Abstract
Numerous antibiotics have proven to be effective at ameliorating the clinical symptoms of urinary tract infections (UTIs), but recurrent and chronic infections continue to plague many individuals. Most UTIs are caused by strains of uropathogenic Escherichia coli (UPEC), which can form both extra- and intracellular biofilm-like communities within the bladder. UPEC also persist inside host urothelial cells in a more quiescent state, sequestered within late endosomal compartments. Here, we tested a panel of 17 different antibiotics, representing seven distinct functional classes, for their effects on the survival of the reference UPEC isolate UTI89 within both biofilms and host bladder urothelial cells. All but one of the tested antibiotics prevented UTI89 growth in broth culture, and most were at least modestly effective against bacteria present within in vitro-grown biofilms. In contrast, only a few of the antibiotics, including nitrofurantoin and the fluoroquinolones ciprofloxacin and sparfloxacin, were able to eliminate intracellular bacteria in bladder cell culture-based assays. However, in a mouse UTI model system in which these antibiotics reached concentrations in the urine specimens that far exceeded minimal inhibitory doses, UPEC reservoirs in bladder tissues were not effectively eradicated. We conclude that the persistence of UPEC within the bladder, regardless of antibiotic treatments, is likely facilitated by a combination of biofilm formation, entry of UPEC into a quiescent or semiquiescent state within host cells, and the stalwart permeability barrier function associated with the bladder urothelium.
Urinary tract infections (UTIs) currently rank among the most prevalent of infectious diseases worldwide, with chronic and recurrent infections being especially problematic (20). The primary etiologic agents associated with UTIs are strains of uropathogenic Escherichia coli (UPEC) (19). Although often categorized as extracellular pathogens, UPEC can in fact invade a number of host cell types, including the terminally differentiated superficial facet cells and less mature intermediate and basal epithelial cells that comprise the stratified layers of the bladder urothelium (9, 45). Host cell invasion is proposed to facilitate both the establishment and persistence of UPEC within the urinary tract.
UPEC entry into bladder epithelial cells occurs via an actin- and microtubule-dependent process that is mediated by type 1 pili, which are filamentous adhesive organelles that are encoded by virtually all UPEC isolates (10, 38, 60). The FimH adhesin associated with the distal tips of type 1 pili binds mannose-containing glycoprotein host receptors, which include uroplakin (specifically UP1a) and α3β1 integrin complexes (16, 63). Uroplakin plaques coat nearly the entire lumenal surface of the bladder, and their internalization likely facilitates UPEC entry into terminally differentiated superficial bladder cells (5, 41, 43, 45, 60, 63). Alternately, α3β1 integrin receptor complexes, which are more widely expressed within the urothelium and elsewhere, can mediate UPEC invasion of less mature bladder cells via a clathrin-dependent pathway (15, 16). Once internalized, UPEC can be either translocated back out of the host cells or trafficked into late endosomal compartments where they can persist for the long term in a seemingly quiescent state, often bound by a meshwork of actin filaments (5, 17, 44, 46, 56). Alternatively, within the superficial facet cells of the bladder where actin filaments are typically sparse, UPEC can break into the host cytosol and rapidly multiply, forming large biofilm-like inclusions in close association with host intermediate filaments (1, 17, 30, 44). These inclusions, known as intracellular bacterial communities (IBCs), have been equated in military parlance to temporary beachheads, foci in which UPEC numbers are amplified before spreading out to infect surrounding superficial cells and the underlying immature cells of the bladder urothelium (53).
As a whole, the urothelium functions as a permeability barrier on par in strength with the blood-brain barrier (2, 4). Disruption of this barrier during the course of a UTI can occur as a consequence of UPEC-induced exfoliation of infected bladder cells and the influx of neutrophils and other inflammatory responses (43, 45). While these events can be viewed as useful host defense mechanisms, they also provide UPEC with greater access to host tissues. The capacity of UPEC to invade all layers of the urothelium, as well as the development of IBCs and extracellular biofilms, is correlated with enhanced levels of UPEC persistence within the host (23, 30, 32, 43, 44, 46, 57, 62). The establishment of quiescent intracellular bacterial reservoirs within either immature or superficial bladder epithelial cells may conceal UPEC from many host immunosurveillance mechanisms, while the development of IBCs and extracellular biofilms may enable UPEC to better resist the antimicrobial activities of neutrophils and other host defenses.
Biofilm formation and host cell invasion may also provide UPEC with enhanced protection against antibiotic treatments. Relative to planktonic bacteria, biofilm-associated microbes are by and large better equipped to survive treatments with antibiotics (21, 58). The inability of many antibiotics to readily cross host membranes may further limit their effectiveness against intracellular bacteria. This problem is likely exacerbated by UPEC infiltration of host cells within the deeper layers of the urothelial barrier. In addition, the quiescent nature of some intracellular UPEC populations could render them resistant to antibiotics that primarily target replicating microbes. The challenge associated with ridding the bladder of UPEC has been illustrated in mouse UTI model systems in which the antibiotics gentamicin, cefuroxime, trimethoprim-sulfamethoxazole (SXT), and the extended-spectrum penicillin drug amdinocillin had little effect on bacterial titers within bladder tissue, even though urine titers were drastically reduced (26, 32, 43, 45, 52). These and related observations indicated that recurrent UTIs in many individuals, including those who receive antibiotic treatments, may in actuality be relapses caused by the resurgence of UPEC from intracellular bacterial reservoirs. Epidemiological studies lend credence to this possibility, demonstrating that the bacteria responsible for recurrent UTIs are identical to the microbes that caused the initial acute infections in up to 68% of patients (6, 8, 27, 28, 31, 42, 51). In light of these findings, it has been suggested that more penetrant antibiotics might better eliminate UPEC reservoirs from the bladder and consequently reduce the incidence of chronic and recurrent UTIs (9, 32). Here, we address this possibility by testing the effects of a range of functionally distinct antibiotics on intracellular and biofilm-associated UPEC using both in vitro and in vivo assays.
MATERIALS AND METHODS
Bacteria and bladder cell culture.
The UPEC cystitis isolate UTI89 and the recombinant strain UTI89/pGEN-GFP(LVA) have been described previously (7, 44, 61). Bacteria were grown from frozen stocks in either Luria-Bertani (LB) broth or M9 minimal medium (6 g/liter Na2HPO4, 3 g/liter KH2PO4, 1 g/liter NH4Cl, 0.5 g/liter NaCl, 1 mM MgSO4, 0.1 mM CaCl2, 0.1% glucose, 0.0025% nicotinic acid, 0.2% casein amino acids, and 16.5 μg/ml thiamine in H2O) at 37°C. The human bladder epithelial cell line 5637 (ATCC HTB-9) was maintained at 37°C and 5% CO2 in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (HyClone).
Antibiotics.
All antibiotics were purchased from Sigma-Aldrich and used at the final concentrations indicated. Stocks were prepared as follows: penicillin (100 mg/ml in H2O), nafcillin (120 mg/ml in H2O), cefadroxil (30 mg/ml in phosphate-buffered saline [PBS] diluted 1:1 with H2O), trimethoprim-sulfamethoxazole (30 and 20 mg/ml, respectively, in H2O), ofloxacin (100 mg/ml in H2O), ciprofloxacin (100 μg/ml in H2O for in vitro assays and 40 mg/ml for in vivo use), norfloxacin (100 mg/ml in H2O), levofloxacin (30 mg/ml in H2O), sparfloxacin (100 μg/ml in H2O for in vitro assays and 14 mg/ml for in vivo use), nalidixic acid (100 mg/ml in H2O), nitrofurantoin (100 mg/ml in dimethyl sulfoxide [DMSO]), fosfomycin (100 mg/ml in 50% methanol for in vitro assays and 20 mg/ml for in vivo use), tetracycline (5 mg/ml in H2O), gentamicin (80 mg/ml in H2O for in vitro assays or 4 mg/ml for in vivo use), kanamycin (50 mg/ml in H2O), and amikacin (200 mg/ml in H2O). NaOH (1 N) was added dropwise to solubilize stocks of trimethoprim-sulfamethoxazole, ofloxacin, ciprofloxacin, norfloxacin, levofloxacin, and sparfloxacin. Diluents alone were used as negative controls in all assays.
Intracellular bacterial survival assays.
UTI89 was grown at 37°C for 24 h in static LB broth to induce type 1 pilus expression. Triplicate sets of confluent 5637 bladder epithelial cell monolayers grown in 24-well tissue culture plates were infected with UTI89 using a multiplicity of infection of ∼15 bacteria per host cell. To facilitate and synchronize bacterial contact with the host cells, plates were centrifuged at 600 × g for 5 min at the start of infection. After a 2-h incubation at 37°C, samples were washed three times with PBS containing Ca2+ and Mg2+ (PBS2+) to remove any nonadherent bacteria. Monolayers were then incubated for another 2 h with complete RPMI medium plus 100 μg/ml of gentamicin to kill extracellular bacteria. Following additional washes with PBS2+, fresh medium containing a lower concentration of gentamicin (10 μg/ml) was added, and incubations were continued for another 14 h. This submaximal concentration of gentamicin was used to prevent extracellular growth of UPEC while limiting possible leaching of the antibiotic into the host cells during longer incubations (13). Monolayers were then washed with PBS2+ and incubated for another 12 h in fresh medium ± the indicated antibiotics. After final washes in PBS2+, host cells were lysed in PBS plus 0.4% Triton X-100, and bacteria present within the lysates were enumerated by plating serial dilutions on LB agar plates.
Cytotoxicity assays.
Luciferase-based lactate dehydrogenase (LDH) cytotoxicity assays were performed using the CytoTox-Glo cytotoxicity assay (Promega), according to the instructions from the manufacturer. Host cell integrity was also assessed using trypan blue (0.4%) exclusion assays, according to standard protocols (Sigma-Aldrich).
Biofilm assays.
In vitro microtiter plate-based biofilm assays were performed as previously described (39). Briefly, UTI89 was diluted 1:100 from overnight shaking cultures into M9 medium, and triplicate 100-μl samples in 96-well pinchbar flat-bottomed polystyrene microtiter plates with lids (Nunc) were incubated for 48 h without shaking at 37°C. Nonadherent bacteria were then removed, and fresh M9 medium was added to each well ± antibiotics, as indicated. After an additional 24 h of incubation, the wells were washed twice with H2O prior to addition of crystal violet (150 μl of a 0.1% solution in water; Sigma-Aldrich). After a 10-min incubation at room temperature, the wells were rinsed twice with H2O and air dried. Dimethyl sulfoxide (200 μl; Sigma-Aldrich) was then added to each well, and the plates were shaken vigorously for 15 min on an orbital shaker to solubilize the dye. A 150-μl aliquot from each well was transferred to a fresh microtiter plate, and A562 was measured using a Synergy HT multidetection microplate reader (BioTek Instruments, Inc.). In separate experiments, viable bacteria within biofilms treated with or without antibiotics were visualized using the Live/Dead BacLight bacterial viability kit (Molecular Probes), according to manufacturer's instructions.
In vivo antibiotic protection assays.
Seven- to eight-week-old female CBA/J mice (Jackson Laboratory) were anesthetized via isoflurane inhalation and slowly inoculated via transurethral catheterization with 50 μl of a bacterial suspension (∼107 CFU from 24-h static LB broth cultures of UTI89) in PBS as previously described (43). Bacterial reflux into the kidneys using this procedure is rare, occurring in less than 1% of the test animals. At 3 days postinoculation, antibiotic treatments were initiated by administering one dose of antibiotic daily for 3 consecutive days. Gentamicin (200 μg) was given subcutaneously, while ciprofloxacin (2 mg), sparfloxacin (700 μg), and fosfomycin (1 mg) were given orally by gavaging. Control animals were given water alone by gavaging. All antibiotics were delivered in 50-μl volumes. Mice were sacrificed 3 days after the final antibiotic treatment (day 9 postinoculation). At this time point, bladders were harvested aseptically, weighed, and homogenized in 1 ml PBS containing 0.025% Triton X-100. Bacterial titers within the homogenates were determined by plating serial dilutions on LB agar plates. Eleven mice total, from two independent experiments, were used for each condition tested. Mock-infected mice were housed in cages with the infected animals.
Disc diffusion assays.
Antibiotic concentrations in mouse urine specimens were determined using disc diffusion assays modified from the Kirby-Bauer method (3). Two hours after administration of antibiotics, urine specimens were collected from mice by gently pressing their bladders over clean plastic wrap. Ten-microliter aliquots of urine obtained from individual mice were spotted onto 7-mm-diameter circular pieces of sterile filter paper, which were then placed on freshly plated lawns of UTI89 spread on LB agar plates. The diameter of clearance around each disc was measured after a 24-h incubation at 37°C. Filter discs containing known antibiotic concentrations were used to generate standard curves from which antibiotic levels in the urine specimens were calculated.
Antibiotic treatment of bladder homogenates.
Three days postinoculation with UTI89, bladders from CBA/J mice were removed and homogenized in 1 ml PBS containing 0.025% Triton X-100. One hundred-microliter aliquots of the homogenates were then incubated for 4 h at 37°C ± gentamicin (400 μg/ml), ciprofloxacin (2 μg/ml), sparfloxacin (12 μg/ml), fosfomycin (700 μg/ml), or a combination of sparfloxacin and fosfomycin. Surviving bacteria present in the homogenates were then pelleted (5 min at 10,000 × g), washed in sterile PBS, and plated.
IBC quantification.
CBA/J mice were infected with UTI89/pGEN-GFP(LVA) via transurethral catheterization. At 6 h postinoculation, mice were sacrificed, and bladders were removed, halved, splayed, and pinned down lumenal side up on silicon disks (Sylgard 184 silicone elastomer; Dow Corning Corp.) under Ringer solution (155 mM NaCl, 3 mM HCl, 2 mM CaCl2, 1 mM MgCl2, 3 mM NaH2PO4, 10 mM glucose, and 5 mM HEPES [pH 7.4]). Sparfloxacin (12 μg/ml)-fosfomycin (700 μg/ml) was then added as indicated. Green fluorescent protein (GFP)-positive IBCs were visualized and enumerated using a SZX10 stereomicroscope (Olympus) equipped with a Canon PowerShot A640 10.1 megapixel camera mounted via a CamAdapter kit.
Statistics.
P values were determined by Mann-Whitney U tests performed using Prism 5.01 software (GraphPad Software). Values of less than 0.05 were defined as significant.
RESULTS
Susceptibility of intracellular UPEC to antibiotics.
The effectiveness of a panel of commonly prescribed antibiotics against intracellular UPEC was assessed using cell culture-based assays. The cystitis isolate UTI89 was allowed to invade monolayers of the bladder epithelial cell line 5637 for 2 h, after which extracellular bacteria were killed by addition of the host cell-impermeable antibiotic gentamicin. Following a 14-h incubation in the continued presence of gentamicin, infected monolayers were washed and treated for another 12 h with fresh gentamicin or 1 of 16 other antibiotics. These antibiotics represent several distinct functional classes, including inhibitors of cell wall synthesis, folic acid production, translation, DNA gyrase, and topoisomerase IV (Table 1). Infected monolayers were treated with antibiotic concentrations that were equal to or above the levels that normally accumulate in the urine of human patients during the course of treatment (36). Of note, in LB broth, UTI89 was sensitive to all of the tested antibiotics, with the exception of nafcillin, which served as a negative control.
TABLE 1.
Antibiotics utilized in this study
| Family/antibiotic | Primary function in E. coli | Effect on bacteria | Urine level (μg/ml)a | Amts tested (μg/ml) |
|---|---|---|---|---|
| β-Lactams | Cell wall synthesis inhibitor | Bactericidal | ||
| Penicillin | 597 | 200, 600, 1,000 | ||
| Nafcillin | UPEC is resistant | 285-1,188 | 120, 1,200, 1,800 | |
| Cefadroxil | 1,200 | 800, 1,200, 1,600 | ||
| Dihydrofolate reductase inhibitor | Folic acid synthesis inhibitor | Bacteriostatic | ||
| Trimethoprimb | 31-165 | 30, 100, 300 | ||
| Sulfonamide | Folic acid synthesis inhibitor | Bacteriostatic | ||
| Sulfamethoxazoleb | 10-133 | 20, 66, 200 | ||
| Quinolones | Topoisomerase IV and DNA gyrase inhibitor | Bactericidal | ||
| Ofloxacin | 126-438 | 100, 400, 700 | ||
| Ciprofloxacin | 2 | 1, 2, 3 | ||
| Norfloxacin | 168-417 | 100, 300, 500 | ||
| Levofloxacin | 286 | 100, 200, 300 | ||
| Sparfloxacin | 12 | 12, 100, 400 | ||
| Nalidixic acid | 63-1,000 | 50, 800, 1,200 | ||
| Tetracyclines | Protein synthesis inhibitor | Bacteriostatic/bactericidal | ||
| Tetracycline | 273 | 100, 250, 300 | ||
| Aminoglycosides | Protein synthesis inhibitor | Bacteriostatic/bactericidal | ||
| Gentamicin | 400-500 | 100, 400, 800 | ||
| Kanamycin | 250-3,100 | 200, 1,000, 3,000 | ||
| Amikacin | 170-1,720 | 150, 1,000, 2,000 | ||
| Miscellaneous | ||||
| Nitrofurantoin | DNA damage | Bactericidal | 25-300 | 15, 300, 500 |
| Fosfomycin | Cell wall synthesis inhibitor | Bactericidal | 706 | 400, 700, 1,000 |
See reference 36.
Used in combination.
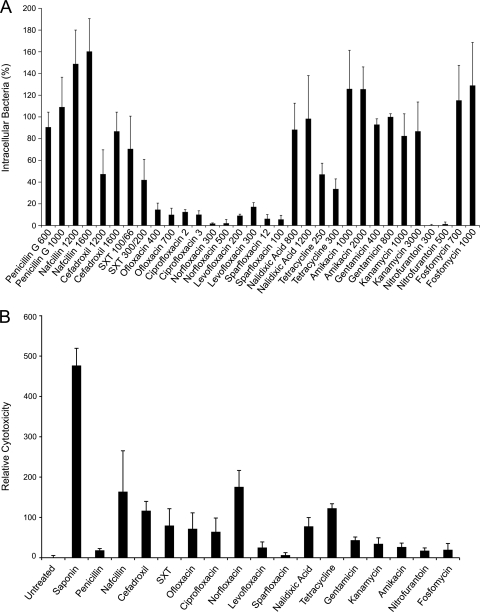
Bacterial titers recovered at the end of the intracellular survival assays were normalized relative to samples treated with 800 μg/ml gentamicin (Fig. 1A). Nitrofurantoin and the quinolones ofloxacin, ciprofloxacin, norfloxacin, levofloxacin, and sparfloxacin were the most effective at eradicating intracellular UPEC. Quinolones are known to accumulate within host cells (48), and this likely contributes to their potency against internalized UPEC in these assays. Penicillin G, fosfomycin, and the drug combination trimethoprim-sulfamethoxazole (SXT) were no more effective than the control membrane-impermeable antibiotic gentamicin or other aminoglycosides. All of the tested antibiotics caused at least a small amount of cytotoxicity to the host cells relative to untreated controls, as determined using trypan blue exclusion assays (data not shown) and quantitation of host LDH release (Fig. 1B). However, the ability of an antibiotic to kill intracellular UPEC did not appear to correlate with cytotoxic effects on the host bladder cells. These results indicate that host cell invasion can provide UPEC with quantifiable survival advantages when confronted with a variety of different antibiotics, except for nitrofurantoin and most of the tested quinolones.
FIG. 1.
Antibiotic effects on intracellular UPEC and host cell cytotoxicity. (A) 5637 bladder epithelial cell monolayers infected with UTI89 were incubated for 14 h in media containing 10 μg/ml gentamicin to prevent extracellular bacterial growth and to allow time for the establishment of UPEC within the host bladder cells. Following washes with PBS2+, infected monolayers were incubated for an additional 12 h with two concentrations (μg/ml) of each of the different antibiotics being tested, as indicated. Surviving bacterial titers are presented relative to reference samples that were treated with 800 μg/ml gentamicin. Data represent the mean results ± SEM from three or more independent assays performed in triplicate. (B) Cytotoxic effects of each antibiotic (used at the higher of the two concentrations indicated in panel A) on 5637 bladder cells were determined after 12 h treatments by measuring LDH release. Cells treated with the pore-forming glycoside saponin were used as positive controls. Results indicate the means ± SEM from three independent experiments performed in duplicate or triplicate.
Antibiotic sensitivity of biofilm-associated UPEC.
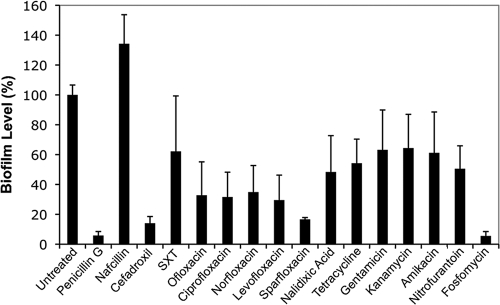
Antibiotic effects on biofilms formed by UTI89 were examined in vitro using standard microtiter plate-based assays (Fig. 2). Notably, biofilm formation in these types of in vitro assays often correlates with the ability of UPEC strains to cause relapsing infections in human patients (57). In our assays, all of the antibiotics, with the exception of nafcillin, appreciably reduced biofilm levels relative to untreated controls. Of the 17 antibiotics tested, the cell wall synthesis inhibitors penicillin G, cefadroxil, and fosfomycin had the most striking inhibitory effects on biofilm persistence, followed closely by the quinolone sparfloxacin (Fig. 2). These antibiotics not only inhibited biofilm growth but also promoted the degeneration of preexisting biofilm communities. Microscopy coupled with Live/Dead staining validated these results and, in addition, confirmed the presence of viable UTI89 within the biofilms that withstood antibiotic treatments (data not shown).
FIG. 2.
Antibiotic effects on UPEC biofilms grown in vitro at 37°C in M9 medium. Biofilm levels were quantified relative to untreated controls following 24-h treatments with the indicated antibiotics, which were used at the higher of the two concentrations specified in Fig. 1A. Data represent the mean results ± SEM from three independent experiments performed in triplicate.
Susceptibility of UPEC to antibiotics in vivo within the bladder.
A subset of the antibiotics used above was tested for effectiveness against UPEC reservoirs in a mouse UTI model system. Adult female CBA/J mice were infected with UTI89 via transurethral inoculation, and antibiotic treatments were initiated 3 days later, allowing time for UTI89 to first establish reservoir populations within the bladder. Fosfomycin was chosen for its superior ability to clear biofilm growth in vitro (Fig. 2). Ciprofloxacin and sparfloxacin were tested as two separate types of quinolones shown to be effective against intracellular UTI89 in our in vitro assays (Fig. 1A). The antibiotic nitrofurantoin, although also very effective at clearing intracellular UPEC, was not used since it has notable toxic side effects in human subjects (55, 59). A combination treatment of sparfloxacin-fosfomycin was also included, with the idea that together these antibiotics might have enhanced activity against UPEC reservoirs within the bladder. Support for this possibility comes from previous work in which fosfomycin used in combination with another quinolone, ulifloxacin, significantly enhanced the clearance of Pseudomonas aeruginosa within biofilms better than either antibiotic on its own (40).
In our assays, antibiotics were administered to infected mice daily for 3 days, with control groups given either water alone or gentamicin. After an additional 3 days without antibiotic treatments (9 days total postinoculation), mice were sacrificed, and bacterial titers present within the bladders were enumerated. By waiting 3 days after cessation of the antibiotic treatments, we minimized the risk that residual levels of antibiotics in the host might kill off any surviving UPEC reservoirs during the bladder tissue homogenization process. Furthermore, this experimental setup represents a convenient model of how relapsing or persistent UTIs may proceed in human populations, with UPEC possibly maintaining a presence in the bladder in the days to weeks after antibiotic treatments have ended. Antibiotic concentrations employed in our in vivo assays were based on standard doses given to human patients, scaled down for use in mice (36). Disc diffusion assays with urine specimens collected from treated mice confirmed that each antibiotic reached concentrations in the urine that were sufficient to kill free-living UTI89. Specifically, within 2 h postadministration, effective antibiotic concentrations in the urine reached 2.0 mg/ml for gentamicin, 1.0 mg/ml for ciprofloxacin, 0.3 mg/ml for sparfloxacin, and 4.0 mg/ml for fosfomycin. These values are all at least 20-fold higher than the levels found in human urine following treatment (36).
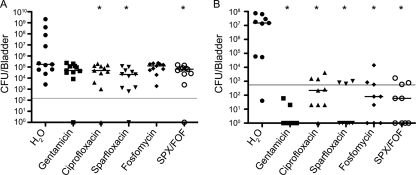
As shown in Fig. 3A, UTI89 was able to persist within the bladder regardless of any of the antibiotic treatments used. While administration of ciprofloxacin, sparfloxacin, or the combination sparfloxacin-fosfomycin significantly reduced bacterial titers relative to controls treated with water alone, these antibiotics were at best only marginally better than gentamicin or fosfomycin. High bacterial titers of greater than 106 CFU/g of bladder tissue, as observed in mice treated with only water, were not recovered in any of the antibiotic-treated mice. Importantly, no bacteria were detected in the bladders of mock-infected mice that were housed in cages with infected animals during the duration of these experiments. These results indicate that reinoculation of UPEC and the dissemination of UTIs between animals are, at best, rare events in these types of studies, as has been reported previously (52).
FIG. 3.
Antibiotic susceptibility of UPEC within mouse bladders. (A) Starting at day 3 postinoculation with UTI89, infected CBA/J mice were treated for 3 consecutive days with the indicated antibiotics. Following an additional 3-day period, surviving bacterial titers present within the bladders were determined. The graph depicts the cumulative results from two independent assays (n = 11). (B) The graph shows bacterial titers in aliquots of tissue homogenates from mouse bladders (n = 9) recovered at 3 days postinoculation and subsequently treated for 4 h with the indicated antibiotics. Bars indicate median values for each group, while the solid lines denote the LOQ. P values of <0.05 (A) or <0.001 (B), relative to the water-treated controls, were determined using Mann-Whitney U tests and are labeled with asterisks. Sparfloxacin-fosfomycin, SPX/FOF.
Microscopic imaging of untreated and antibiotic-treated mouse bladders alike shows that UTI89 can invade all layers of the urothelium by 9 days postinoculation, in line with previous observations (17, 44, 46; data not shown). Bacterial penetration of the urothelial permeability barrier likely renders UPEC less susceptible to antibiotic treatments in vivo. To better address this possibility, bladders from UTI89-infected mice were homogenized in the presence of 0.025% Triton X-100 detergent to disrupt the urothelial barrier function, as well as individual host cells, prior to antibiotic treatments. In this situation, all of the antibiotics tested significantly reduced bacterial numbers to levels near or below the limit of quantitation (LOQ) (Fig. 3B). Lack of accessibility to UPEC within urothelial barriers thus appears to reduce the effectiveness of antibiotics, including quinolones that can readily penetrate host cell monolayers in cell culture-based assays. Of note, antibiotic treatments of homogenates from infected bladders did not completely eradicate UPEC in all cases, reaffirming the possible development during UTIs of metabolically quiescent reservoirs, or persister cells, that may be innately less susceptible to antibiotics regardless of antibiotic accessibility issues (11, 37, 44, 46).
Antibiotic effects on IBCs.
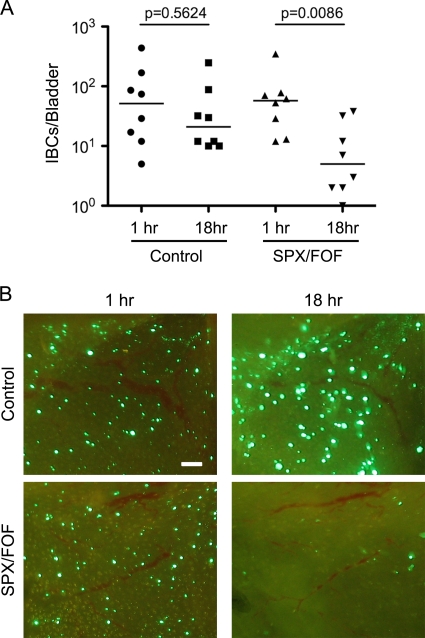
The persistence of UPEC reservoir populations in the bladder following antibiotic treatments, as seen in Fig. 3A, may also be in part attributable to the presence of IBCs. If so, IBCs should be resistant to the antibiotics used in our in vivo assays. To address this possibility, CBA/J mice were inoculated with UTI89 carrying pGEN-GFP(LVA), a high-retention, low-copy-number plasmid that constitutively expresses a destabilized variant of GFP (61). Use of this strain allows for direct observation by fluorescent microscopy of viable, or recently viable, bacteria. Within 6 h postinoculation into the bladder, UTI89/pGEN-GFP(LVA) is able to invade the superficial cells of the bladder urothelium and form IBCs that can be enumerated by imaging bladder explants. Incubation of these explants in the absence of antibiotics for 18 h does not appreciably affect the numbers of IBCs (Fig. 4A), although the overall sizes and intensities of the IBCs do increase (Fig. 4B). In contrast, incubation in the presence of the drug combination sparfloxacin-fosfomycin leads to significantly reduced IBC numbers (Fig. 4A and B). Similar results were obtained in experiments using only sparfloxacin (data not shown). These data indicate that IBCs can be fairly sensitive to antibiotic treatments. Microscopic analysis of bladders recovered from antibiotic-treated mice at 9 days postinoculation support this conclusion, revealing a scarcity of IBCs at this late time point (data not shown). Even in the absence of antibiotic treatments, IBCs do not appear to be particularly long-lived, being key targets of infiltrating neutrophils and subject to attrition as the host cells that they occupy fall apart and/or exfoliate (30, 44, 45; our unpublished observations). Together, these observations suggest that IBCs do not act as primary reservoirs for the long-term persistence of UPEC within the bladder, although they likely function in the establishment of reservoir populations.
FIG. 4.
Antibiotic susceptibility of IBCs. At 6 h postinoculation with UTI89/pGEN-GFP(LVA), bladders obtained from CBA/J mice were collected, splayed, and incubated in the presence or absence of the antibiotic combination sparfloxacin-fosfomycin. (A) Using fluorescence microscopy, IBCs were enumerated at the indicated times after addition of the antibiotics. Bars denote median values. P values were calculated using the Mann-Whitney U test (n = 8 mice per group). (B) Representative images showing IBCs in control and sparfloxacin-fosfomycin-treated bladder explants at the 1- and 18-h time points. Scale bar = 100 μm.
DISCUSSION
In human studies, recurrent UTI rates in untreated individuals have been reported to range from about 25% to greater than 40% (Table 2). Administration of commonly used antibiotics, including several of the ones used here, can reportedly reduce UTI recurrence rates. Some alternative therapeutic approaches may also be of benefit, as suggested by recent meta-analyses showing that the long-term intake of cranberry juice or cranberry juice extracts can reduce recurrent UTI rates in young to middle-aged women by about 35% (22). Variations in study design and execution make direct comparison of results from different treatment approaches difficult. However, it is clear from the literature that recurrent UTIs remain problematic for many individuals, regardless of therapeutic intervention (Table 2).
TABLE 2.
Recurrence after antibiotic treatments in human studiesa
| Treatment | Abbreviation | Treatment duration | Recurrence rate | Time of recurrence | Reference |
|---|---|---|---|---|---|
| Untreated | 27.8 | <3 mo | 8 | ||
| 44 | >1 mo | 27 | |||
| 27 | <6 mo | 20 | |||
| 36 | 12 mo | 33 | |||
| Cranberry juice | 6 mo | 16 | 12 mo | 33 | |
| Trimethoprim-sulfamethoxazole | SXT | 7 d | 2 | 5 wk | 25 |
| NR | 43 | <5 mo | 35 | ||
| NR | 7.1 | NR | 54 | ||
| Ofloxacin | OFX | 1 d | 19 | 5 wk | 25 |
| 3 d | 11 | 5 wk | 25 | ||
| Ciprofloxacin | CIP | 7 d | 7.7 | 4-6 wk | 24 |
| NR | 4.7 | NR | 54 | ||
| Norfloxacin | NOR | NR | 14.8 | NR | 54 |
| NR | 11 | 4-6 wk | 50 | ||
| Levofloxacin | LVX | 1 d | 17.4 | <3 mo | 34 |
| 3 d | 5.6 | <3 mo | 34 | ||
| Sparfloxacin | SPX | 1 d | 12 | 4-6 wk | 24 |
| 3 d | 8.1 | 4-6 wk | 24 | ||
| Nalidixic acid/citrate | NAL | 5 d | 17 | 1 mo | 18 |
| NR | 33 | 4-6 wk | 50 | ||
| Nitrofurantoin | NIT | NR | 35.3 | NR | 54 |
| Fosfomycin | FOF | NR | 0.2 | NR | 54 |
| Fosfomycin trometamol | FT | 1 d | 14 | 1 mo | 12 |
| Gentamicin | GEN | 10 d | 42 | 30 d | 49 |
NR = not reported.
A growing number of studies indicate that many recurrent UTIs may in effect be relapses caused by the resurgence of intracellular bacterial reservoirs that can persist for many weeks to months within the urothelium (26, 32, 43-45, 52). Here, using bladder cell culture-based assays, we found that intracellular UPEC strains are protected against several different classes of antibiotics, with the exceptions of nitrofurantoin and several fluoroquinolones (Fig. 1A). These antibiotics are known to penetrate host cell membranes and accumulate to high concentrations intracellularly (48). Interestingly, in human studies, patients who received fluoroquinolones often appeared to have fewer recurrent UTIs overall, in comparison with patients who were given other antibiotic treatments (Table 2). However, with respect to bacterial clearance from bladder tissues in our mouse UTI model system, the fluoroquinolones ciprofloxacin and sparfloxacin were statistically no better than the membrane-impermeable antibiotic gentamicin, although all three of these antibiotics were significantly better than no treatment at all (Fig. 3A).
Surprisingly, none of the antibiotics tested in mice, regardless of their effectiveness against internalized UPEC in cell culture work, were able to completely eradicate UPEC reservoir populations in the mouse bladder. The redoubtable permeability barrier function of the bladder urothelium likely limits the effectiveness of these antibiotics in vivo. The increased susceptibility of UPEC to ex vivo antibiotic treatments following disruption of bladder tissue and cells supports this conclusion (Fig. 2). These observations also raise the intriguing possibility that host cell cytotoxic effects associated with some antibiotics could enhance their efficacy against internalized UPEC by disrupting cell and tissue barriers, although increased host cytotoxicity did not necessarily correlate with improved bacterial killing by antibiotics in our cell culture-based assays (Fig. 1B).
In addition to host cell invasion, IBC formation within superficial bladder cells and the development of biofilms in association with catheter and urothelial surfaces may also render UPEC resistant to antibiotic treatments. Our in vitro biofilm assays indicated that only a limited subset of the tested antibiotics were able to effectively eradicate UPEC growing in biofilms. The most potent antibiotics in these assays were the cell wall synthesis inhibitor fosfomycin, the fluoroquinolone sparfloxacin, and the β-lactam antibiotics penicillin G and cefadroxil. These antibiotics prevented growth of new biofilms and disrupted preexisting biofilm communities. In contrast, the β-lactam antibiotic nafcillin appeared to significantly enhance biofilm formation relative to untreated controls (Fig. 2). However, if nafcillin was added at the start of our biofilm assays, UTI89 was not able to initiate biofilm formation. This antibiotic has only a slight effect on UTI89 growth rates, but it does alter the morphology of the bacteria substantially, causing them to become more elongated and crescent shaped (data not shown). This observation suggests that nafcillin causes significant stress to UTI89, even though UTI89 and most other E. coli isolates are inherently resistant to this antibiotic (47). The apparent stimulation of biofilm formation by nafcillin may be a consequence of this stress, morphological alterations of the bacteria, and/or other as yet undefined factors.
Ineffective antibiotic diffusion within biofilms, as well as alterations in the metabolic state of biofilm-associated microbes (21, 58), likely decreases the sensitivity of UTI89 to many of the antibiotics used in our assays. Consequently, the development of biofilm-like communities, including IBCs, may promote UPEC persistence within the urinary tract, despite the administration of antibiotics. However, while it is probable that IBCs contribute to the establishment of long-term bacterial reservoirs within the bladder, we propose that IBCs are likely not the major embodiment of the reservoir populations. This conclusion is based on results showing that IBCs are rather sensitive in ex vivo assays to antibiotics like sparfloxacin, which nonetheless fail to eradicate UPEC reservoirs in vivo. Furthermore, previous work from our lab and others indicates that IBCs do not persist in the bladder for long durations even in the absence of antibiotic treatments (30, 44, 45).
In total, our data indicate that bacterial reservoir populations within the bladder are protected from even highly membrane-permeable antibiotics such as quinolones, as well as drugs like fosfomycin that have the ability to disrupt UPEC biofilms and IBCs. The results presented here are in agreement with previous findings suggesting that long-lived UPEC reservoirs within the bladder consist primarily of small bacterial clusters or single microbes that are bound by actin and compartmentalized within the urothelium barrier (17, 30, 46). In addition to their localization within the urothelial barrier, the quiescent nature of the UPEC reservoirs and, perhaps, the generation of so-called persister bacterial cells may provide further protection against antibiotics (11, 17, 29, 37). Interestingly, it has been reported that fluoroquinolones like sparfloxacin have some bactericidal activity against nonreplicating and slowly growing E. coli (14), characteristics that may contribute to the effectiveness of sparfloxacin and related antibiotics against intracellular and biofilm-associated UPEC.
It is possible that longer-term antibiotic treatments, beyond the 3-day regimen common in the clinic and used here with infected mice, may better eliminate UPEC reservoir populations and potentially reduce recurrent UTI rates. However, longer-term antibiotic treatments may be associated with increased financial costs, decreased patient compliance, and increased risks for selection of antibiotic-resistant pathogens. The effectiveness of longer-term treatments will also likely be critically dependent upon the permeability and bactericidal properties of the antibiotics used. Of note, with respect to the long-term persistence of UPEC within the urinary tract, previous work indicated that mice treated for 3 days with the host membrane-impermeable antibiotic combination trimethoprim-sulfamethoxazole were not significantly better off than mice treated for 10 days (52). Likewise, in human studies, children treated with gentamicin for 10 days still had high rates of recurrence (Table 2) (49). Ultimately, the development of antimicrobials that can both readily penetrate tissue barriers like the urothelium and target nonreplicating microbes, coupled with optimized treatment regimes, may provide substantially improved protection against chronic and recurrent UTIs.
Acknowledgments
This work was supported by Public Health Service grant DK068585 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Published ahead of print on 15 March 2010.
REFERENCES
- 1.Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. [DOI] [PubMed] [Google Scholar]
- 2.Apodaca, G. 2004. The uroepithelium: not just a passive barrier. Traffic 5:117-128. [DOI] [PubMed] [Google Scholar]
- 3.Bauer, A. W., W. M. Kirby, J. C. Sherris, and M. Turck. 1966. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 45:493-496. [PubMed] [Google Scholar]
- 4.Beckel, J. M., A. Kanai, S. J. Lee, W. C. de Groat, and L. A. Birder. 2006. Expression of functional nicotinic acetylcholine receptors in rat urinary bladder epithelial cells. Am. J. Physiol. Renal Physiol. 290:F103-F110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishop, B. L., M. J. Duncan, J. Song, G. Li, D. Zaas, and S. N. Abraham. 2007. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat. Med. 13:625-630. [DOI] [PubMed] [Google Scholar]
- 6.Brauner, A., S. H. Jacobson, and I. Kuhn. 1992. Urinary Escherichia coli causing recurrent infections—a prospective follow-up of biochemical phenotypes. Clin. Nephrol. 38:318-323. [PubMed] [Google Scholar]
- 7.Chen, S. L., C. S. Hung, J. Xu, C. S. Reigstad, V. Magrini, A. Sabo, D. Blasiar, T. Bieri, R. R. Meyer, P. Ozersky, J. R. Armstrong, R. S. Fulton, J. P. Latreille, J. Spieth, T. M. Hooton, E. R. Mardis, S. J. Hultgren, and J. I. Gordon. 2006. Identification of genes subject to positive selection in uropathogenic strains of Escherichia coli: a comparative genomics approach. Proc. Natl. Acad. Sci. U. S. A. 103:5977-5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czaja, C. A., W. E. Stamm, A. E. Stapleton, P. L. Roberts, T. R. Hawn, D. Scholes, M. Samadpour, S. J. Hultgren, and T. M. Hooton. 2009. Prospective cohort study of microbial and inflammatory events immediately preceding Escherichia coli recurrent urinary tract infection in women. J. Infect. Dis. 200:528-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhakal, B. K., R. R. Kulesus, and M. A. Mulvey. 2008. Mechanisms and consequences of bladder cell invasion by uropathogenic Escherichia coli. Eur. J. Clin. Invest. 38(Suppl. 2):2-11. [DOI] [PubMed] [Google Scholar]
- 10.Dhakal, B. K., and M. A. Mulvey. 2009. Uropathogenic Escherichia coli invades host cells via an HDAC6-modulated microtubule-dependent pathway. J. Biol. Chem. 284:446-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorr, T., K. Lewis, and M. Vulic. 2009. SOS response induces persistence to fluoroquinolones in Escherichia coli. PLoS Genet. 5:e1000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elhanan, G., H. Tabenkin, R. Yahalom, and R. Raz. 1994. Single-dose fosfomycin trometamol versus 5-day cephalexin regimen for treatment of uncomplicated lower urinary tract infections in women. Antimicrob. Agents Chemother. 38:2612-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elsinghorst, E. A. 1994. Measurement of invasion by gentamicin resistance. Methods Enzymol. 236:405-420. [DOI] [PubMed] [Google Scholar]
- 14.Eng, R. H., F. T. Padberg, S. M. Smith, E. N. Tan, and C. E. Cherubin. 1991. Bactericidal effects of antibiotics on slowly growing and nongrowing bacteria. Antimicrob. Agents Chemother. 35:1824-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eto, D. S., H. B. Gordon, B. K. Dhakal, T. A. Jones, and M. A. Mulvey. 2008. Clathrin, AP-2, and the NPXY-binding subset of alternate endocytic adaptors facilitate FimH-mediated bacterial invasion of host cells. Cell. Microbiol. 10:2553-2567. [DOI] [PubMed] [Google Scholar]
- 16.Eto, D. S., T. A. Jones, J. L. Sundsbak, and M. A. Mulvey. 2007. Integrin-mediated host cell invasion by type 1-piliated uropathogenic Escherichia coli. PLoS Pathog. 3:e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eto, D. S., J. L. Sundsbak, and M. A. Mulvey. 2006. Actin-gated intracellular growth and resurgence of uropathogenic Escherichia coli. Cell. Microbiol. 8:704-717. [DOI] [PubMed] [Google Scholar]
- 18.Ferry, S., L. G. Burman, B. Widberg, and C. Calmenius. 1987. Short-term nalidixic acid plus sodium citrate in acute lower urinary tract infection. Scand. J. Infect. Dis. 19:469-477. [DOI] [PubMed] [Google Scholar]
- 19.Foxman, B. 2003. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis. Mon. 49:53-70. [DOI] [PubMed] [Google Scholar]
- 20.Foxman, B. 1990. Recurring urinary tract infection: incidence and risk factors. Am. J. Public Health 80:331-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fux, C. A., J. W. Costerton, P. S. Stewart, and P. Stoodley. 2005. Survival strategies of infectious biofilms. Trends Microbiol. 13:34-40. [DOI] [PubMed] [Google Scholar]
- 22.Guay, D. R. 2009. Cranberry and urinary tract infections. Drugs 69:775-807. [DOI] [PubMed] [Google Scholar]
- 23.Hatt, J. K., and P. N. Rather. 2008. Role of bacterial biofilms in urinary tract infections. Curr. Top. Microbiol. Immunol. 322:163-192. [DOI] [PubMed] [Google Scholar]
- 24.Henry, D. C., R. C. Nenad, A. Iravani, A. D. Tice, D. L. Mansfield, D. J. Magner, M. B. Dorr, and G. H. Talbot. 1999. Comparison of sparfloxacin and ciprofloxacin in the treatment of community-acquired acute uncomplicated urinary tract infection in women. Clin. Ther. 21:966-981. [DOI] [PubMed] [Google Scholar]
- 25.Hooton, T. M., C. Johnson, C. Winter, L. Kuwamura, M. E. Rogers, P. L. Roberts, and W. E. Stamm. 1991. Single-dose and three-day regimens of ofloxacin versus trimethoprim-sulfamethoxazole for acute cystitis in women. Antimicrob. Agents Chemother. 35:1479-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hvidberg, H., C. Struve, K. A. Krogfelt, N. Christensen, S. N. Rasmussen, and N. Frimodt-Moller. 2000. Development of a long-term ascending urinary tract infection mouse model for antibiotic treatment studies. Antimicrob. Agents Chemother. 44:156-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikäheimo, R., A. Siitonen, T. Heiskanen, U. Kärkkäinen, P. Kuosmanen, P. Lipponen, and P. H. Mäkelä. 1996. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin. Infect. Dis. 22:91-99. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson, S. H., I. Kuhn, and A. Brauner. 1992. Biochemical fingerprinting of urinary Escherichia coli causing recurrent infections in women with pyelonephritic renal scarring. Scand. J. Urol. Nephrol. 26:373-377. [DOI] [PubMed] [Google Scholar]
- 29.Jayaraman, R. 2008. Bacterial persistence: some new insights into an old phenomenon. J. Biosci. 33:795-805. [DOI] [PubMed] [Google Scholar]
- 30.Justice, S. S., C. Hung, J. A. Theriot, D. A. Fletcher, G. G. Anderson, M. J. Footer, and S. J. Hultgren. 2004. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 101:1333-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karkkainen, U. M., R. Ikaheimo, M. L. Katila, and A. Siitonen. 2000. Recurrence of urinary tract infections in adult patients with community-acquired pyelonephritis caused by E. coli: a 1-year follow-up. Scand. J. Infect. Dis. 32:495-499. [DOI] [PubMed] [Google Scholar]
- 32.Kerrn, M. B., C. Struve, J. Blom, N. Frimodt-Møller, and K. A. Krogfelt. 2005. Intracellular persistence of Escherichia coli in urinary bladders from mecillinam-treated mice. J. Antimicrob. Chemother. 55:383-386. [DOI] [PubMed] [Google Scholar]
- 33.Kontiokari, T., K. Sundqvist, M. Nuutinen, T. Pokka, M. Koskela, and M. Uhari. 2001. Randomised trial of cranberry-lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. BMJ 322:1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koyama, Y., O. Mikami, T. Matsuda, T. Murota, T. Ohara, H. Kawamura, K. Amazutsumi, J. Uchida, and T. Harada. 2000. Efficacy of single-dose therapy with levofloxacin for acute cystitis: comparison to three-day therapy. Hinyokika Kiyo 46:49-52. (In Japanese.) [PubMed] [Google Scholar]
- 35.Lemieux, G. 1974. Trimethoprim-sulfamethoxazole compared with sulfamethoxazole in urinary tract infection. Can. Med. Assoc. J. 110:910-912. [PMC free article] [PubMed] [Google Scholar]
- 36.Lorian, V. 2005. Antibiotics in laboratory medicine, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 37.Ma, C., S. Sim, W. Shi, L. Du, D. Xing, and Y. Zhang. 17 November 2009, posting date. Energy production genes sucB and ubiF are involved in persister survival and tolerance to multiple antibiotics and stresses in Escherichia coli. FEMS Microbiol. Lett. doi: 10.1111/j.1574-6968.2009.01857.x. [DOI] [PubMed]
- 38.Martinez, J. J., M. A. Mulvey, J. D. Schilling, J. S. Pinkner, and S. J. Hultgren. 2000. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 19:2803-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merritt, J. H., D. E. Kadouri, and G. A. O'Toole. 2005. Growing and analyzing static biofilms. Curr. Protoc. Microbiol. Chapter 1:Unit 1B.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mikuniya, T., Y. Kato, R. Kariyama, K. Monden, M. Hikida, and H. Kumon. 2005. Synergistic effect of fosfomycin and fluoroquinolones against Pseudomonas aeruginosa growing in a biofilm. Acta Med. Okayama 59:209-216. [DOI] [PubMed] [Google Scholar]
- 41.Min, G., M. Stolz, G. Zhou, F. Liang, P. Sebbel, D. Stoffler, R. Glockshuber, T. T. Sun, U. Aebi, and X. P. Kong. 2002. Localization of uroplakin Ia, the urothelial receptor for bacterial adhesin FimH, on the six inner domains of the 16 nm urothelial plaque particle. J. Mol. Biol. 317:697-706. [DOI] [PubMed] [Google Scholar]
- 42.Mulvey, M. A. 2002. Adhesion and entry of uropathogenic Escherichia coli. Cell. Microbiol. 4:257-271. [DOI] [PubMed] [Google Scholar]
- 43.Mulvey, M. A., Y. S. Lopez-Boado, C. L. Wilson, R. Roth, W. C. Parks, J. Heuser, and S. J. Hultgren. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282:1494-1497. [DOI] [PubMed] [Google Scholar]
- 44.Mulvey, M. A., J. D. Schilling, and S. J. Hultgren. 2001. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 69:4572-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mulvey, M. A., J. D. Schilling, J. J. Martinez, and S. J. Hultgren. 2000. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc. Natl. Acad. Sci. U. S. A. 97:8829-8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mysorekar, I. U., and S. J. Hultgren. 2006. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc. Natl. Acad. Sci. U. S. A. 103:14170-14175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nikaido, H. 1998. Multiple antibiotic resistance and efflux. Curr. Opin. Microbiol. 1:516-523. [DOI] [PubMed] [Google Scholar]
- 48.Oliphant, C. M., and G. M. Green. 2002. Quinolones: a comprehensive review. Am. Fam. Physician 65:455-464. [PubMed] [Google Scholar]
- 49.Principi, N., A. Gervasoni, E. Reali, and P. Tagliabue. 1977. Treatment of urinary tract infections in children with a single daily dose of gentamicin. Helv. Paediatr. Acta 32:343-350. [PubMed] [Google Scholar]
- 50.Reeves, D. S., R. W. Lacey, R. V. Mummery, M. Mahendra, A. J. Bint, and S. W. Newsom. 1984. Treatment of acute urinary infection by norfloxacin or nalidixic acid/citrate: a multi-centre comparative study. J. Antimicrob. Chemother. 13(Suppl. B):99-105. [DOI] [PubMed] [Google Scholar]
- 51.Russo, T. A., A. Stapleton, S. Wenderoth, T. M. Hooton, and W. E. Stamm. 1995. Chromosomal restriction fragment length polymorphism analysis of Escherichia coli strains causing recurrent urinary tract infections in young women. J. Infect. Dis. 172:440-445. [DOI] [PubMed] [Google Scholar]
- 52.Schilling, J. D., R. G. Lorenz, and S. J. Hultgren. 2002. Effect of trimethoprim-sulfamethoxazole on recurrent bacteriuria and bacterial persistence in mice infected with uropathogenic Escherichia coli. Infect. Immun. 70:7042-7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schilling, J. D., M. A. Mulvey, and S. J. Hultgren. 2001. Structure and function of Escherichia coli type 1 pili: new insight into the pathogenesis of urinary tract infections. J. Infect. Dis. 183(Suppl. 1):S36-S40. [DOI] [PubMed] [Google Scholar]
- 54.Schneeberger, C., R. P. Stolk, J. H. Devries, P. M. Schneeberger, R. M. Herings, and S. E. Geerlings. 2008. Differences in the pattern of antibiotic prescription profile and recurrence rate for possible urinary tract infections in women with and without diabetes. Diabetes Care 31:1380-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharp, J. R., K. G. Ishak, and H. J. Zimmerman. 1980. Chronic active hepatitis and severe hepatic necrosis associated with nitrofurantoin. Ann. Intern. Med. 92:14-19. [DOI] [PubMed] [Google Scholar]
- 56.Song, J., B. L. Bishop, G. Li, R. Grady, A. Stapleton, and S. N. Abraham. 2009. TLR4-mediated expulsion of bacteria from infected bladder epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 106:14966-14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soto, S. M., A. Smithson, J. P. Horcajada, J. A. Martinez, J. P. Mensa, and J. Vila. 2006. Implication of biofilm formation in the persistence of urinary tract infection caused by uropathogenic Escherichia coli. Clin. Microbiol. Infect. 12:1034-1036. [DOI] [PubMed] [Google Scholar]
- 58.Stewart, P. S., and J. W. Costerton. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135-138. [DOI] [PubMed] [Google Scholar]
- 59.Suntres, Z. E., and P. N. Shek. 1992. Nitrofurantoin-induced pulmonary toxicity. In vivo evidence for oxidative stress-mediated mechanisms. Biochem. Pharmacol. 43:1127-1135. [DOI] [PubMed] [Google Scholar]
- 60.Wang, H., F. X. Liang, and X. P. Kong. 2008. Characteristics of the phagocytic cup induced by uropathogenic Escherichia coli. J. Histochem. Cytochem. 56:597-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wiles, T. J., J. M. Bower, M. J. Redd, and M. A. Mulvey. 2009. Use of zebrafish to probe the divergent virulence potentials and toxin requirements of extraintestinal pathogenic Escherichia coli. PLoS Pathog. 5:e1000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wright, K. J., P. C. Seed, and S. J. Hultgren. 2007. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell. Microbiol. 9:2230-2241. [DOI] [PubMed] [Google Scholar]
- 63.Zhou, G., W. J. Mo, P. Sebbel, G. Min, T. A. Neubert, R. Glockshuber, X. R. Wu, T. T. Sun, and X. P. Kong. 2001. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: evidence from in vitro FimH binding. J. Cell Sci. 114:4095-4103. [DOI] [PubMed] [Google Scholar]