Abstract
A-type cranberry proanthocyanidins (AC-PACs) have recently been reported to be beneficial for human health, especially urinary tract health. The effect of these proanthocyanidins on periodontitis, a destructive disease of tooth-supporting tissues, needs to be investigated. The purpose of this study was to investigate the effects of AC-PACs on various virulence determinants of Porphyromonas gingivalis as well as on the inflammatory response of oral epithelial cells stimulated by this periodontopathogen. We examined the effects of AC-PACs on P. gingivalis growth and biofilm formation, adherence to human oral epithelial cells and protein-coated surfaces, collagenase activity, and invasiveness. We also tested the ability of AC-PACs to modulate the P. gingivalis-induced inflammatory response by human oral epithelial cells. Our results showed that while AC-PACs neutralized all the virulence properties of P. gingivalis in a dose-dependent fashion, they did not interfere with growth. They also inhibited the secretion of interleukin-8 (IL-8) and chemokine (C-C motif) ligand 5 (CCL5) but did not affect the secretion of IL-6 by epithelial cells stimulated with P. gingivalis. This anti-inflammatory effect was associated with reduced activation of the nuclear factor-κB (NF-κB) p65 pathway. AC-PACs may be potentially valuable bioactive molecules for the development of new strategies to treat and prevent P. gingivalis-associated periodontal diseases.
Periodontitis is a multifactorial polymicrobial infection characterized by a destructive inflammatory process resulting in the loss of tooth-supporting tissues. Approximately 5 to 15% of the population is affected by severe forms of the disease which, if left untreated, may result in tooth loss and systemic complications (8). The Gram-negative bacterium Porphyromonas gingivalis is a key etiologic agent of periodontitis, more particularly the chronic form (41). P. gingivalis is able to adhere to cellular and acellular surfaces (32) and form a biofilm (33), both of which contribute to its establishment in and colonization of the oral cavity. P. gingivalis also produces soluble and cell-bound proteases (24) that can degrade various tissue and plasma proteins and that contribute to the invasion of periodontal tissues (1).
The gingival epithelium has a stratified squamous structure that is an interface between the external environment, with its complex bacterial ecosystem, and the underlying periodontal tissue. P. gingivalis has developed different strategies to colonize and perturb the structural and functional integrity of the gingival epithelium (2). It is capable of inducing a strong proinflammatory cytokine response in gingival epithelial cells in vitro, which has been correlated with the adhesive/invasive potential of P. gingivalis (39, 40).
The cranberry (Vaccinium macrocarpon Ait.) is a native North American fruit that has recently received considerable attention in health research, particularly in the field of infectious diseases. While proanthocyanidins are found in all berry fruits, those isolated from cranberry possess unusual structures with A-type linkages, with a second ether linkage between an A-ring of the lower unit and the C-2 ring of the upper unit (O7 → C2) (14). These A-type cranberry proanthocyanidins (AC-PACs) are composed mainly of epicatechin units, with at least one A-type linkage (13). A-type linkages can occur at the terminal unit or between the extension units (18). AC-PAC oligomers and polymers with up to 12 degrees of polymerization and as many as four A-type linkages have been detected using matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF MS) (37). While AC-PACs inhibit the adherence properties of uropathogenic Escherichia coli (23), which may explain the beneficial effect of cranberry for preventing urinary tract infections in children (12) and aging patients (36), the B-linked proanthocyanidins isolated from apple juice, green tea, and dark chocolate have no antiadhesion activity (23). A number of studies have suggested that cranberry polyphenols may promote oral health by inhibiting dental biofilm formation (31, 42, 44), acid production by Streptococcus mutans (11), periodontopathogen-derived proteolytic enzymes (6), and host inflammatory responses (4), but they suffer from the fact that poorly characterized fractions have been used.
In the present study, we hypothesized that AC-PACs may have beneficial effects for P. gingivalis-associated periodontal diseases. We thus investigated the effects of AC-PACs on P. gingivalis growth and biofilm formation, adherence to human oral epithelial cells and protein-coated surfaces, collagenase activity, and invasiveness. We also investigated the anti-inflammatory effects of AC-PACs in oral epithelial cells stimulated by P. gingivalis.
MATERIALS AND METHODS
A-type cranberry proanthocyanidins.
Cranberry proanthocyanidins were isolated from cranberry fruit (Vaccinium macrocarpon Ait.) using solid-phase chromatography according to a well-established method for proanthocyanidin isolation (23). Briefly, cranberry fruit was homogenized with 70% aqueous acetone and filtered, and the pulp was discarded. The collected extract was concentrated under reduced pressure to remove acetone. The cranberry extract was suspended in water, applied to a preconditioned C18 solid-phase chromatography column, and washed with water to remove sugars, followed by acidified aqueous methanol to remove acids. The fats and waxes retained on the C18 sorbent were discarded. The polyphenolic fraction containing anthocyanins, flavonol glycosides, and proanthocyanidins (confirmed using reverse-phase high-pressure liquid chromatography [HPLC] with diode array detection) was eluted with 100% methanol and dried under reduced pressure. This fraction was suspended in 50% ethanol (EtOH) and applied to a preconditioned Sephadex LH-20 column which was washed with 50% EtOH to remove low-molecular-weight anthocyanins and flavonol glycosides. Proanthocyanidins adsorbed to the LH-20 were eluted from the column with 70% aqueous acetone and monitored using diode array detection at 280 nm. The absence of absorption at 360 nm and 450 nm confirmed that anthocyanins and flavonol glycosides were removed. Acetone was removed under reduced pressure and the resulting purified proanthocyanidin extract freeze-dried. 13C nuclear magnetic resonance (NMR), electrospray mass spectrometry, matrix-assisted laser desorption ionization-time-of-flight mass spectrometry, and acid-catalyzed degradation with phloroglucinol have all been utilized to confirm the presence of A-type linkages and concentration of proanthocyanidins present in the extract (13, 14, 23).
Bacterium.
P. gingivalis ATCC 33277 was grown in Todd-Hewitt broth (THB) (BBL Microbiology Systems, Cockeysville, MD) supplemented with 0.001% hemin and 0.0001% vitamin K (THB-HK). Cultures were incubated at 37°C under anaerobic conditions (N2-H2-CO2, 80:10:10).
Growth and biofilm formation.
A 24-h culture of P. gingivalis in THB-HK was diluted in fresh broth medium to obtain an optical density at 655 nm (OD655) of 0.2. Equal volumes (100 μl) of P. gingivalis and AC-PACs (0, 50, 100, and 200 μg/ml) in THB-HK were mixed in the wells of 96-well plates (Sarstedt, Newton, NC). Control wells with no P. gingivalis were also prepared. After a 24-h incubation at 37°C under anaerobic conditions, bacterial growth was recorded by measuring the OD655 using a microplate reader. The spent medium and free-floating bacteria were then removed by aspiration, and the wells were washed three times with distilled water. The P. gingivalis biofilms were stained with 100 μl of 0.4% crystal violet for 15 min. The wells were washed four times with distilled water to remove the unbound crystal violet dye and were dried for 2 h at 37°C. After addition of 100 μl of 95% ethanol to each well, the plate was shaken for 10 min to release the stain from the biofilms. The absorbance at 550 nm (A550) was measured to quantify biofilm formation.
Adherence to human oral epithelial cells and extracellular matrix proteins.
P. gingivalis cells were radiolabeled by incubating a mid-log-phase culture (OD655 of 0.5) with a mixture of 14C-labeled amino acids (Amersham Biosciences UK Ltd., Little Chalfont, Buckinghamshire, United Kingdom) at a final concentration of 20 μCi/ml at 37°C for 16 h in an anaerobic chamber. Cells were harvested by centrifugation at 10,000 × g for 10 min, washed three times in 10 mM phosphate-buffered saline (PBS) (pH 7.2), and suspended to an OD655 of 0.8 in PBS prereduced by overnight incubation in the anaerobic chamber. The immortalized human oral epithelial cell line GMSM-K (developed by Valerie Murrah, Department of Diagnostic Sciences and General Dentistry, University of North Carolina at Chapel Hill), which has an epithelial phenotype (17), was used to investigate the effect of AC-PACs on the adherence of P. gingivalis. GMSM-K cells were cultured onto wells of 96-well plates (Sarstedt) in Dulbecco's modified Eagle's medium (DMEM) to form a confluent monolayer as reported elsewhere (30). The wells were washed three times with PBS prior to performance of the adherence assay. Other wells of 96-well flat-bottomed microtiter plates were filled with 100 μl of Matrigel (Sigma-Aldrich, St. Louis, MO) diluted 1/10 in ice-cold PBS. The Matrigel was allowed to settle at room temperature for 2 h and was then washed three times with distilled water prior to the adherence assay. Matrigel is composed of several extracellular matrix proteins, including laminin, type IV collagen, heparan sulfate proteoglycans, and entactin. Mixtures containing 20 μl of 14C-labeled P. gingivalis (OD655 of 0.8) and 30 μl of AC-PACs in PBS were prepared to obtain a final AC-PAC concentration of 0, 25, 50, or 100 μg/ml. The mixtures were placed in wells coated with epithelial cells or extracellular matrix proteins. After a 30-min incubation at 37°C, the wells were washed three times with PBS to remove unbound bacteria. Adhered radiolabeled bacteria were detached by adding 100 μl of 0.5 M NaOH and incubating the plates at room temperature for 15 min on an orbital shaker. The bacteria were suspended in EcoLite scintillation liquid (ICN, Costa Mesa, CA), and the radioactivity was counted using a multipurpose scintillation counter (Beckman Coulter, Fullerton, CA).
Degradation of type I collagen.
A cell-free culture supernatant of P. gingivalis was obtained from a 36-h culture. Aliquots of supernatant (7.5 μl) were mixed with 127.5 μl of TCNB (50 mM Tris HCl, 10 mM CaCl2, 150 mM NaCl, and 0.05% Brij 35, pH 7.5) buffer containing increasing concentrations of AC-PACs (final concentrations of 0, 25, 50, and 100 μg/ml) and 15 μl of 1-mg/ml fluorogenic substrate (fluorescein-conjugated DQ type I collagen; Molecular Probes, Eugene, OR). The assay mixtures were incubated in the dark at room temperature, and the fluorescence was measured every hour for 4 h using a fluorometer with the excitation and emission wavelengths set at 490 nm and 520 nm, respectively.
Invasion of a reconstituted basement membrane model.
One hundred microliters of Matrigel diluted 1/2 in ice-cold PBS was placed on 8-μm-pore-size filters in Transwell cell culture chamber inserts (Costar, Cambridge, MA), while 400 μl of PBS was added to the lower chamber. The Matrigel was allowed to settle at 4°C for 30 min and then to form a gel at 37°C for 2 h in an anaerobic chamber (N2-H2-CO2, 80:10:10) prior to performing the invasion assay. Fifty microliters of 14C-labeled P. gingivalis cells (OD655 of 0.16) was pretreated with AC-PACs at a final concentration of 0, 25, 50, or 100 μg/ml for 30 min and then placed on top of the Matrigel in the double-chamber system, which was incubated in the anaerobic chamber at 37°C for 48 h. The migration of P. gingivalis cells through the reconstituted basement membrane model was monitored by measuring the radioactivity in the buffer recovered from the lower chamber after the incubation period.
Inflammatory response of an oral epithelial cell model.
The oral epithelial cells (GMSM-K) were grown in DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS) and antibiotics, harvested by gentle trypsinization (0.05% trypsin-EDTA; Gibco-BRL, Grand Island, NY), washed once in DMEM-FBS, and suspended at a density of 4 × 105 cells per ml in DMEM with 1% heat-inactivated FBS (30). Cells were seeded in a 12-well plate (4 × 105 cells/well in 1 ml) and cultured overnight at 37°C in a 5% CO2 atmosphere to allow cell adhesion prior to the stimulation with P. gingivalis. The epithelial cells were pretreated with increasing concentrations of AC-PACs (0, 25, 50, and 100 μg/ml) and were incubated at 37°C in 5% CO2 for 2 h before the stimulation with P. gingivalis cells at a multiplicity of infection (MOI) of 125. This MOI was selected on the basis of preliminary experiments showing that it induced cytokine secretion by epithelial cells while not affecting their viability, as determined by an MTT (3-[4,5-diethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay (data not shown). After a 24-h incubation at 37°C in 5% CO2, cell-free supernatants were collected and stored at −20°C until used. Commercial enzyme-linked immunosorbent assay (ELISA) kits (R & D Systems, Minneapolis, MN) were used to quantify interleukin-6 (IL-6), interleukin-8 (IL-8), and chemokine (C-C motif) ligand 5 (CCL5) concentrations in the free-cell supernatants according to the manufacturer's protocols. The absorbance at 450 nm was read using a microplate reader with the wavelength correction set at 550 nm. The rated sensitivities of the commercial ELISA kits were 9.3 pg/ml for IL-6, 31.2 pg/ml for IL-8, and 15.6 pg/ml for CCL5.
To understand the mechanism of action of AC-PACs, their effect on nuclear factor-κB (NF-κB) p65 activation was investigated. Epithelial cells prepared as described above were incubated in the absence or presence of AC-PACs (50 μg/ml) for 1 h prior to being stimulated with P. gingivalis cells at an MOI of 125 for 30 min. Whole-cell extracts were then prepared using nuclear extract kits (Active Motif, Carlsbad, CA) according to the manufacturer's protocol and were adjusted to a protein concentration of 1 mg/ml. NF-κB p65 binding to DNA in the cell extracts was determined using TransAm NF-κB p65 kits (Active Motif) according to the manufacturer's protocol.
Statistical analysis.
Data are expressed as the means ± standard deviations (SD) from at least three assays. The statistical analysis was conducted using the Student t test with Bonferroni corrections. A P value of <0.05 was considered statistically significant.
RESULTS
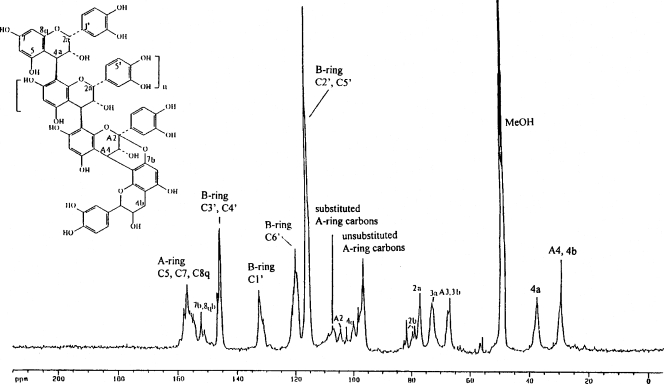
The characterization of the AC-PAC fraction was made by 13C NMR. As shown in Fig. 1, the proanthocyanidin molecules consist of epicatechin units with degrees of polymerization (DP) mainly of 4 and 5 containing at least one A-type linkage, as previously reported (13) (Fig. 1).
FIG. 1.
13C nuclear magnetic resonance (NMR) spectrum of cranberry proanthocyanidins, showing the presence of A-type linkages.
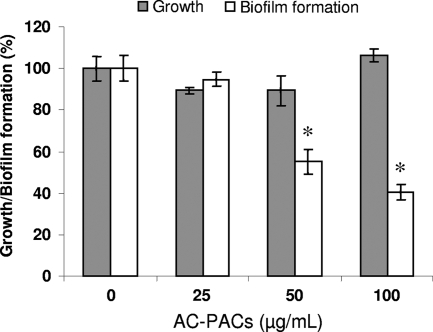
To investigate the effect of AC-PACs on various pathogenic properties of P. gingivalis, up to 100 μg/ml of AC-PACs was used, since this concentration has been reported to be nontoxic for human cells (29). Biofilm formation by P. gingivalis was significantly decreased by 50 and 100 μg/ml AC-PACs. More specifically, AC-PACs inhibited biofilm formation by 45% ± 6% and 60% ± 4% at concentrations of 50 and 100 μg/ml, respectively (Fig. 2). AC-PACs at all the concentrations tested did not significantly affect the growth of P. gingivalis.
FIG. 2.
Effect of AC-PACs on the growth of and biofilm formation by P. gingivalis. AC-PACs (0, 25, 50, and 100 μg/ml) were added to the growth medium, and the cultures were incubated under anaerobic conditions at 37°C for 24 h. Bacterial growth was assessed by measuring the OD655 using a microplate reader. Biofilm formation was assessed by staining with 0.4% crystal violet and measuring the A550. Assays were run in quadruplicate, and the means ± SD from three independent assays were calculated. A value of 100% was assigned to growth and biofilm formation in the absence of AC-PACs. *, significantly lower than the value for the control (no AC-PACs) (P < 0.05).
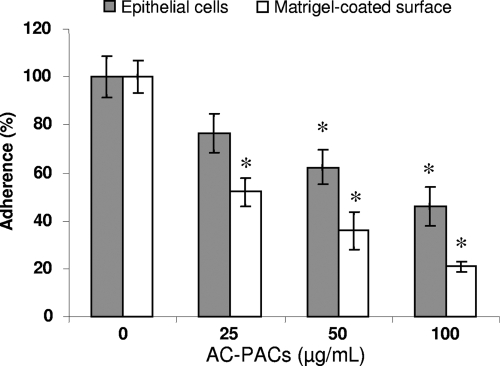
The effect of AC-PACs on the adherence properties of P. gingivalis was investigated using microplate wells covered with confluent oral epithelial cells or coated with extracellular matrix proteins (Matrigel). The relative adherence of 14C-labeled P. gingivalis to oral epithelial cells was not significantly decreased at the lowest concentration of AC-PACs tested (25 μg/ml). However, 50 and 100 μg/ml of AC-PACs inhibited P. gingivalis adherence to epithelial cells by 37.5% ± 7.0% and 54.1% ± 8.2%, respectively. AC-PACs also inhibited the adherence of P. gingivalis to Matrigel-coated polystyrene surfaces, with 25, 50, and 100 μg/ml inhibiting adherence by 48.0% ± 5.8%, 64.1% ± 7.7%, and 78.9% ± 2.2%, respectively (Fig. 3).
FIG. 3.
Effect of AC-PACs on the adherence of P. gingivalis to human oral epithelial cells and a Matrigel-coated polystyrene surface. 14C-labeled P. gingivalis cells in the presence of AC-PACs (0, 25, 50, and 100 μg/ml) were added to wells coated with epithelial cells or Matrigel. After 30 min of incubation at 37°C, the wells were extensively washed and the quantity of adhered 14C-labeled bacteria was determined by gamma counting using a multipurpose scintillation counter. Assays were run in triplicate, and the means ± SD from three independent assays were calculated. A value of 100% was assigned to the amount of 14C bound in the absence of AC-PACs. *, significantly lower than the value for the untreated control (P < 0.05).
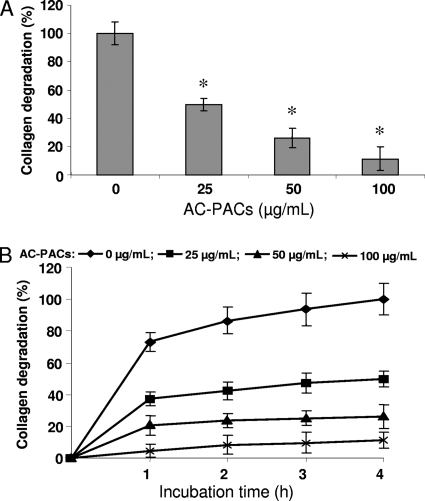
The ability of AC-PACs to inhibit type I collagen degradation by extracellular proteinases produced by P. gingivalis was also tested. Significant inhibition was observed at all the concentrations tested, with 25, 50, and 100 μg/ml of AC-PACs inhibiting type I collagen degradation by a P. gingivalis culture supernatant by 50.2% ± 4.4%, 73.7% ± 6.8%, and 88.7% ± 8.5%, respectively (Fig. 4A). This inhibitory effect of AC-PACs on type I collagen degradation was time dependent (Fig. 4B).
FIG. 4.
Effect of AC-PACs on the degradation of type I collagen by extracellular proteases of P. gingivalis. Assays were run in quadruplicate, and the means ± SD from three independent assays were calculated. A value of 100% was assigned to the degradation obtained after a 4-h incubation at room temperature in the absence of AC-PACs (A). The inhibition of collagen degradation was also measured as function of time (B). *, significantly lower than the value for the untreated control (P < 0.05).
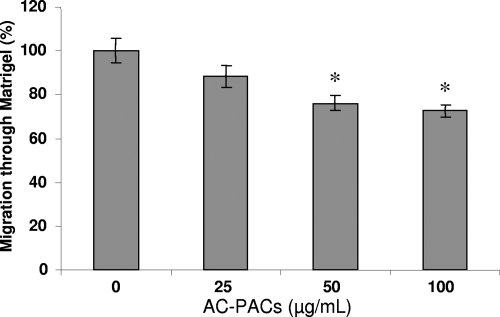
The effect of AC-PACs on the invasiveness of P. gingivalis was investigated using a reconstituted basement membrane model (Matrigel) in a double-chamber system. The migration of 14C-labeled P. gingivalis cells through the Matrigel was reduced by 23.9% ± 3.6% and 27.5% ± 3.0% in the presence of 50 and 100 μg/ml AC-PACs, respectively (Fig. 5).
FIG. 5.
Effect of AC-PACs on the migration of P. gingivalis through a reconstituted basement membrane model (Matrigel). 14C-labeled P. gingivalis cells were preincubated with AC-PACs (0, 25, 50, and 100 μg/ml) for 30 min. They were then placed on the Matrigel in the top of a double-chamber system. After a 48-h incubation at 37°C under anaerobic conditions, the number of 14C-labeled P. gingivalis organisms recovered in the lower chamber was determined by gamma counting. Assays were run in triplicate, and the means ± SD from three independent assays were calculated. A value of 100% was assigned to the amount of radioactivity counted in the absence of AC-PACs. *, significantly lower than the value for the untreated control (P < 0.05).
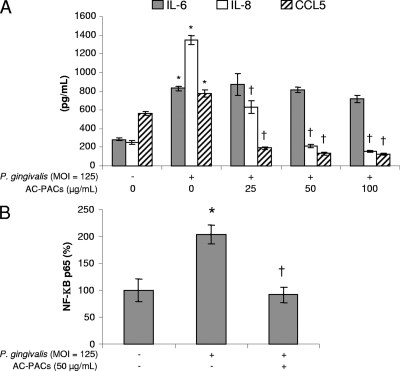
The ability of AC-PACs to modulate the P. gingivalis-induced inflammatory response of oral epithelial cells was then examined. Epithelial cells were treated with AC-PACs for 2 h prior to being stimulated with cells of P. gingivalis to investigate their effects on IL-6, IL-8, and CCL5 secretion. At an MOI of 125, P. gingivalis significantly increased the secretion of IL-6, IL-8, and CCL5 (Fig. 6A), while having no effect on epithelial cell viability. AC-PACs significantly decreased the secretion of IL-8 and CCL5 at all concentrations tested in a dose-dependent fashion. Decreased secretion of IL-8 and CCL5 was not related to loss of cell viability, since AC-PACs did not show any cytotoxicity at up to 200 μg/ml (data not shown). However, AC-PACs did not affect the secretion of IL-6. More specifically, 25, 50, and 100 μg/ml of AC-PACs reduced the secretion of IL-8 by 53.2% ± 5.3%, 84.1% ± 2.0%, and 88.3% ± 1.4%, respectively, and that of CCL5 by 63.6% ± 4.1%, 82.8% ± 2.8%, and 83.7% ± 1.4%, respectively (Fig. 6A). The relative DNA-binding activity of nuclear transcription factor NF-κB p65 in oral epithelial cells treated with P. gingivalis at an MOI of 125 increased to 203.9% ± 17.4% compared to an unstimulated control. A pretreatment of epithelial cells with 50 μg/ml of AC-PACs prior to the stimulation with P. gingivalis significantly decreased the P. gingivalis-induced activity of NF-κB p65 to 91% ± 14.5% (Fig. 6B).
FIG. 6.
Effect of AC-PACs on the P. gingivalis-induced inflammatory response in oral epithelial cells. Oral epithelial cells were pretreated with AC-PACs (0, 25, 50, and 100 μg/ml) for 2 h (1 h for the NF-κB p65 assay). They were then stimulated with P. gingivalis at an MOI of 125 for 24 h (30 min for the NF-κB p65 assay). Cell-free supernatants were collected to determine IL-6, IL-8, and CCL5 concentrations by ELISA (A). The activation of NF-κB p65 in the cellular extract was determined by an ELISA-based assay (B). Assays were run in triplicate, and the means ± SD from three independent assays were calculated. *, significantly higher than the value for the unstimulated (P. gingivalis) control (P < 0.05); †, significantly lower than the value for the untreated (AC-PACs) control (P < 0.05).
DISCUSSION
The proanthocyanidins isolated from the American cranberry (V. macrocarpon) are composed of oligomers containing at least one A-type interflavan bond, but there are often multiple A-type interflavan linkages at each degree of polymerization within the proanthocyanidin oligomeric series (23). This unique structural feature has been associated with its antiadhesion activity and its beneficial effects for prevention of urinary tract infections (23). While the A-type linkage in cranberry proanthocyanidins is an important structural feature in terms of bacterial antiadhesion activity, very little is known about other health benefits. In the present study, we investigated the effects of AC-PACs on the pathogenic properties of P. gingivalis and the P. gingivalis-induced inflammatory response in oral epithelial cells.
The ability to grow and form biofilms is likely critical for the establishment and persistence of P. gingivalis in subgingival sites. In addition, biofilms allow bacterial pathogens to evade immune defenses and better resist mechanical removal and chemotherapeutic agents. While AC-PACs did not have any effect on P. gingivalis growth, they did inhibit biofilm formation. This result is of interest because AC-PACs did not seem to have antibiotic properties, even at the highest concentration tested, which would limit the development of resistance to them and would not disturb the oral ecology. The inhibition of biofilm formation by an uncharacterized polyphenol extract prepared from cranberry has been previously reported for Streptococcus mutans and P. gingivalis (5, 11, 31, 45).
The adherence of bacteria to mucosal cells is a critical step in the development of infections. The prevention of adhesion may represent a potentially valuable approach for the development of new approaches to prevent infectious diseases, particularly infections of mucosal surfaces (3). The ability of P. gingivalis to attach to human gingival epithelial cells has been previously reported (43, 46). The adhesion of P. gingivalis to host cells is multimodal (32) and involves a variety of cell surface and extracellular components, including fimbriae, proteinases, hemagglutinins, and lipopolysaccharides (10). The major fimbriae (FimA), as well as cysteine proteinases (gingipains), contribute to the attachment to and invasion of oral epithelial cells via different receptors (9, 43). The adhesion to and subsequent invasion of epithelial cells by P. gingivalis are likely critical in the pathogenesis of periodontitis, especially during the initial stages of the infection. In the present study, AC-PACs inhibited the adhesion of P. gingivalis to oral epithelial cells and extracellular matrix protein-coated surfaces in a dose-dependent fashion. These results are consistent with the previously reported inhibitory effect of AC-PACs on the adhesion of uropathogens to primary cultured bladder and vaginal epithelial cells (19, 23). Since proanthocyanidins are known to bind proteins (20), it has been suggested that AC-PACs may bind to the proteinaceous fimbriae on E. coli, thus inhibiting adherence to uroepithelial cells via the specific receptor ligand (22).
Type I collagen makes up approximately 60% of the tissue volume of periodontal tissues. Collagen degradation by periodontopathogens may thus be involved in gingival tissue destruction. The collagenolytic activity of P. gingivalis has been attributed to various proteinases (21, 27). In the present study, we showed that AC-PACs inhibited collagen degradation by P. gingivalis in a dose-dependent manner, suggesting that AC-PACs may contribute to reducing the destructive process. Interestingly, we previously showed that AC-PACs have the ability to inhibit the activity of recombinant human matrix metalloproteinases (MMP-1 and MMP-9) and the secretion of various MMPs by macrophages (29). Using a reconstituted basement membrane model composed of laminin, type IV collagen, proteoglycans, and entactin, we also showed that AC-PACs could inhibit the invasiveness of P. gingivalis. Since we previously reported that P. gingivalis proteinases contribute to the ability of this bacterium to penetrate this model (1), the inhibitory effect of AC-PACs is likely related to its antiproteinase effect.
The host inflammatory response is a critical factor in the destruction of tooth-supporting tissue during periodontitis. Therapeutic agents that modulate host inflammatory mediators have thus shown promise for managing adult periodontitis and may be very useful for treating individuals with a substantially increased risk for periodontitis (28). In addition to providing a physical barrier against invading P. gingivalis, epithelial cells play an important role in innate host immune defenses. P. gingivalis adheres to and invades epithelial cells by targeting specific host receptors, modulating host signaling events, and deregulating the host cytokine network (2). We showed that P. gingivalis increased the secretion of inflammatory mediators IL-6, IL-8, and CCL5 by oral epithelial cells. AC-PACs significantly decreased the secretion of IL-8 and CCL5 at all concentrations tested in a dose-dependent fashion but did not affect the secretion of IL-6. In an ongoing study, we showed that AC-PACs could also decrease the secretion of IL-8 and CCL5 by oral epithelial cells stimulated with purified lipopolysaccharides from P. gingivalis and Aggregatibacter actinomycetemcomitans (data not shown). IL-8 is an important chemoattractant that enhances the recruitment and infiltration of neutrophils to sites of inflammation (35), while CCL5 has significant chemotactic activity for basophils, eosiniphils, monocytes, and T helper type 1 cells (34). The stimulation of chemokine production by periodontopathogens is believed to play an important role in initiating inflammatory reactions in gingival tissue. Interestingly, the concentration of IL-8 in the gingival crevicular fluid of inflamed periodontal sites has been correlated with the severity of periodontitis (25). In addition, treating periodontitis reduces the number of immune cells and the levels of IL-8 infiltrates, suggesting that this chemokine plays a role in periodontal status (15). Similarly, high levels of CCL5 have been detected in the gingival crevicular fluid and inflamed gingival tissues adjacent to periodontal pockets, indicating that this chemokine may be important in the initiation and progression of periodontitis (15, 16, 26). The fact that AC-PACs inhibited the secretion of IL-8 and CCL5 in human oral epithelial cells stimulated by P. gingivalis suggests that they have the potential to reduce the influx of inflammatory cells to diseased sites and the amplification of P. gingivalis-induced inflammatory processes. We also provided evidence that AC-PACs inhibited, at least in part, the P. gingivalis-induced secretion of IL-8 and CCL5 by their ability to inhibit the activation of NF-κB p65, which is an activator of many cytokines and inflammatory processes (38). This is in agreement with previous studies showing that natural products, including plant polyphenols, with anti-inflammatory properties exert their effect by modulating the NF-κB pathway (7). The fact that IL-6 secretion by P. gingivalis-stimulated epithelial cells was not reduced by AC-PACs suggests that different cascades of kinases leading to cytokine secretion are involved and that AC-PACs may act on specific kinases.
In conclusion, the present in vitro study provided clear evidence that AC-PACs possess interesting therapeutic properties for the treatment of periodontal disease because of their ability to affect the etiology of periodontitis by acting on periodontopathogens and host responses. On the one hand, AC-PACs reduce the virulence properties of P. gingivalis by inhibiting biofilm formation, adhesion, proteinase activity, and invasiveness. On the other, AC-PACs exert anti-inflammatory activity by inhibiting the P. gingivalis-induced inflammatory response in human oral epithelial cells. As such, they may be of interest in the prevention and treatment of P. gingivalis-associated periodontal diseases. Further studies will be required to investigate the exact mechanisms by which AC-PACs neutralize the virulence properties of this bacterium.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (CIHR). V. D. La holds a fellowship from the Training Program in Applied Oral Health Research (CIHR).
We thank V. Murrah (University of North Carolina at Chapel Hill) and J. M. DiRienzo (University of Pennsylvania) for providing the GMSM-K epithelial cell line.
Footnotes
Published ahead of print on 22 February 2010.
REFERENCES
- 1.Andrian, E., D. Grenier, and M. Rouabhia. 2004. In vitro models of tissue penetration and destruction by Porphyromonas gingivalis. Infect. Immun. 72:4689-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrian, E., D. Grenier, and M. Rouabhia. 2006. Porphyromonas gingivalis-epithelial cell interactions in periodontitis. J. Dent. Res. 85:392-403. [DOI] [PubMed] [Google Scholar]
- 3.Bavington, C., and C. Page. 2005. Stopping bacterial adhesion: a novel approach to treating infections. Respiration 72:335-344. [DOI] [PubMed] [Google Scholar]
- 4.Bodet, C., F. Chandad, and D. Grenier. 2006. Anti-inflammatory activity of a high-molecular-weight cranberry fraction on macrophages stimulated by lipopolysaccharides from periodontopathogens. J. Dent. Res. 85:235-239. [DOI] [PubMed] [Google Scholar]
- 5.Bodet, C., D. Grenier, F. Chandad, I. Ofek, D. Steinberg, and E. I. Weiss. 2008. Potential oral health benefits of cranberry. Crit. Rev. Food Sci. Nutr. 48:672-680. [DOI] [PubMed] [Google Scholar]
- 6.Bodet, C., M. Piche, F. Chandad, and D. Grenier. 2006. Inhibition of periodontopathogen-derived proteolytic enzymes by a high-molecular-weight fraction isolated from cranberry. J. Antimicrob. Chemother. 57:685-690. [DOI] [PubMed] [Google Scholar]
- 7.Bremner, P., and M. Heinrich. 2002. Natural products as targeted modulators of the nuclear factor-kappa B pathway. J. Pharm. Pharmacol. 54:453-472. [DOI] [PubMed] [Google Scholar]
- 8.Burt, B. 2005. Epidemiology of periodontal diseases. J. Periodontol. 76:1406-1419. [DOI] [PubMed] [Google Scholar]
- 9.Chen, T., K. Nakayama, L. Belliveau, and M. J. Duncan. 2001. Porphyromonas gingivalis gingipains and adhesion to epithelial cells. Infect. Immun. 69:3048-3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cutler, C. W., J. R. Kalmar, and C. A. Genco. 1995. Pathogenic strategies of the oral anaerobe, Porphyromonas gingivalis. Trends Microbiol. 3:45-51. [DOI] [PubMed] [Google Scholar]
- 11.Duarte, S., S. Gregoire, A. P. Singh, N. Vorsa, K. Schaich, W. H. Bowen, and H. Koo. 2006. Inhibitory effects of cranberry polyphenols on formation and acidogenicity of Streptococcus mutans biofilms. FEMS Microbiol. Lett. 257:50-56. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara, P., L. Romaniello, O. Vitelli, A. Gatto, M. Serva, and L. Cataldi. 2009. Cranberry juice for the prevention of recurrent urinary tract infections: a randomized controlled trial in children. Scand. J. Urol. Nephrol. 9:1-5. [DOI] [PubMed] [Google Scholar]
- 13.Foo, L. Y., Y. Lu, A. B. Howell, and N. Vorsa. 2000. The structure of cranberry proanthocyanidins which inhibit adherence of uropathogenic P-fimbriated Escherichia coli in vitro. Phytochemistry 54:173-181. [DOI] [PubMed] [Google Scholar]
- 14.Foo, L. Y., Y. Lu, A. B. Howell, and N. Vorsa. 2000. A-type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbriated Escherichia coli. J. Nat. Prod. 63:1225-1228. [DOI] [PubMed] [Google Scholar]
- 15.Gamonal, J., A. Acevedo, A. Bascones, O. Jorge, and A. Silva. 2001. Characterization of cellular infiltrate, detection of chemokine receptor CCR5 and interleukin-8 and CCL5 chemokines in adult periodontitis. J. Periodontal Res. 36:194-203. [DOI] [PubMed] [Google Scholar]
- 16.Gamonal, J., A. Bascones, O. Jorge, and A. Silva. 2000. Chemokine CCL5 in gingival crevicular fluid of adult patients with periodontitis. J. Clin. Periodontol. 27:675-681. [DOI] [PubMed] [Google Scholar]
- 17.Gilchrist, E. P., M. P. Moyer, E. J. Shillitoe, N. Clare, and V. A. Murrah. 2000. Establishment of a human polyclonal oral epithelial cell line. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 90:340-347. [DOI] [PubMed] [Google Scholar]
- 18.Gu, L., M. A. Kelm, J. F. Hammerstone, G. Beecher, J. Holden, D. Haytowitz, and R. L. Prior. 2003. Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. J. Agric. Food Chem. 51:7513-7521. [DOI] [PubMed] [Google Scholar]
- 19.Gupta, K., M. Y. Chou, A. Howell, C. Wobbe, R. Grady, and A. E. Stapleton. 2007. Cranberry products inhibit adherence of p-fimbriated Escherichia coli to primary cultured bladder and vaginal epithelial cells. J. Urol. 177:2357-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagerman, A. E., and L. G. Butler. 1981. The specificity of proanthocyanidin-protein interactions. J. Biol. Chem. 256:4494-4497. [PubMed] [Google Scholar]
- 21.Houle, M. A., D. Grenier, P. Plamondon, and K. Nakayama. 2003. The collagenase activity of Porphyromonas gingivalis is due to Arg-gingipain. FEMS Microbiol. Lett. 221:181-185. [DOI] [PubMed] [Google Scholar]
- 22.Howell, A. B. 2007. Bioactive compounds in cranberries and their role in prevention of urinary tract infections. Mol. Nutr. Food Res. 51:732-737. [DOI] [PubMed] [Google Scholar]
- 23.Howell, A. B., J. D. Reed, C. G. Krueger, R. Winterbottom, D. G. Cunningham, and M. Leahy. 2005. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry 66:2281-2291. [DOI] [PubMed] [Google Scholar]
- 24.Imamura, T. 2003. The role of gingipains in the pathogenesis of periodontal disease. J. Periodontol. 74:111-118. [DOI] [PubMed] [Google Scholar]
- 25.Jin, L. J., W. K. Leung, E. F. Corbet, and B. Soder. 2002. Relationship of changes in interleukin-8 levels and granulocyte elastase activity in gingival crevicular fluid to subgingival periodontopathogens following non-surgical periodontal therapy in subjects with chronic periodontitis. J. Clin. Periodontol. 29:604-614. [DOI] [PubMed] [Google Scholar]
- 26.Johnson, R. B., N. Wood, and F. G. Serio. 2004. Interleukin-11 and IL-17 and the pathogenesis of periodontal disease. J. Periodontol. 75:37-43. [DOI] [PubMed] [Google Scholar]
- 27.Kato, T., N. Takahashi, and H. K. Kuramitsu. 1992. Sequence analysis and characterization of the Porphyromonas gingivalis prtC gene, which expresses a novel collagenase activity. J. Bacteriol. 174:3889-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kornman, K. S. 1999. Host modulation as a therapeutic strategy in the treatment of periodontal disease. Clin. Infect. Dis. 28:520-526. [DOI] [PubMed] [Google Scholar]
- 29.La, V. D., A. B. Howell, and D. Grenier. 2009. Cranberry proanthocyanidins inhibit MMP production and activity. J. Dent. Res. 88:627-632. [DOI] [PubMed] [Google Scholar]
- 30.La, V. D., J. Labrecque, and D. Grenier. 2009. Cytoprotective effect of proanthocyanidin-rich cranberry fraction against bacterial cell wall-mediated toxicity in macrophages and epithelial cells. Phytother Res. 23:1449-1452. [DOI] [PubMed] [Google Scholar]
- 31.Labrecque, J., C. Bodet, F. Chandad, and D. Grenier. 2006. Effects of a high-molecular-weight cranberry fraction on growth, biofilm formation and adherence of Porphyromonas gingivalis. J. Antimicrob. Chemother. 58:439-443. [DOI] [PubMed] [Google Scholar]
- 32.Lamont, R. J., and H. F. Jenkinson. 1998. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 62:1244-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamont, R. J., and H. F. Jenkinson. 2000. Subgingival colonization by Porphyromonas gingivalis. Oral Microbiol. Immunol. 15:341-349. [DOI] [PubMed] [Google Scholar]
- 34.Luster, A. D. 1998. Chemokines—chemotactic cytokines that mediate inflammation. N Engl. J. Med. 338:436-445. [DOI] [PubMed] [Google Scholar]
- 35.Madianos, P. N., Y. A. Bobetsis, and D. F. Kinane. 2005. Generation of inflammatory stimuli: how bacteria set up inflammatory responses in the gingiva. J. Clin. Periodontol 32(Suppl. 6):57-71. [DOI] [PubMed] [Google Scholar]
- 36.McMurdo, M. E., I. Argo, G. Phillips, F. Daly, and P. Davey. 2009. Cranberry or trimethoprim for the prevention of recurrent urinary tract infections? A randomized controlled trial in older women. J. Antimicrob. Chemother. 63:389-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neto, C. C., G. C. Krueger, L. T. Lamoureaux, M. Kondo, J. A. Vaisberg, R. A. R. Hurta, S. Curtis, D. M. Matchett, H. Yeung, M. I. Sweeney, and D. J. Reed. 2006. MALDI-TOF MS characterization of proanthocyanidins from cranberry fruit (Vaccinium macrocarpon) that inhibit tumor cell growth and matrix metalloproteinase expression in vitro. J. Sci. Food Agric. 86:18-25. [Google Scholar]
- 38.Nichols, T. C., T. H. Fischer, E. N. Deliargyris, and A. S. Baldwin, Jr. 2001. Role of nuclear factor-kappa B (NF-kappa B) in inflammation, periodontitis, and atherogenesis. Ann. Periodontol 6:20-29. [DOI] [PubMed] [Google Scholar]
- 39.Njoroge, T., R. J. Genco, H. T. Sojar, N. Hamada, and C. A. Genco. 1997. A role for fimbriae in Porphyromonas gingivalis invasion of oral epithelial cells. Infect. Immun. 65:1980-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandros, J., C. Karlsson, D. F. Lappin, P. N. Madianos, D. F. Kinane, and P. N. Papapanou. 2000. Cytokine responses of oral epithelial cells to Porphyromonas gingivalis infection. J. Dent. Res. 79:1808-1814. [DOI] [PubMed] [Google Scholar]
- 41.Slots, J., and M. Ting. 1999. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in human periodontal disease: occurrence and treatment. Periodontology 2000. 20:82-121. [DOI] [PubMed] [Google Scholar]
- 42.Steinberg, D., M. Feldman, I. Ofek, and E. I. Weiss. 2004. Effect of a high-molecular-weight component of cranberry on constituents of dental biofilm. J. Antimicrob. Chemother. 54:86-89. [DOI] [PubMed] [Google Scholar]
- 43.Weinberg, A., C. M. Belton, Y. Park, and R. J. Lamont. 1997. Role of fimbriae in Porphyromonas gingivalis invasion of gingival epithelial cells. Infect. Immun. 65:313-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamanaka, A., R. Kimizuka, T. Kato, and K. Okuda. 2004. Inhibitory effects of cranberry juice on attachment of oral streptococci and biofilm formation. Oral Microbiol. Immunol. 19:150-154. [DOI] [PubMed] [Google Scholar]
- 45.Yamanaka, A., T. Kouchi, K. Kasai, T. Kato, K. Ishihara, and K. Okuda. 2007. Inhibitory effect of cranberry polyphenol on biofilm formation and cysteine proteases of Porphyromonas gingivalis. J. Periodontal Res. 42:589-592. [DOI] [PubMed] [Google Scholar]
- 46.Yilmaz, O., K. Watanabe, and R. J. Lamont. 2002. Involvement of integrins in fimbriae-mediated binding and invasion by Porphyromonas gingivalis. Cell. Microbiol. 4:305-314. [DOI] [PubMed] [Google Scholar]