds, J. J. Engemann, S. G. Weber, and Y. Carmeli. 2006. Differential effects of levofloxacin and ciprofloxacin on the risk for isolation of quinolone-resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 50:2192-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim, J. Y., J. W. Sohn, D. W. Park, Y. K. Yoon, Y. M. Kim, and M. J. Kim. 2008. Control of extended-spectrum β-lactamase-producing Klebsiella pneumoniae using a computer-assisted management program to restrict third-generation cephalosporin use. J. Antimicrob. Chemother. 62:416-421. [DOI] [PubMed] [Google Scholar]
- 24.Kim, S. H., W. B. Park, C. S. Lee, C. I. Kang, J. W. Bang, H. B. Kim, N. J. Kim, E. C. Kim, M. D. Oh, and K. W. Choe. 2006. Outcome of inappropriate empirical antibiotic therapy in patients with Staphylococcus aureus bacteraemia: analytical strategy using propensity scores. Clin. Microbiol. Infect. 12:13-21. [DOI] [PubMed] [Google Scholar]
- 25.Korten, V., and B. E. Murray. 1993. Impact of the fluoroquinolones on gastrointestinal flora. Drugs 45(Suppl. 3):125-133. [DOI] [PubMed] [Google Scholar]
- 26.Landman, D., M. Chockalingam, and J. M. Quale. 1999. Reduction in the incidence of methicillin-resistant Staphylococcus aureus and ceftazidime-resistant Klebsiella pneumoniae following changes in a hospital antibiotic formulary. Clin. Infect. Dis. 28:1062-1066. [DOI] [PubMed] [Google Scholar]
- 27.Lautenbach, E., J. P. Metlay, M. G. Weiner, W. B. Bilker, P. Tolomeo, X. Mao, I. Nachamkin, and N. O. Fishman. 2009. Gastrointestinal tract colonization with fluoroquinolone-resistant Escherichia coli in hospitalized patients: changes over time in risk factors for resistance. Infect. Control Hosp. Epidemiol. 30:18-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lautenbach, E., J. B. Patel, W. B. Bilker, P. H. Edelstein, and N. O. Fishman. 2001. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin. Infect. Dis. 32:1162-1171. [DOI] [PubMed] [Google Scholar]
- 29.Lautenbach, E., B. L. Strom, W. B. Bilker, J. B. Patel, P. H. Edelstein, and N. O. Fishman. 2001. Epidemiological investigation of fluoroquinolone resistance in infections due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Clin. Infect. Dis. 33:1288-1294. [DOI] [PubMed] [Google Scholar]
- 30.Lee, S. O., E. S. Lee, S. Y. Park, S. Y. Kim, Y. H. Seo, and Y. K. Cho. 2004. Reduced use of third-generation cephalosporins decreases the acquisition of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae. Infect. Control Hosp. Epidemiol. 25:832-837. [DOI] [PubMed] [Google Scholar]
- 31.Lin, M. F., M. L. Huang, and S. H. Lai. 2003. Risk factors in the acquisition of extended-spectrum beta-lactamase Klebsiella pneumoniae: a case-control study in a district teaching hospital in Taiwan. J. Hosp. Infect. 53:39-45. [DOI] [PubMed] [Google Scholar]
- 32.Navon-Venezia, S., I. Chmelnitsky, A. Leavitt, and Y. Carmeli. 2008. Dissemination of the CTX-M-25 family beta-lactamases among Klebsiella pneumoniae, Escherichia coli and Enterobacter cloacae and identification of the novel enzyme CTX-M-41 in Proteus mirabilis in Israel. J. Antimicrob. Chemother. 62:289-295. [DOI] [PubMed] [Google Scholar]
- 33.Neuhauser, M. M., R. A. Weinstein, R. Rydman, L. H. Danziger, G. Karam, and J. P. Quinn. 2003. Antibiotic resistance among gram-negative bacilli in US intensive care units: implications for fluoroquinolone use. JAMA 289:885-888. [DOI] [PubMed] [Google Scholar]
- 34.Paterson, D. L. 2004. “Collateral damage” from cephalosporin or quinolone antibiotic therapy. Clin. Infect. Dis. 38(Suppl. 4):S341-S345. [DOI] [PubMed] [Google Scholar]
- 35.Paterson, D. L. 2002. Looking for risk factors for the acquisition of antibiotic resistance: a 21st-century approach. Clin. Infect. Dis. 34:1564-1567. [DOI] [PubMed] [Google Scholar]
- 36.Paterson, D. L., and R. A. Bonomo. 2005. Extended-spectrum beta-lactamases: a clinical update. Clin. Microbiol. Rev. 18:657-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paterson, D. L., W. C. Ko, A. Von Gottberg, S. Mohapatra, J. M. Casellas, H. Goossens, L. Mulazimoglu, G. Trenholme, K. P. Klugman, R. A. Bonomo, L. B. Rice, M. M. Wagener, J. G. McCormack, and V. L. Yu. 2004. Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum beta-lactamases. Clin. Infect. Dis. 39:31-37. [DOI] [PubMed] [Google Scholar]
- 38.Paterson, D. L., W. C. Ko, A. Von Gottberg, S. Mohapatra, J. M. Casellas, H. Goossens, L. Mulazimoglu, G. Trenholme, K. P. Klugman, R. A. Bonomo, L. B. Rice, M. M. Wagener, J. G. McCormack, and V. L. Yu. 2004. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial infections. Ann. Intern. Med. 140:26-32. [DOI] [PubMed] [Google Scholar]
- 39.Paterson, D. L., L. Mulazimoglu, J. M. Casellas, W. C. Ko, H. Goossens, A. Von Gottberg, S. Mohapatra, G. M. Trenholme, K. P. Klugman, J. G. McCormack, and V. L. Yu. 2000. Epidemiology of ciprofloxacin resistance and its relationship to extended-spectrum beta-lactamase production in Klebsiella pneumoniae isolates causing bacteremia. Clin. Infect. Dis. 30:473-478. [DOI] [PubMed] [Google Scholar]
- 40.Patterson, J. E., T. C. Hardin, C. A. Kelly, R. C. Garcia, and J. H. Jorgensen. 2000. Association of antibiotic utilization measures and control of multiple-drug resistance in Klebsiella pneumoniae. Infect. Control Hosp. Epidemiol. 21:455-458. [DOI] [PubMed] [Google Scholar]
- 41.Pena, C., C. Gudiol, L. Calatayud, F. Tubau, M. A. Dominguez, M. Pujol, J. Ariza, and F. Gudiol. 2008. Infections due to Escherichia coli producing extended-spectrum beta-lactamase among hospitalised patients: factors influencing mortality. J. Hosp. Infect. 68:116-122. [DOI] [PubMed] [Google Scholar]
- 42.Pitout, J. D., and K. B. Laupland. 2008. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect. Dis. 8:159-166. [DOI] [PubMed] [Google Scholar]
- 43.Quale, J. M., D. Landman, P. A. Bradford, M. Visalli, J. Ravishankar, C. Flores, D. Mayorga, K. Vangala, and A. Adedeji. 2002. Molecular epidemiology of a citywide outbreak of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae infection. Clin. Infect. Dis. 35:834-841. [DOI] [PubMed] [Google Scholar]
- 44.Rahal, J. J., C. Urban, D. Horn, K. Freeman, S. Segal-Maurer, J. Maurer, N. Mariano, S. Marks, J. M. Burns, D. Dominick, and M. Lim. 1998. Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella. JAMA 280:1233-1237. [DOI] [PubMed] [Google Scholar]
- 45.Reeves, D. S. 1986. The effect of quinolone antibacterials on the gastrointestinal flora compared with that of other antibacterials. J. Antimicrob. Chemother. 18(Suppl. D):89-102. [DOI] [PubMed] [Google Scholar]
- 46.Rice, L. B., E. C. Eckstein, J. DeVente, and D. M. Shlaes. 1996. Ceftazidime-resistant Klebsiella pneumoniae isolates recovered at the Cleveland Department of Veterans Affairs Medical Center. Clin. Infect. Dis. 23:118-124. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez-Bano, J., M. D. Navarro, L. Romero, M. A. Muniain, M. Cueto, J. Galvez, E. J. Perea, and A. Pascual. 2008. Risk-factors for emerging bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Clin. Microbiol. Infect. 14:180-183. [DOI] [PubMed] [Google Scholar]
- 48.Rubin, D. B. 1997. Estimating causal effects from large data sets using propensity scores. Ann. Intern. Med. 127:757-763. [DOI] [PubMed] [Google Scholar]
- 49.Schwaber, M. J., S. Navon-Venezia, K. S. Kaye, R. Ben-Ami, D. Schwartz, and Y. Carmeli. 2006. Clinical and economic impact of bacteremia with extended-spectrum-beta-lactamase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 50:1257-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwaber, M. J., S. Navon-Venezia, D. Schwartz, and Y. Carmeli. 2005. High levels of antimicrobial coresistance among extended-spectrum-beta-lactamase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 49:2137-2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steward, C. D., J. K. Rasheed, S. K. Hubert, J. W. Biddle, P. M. Raney, G. J. Anderson, P. P. Williams, K. L. Brittain, A. Oliver, J. E. McGowan, Jr., and F. C. Tenover. 2001. Characterization of clinical isolates of Klebsiella pneumoniae from 19 laboratories using the National Committee for Clinical Laboratory Standards extended-spectrum beta-lactamase detection methods. J. Clin. Microbiol. 39:2864-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stone, S. P., B. S. Cooper, C. C. Kibbler, B. D. Cookson, J. A. Roberts, G. F. Medley, G. Duckworth, R. Lai, S. Ebrahim, E. M. Brown, P. J. Wiffen, and P. G. Davey. 2007. The ORION statement: guidelines for transparent reporting of outbreak reports and intervention studies of nosocomial infection. Lancet Infect. Dis. 7:282-288. [DOI] [PubMed] [Google Scholar]
- 53.Tumbarello, M., M. Sanguinetti, E. Montuori, E. M. Trecarichi, B. Posteraro, B. Fiori, R. Citton, T. D'Inzeo, G. Fadda, R. Cauda, and T. Spanu. 2007. Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob. Agents Chemother. 51:1987-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turner, P. J. 2005. Extended-spectrum beta-lactamases. Clin. Infect. Dis. 41(Suppl. 4):S273-S275. [DOI] [PubMed] [Google Scholar]
- 55.Wiener, J., J. P. Quinn, P. A. Bradford, R. V. Goering, C. Nathan, K. Bush, and R. A. Weinstein. 1999. Multiple antibiotic-resistant Klebsiella and Escherichia coli in nursing homes. JAMA 281:517-523. [DOI] [PubMed] [Google Scholar]
- 56.World Health Organization. 1977. Manual of international statistical classification of diseases, injuries, and causes of death, vol. 1. World Health Organization, Geneva, Switzerland.
- 57.Zimhony, O., I. Chmelnitsky, R. Bardenstein, S. Goland, O. Hammer Muntz, S. Navon Venezia, and Y. Carmeli. 2006. Endocarditis caused by extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae: emergence of resistance to ciprofloxacin and piperacillin-tazobactam during treatment despite initial susceptibility. Antimicrob. Agents Chemother. 50:3179-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]