Abstract
We studied three clinical isolates of Candida spp. (one C. tropicalis isolate and two C. glabrata isolates) from patients with invasive candidiasis. The first isolate emerged during echinocandin treatment, while the others emerged after the same treatment. These strains harbored an amino acid substitution in Fksp never linked before with reduced echinocandin susceptibility in C. tropicalis or in C. glabrata. The molecular mechanism of reduced susceptibility was confirmed using a 1,3-β-d-glucan synthase inhibition assay.
Since the introduction of the echinocandin drugs in 2001, these antifungals are becoming the preferred choice for invasive candidiasis treatment (13, 14, 16, 24). Candida tropicalis and Candida glabrata are important pathogens causing invasive disease, especially in immunocompromised patients (12, 22). Echinocandin drugs target the Fksp subunits of the 1,3-β-d-glucan synthase complex and inhibit fungal cell wall biosynthesis (4). Echinocandin clinical failures are rare events. Yet, as the number of patients exposed to echinocandin therapy increases, the probability for resistance increases. In all the published cases of C. tropicalis and C. glabrata with a reduced echinocandin susceptibility (RES) phenotype, amino acid substitutions in two conserved regions of the Fksp (hot spots) were reported (2, 6, 11, 21, 25).
From March 2004 to March 2009, 285 cases of candidemia were identified at Summa Health System's hospitals. Eighty-nine cases were identified as C. glabrata and 17 as C. tropicalis. During this period, two C. glabrata isolates and one C. tropicalis isolate were considered nonsusceptible to caspofungin by the CLSI susceptibility breakpoint (MIC ≥ 2 μg/ml) (3, 23), representing an RES incidence of 2.2% (2/89) and 5.8% (1/17) for C. glabrata and C. tropicalis, respectively. In this work, we describe the molecular mechanism responsible for the RES phenotype observed in these three clinical cases.
Case 1 (C. tropicalis strain CT-C1).
A 28-year-old female with acute myelogenous leukemia receiving broad-spectrum antibacterials showed persistent fever. An empirical caspofungin treatment was started (5th hospital day). A set of cultures were obtained. On her 22nd hospital day, while she was on caspofungin (50 mg/daily) (16th day), her esophageal biopsy specimen indicated severe invasive Candida esophagitis and her blood cultures were positive for C. tropicalis. Echinocandin and fluconazole MICs were determined (Table 1). Caspofungin was discontinued, and she was switched to fluconazole (MIC, 1 μg/ml), with resolution of symptoms. She completed 4 weeks of fluconazole, and she was discharged after 56 days.
TABLE 1.
In vitro susceptibility testing and 1,3-β-d-glucan synthase complex inhibition profiles for anidulafungin, caspofungin, and micafungin drugsd
| Strain | Organism | Wild-type FKS or FKS with nucleotide substitution | Fksp hot spot 1a | MIC (μg/ml)b |
IC50 (ng/ml)c |
||||
|---|---|---|---|---|---|---|---|---|---|
| ANF | CSF | MCF | ANF | CSF | MCF | ||||
| ATCC 750 | C. tropicalis | Wild type | FLTLSLRDP | 0.06 | 0.25 | 0.06 | 4.66 | 2.33 | 7.75 |
| CT-C1 | C. tropicalis | FKS1-T227C | SLTLSLRDP | 1.00 | 4.00 | 1.00 | 122.7 | 61.28 | 405.9 |
| ATCC 90030 | C. glabrata | Wild type | FLILSLRDP | <0.03 | 0.03 | 0.03 | 1.91 | 1.92 | 0.71 |
| CG-C2 | C. glabrata | FKS1-T1885C | FLILPLRDP | 4.00 | 8.00 | 2.00 | 13,998 | 12,778 | 15,144 |
| CG-C3 | C. glabrata | FKS2-T1987C | FLILPLRDP | 8.00 | >16.0 | >16.0 | 13,351 | 16,882 | 11,422 |
| ATCC 90028 | C. albicans | Wild type | FLTLSLRDP | 0.03 | 0.12 | 0.03 | 1.83 | 0.50 | 18.80 |
| 177 | C. albicans | FKS1-T1922C | SLTLSLRDP | 0.83 | 4.00 | 1.00 | 2,622 | 1,091 | 1,435 |
| 205 | C. albicans | FKS1-T1933C | FLTLPLRDP | 1.33 | 8.00 | 4.00 | 989 | 245.4 | 1,088 |
Bold letters represent amino acid changes. Shown are the sequences of Fks1p hot spot 1 for all strains except 03-Cg, in which the mutation was present in Fks2p.
MIC values represent the geometric means of results from three repetitions.
IC50 values represent the arithmetical means of results from three repetitions.
ANF, anidulafungin; CSF, caspofungin; MCF, micafungin.
Case 2 (C. glabrata strain CG-C2).
A 49-year-old female diabetic and renal transplant recipient showed erosive esophagitis. She developed respiratory failure (3rd hospital day) and was treated empirically with broad-spectrum antibacterials. Her tracheal aspirate culture was positive for Candida spp., and her urine culture was positive for C. glabrata. She was accordingly started on caspofungin (50 mg/daily) (12th hospital day). After 2 weeks, her condition stabilized but did not improve. On her 55th hospital day, 24 days after completing the initial caspofungin course, she developed low-grade fever. Her blood, urine, and central line catheter tip cultures grew C. glabrata, and caspofungin was reinitiated. MICs were determined (Table 1), and caspofungin was switched to liposomal amphotericin B (58th hospital day). She completed a 3-week course, with noted clinical improvement and clearing of her fungemia.
Case 3 (C. glabrata strain CG-C3).
A 53-year-old male who received a kidney transplant was admitted for gastrointestinal bleeding and pulmonary infiltrates. He developed candiduria 2 months prior to this admission, which was treated with fluconazole. Upon admission, the patient was initially treated with broad-spectrum antibacterials for 2 weeks. His pulmonary infiltrates progressed. His respiratory cultures showed C. glabrata and C. albicans. Anidulafungin (100 mg/daily) was started (21st hospital day) and continued for 3 weeks, with no clinical improvement. After a respiratory failure, his blood and urine cultures were positive for Klebsiella pneumoniae and C. glabrata (>100,000 CFU/ml), respectively. Echinocandin MICs were determined (Table 1), and the treatment was switched to liposomal amphotericin B, with significant clinical improvement.
Characterization of clinical isolates.
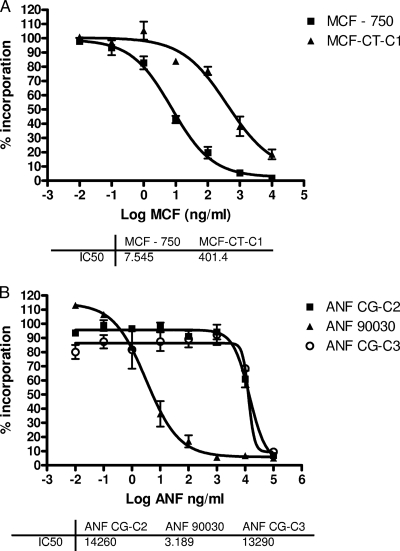
Susceptibility testing was performed according to the recommendations of CLSI document M27-A3 (3). C. glabrata ATCC 90030, C. tropicalis ATCC 750, C. albicans ATCC 90028, C. albicans 205, and C. albicans 177 (3, 7) were used to compare the MICs for our clinical isolates. The strains CT-C1, CG-C2, and CG-C3 showed 16- to >500-fold-higher MICs than the reference strains (Table 1). 1,3-β-d-Glucan synthase complexes were isolated, and inhibition kinetics values (50% inhibitory concentrations [IC50s]) were obtained to confirm the RES phenotype (5, 6, 18). The 1,3-β-d-glucan synthase complexes isolated from strains CT-C1, CG-C2, and CG-C3 yielded higher IC50s (from 26- to >20,000-fold) for all echinocandins than wild-type 1,3-β-d-glucan synthase complexes (Fig. 1; Table 1). Consistent with this biochemical data, DNA sequence analysis of the FKS1 gene (GenBank accession no. EU676168) of the CT-C1 strain showed a homozygous T227C mutation, resulting in a deduced F76S amino acid substitution. This is the only sequence available, and it is partial. Its first amino acid is equivalent to Ala566 of the C. albicans Fks1p (GenBank accession no. XM_716336). Thus, the F76S amino acid substitution in strain 01-Ct is equivalent to a F641S substitution in C. albicans (Table 1) (6).
FIG. 1.
Echinocandin inhibition profiles for product-entrapped 1,3-β-d-glucan synthase complexes obtained using a sigmoidal-response (variable-slope) curve. Echinocandin inhibition kinetics yielding 50% inhibitory concentrations (IC50s) were obtained and are expressed in nanograms per milliliter. (A) Inhibition curves and 50% inhibitory concentrations (IC50) for micafungin (MCF) and for 1,3-β-d-glucan synthase complexes obtained from reference C. tropicalis ATCC 750 (750) and C. tropicalis CT-C1 strains. (B) Anidulafungin (ANF) titration curves for 1,3-β-d-glucan synthase complexes isolated from reference C. glabrata ATCC 90030, C. glabrata CG-C2, and C. glabrata CG-C3 strains.
The C. glabrata FKS1 and FKS2 genes (GenBank accession no. XM_446406 and XM_448401, respectively) from strains CG-C2 and CG-C3 were sequenced. These isolates showed S629P and S663P amino acid substitutions in Fks1p and Fks2p, respectively (Table 1). These amino acid substitutions have not been previously reported for C. tropicalis or C. glabrata. However, equivalent substitutions were described for C. albicans (7, 11, 18) and are located in the highly conserved hot spot 1 region of the Fksp associated with RES in Candida spp. (21).
Our isolates showed MIC values comparable with those of clinical C. albicans strains harboring equivalent FKS mutations (strains 177 and 205) (Table 1). Also, the 1,3-β-d-glucan synthase complexes isolated from our strains showed decreased echinocandin sensitivity (a higher IC50), demonstrating that FKS hot spot mutations are sufficient and necessary to produce an RES phenotype. However, the IC50 shifts did not show a strict correlation with MIC values, especially for the 01-Ct strain. This phenomenon was observed before for C. albicans and C. tropicalis, suggesting that factors other than 1,3-β-d-glucan synthase complex inhibition may influence echinocandin MIC values (1, 6, 7, 17).
In summary, we report three patients who developed invasive Candida species infection despite echinocandin treatment, showing that RES is an emerging problem as echinocandin use continues to increase (2, 6, 8-11, 15, 18-20, 25). Furthermore, we demonstrate the clear linkage between Fksp substitutions, 1,3-β-d-glucan synthase complex resistance to echinocandin drugs, elevated MIC, and echinocandin clinical failure.
Acknowledgments
This work was supported by a grant to D.S.P. from the NIH (AI069397).
Footnotes
Published ahead of print on 9 February 2010.
REFERENCES
- 1.Chamilos, G., R. E. Lewis, and D. P. Kontoyiannis. 2006. Inhibition of Candida parapsilosis mitochondrial respiratory pathways enhances susceptibility to caspofungin. Antimicrob. Agents Chemother. 50:744-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cleary, J. D., G. Garcia-Effron, S. W. Chapman, and D. S. Perlin. 2008. Reduced Candida glabrata susceptibility secondary to an FKS1 mutation developed during candidemia treatment. Antimicrob. Agents Chemother. 52:2263-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A3, 3rd ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Douglas, C. M., F. Foor, J. A. Marrinan, N. Morin, J. B. Nielsen, A. M. Dahl, P. Mazur, W. Baginsky, W. Li, and M. el Sherbeini. 1994. The Saccharomyces cerevisiae FKS1 (ETG1) gene encodes an integral membrane protein which is a subunit of 1,3-β-d-glucan synthase. Proc. Natl. Acad. Sci. U. S. A. 91:12907-12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Effron, G., S. K. Katiyar, S. Park, T. D. Edlind, and D. S. Perlin. 2008. A naturally occurring proline-to-alanine amino acid change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 52:2305-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Effron, G., D. P. Kontoyiannis, R. E. Lewis, and D. S. Perlin. 2008. Caspofungin-resistant Candida tropicalis strains causing breakthrough fungemia in patients at high risk for hematologic malignancies. Antimicrob. Agents Chemother. 52:4181-4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Effron, G., S. Park, and D. S. Perlin. 2009. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrob. Agents Chemother. 53:112-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hakki, M., J. F. Staab, and K. A. Marr. 2006. Emergence of Candida krusei isolated with reduced susceptibility to caspofungin during therapy. Antimicrob. Agents Chemother. 50:2522-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez, S., J. L. Lopez-Ribot, L. K. Najvar, D. I. McCarthy, R. Bocanegra, and J. R. Graybill. 2004. Caspofungin resistance in Candida albicans: correlating clinical outcome with laboratory susceptibility testing of three isogenic isolates serially obtained from a patient with progressive Candida esophagitis. Antimicrob. Agents Chemother. 48:1382-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahn, J. N., G. Garcia-Effron, M. J. Hsu, S. Park, K. A. Marr, and D. S. Perlin. 2007. Acquired echinocandin resistance in a Candida krusei isolate due to modification of glucan synthase. Antimicrob. Agents Chemother. 51:1876-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katiyar, S., M. Pfaller, and T. Edlind. 2006. Candida albicans and Candida glabrata clinical isolates exhibiting reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 50:2892-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kontoyiannis, D. P., I. Vaziri, H. A. Hanna, M. Boktour, J. Thornby, R. Hachem, G. P. Bodey, and I. I. Raad. 2001. Risk factors for Candida tropicalis fungemia in patients with cancer. Clin. Infect. Dis. 33:1676-1681. [DOI] [PubMed] [Google Scholar]
- 13.Krause, D. S., A. E. Simjee, C. van Rensburg, J. Viljoen, T. J. Walsh, B. P. Goldstein, M. Wible, and T. Henkel. 2004. A randomized, double-blind trial of anidulafungin versus fluconazole for the treatment of esophageal candidiasis. Clin. Infect. Dis. 39:770-775. [DOI] [PubMed] [Google Scholar]
- 14.Kuse, E. R., P. Chetchotisakd, C. A. Cunha, C. Barrios, D. Raghunadharao, J. S. Sekhon, V. Ramasubramanian, I. Demeyer, M. Nucci, A. Leelarasamee, F. Jacobs, J. Decruyenaere, D. Pittet, A. J. Ullmann, L. Ostrosky-Zeicher, O. Lortholary, S. Koblinger, H. Diekmann-Berndt, and O. A. Cornely for the Micafungin Invasive Candidiasis Working Group. 2007. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomized double-blind trial. Lancet 369:519-1526. [DOI] [PubMed] [Google Scholar]
- 15.Laverdiere, M., R. G. Lalonde, J. G. Baril, D. C. Sheppard, S. Park, and D. S. Perlin. 2006. Progressive loss of echinocandin activity following prolonged use for treatment of Candida albicans esophagitis. J. Antimicrob. Chemother. 57:705-708. [DOI] [PubMed] [Google Scholar]
- 16.Mora-Duarte, J., R. Betts, C. Rotstein, A. L. Colombo, L. Thompson-Moya, J. Smietana, R. Lupinacci, C. Sable, N. Kartsonis, and J. Perfect. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N. Engl. J. Med. 347:2020-2029. [DOI] [PubMed] [Google Scholar]
- 17.Osherov, N., G. S. May, N. D. Albert, and D. P. Kontoyiannis. 2002. Overexpression of Sbe2p, a Golgi protein, results in resistance to caspofungin in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 46:2462-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park, S., R. Kelly, J. N. Kahn, J. Robles, M. J. Hsu, E. Register, W. Li, V. Vyas, H. Fan, G. Abruzzo, A. Flattery, C. Gill, G. Chrebet, S. A. Parent, M. Kurtz, H. Teppler, C. M. Douglas, and D. S. Perlin. 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 49:3264-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasquale, T., J. R. Tomada, M. Ghannoun, J. Dipersio, and H. Bonilla. 2008. Emergence of Candida tropicalis resistant to caspofungin. J. Antimicrob. Chemother. 61:219. [DOI] [PubMed] [Google Scholar]
- 20.Pelletier, R., I. Alarie, R. Lagace, and T. Walsh. 2005. Emergence of disseminated candidiasis caused by Candida krusei during treatment with caspofungin: case report and review of literature. Med. Mycol. 43:559-564. [DOI] [PubMed] [Google Scholar]
- 21.Perlin, D. S. 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updat. 10:121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfaller, M. A., and D. J. Diekema. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfaller, M. A., D. J. Diekema, L. Ostrosky-Zeichner, J. H. Rex, B. D. Alexander, D. Andes, S. D. Brown, V. Chaturvedi, M. A. Ghannoum, C. C. Knapp, D. J. Sheehan, and T. J. Walsh. 2008. Correlation of MIC with outcome for Candida species tested against caspofungin, anidulafungin, and micafungin: analysis and proposal for interpretive MIC breakpoints. J. Clin. Microbiol. 46:2620-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reboli, A. C., C. Rotstein, P. G. Pappas, S. W. Chapman, D. H. Kett, D. Kumar, R. Betts, M. Wible, B. P. Goldstein, J. Schranz, D. S. Krause, and T. J. Walsh for the Anidulafungin Study Group. 2007. Anidulafungin versus fluconazole for invasive candidiasis. N. Engl. J. Med. 356:2472-2482. [DOI] [PubMed] [Google Scholar]
- 25.Thompson, G. R., III, N. P. Wiederhold, A. C. Vallor, N. C. Villareal, J. S. Lewis II, and T. F. Patterson. 2008. Development of caspofungin resistance following prolonged therapy for invasive candidiasis secondary to Candida glabrata infection. Antimicrob. Agents Chemother. 52:3783-3785. [DOI] [PMC free article] [PubMed] [Google Scholar]