Abstract
Artemisinin (ART)-based combination therapies (ACTs) are the first-line drugs—and often the last treatments—that can effectively cure Plasmodium falciparum infections. Unfortunately, the decreased clinical efficacy of artesunate, one of the major ART derivatives, was recently reported along the Thailand-Cambodia border. Through long-term artemisinin pressure in vitro, we have obtained an ART-tolerant strain that can survive extremely high doses of ART. We showed that drug pressure could induce a subpopulation of ring stages into developmental arrest, which can explain the ART tolerance in P. falciparum. We also observed interesting transcriptomic modifications possibly associated with the acquisition of ART tolerance. These modifications include the overexpression of heat shock and erythrocyte surface proteins and the downexpression of a cell cycle regulator and a DNA biosynthesis protein. This study highlights a new phenomenon in the Plasmodium response to ART that may explain the delayed clearance of parasites after artesunate treatment observed on the Thailand-Cambodia border and that provides important information for achieving a better understanding of the mechanisms of antimalarial resistance.
Plasmodium falciparum malaria remains the worst parasitic disease in developing countries, with nearly 200 million cases and 800,000 deaths being reported each year (28). Quinolines (mefloquine, amodiaquine, chloroquine, quinine) and antifolate (pyrimethamine, proguanil, sulfadoxine) have been the major antimalarial drugs in areas of endemicity for decades. Nevertheless, the emergence of multidrug-resistant P. falciparum parasites has made these drugs useless in many areas where they are endemic. This situation was particularly threatening, until the introduction of artemisinin (ART) and its derivatives. ART combination therapy (ACT) has become the first-line treatment of uncomplicated falciparum malaria in most countries where it is endemic. More than a million doses of ACT are administered to treat malaria each year, particularly after the recommendation of World Health Organization (WHO) in 2005 (26).
Artemisinin is isolated from a plant (Artemisia annua) that has been used for the treatment of fever for more than 2,000 years in Chinese traditional medicine. ART shows rapid antimalarial activity, has few side effects, and is active against parasite strains that are resistant to many traditional antimalarial drugs, such as quinolines and antifolates. To reduce the risk of drug resistance, ART and its derivatives are generally used in combination with other antimalarial agents. Although it is still being debated whether there are parasites that can be considered to have real resistance to ART (23), e.g., greatly increased 50% inhibitory concentrations (IC50s), the delayed clearance of parasites has been reported (6, 16, 17). Delayed clearance suggests that some parasites can survive ART treatment for a longer period of time than expected, but they are eventually killed by the drug, raising the possibility of a survival mechanism different from that of a classical drug resistance phenotype.
To better understand how parasites circumvent the action of ART, we placed a P. falciparum strain (F32-Tanzania) under ART pressure over a period of 3 years and selected an ART-tolerant parasite line (F32-ART) that can survive a drug dose up to 7,000-fold the initial IC50. In contrast to the known resistance mechanisms through mutations and/or amplification in drug transporters (quinolines) or drug targets (antifolates) (27), we demonstrate that P. falciparum employs a quiescence mechanism that allows it to survive ART treatment. We show that the parasite is arrested at the ring stage when it is under the pressure of high doses of ART but can resume its regular cycle after removal of the drug. This observation demonstrates a new mechanism of drug tolerance in P. falciparum that could greatly influence our use of drug tests and our disease control strategies.
MATERIALS AND METHODS
Parasites and parasite culture.
Mycoplasma-free strain F32-Tanzania was cultivated as described by Trager and Jensen (24), with modifications (2). Briefly, parasites were maintained in type O-positive human red blood cells (French Blood Bank) diluted to 2.5% hematocrit in RPMI medium supplemented with 5% human serum (Invitrogen, San Diego, CA). The culture was maintained at 37°C with 5% CO2. The level of parasitemia was checked each day and was not allowed to be over 10%. ART and chloroquine diphosphate were purchased from Sigma-Aldrich (St. Louis, MO), and artesunate was provided by Sanofi-Aventis (Labège, France).
Selection of ART-tolerant strain.
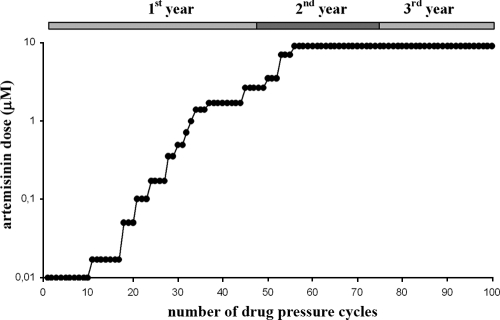
Parasites were adjusted to 5% to 7% parasitemia (asynchronous) and were grown in the presence of ART for 24 h. The medium was discarded and replaced by human serum-supplemented (20%) drug-free RPMI medium. The level of parasitemia was monitored until it reached 5%. At that time, drug pressure was reapplied. This discontinuous drug pressure (27) was applied for more than 3 years with a starting ART dose of 10 nM and to a maximum dose of 9 μM (Fig. 1). The parasite strain obtained after ART selection was named F32-ART. In parallel, as a control, parental strain F32-Tanzania, which was not submitted to ART treatment pressure, was kept in continuous culture.
FIG. 1.
Stepwise drug selection of an artemisinin-tolerant Plasmodium falciparum parasite. Dots represent the number of 24-h cycles of drug pressure applied to F32-Tanzania to select for strain F32-ART. The level of parasitemia was fixed to 5 to 7% before drug exposure, and new pressure was reapplied when the level of parasitemia reached 5% or higher. The duration of the experiment was defined by the time scale at the top.
To ensure that the 3-year ART selection pressure had not selected a minority clone(s) and that F32-ART and F32-Tanzania had not been contaminated by other parasites, assessment of the clonality was performed by determining the fragment lengths of the PCR amplicons of seven microsatellite loci, POLYa (chromosome 4), TA60 (chromosome 13), ARA2 (chromosome 11), P. falciparum g377 (Pfg377) and P. falciparum PK2 (PfPK2) (chromosome 12), and TA87 and TA109 (chromosome 6), by the methodology published by Annan et al. (1).
Evaluation of chemosensitivity.
A semiautomated radioactive microplate method was used to measure the IC50s, as described by Desjardins et al. (5), with some minor modifications (2). Briefly, ring-form parasites obtained after synchronization with 5% d-sorbitol were placed into drug-prefilled 96-well plates for a final volume of 200 μl (1% hematocrit, 1% parasitemia). The parasite culture was incubated with ART at various concentrations (10 pM to 100 nM) at 37°C with 5% CO2 for 24 h before addition of tritiated hypoxanthine (50 μl/0.25 μCi; Perkin-Elmer, Foster City, CA). The control parasite cultures devoid of drug were referred to as having 100% growth. The plates were incubated under the same conditions for another 24 h and were then submitted to a freeze-thaw cycle to release the nucleic acids, which were collected on fiberglass filters. The filters were dried and placed in vials containing 6 ml scintillation fluid (Optiphase Supermix; Perkin-Elmer). Tritium incorporation was determined with a β-counter (Wallac 1450 microbeta trilux; Perkin-Elmer), and the IC50 was determined after the drug concentration was plotted against the radioactivity (2).
Survival assay.
A survival assay was performed to evaluate the abilities of different strains to survive drug pressure. A ring-form culture with 5% parasitemia (2.5% hematocrit) was obtained by sorbitol synchronization (12). The culture medium was replaced by fresh medium containing different amounts of drug for 48 h or 96 h. At the end point, the cultures were washed with RPMI medium and returned to normal conditions. The level of parasitemia was monitored to assess the time required for each parasite culture to reach 5% parasitemia. The cultures were kept for 21 days; if no parasites were observed, the parasite culture was considered to have “no recovery” and was discarded.
Viability staining.
Rhodamine 123 (R123; Fluka Chemical Corp., Ronkonkoma, NY) was used to stain the parasites to evaluate their viability (10) after drug pressure. R123 stains the mitochondria green only if the parasite is viable (i.e., has polarized mitochondria). Infected red blood cells were stained at 37°C (1 μg/ml R123 in RPMI medium) for 5 min, washed with RPMI medium, and incubated for another 30 min under standard culture conditions. Counterstaining of the nuclei was carried out with 4′,6-diamidino-2-phenylindole (DAPI; 1 μg/ml; Sigma Chemical Corp., St. Louis, MO) at 37°C for 20 min. All thin blood smears were observed on the same day of preparation under a Leica DMIRB-E epifluorescence microscope at the appropriate excitation wavelength.
Flow cytometry.
Fluorescence-activated cell sorting (FACS) was carried out with the ART-treated culture to show that quiescent ring forms were present during drug pressure and that drug removal permitted restoration of the cell cycle. The culture was stained with 1 μg/ml DAPI, and analysis with an LSRII cytometer and FACSDiva software (Becton Dickinson and Company, San José, CA) was immediately performed for 400,000 events. Calibration was done by analyzing uninfected red blood cells, ring-form parasites obtained after sorbitol synchronization, and trophozoites and schizonts isolated by using magnetic-activated cell sorting columns (Miltenyi Biotec, Sunnyvale, CA) (21).
Microarray experiment and sample preparation.
The parasites were synchronized (12), and the cultures were tested in quadruplicate. Two RNA extraction points were selected: 16 to 20 h and 36 to 40 h. Infected erythrocytes (900 μl) were collected after centrifugation (1,000 × g for 5 min), lysed with phosphate-buffered saline (PBS) containing 0.05% saponin for 10 min, and centrifuged (at 1,500 × g for 3 min) to remove the supernatant. The lysed cells were washed twice with PBS, and finally, the pellet was resuspended in 1 ml of Trizol (Invitrogen, San Diego, CA). Chloroform (200 μl) was added, and the entire mixture was centrifuged (at 14,000 × g at 4°C for 20 min). The aqueous phase was mixed (vol/vol) with 70% ethanol, and RNA extraction was carried out with a Mini Elute RNA cleanup kit (Qiagen, Santa Clara, CA).
Pfmdr1 copy number and pfatp6 sequencing.
The copy number of P. falciparum mdr1 (pfmdr1) was determined by quantitative PCR. Primers MDR1 (5′-GCA-TTT-GTG-GGA-GAA-TCA-GG) and MDR2 (5′-CAA-CTC-CAA-TTT-TTG-ATC-TCC-A) and primers TUB1 (5′-ACA-ACG-AAG-CAA-CAG-GAG-GT) and TUB2 (5′-AGC-CCA-ATT-ATT-TCC-TGC-AC) were designed to amplify pfmdr1 and the gene for β-tubulin (a single-copy housekeeping gene), respectively. Reverse transcription-PCR (RT-PCR) was performed on a LightCycler instrument (version 1.5; Roche Laboratories, Nutley, NJ) with a 10-μl final reaction mixture containing 2.5 μl of the DNA template, 0.5 mM primers (Eurogenetec, Belgium), 4 mM MgCl2, and 1 μl Fast Start SYBR green (Roche Diagnostics, France). The gene amplification copy number was estimated by comparison of the threshold cycle (CT) value of β-tubulin with the CT value of pfmdr1 by the 2−ΔΔCT method (13). Reference strains Dd2 (which carries two to three pfmdr1 copies) and FcM29 (which carries one pfmdr1 copy) were used as controls. Each reaction was performed in triplicate, and the experiment was repeated three times.
Regions of interest in the pfatp6 gene were amplified by using four pairs of primers (SERCA1, 5′-TCA-GTT-CTA-CAG-AAA-ACA-ATT-CTT-GG and 5′-GAG-CAT-GGC-ACA-AGT-TTT-GA; SERCA2, 5′-CCA-AGA-AGA-AAT-ACA-TTC-ACT-TGG-A and 5′-CGG-ATG-ATG-GAG-AAG-AAG-GA; SERCA3, 5′-ATT-CCT-CTT-AGC-ACC-ACT-CCT and 5′-TCA-TCT-ACC-GCT-ATT-GTA-TGT-GG; and SERCA4, 5′-TCG-ATT-TTT-ATT-TGT-AAA-GGT-GTT-TG and 5′-TCA-TAC-ATA-CGA-TGT-TGA-GGA-TG). P. falciparum atp6 (pfatp6) sequencing operations were carried out by the Millegene Company (Labège, France). The sequences were analyzed with Seqman-DNAStar Lasergene (version 8) software (DNAStar, Inc., Madison, WI).
Microarray experiment and analysis.
Total RNA isolated from the P. falciparum parasites receiving the different drug treatments was used for cDNA synthesis and amplification by use of an Ovation aminoallyl RNA amplification and labeling system (version 1.1; NuGEN Technologies, Inc., San Carlos, CA). The amplified cDNA was labeled by use of a Cy3′ and Cy5′ postlabeling reactive dye pack (GE Healthcare, Piscataway, NJ). A SurePrint custom array platform (Agilent Technologies, Santa Clara, CA) was used in the present study and consisted of 7,462 individual 70-mer oligonucleotides representing 4,488 of the 5,409 open reading frames annotated by the malaria genome sequencing consortium (3). The Cy5- and Cy3-labeled probes were mixed and hybridized to the DNA microarrays by using a MAUI hybridization system (BioMicro Systems, Salt Lake City, UT). The slides were scanned with a GenePix 4000B array scanner (Axon Instruments, Union City, CA). The data were transformed into the log2(Cy3/Cy5) ratio for statistical analysis. The Pearson correlation between data sets was performed by using the National Institute of Allergy and Infectious Diseases (NIAID) μArray Center mAdb Gateway program.
Quantitative PCR.
To verify the changes in the expression of some genes from the microarray analyses, we synchronized F32-ART and F32-Tanzania and harvested the parasites at 16 to 20 h and 36 to 40 h postreinvasion for total RNA extraction. cDNA was synthesized from 4 μg of total RNA by using a QuantiTect reverse transcription kit (Qiagen), according to the manufacturer's instructions. Three genes were tested (with primers PF10_0121 [5′-TGT-GGA-ATG-GTT-TTA-AAG-CTG-A and 5′-AAC-CAA-ACA-ACA-ATG-GTC-AAG-A] PFE1415w [5′-GAG-ATG-GGG-GTT-TTT-CAT-TTT-T and 5′-TTC-TTG-AAA-ATC-ATT-CAA-AGA-CCA], and PF08_0054 [5′-GAA-ACT-GCT-GGT-GGT-GTT-ATG-A and 5′-GGT-TAA-GGC-TCT-TTC-ACC-TTC-A]) by real-time PCR with a QuantiTect SYBR green PCR kit (Qiagen). The efficiency of amplification of the individual genes was normalized to that of the reference 60S ribosomal protein gene (with primer MAL13P1.209 [5′-GTG-GAG-AAT-GCT-TGA-CAT-TTG-A and 5′-CTG-GAG-CTT-TTC-CAA-AGT-GTT-T]). The relative levels of expression of the tested genes were determined by the 2−ΔΔCT method, where ΔΔCT = (CT of F32-ART parasite target gene − CT of control reference gene) − (CT of F32-Tanzania parasite target gene − CT of control reference gene) (13).
RESULTS
Selection of ART-tolerant parasites.
After 3 years of applying ART pressure, we selected a P. falciparum line from strain F32-Tanzania. Selection was performed over 3 years with more than 100 drug pressure cycles (Fig. 1). The chemosensitivity of the parasites was evaluated at the end of each step and did not show significant alterations (see below). When the experiment reached 100 drug pressure cycles, an alternative method was used to assay the parasite response to ART. The parasites were treated with high doses of ART for 48 h to 96 h and were returned to normal culture without the drug for 21 days or until the level of parasitemia reached 5%. Drug-selected parasite F32-ART was able to recover from ART treatment in a much shorter time than strain F32-Tanzania. The results of the survival assays (Table 1) showed a significant difference in the time to reach 5% parasitemia between F32-ART and F32-Tanzania: a minimum 10-day difference in the time required to reach 5% parasitemia at doses of 9 μM and 18 μM between F32-Tanzania and F32-ART was observed. At higher doses (35 μM and 70 μM) or with a longer length of drug pressure (96 h), only F32-ART recovered, indicating that this tolerant strain managed to survive at a dose equivalent to 7,000-fold the initial IC50 (9.9 ± 1 nM). In comparison to the results for F32-Tanzania, strain F32-ART was also more tolerant to artesunate (at 2.6 μM and 9 μM), even though F32-ART was never exposed to this compound. This tolerance appeared to be limited to ART and its derivatives, as F32-ART and F32-Tanzania showed no difference in their responses to chloroquine (Table 1).
TABLE 1.
Drug survival assays comparing strains F32-Tanzania and F32-ART
| Drug | Treatment duration (h)d | Drug dose (μM) | Time (days) to 5% parasitemiae |
|
|---|---|---|---|---|
| F32-Tanzania | F32-ART | |||
| ARTa | 48 | 9 | 16 ± 2f | 6 ± 1.5 |
| ART | 48 | 18 | 17g | 7 ± 1 |
| ART | 48 | 35 | NGh | 11 ± 2 |
| ART | 48* | 70 | NG | 17 |
| ART | 96* | 0.1 | NG | 15 |
| ART | 96* | 1 | NG | 16 |
| ATSb | 48 | 2.6 | 14 ± 0.5 | 6 ± 0 |
| ATS | 48* | 9 | 15 | 8 |
| CQc | 48 | 0.2 | 14 ± 0 | 14 ± 2 |
ART, artemisinin.
ATS, artesunate.
The dose tested corresponds to 10-fold the IC50 of chloroquine (CQ) for F32-Tanzania.
All experiments were carried out at least three times independently except those marked with an asterisk, which were carried out once.
Values express the time to reach 5% parasitemia in days ± standard deviations.
At day 21, two of three cultures recovered.
At day 21, only one of three cultures recovered.
NG, no growth at day 21.
In parallel, isotopic evaluation of chemosensitivity was routinely performed over the 3-year period of ART pressure. IC50s were measured before, during, and after the selection process. Surprisingly, no changes in the IC50s were observed for strain F32-ART before drug selection (day 0, 9.9 nM ± 1.0, which was the same as that for F32-Tanzania) and after drug selection (day 1,000; 9.9 nM ± 1.4).
The clonality results indicated that only one allele size was detected from each locus in the initial strain, F32-Tanzania, showing its clonality. Analysis of F32-ART showed that it had the same allelic pattern as F32-Tanzania. This demonstrates that F32-ART is also clonal and was obtained from F32-Tanzania.
Mechanism and parasite stage implicated in ART tolerance.
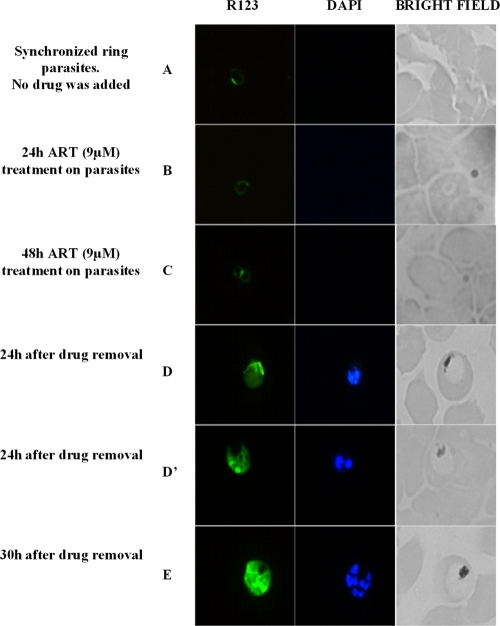
Direct observation of the parasites after drug pressure was difficult, as the majority died after drug treatment (and had a pycnotic morphology). To improve the ability to detect viable parasites, a mitochondrial dye (rhodamine 123) was used to stain live parasites. A kinetic survey was performed during the period that F32-ART underwent drug pressure (48 h, 9 μM), and only some rare ring forms (∼0.1% parasitemia) persisted in the culture; there were no signs of late trophozoites or schizonts (Fig. 2B and C). Notably, at 24 h and then at 30 h after removal of the drug, these ring forms were able to return to a normal cell cycle and developed into late trophozoites and schizonts (Fig. 2D, D′, and E), which then reinvaded erythrocytes and started a new cycle. These observations indicate that drug tolerance is mediated by cell cycle arrest at the ring stage, as they were the only viable forms observed during the 48-h ART pressure period. On the contrary, no quiescent rings could be observed in the F32-Tanzania culture under drug pressure (9 μM ART, 48 h). Additionally, no mature forms could be observed in the F32-Tanzania culture 24 h and 30 h after ART removal.
FIG. 2.
Epifluorescence observations of parasites with or without drug pressure. (A) Before drug pressure. Ring forms are present (R123 positive; not detectable with DAPI; no malaria pigment). (B) After 24 h of drug pressure. Quiescent ring forms are the only viable stage present (R123 positive; not detectable with DAPI; no malaria pigment). (C) After 48 h of drug pressure. Quiescent ring forms are the only viable stage present (R123 positive; not detectable with DAPI; no malaria pigment). (D and D′) Restoration of the parasite cycle with the presence of mature trophozoites and young schizonts, respectively, 24 h after drug removal (R123 positive; nuclei detectable with DAPI; presence of malaria pigment). (E) The presence of mature schizonts 30 h after drug removal (R123 positive; multiple nuclei detectable with DAPI; presence of malaria pigment).
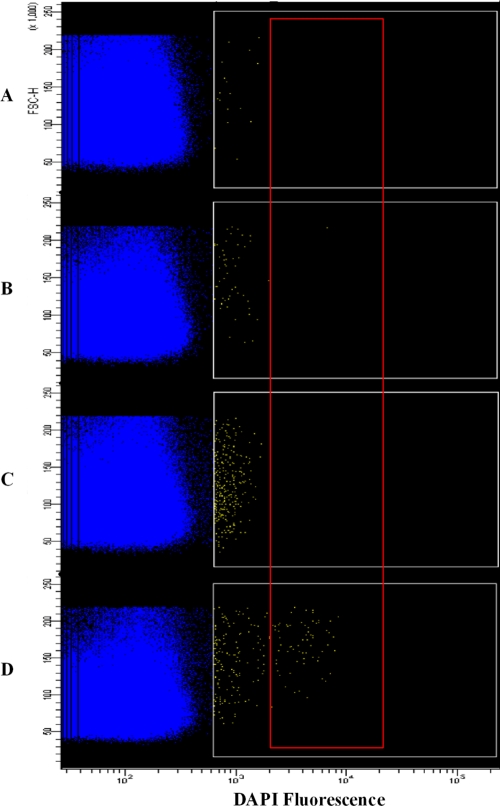
Flow cytometry analysis reinforced the microscopy results, as no F32-ART late-stage parasites were present after 24 h and 48 h of ART pressure (Fig. 3B and C). Twenty-four hours after drug removal, late stages of the parasite were present in the culture (Fig. 3D), as the cell cycle was restored.
FIG. 3.
Flow cytometry analysis of strain F32-ART submitted to 9 μM artemisinin treatment. Yellow dots (white gate) correspond to DAPI-positive events, and blue dots correspond to DAPI-negative events (early rings and erythrocytes). The progressive increase in the number of DAPI-positive events during pressure (B and C) was due to dead forms, which incorporate DAPI better than ring forms; however, fluorescence was limited to 103, while schizont forms (D) exhibited high levels of DAPI incorporation, with fluorescence ranging from 2 × 103 to 2 × 104 (red gate). Dead forms progressively disappeared from the culture after pressure, corresponding to the relative decrease in the numbers of DAPI-positive events after the release of the artemisinin pressure. (A) Before drug pressure. Ring forms (0 to 12 h; 5% parasitemia) are the selected stage. (B) After 24 h of drug pressure. Quiescent ring forms are the only stage present with dead parasites. (C) After 48 h of drug pressure. Quiescent ring forms are the only stage present with dead parasites. (D) Twenty-four hours after drug removal. Restoration of the parasite cycle with the presence of trophozoites and schizonts is shown (parasites in the red square).
To further confirm that only ring forms were responsible for recrudescence, scheduled sorbitol treatments that selectively kill all late-parasite stages except ring forms (12) were performed five times during the 48-h ART pressure (at five time points: 0, 12, 24, 36, and 48 h). These treatments did not affect parasite survival. Moreover, when the effect of ART pressure (9 μM, 48 h) on late-stage parasites (24 to 30 h) was assessed, all parasites were killed and no parasites were recovered (data not shown).
No association of reported drug resistance markers with ART tolerance.
We monitored two reported molecular markers that have been associated with decreased sensitivity to ART and its derivatives, such as mutations in the pfatp6 gene (8, 25) and variations in the pfmdr1 copy number (20). Sequencing was used to assess pfatp6 for the single-nucleotide polymorphisms associated with ART sensitivity. None of the reported mutations at codons 263, 431, 623, and 769 in pfatp6 was detected in either F32-ART or F32-Tanzania (data not shown). Quantitative PCR was performed with DNA from F32-ART and F32-Tanzania, and again, no amplification of the pfmdr1 copy number was observed. Additionally, addition of 0.5 μM penfluridol (a potent PfMDR1 inhibitor [18]) during the 48-h drug pressure (ART at 9 μM) did not alter the F32-ART recovery time (data not shown).
Transcriptome analysis.
Microarray analysis was carried out to determine whether transcriptional upregulation and/or downregulation could be associated with tolerance of ART. Comparison of the levels of transcription of F32-ART and F32-Tanzania revealed differences in the level of expression of some genes in response to ART selection (Table 2). The transcription of six genes was significantly (P < 0.01) upregulated at both the early phase (16 to 20 h) and the late phase (36 to 40 h) of the F32-ART parasite cycle. Notably, all these highly expressed genes encode exported protein families: KAHRP (PFB0100c), PHISTc (PFB0105c), RIFIN (MAL13P1.495 and PFC0045w), STEVOR (MAL13P1.490), and the Plasmodium exported protein PFC0085c. Other genes that showed slightly (P < 0.05) altered expression in F32-ART included genes encoding hypoxanthine phosphoribosyl transferase (PF10_0121) and HSP70 (PF08_0054) and the cell cycle regulator (PFE1415w). The changes in the levels of expression for the last three genes were confirmed by RT-PCR.
TABLE 2.
Genes with significantly altered expression in F32-ART compared with the level of expression in F32-Tanzania
| Gene | Expression level ± SDa | P value | Location (chromosome, positions) | Predicted gene function |
|---|---|---|---|---|
| PFB0100c | 2.91 ± 0.43 | 4.4E-07 | chr2, 103038-105796 | Knob-associated histidine-rich protein (KAHRP) |
| PFB0105c | 2.02 ± 0.58 | 1.4E-04 | chr2, 109564-110580 | Plasmodium exported protein (PHISTc) |
| PFC0085c | 2.75 ± 0.51 | 1.2E-05 | chr3, 93248-94587 | Plasmodium exported protein, unknown function |
| MAL13P1.495 | 2.18 ± 0.69 | 2.5E-03 | chr13, 2828796-2830236 | Rifin protein |
| MAL13P1.490 | 2.44 ± 0.81 | 3.2E-03 | chr13, 2826695-2827778 | Stevor protein |
| PFC0045w | 2.19 ± 0.72 | 1.0E-02 | chr3, 64604-65508 | Rifin protein |
| PF08_0054b | 1.33 ± 0.67c | 4.2E-02 | chr8, 861481-863514 | 70-kDa heat shock protein |
| PF10_0121b | −1.26 ± 0.64d | 4.3E-02 | chr10, 475326-476562 | Hypoxanthine phosphoribosyltransferase |
| PFE1415wb | −1.31 ± 0.43c | 1.3E-02 | chr5, 1171653-1173884 | Cell cycle regulator with zinc finger domain |
Values are means ± standard deviations from four biological replicates.
Genes for which transcription levels were checked by quantitative PCR.
Altered transcription only at late stage.
Altered transcription only at early stage.
DISCUSSION
To our knowledge, this work describes for the first time a culture-adapted P. falciparum strain with a ring stage that can survive high-dose ART pressure. We showed that a P. falciparum ring-stage subpopulation persists in culture under a very high dose of ART for at least 96 h, suggesting cell cycle arrest under drug pressure. This quiescence phenomenon is temporary, as the parasite can usually continue its cycle development after drug removal and may explain the observation of delayed clearance after treatment (6, 16, 17). Full elucidation of the signaling pathway trigger and the genetic alterations leading to the cell cycle arrest may permit the development of new antiplasmodial drugs.
Interestingly, ART tolerance did not influence the IC50s of the parasites, and measurement of the IC50 of ART did not reflect the ability of the parasite to survive ART treatment. This raises the question of the suitability of using the classic chemosensitivity monitoring methods to determine the response to ART. The ability of strain F32-ART to tolerate ART by a quiescence mechanism would be invisible by use of a radioisotopic sensitivity evaluation, as this method determines the rate of DNA synthesis of Plasmodium continuously exposed to drug. The quiescence of the ring stage could permit parasite survival by slowing its metabolism and limiting the effects of the drug. As no DNA was synthesized by quiescent parasites, drug-tolerant strain F32-ART appeared to be as sensitive as control strain F32-Tanzania in an IC50 assay. These data corroborate the findings of a study by Dondorp et al., in which resistant isolates from western Cambodia did not present higher IC50s (6). Although delayed clearance after ART treatment is not yet commonly observed in the field, its emergence in southeast Asia represents a glimpse of its global spread (6, 16), and a protocol better than the traditionally used hypoxanthine microplate method for monitoring of survival during ART treatment is thus urgently needed.
ART acts on F32-ART tolerant parasites via a cytostatic mechanism. In the study of Dondorp et al. (6), the ring forms were envisaged to be responsible for resistance, whereas they were previously described to be the most sensitive stages (22). Our data support a new mechanism for P. falciparum ART tolerance by cell cycle arrest under ART pressure and further parasite development after drug removal.
Our investigations of the molecular markers associated with decreased sensitivity to ART (pfatp6, pfmdr1) showed a lack of association of the known genes with ART tolerance mechanism, as reported in the results of clinical studies (6, 16). Nevertheless, we highlighted evidence of new potential markers of ART tolerance through microarray studies. Transcriptional analyses suggested a potential role of P. falciparum exported proteins (KAHRP, PHISTc, RIFIN, STEVOR, and an exported protein of unknown function) in transporting substances from the red blood cell into the parasite (14). The 70-kDa heat shock protein (HSP70) and the hypoxanthine phosphoribosyltransferase have previously been associated with the P. falciparum response to lethal doses of artesunate (15). HSP70 has also been proposed to play an important role during parasite adaptation to different environments (11), which might be correlated with the physiologic changes that the parasite may undergo when it restarts its cell cycle following its quiescence stage. The PF10_0121 gene product (hypoxanthine phosphoribosyltransferase), an enzyme involved in purine biosynthesis, is essential for DNA synthesis. The modified transcription of PFE1415w supports its role as a cell cycle regulator potentially involved in the quiescence phenomenon in response to ART pressure. PFE1415w could thus be implicated not only in the parasite life cycle but also in ART tolerance; however, because these changes were relatively small and the genes with changed levels of transcription could represent those encoding variant and stress proteins whose levels of expression could change after drug pressure, further investigation is necessary to determine whether these genes play a direct role in the response to ART.
Cell cycle arrest or quiescence is a physiologic feature previously found in other Plasmodium species, such as P. vivax and P. ovale, during hepatic stages, but it has never been demonstrated in P. falciparum. Quiescence is a phenomenon also found in a wide range of organisms. For example, varicella-zoster virus is able to resist antiviral agents, bacteria in stationary growth phase are resistant to antibiotics, and human cancer cells can survive the assault of chemotherapy by arresting the cell cycle in the G1 phase (19). In mammalian cells, P27kip1 can inhibit cyclin-dependent kinase 2 (CDK2), which is indispensable for progression of the cell cycle from G1 phase to S phase (9), leading to quiescent cells. CDKs exist in P. falciparum, such as Pfpk6 (whose structure is related to that of human CDK2), and their overexpression was noted in the transition from the ring to the mature trophozoite form (4). We hypothesize that the quiescence observed here could result in alteration of the cell cycle regulation mediated by CDK and/or CDK inhibitors. Further investigations of P. falciparum cell cycle regulators (7) may shed new light on the association between drug tolerance and quiescence.
Finally, our P. falciparum ART-tolerant model offers a major tool for research and screening of molecules active against resistant P. falciparum strains and for a better understanding of the atypical mechanism survival of malaria parasites when they are exposed to ART and its derivatives.
Acknowledgments
J.L. is indebted to the EU program for a Ph.D. fellowship, and B.W. received a Ph.D. grant from the Région Midi-Pyrénées. ANR (grant ANR-06-RIB-020) is acknowledged for financial support. This work was supported in part by the Intramural Research Program of the Division of Intramural Research, NIAID, National Institutes of Health.
We thank I. Morlais and L. Abate from IRD-UR016, Montpellier, France, for microsatellite genotyping to check the clonality of the P. falciparum strains used; the NIAID Microarray Research Facility for array fabrication and hybridizations; the IFR40 Microscopy Facility (INRA, Auzeville, France); Fatima-Ezzahra L'Faqihi-Olive from the IFR150 Cytometry Facility (INSERM, Toulouse, France); and NIAID intramural editor Brenda Rae Marshall.
Footnotes
Published ahead of print on 16 February 2010.
REFERENCES
- 1.Annan, Z., P. Durand, F. J. Ayala, C. Arnathau, P. Awono-Ambene, F. Simard, F. G. Razakandrainibe, J. C. Koella, D. Fontenille, and F. Renaud. 2007. Population genetic structure of Plasmodium falciparum in the two main African vectors, Anopheles gambiae and Anopheles funestus. Proc. Natl. Acad. Sci. U. S. A. 104:7987-7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benoit-Vical, F., J. Lelievre, A. Berry, C. Deymier, O. Dechy-Cabaret, J. Cazelles, C. Loup, A. Robert, J. Magnaval, and B. Meunier. 2007. Trioxaquines: new antimalarial agents active on all erythrocytic forms including gametocytes. Antimicrob. Agents Chemother. 51:1463-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bozdech, Z., M. Llinas, B. L. Pulliam, E. D. Wong, J. Zhu, and J. L. DeRisi. 2003. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. 1:E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bracchi-Ricard, V., S. Barik, C. Delvecchio, C. Doerig, R. Chakrabarti, and D. Chakrabarti. 2000. PfPK6, a novel cyclin-dependent kinase/mitogen-activated protein kinase-related protein kinase from Plasmodium falciparum. Biochem. J. 347(Pt. 1):255-263. [PMC free article] [PubMed] [Google Scholar]
- 5.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dondorp, A. M., F. Nosten, P. Yi, D. Das, A. P. Phyo, J. Tarning, K. M. Lwin, F. Ariey, W. Hanpithakpong, S. J. Lee, P. Ringwald, K. Silamut, M. Imwong, K. Chotivanich, P. Lim, T. Herdman, S. S. An, S. Yeung, P. Singhasivanon, N. P. Day, N. Lindegardh, D. Socheat, and N. J. White. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361:455-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geyer, J. A., S. T. Prigge, and N. C. Waters. 2005. Targeting malaria with specific CDK inhibitors. Biochim. Biophys. Acta 1754:160-170. [DOI] [PubMed] [Google Scholar]
- 8.Jambou, R., E. Legrand, M. Niang, N. Khim, P. Lim, B. Volney, M. T. Ekala, C. Bouchier, P. Esterre, T. Fandeur, and O. Mercereau-Puijalon. 2005. Resistance of Plasmodium falciparum field isolates to in-vitro artemether and point mutations of the SERCA-type PfATPase6. Lancet 366:1960-1963. [DOI] [PubMed] [Google Scholar]
- 9.Jin, K., D. Z. Ewton, S. Park, J. Hu, and E. Friedman. 2009. Mirk regulates the exit of colon cancer cells from quiescence. J. Biol. Chem. 284:22916-22925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kato, M., A. Izumo, and K. Tanabe. 1987. Vital staining of Plasmodium falciparum with cationic fluorescent rhodamine dyes. J. Parasitol. 73:1058-1059. [PubMed] [Google Scholar]
- 11.Kumar, N., and H. Zheng. 1992. Nucleotide sequence of a Plasmodium falciparum stress protein with similarity to mammalian 78-kDa glucose-regulated protein. Mol. Biochem. Parasitol. 56:353-356. [DOI] [PubMed] [Google Scholar]
- 12.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 13.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 14.Marti, M., R. T. Good, M. Rug, E. Knuepfer, and A. F. Cowman. 2004. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science 306:1930-1933. [DOI] [PubMed] [Google Scholar]
- 15.Natalang, O., E. Bischoff, G. Deplaine, C. Proux, M. A. Dillies, O. Sismeiro, G. Guigon, S. Bonnefoy, J. Patarapotikul, O. Mercereau-Puijalon, J. Y. Coppee, and P. H. David. 2008. Dynamic RNA profiling in Plasmodium falciparum synchronized blood stages exposed to lethal doses of artesunate. BMC Genomics 9:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noedl, H., Y. Se, K. Schaecher, B. L. Smith, D. Socheat, and M. M. Fukuda. 2008. Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 359:2619-2620. [DOI] [PubMed] [Google Scholar]
- 17.Noedl, H., D. Socheat, and W. Satimai. 2009. Artemisinin-resistant malaria in Asia. N. Engl. J. Med. 361:540-541. [DOI] [PubMed] [Google Scholar]
- 18.Oduola, A. M., G. O. Omitowoju, L. Gerena, D. E. Kyle, W. K. Milhous, A. Sowunmi, and L. A. Salako. 1993. Reversal of mefloquine resistance with penfluridol in isolates of Plasmodium falciparum from south-west Nigeria. Trans. R. Soc. Trop. Med. Hyg. 87:81-83. [DOI] [PubMed] [Google Scholar]
- 19.Phadke, M. S., N. F. Krynetskaia, A. K. Mishra, and E. Krynetskiy. 2009. Glyceraldehyde 3-phosphate dehydrogenase depletion induces cell cycle arrest and resistance to antimetabolites in human carcinoma cell lines. J. Pharmacol. Exp. Ther. 331:77-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price, R. N., C. Cassar, A. Brockman, M. Duraisingh, M. van Vugt, N. J. White, F. Nosten, and S. Krishna. 1999. The pfmdr1 gene is associated with a multidrug-resistant phenotype in Plasmodium falciparum from the western border of Thailand. Antimicrob. Agents Chemother. 43:2943-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ribaut, C., A. Berry, S. Chevalley, K. Reybier, I. Morlais, D. Parzy, F. Nepveu, F. Benoit-Vical, and A. Valentin. 2008. Concentration and purification by magnetic separation of the erythrocytic stages of all human Plasmodium species. Malar. J. 7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skinner, T., L. Manning, W. Johnston, and T. Davis. 1996. In vitro stage-specific sensitivity of Plasmodium falciparum to quinine and artemisinin drugs. Int. J. Parasitol. 26:519-525. [DOI] [PubMed] [Google Scholar]
- 23.Taylor, S. M., J. J. Juliano, and S. R. Meshnick. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361:1807. [DOI] [PubMed] [Google Scholar]
- 24.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 25.Uhlemann, A. C., A. Cameron, U. Eckstein-Ludwig, J. Fischbarg, P. Iserovich, F. A. Zuniga, M. East, A. Lee, L. Brady, R. K. Haynes, and S. Krishna. 2005. A single amino acid residue can determine the sensitivity of SERCAs to artemisinins. Nat. Struct. Mol. Biol. 12:628-629. [DOI] [PubMed] [Google Scholar]
- 26.White, N. J. 2008. Qinghaosu (artemisinin): the price of success. Science 320:330-334. [DOI] [PubMed] [Google Scholar]
- 27.Witkowski, B., A. Berry, and F. Benoit-Vical. 2009. Resistance to antimalarial compounds: methods and applications. Drug Resist. Update 12:42-50. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. 2008. World malaria report 2008. Report WHO/HTM/GMP/2008.1. World Health Organization, Geneva, Switzerland.