Abstract
Cyclotides are a large family of cyclic cystine knot-containing plant peptides that have anthelminthic activities against Haemonchus contortus and Trichostrongylus colubriformis, two important gastrointestinal nematodes of sheep. In this study, we investigated the interaction of the prototypic cyclotide kalata B1 with the external surface of H. contortus larvae and adult worms. We show that cyclotides do not need to be ingested by the worms to exert their toxic effects but that an interaction with the external surface alone is toxic. Evidence for this was the toxicity toward adult worms in the presence of a chemically induced pharyngeal ligature and toxicity of cyclotides toward nonfeeding larval life stages. Uptake of tritiated inulin in ligated adult worms was increased in the presence of cyclotide, suggesting that cyclotides increase the permeability of the external membranes of adult nematodes. Polyethylene glycols of various sizes showed protective effects on the nonfeeding larval life stage, as well as in hemolytic activity assays, suggesting that discrete pores are formed in the membrane surfaces by cyclotides and that these can be blocked by polyethylene glycols of appropriate size. This increased permeability is consistent with recently reported effects of cyclotides on membranes in which kalata B1 was demonstrated to form pores and cause leakage of vesicle/cellular contents. Our data, together with known size constraints on the movement of permeants across nematode cuticle layers, suggest that one action of the cyclotides involves an interaction with the lipid-rich epicuticle layer at the surface of the worm.
Cyclotides are disulfide-rich peptides from plants that are characterized by their cyclic peptide backbone; they are the largest known family of genetically encoded cyclic proteins (11, 23, 46). More than 140 cyclotides have been reported to exist in the Violaceae and Rubiaceae plant families, and this number is expanding rapidly. Recent investigations suggest that thousands more cyclotides have yet to be discovered (46). Cyclotides exhibit a diverse range of biological activities, including anti-HIV (17, 22), antimicrobial (50), antineurotensin (52), cytotoxic (28, 49), and hemolytic (3, 40) activities. It has been postulated that the natural function of cyclotides is one of defense against plant pests and pathogens (2, 23, 24). The cyclotides kalata B1 (kB1) and kalata B2 (kB2) have a dramatic effect on growth and development of Helicoverpa punctigera (23) and Helicoverpa armigera larvae (24), two major pests of cotton. Recently, we demonstrated that cyclotides isolated from the plant Oldenlandia affinis display anthelminthic activity toward the gastrointestinal parasitic nematodes Haemonchus contortus and Trichostrongylus colubriformis (8). Subsequent studies found that cycloviolacins (a group of cyclotides isolated from Viola odorata) showed up to an 18-fold-increased potency against these nematodes relative to the prototypic cyclotide, kB1 (9). More recently, selected cyclotides have been shown to exert a toxic effect on canine and human hookworms (10).
Cyclotides incorporate a cystine knot motif (12) that interlocks three conserved disulfide bonds and have a head-to-tail cyclized peptide backbone, resulting in a structure that imparts to them exceptional stability (7). The cystine knot motif occupies the molecular core of cyclotides, with the result that hydrophobic residues, which are typically in the core of most proteins, are exposed on the surface of cyclotides, a factor that is responsible for their unusual biophysical properties. The three-dimensional structures and locations of the characteristic surface-exposed hydrophobic patch of cyclotides have been delineated by nuclear magnetic resonance (NMR) (37) and are shown in Fig. 1 for the prototypic cyclotide, kB1. Analytical ultracentrifugation has demonstrated that the cyclotides kB1 and kB2 form oligomers (tetramers and octamers) in solution (30), and this has been postulated to be important in their mode of action. Both kB1 and kB7 bind to the surface of dodecylphosphocholine micelles via two hydrophobic loops with relatively little perturbation of their structure on interaction with the micelles (43, 44). The patch of hydrophobic residues on the surface of kB1 is shown in Fig. 1. Disruption of this hydrophobic patch by point mutation has been shown to abolish nematocidal activity (20). Recently, kB1 has been shown to induce leakage of fluorescent dye from phospholipid vesicles and induce a current in patch-clamping experiments on asolectin bilayers (21). Similarly, cycloviolacin O2 from Viola odorata was shown to cause leakage from liposomes as well as from whole cells (49). The exact mode of action of cyclotides has not been confirmed, but they are thought to act via a mechanism involving pore formation (21).
FIG. 1.
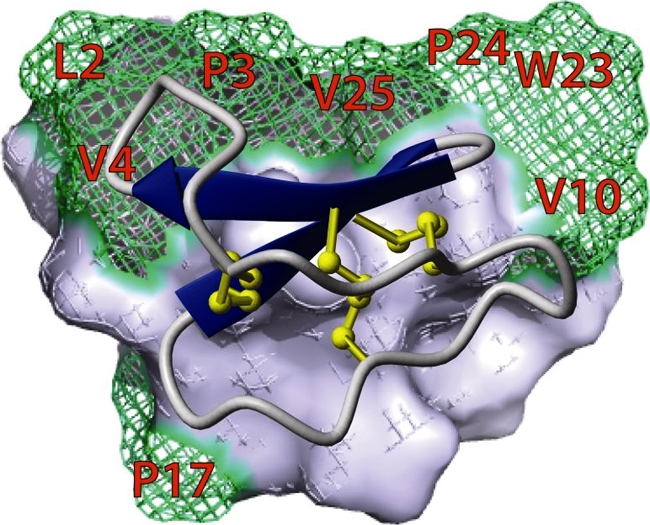
Surface representation of kalata B1. Hydrophobic residues are highlighted in green. The overlaid ribbon representation indicates the cystine knot motif characteristic of cyclotides.
The aim of the current study was to investigate the action of the cyclotides on larval and adult H. contortus. The prototypic cyclotide kB1 was selected, as it has been most extensively tested in both insecticidal assays as well as mode-of-action studies. We were interested in the site of action of the peptide and particularly in whether it required ingestion to exert its toxic effects. This information is important for understanding the requirements for the effective delivery of cyclotides to parasites in the host intestinal tract. Because many of the other known activities, aside from anthelminthic activity, of cyclotides appear to be associated with membrane disruption, the findings of the current study are of broader importance to understanding the mechanism of action of cyclotides in general.
MATERIALS AND METHODS
Cyclotide isolation.
kB1 and other natural cyclotide variants were isolated from the above-ground parts of the plant Oldenlandia affinis. Fresh plant material (500 g) was ground and extracted with 50/50 (vol/vol) dichloromethane-methanol, and the crude extract was partially purified by reversed-phase (RP) flash chromatography, yielding a fraction containing predominantly cyclotides (5 g). This sample was purified further by preparative RP-high-performance liquid chromatography (HPLC) to yield pure kB1 (125 mg), together with smaller amounts of the other natural variants as described previously (11).
Synthesis and biotinylation of a kB1 lysine mutant.
Incorporation of a lysine residue into kB1 enabled the biotinylation of the peptide via the ɛ-amino group of the lysine residue. The linear precursor with a single-point Lys substitution of Asn at position 29 (named [N29K]-kB1) was synthesized using manual solid-phase peptide synthesis with an in situ neutralization/2-(1H-benzotriazolyl)-1,1,3,3-tetramethyluronium hexafluorophosphate protocol for Boc chemistry, as described previously (14, 39, 47). The peptide was cyclized and oxidized in a standard folding buffer (0.5 mg/ml) containing 50% (vol/vol) isopropyl alcohol in 0.1 M ammonium bicarbonate (pH 8.2) with 2 mM reduced glutathione and 0.4 mM oxidized glutathione added. The mixture was stirred for 48 h at room temperature. The reduced peptide was purified by RP-HPLC on a Phenomenex C18 column and confirmed by electrospray ionization with mass spectrometry (ESI-MS). The purity of [N29K]-kB1 was confirmed by its late elution as a single peak under reversed-phase conditions, and the 1H-NMR spectrum recorded on a Bruker ARX 500 MHz spectrometer confirmed the correctly folded state (6, 13).
An equal volume of [N29K]-kB1 solution (5 mg/ml in 50 mM phosphate buffer, pH 8.5) was mixed with 20 mM N-hydroxysuccinimidobiotin (NHS-biotin; Pierce) prepared in dimethyl sulfoxide (DMSO), and the reaction mixture was incubated at room temperature for 30 min (42). NHS-activated biotins form stable amide bonds with the primary amino group of lysine residues. The nonreacted NHS-biotin and by-products were removed by analytical HPLC (1%/min in a gradient).
Larval and adult nematode life stages.
Feces were collected from sheep infected with H. contortus and housed at the McMaster Laboratory, CSIRO Livestock Industries, Armidale, NSW, Australia. The worms belonged to the Kirby isolate (1), which was isolated from the field at the University of New England Kirby Research Farm in 1986. The feces were maintained in 2-liter flasks at 27°C for approximately 7 days, and infective-stage L3 larvae were collected as they migrated up the sides of the flasks. These worms were designated as L3 worms for the migration assays (see below).
The adult worm recovery and culture methods were as described previously (25). Briefly, adult nematodes were removed from sheep abomasa approximately 10 to 15 weeks postinfection and placed into RPMI 1640 medium containing 1% glucose, 0.25 μg/ml amphotericin B, 10 U/ml penicillin, 10 μg/ml streptomycin, and 10 mM HEPES buffer (pH 6.8) at approximately 37°C. Nematodes were removed from digesta material using forceps and were held in the medium for 3 to 4 h while removal from digesta continued. They were then transferred to a medium containing the antimicrobial and antifungal agents described above at 10-fold-higher concentrations and left for 1 to 2 h. Groups of 10 female worms were then placed into 0.5 ml of the culture medium in separate glass vials and held at 37°C before use in peptide localization experiments and adult motility assays.
Localization of biotinylated [N29K]-kB1 in adult nematodes.
A modified method from that of Redmond et al. (35) was utilized to prepare cross-sections of adult H. contortus. After 24 h of incubation in RPMI-based medium at 37°C in the presence or absence of 84 μg/ml biotinylated [N29K]-kB1, six peptide-treated and six control H. contortus adults were cut into three segments in ice-cold phosphate-buffered saline (PBS). The segments were placed in 3% (wt/vol) agar, fixed in Bouin's solution (Sigma-Aldrich) overnight, transferred to a tissue processor for dehydration, clearing, and infiltration with paraffin, and embedded in paraffin wax. The paraffinized nematode tissue was cut using a microtome to a thickness of 5 μm, and the thin sections were fixed to slides. The sections on slides were dewaxed in xylene and rehydrated with serial ethanol incubations (100%, 80%, 70%, and distilled H2O, followed by 10 mM HEPES buffer containing 0.15 M NaCl, pH 8.2) before probing with 50 μg/ml fluorescein isothiocyanate-conjugated avidin (avidin-FITC; Thermo Scientific). After being washed, coverslips were mounted on slides with the addition of antifading mounting medium (Dako). Slides were visualized by using a fluorescence microscope (Olympus BX51).
Adult motility and feeding assays.
The effects of cyclotides, and of the anthelminthic drug ivermectin, on the adult stages of H. contortus were assessed by observing the degree of motility shown by worms after a period of exposure to the compounds in vitro. In addition, the uptake of [3H]inulin was used as a measure of the level of feeding activity in adult worms (16).
We initially determined the most appropriate ivermectin concentration to use in ligation experiments by measuring the effects of a range of ivermectin concentrations on adult worm motility and uptake of radiolabeled inulin. Adult worms were harvested and placed into vials in groups of 10. Ivermectin (dissolved in DMSO) was added to the vials at concentrations of 0.1, 1, 10, and 100 nM, while control worms received DMSO only (final concentration, 0.2%). After 6 h, [3H]inulin was added (final concentration, 4 μCi/ml). After a further 24 h, the nematodes were scored for motility using the system described by O'Grady and Kotze (31). Briefly, each assay tube was swirled to thoroughly disturb the nematodes and was then scored according to the degree of motility shown by the worms, as follows: 3, most individuals showing significant smooth sinusoidal motion, similar to motion at the start of the culture period; 2, significant movement shown by a small number of individuals, at least one individual able to move in a normal sinusoidal fashion; 1, only very limited movement in a small number of individuals, no sinusoidal motion; 0, no movement. The nematodes were then removed and washed by suspension in three successive 200-ml volumes of saline, followed by rinsing under vacuum with five 30-ml volumes of saline to remove any unbound inulin. The worms were transferred into scintillation vials and solubilized by incubation in 1 ml of 0.5 M KOH at 50°C overnight. The digests were neutralized with 25 μl acetic acid, scintillant fluid was added, and the radioactivity was counted. Each treatment was conducted in triplicate.
In a separate experiment, the effectiveness of the ligature was confirmed using uptake of FITC-labeled dextran (molecular mass, 40 kDa) as a measure of feeding activity. Groups of approximately 10 worms were incubated for 6 h in the absence or presence of ivermectin at 10 nM and then dextran was added (final concentration, 5 mg/ml). After 24 h worms were washed three times in 50 ml with PBS, fixed in 10% formalin, and photographed under white and UV light.
The effects of the cyclotide kB1 on worm motility and [3H]inulin uptake were then examined in worms with or without a functioning pharynx (absence or presence of ivermectin). Worms were maintained for 6 h in the presence or absence of ivermectin, then kB1 (200 μg/ml) and inulin were added and the assay continued for another 24 h. Worms were then scored for motility, and radioactivity recovered with the worms was measured as described above. Assays were conducted in triplicate.
Larval migration assays.
The effect of kB1 on the ability of L3-stage larvae to migrate through a 20-μm filter mesh was assessed using a method modified slightly from that of Kotze et al. (26). The larvae were incubated in PBS containing a range of peptide concentrations for 24 h in the dark in a 96-well plate format. The solutions containing worms were then transferred to 20-μm filters, allowing motile larvae to migrate to the wells below. The number of worms that had migrated after 24 h were counted, corrected by comparison to migration rates in control (no-peptide) wells, and plotted as a function of kB1 concentration.
The ability of osmotic protection agents to influence the observed anthelminthic activity was examined using polyethylene glycols (PEGs) of different sizes. Larvae were incubated for 24 h in a 96-well plate format with a range of kB1 concentrations alone, as described above, or in the presence of 30 mM PEG solution (either PEG 1000, PEG 4000, or PEG 6000). Controls containing PEG solution but no cyclotide were also included. After 24 h, the solutions containing worms were transferred to 20-μm filters, allowing motile larvae to migrate to the wells below, and the percent migration in each well was determined.
Osmotic protection in hemolytic activity assays.
Cyclotides were dissolved in water and serially diluted in PBS to give 20-μl test solutions in a 96-well U-bottomed microtiter plate (Nunc). Human type A red blood cells (RBCs) were washed with PBS and centrifuged at 1,500 × g for 60 s in a microcentrifuge several times until a clear supernatant was obtained. A 0.25% suspension of washed RBCs in PBS (100 μl) containing 30 mM PEG solution (either PEG 1000, PEG 4000, or PEG 6000) was added to the peptide solutions. The plate was incubated at 37°C for 1 h and centrifuged at 150 × g for 5 min. Aliquots of 100 μl were transferred to a 96-well flat-bottomed microtiter plate (Falcon), and the absorbance was measured at 405 nm with an automatic Multiskan Ascent plate reader (Labsystems). The amount of hemolysis was calculated as the percentage of maximum lysis (of the 1% Triton X-100 control) after adjusting for minimum lysis (of the PBS control).
RESULTS
Fluorescence microscopy of nematode sections.
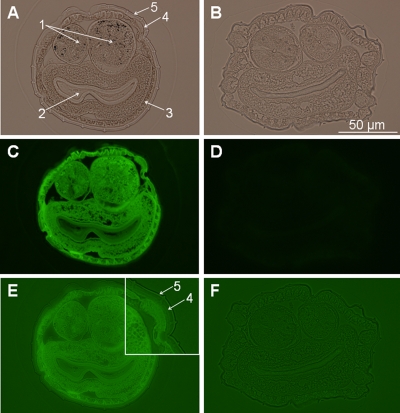
A synthetic lysine mutant of kB1 in which the asparagine at position 29 had been replaced with lysine was produced. The inclusion of a lysine residue in kB1 enabled labeling with biotin using the NHS chemistry of this cyclotide, which is otherwise deficient for a primary amine. The nematocidal activity of biotinylated [N29K]-kB1 was determined using a larval development assay as described previously (8). The incorporation of lysine into position 29 had been previously shown to increase the anthelminthic activity of kB1 (20); however, biotinylation of the mutant peptide in the present study reduced the activity to a level comparable with wild-type kB1 as judged by larval development assays (data not shown). Adult female nematodes were incubated in either the presence or absence of biotinylated [N29K]-kB1 for 24 h. Transverse sections were then prepared and probed with fluorescein-conjugated avidin to determine the location of the labeled cyclotide. Figure 2 shows the sections under white light (A and B) and under fluorescent light (C to F). No staining was observed in control worms, confirming that there was not any nonspecific labeling (Fig. 2D and F). In the peptide-treated nematodes, fluorescence was observed on all internal organs (ovaries, intestine, muscle, and hypodermis). In addition, a thin layer of fluorescence was observed at the surface of the worm (Fig. 2E, inset, arrow 5). The cuticle below this surface layer remained unstained (Fig. 2E, inset, arrow 4).
FIG. 2.
Transverse sections through adult Haemonchus contortus probed with fluorescein-conjugated avidin. Sections of adult nematodes following incubation with a biotinylated form of [N29K]-kalata B1 (A, C, and E) and control nematodes (B, D, and F) are shown. (A and B) Nematode sections under white light, enabling visualization of the nematode structural features as indicated by the arrows: (1) ovaries; (2) intestine; (3) hypodermis; (4) cuticle; (5) epicuticle. (C and D) Sections under fluorescent light. (E and F) Overexposed fluorescent images. The inset to panel E shows the external layers at higher magnification. The diameter of the sections is approximately 150 to 200 μm.
Adult motility and feeding assays.
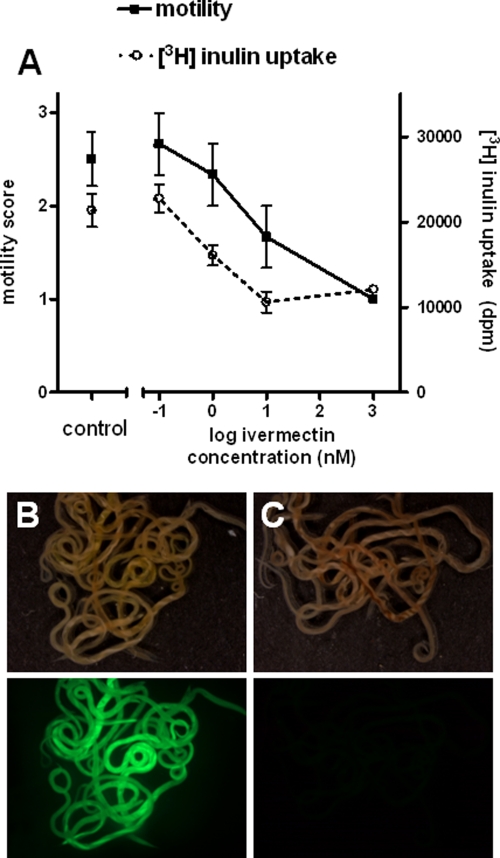
It was apparent from the localization experiments that kB1 was present throughout the internal areas of the worm, as well as in a thin layer on the worm's surface, following a period of incubation. In order to examine one aspect of the possible multiple interactions that this localization pattern indicated might be involved in the action of the cyclotide and to determine whether ingestion was a requirement for it to be toxic, we utilized the availability of a nonfeeding life stage, and an ability to ligate the pharynx of adult worms with ivermectin to prevent ingestion, to examine the effects of the peptide on worm external surfaces alone. Initial experiments examined the effectiveness of the ivermectin ligature. Figure 3A shows the motility scores and radioactivity levels of worms held for 30 h in the presence of ivermectin, with [3H]inulin present for the final 24 h of this incubation period, compared to control (no-ivermectin) worms. Motility decreased as the ivermectin concentration increased. [3H]inulin uptake decreased up to an ivermectin concentration of 10 nM and then remained constant as the ivermectin concentration was increased to 1,000 nM.
FIG. 3.
Ivermectin as a chemical ligature. (A) Motility and [3H]inulin uptake in adult worms exposed to various concentrations of ivermectin. Assays received ivermectin (or DMSO alone) at the start of the experiment, then [3H]inulin was added after 6 h, and motility was scored and worms processed for radioactivity measurements after a further 24 h. In a separate experiment, worms were maintained for 6 h in the absence (B) or presence (C) of 10 nM ivermectin, then incubated in 5 mg/ml FITC-labeled dextran for 24 h, and then photographed using white light (upper panel) or UV light (lower panel).
As with other studies utilizing inulin as a marker of ingestion (16, 36), the continued recovery of some inulin as the ivermectin concentration increased above 10 nM was expected and is probably due to adherence of the carbohydrate to the external surface of the worm rather than ingestion. The effectiveness of 10 nM ivermectin in preventing uptake by ingestion was confirmed using FITC-labeled dextran (molecular mass, 40 kDa) as shown in Fig. 3B (no ivermectin) and Fig. 3C (plus ivermectin). Pretreatment with ivermectin reduced the uptake of the FITC-dextran to negligible levels compared to control worms. Based on the effectiveness of 10 nM in inhibiting pharyngeal uptake while not being lethal to the worms (mean motility score, approximately 1.6 [Fig. 3A]), this concentration was used in combination with kB1 for the subsequent experiments examining the effects of the cyclotide on adult worms.
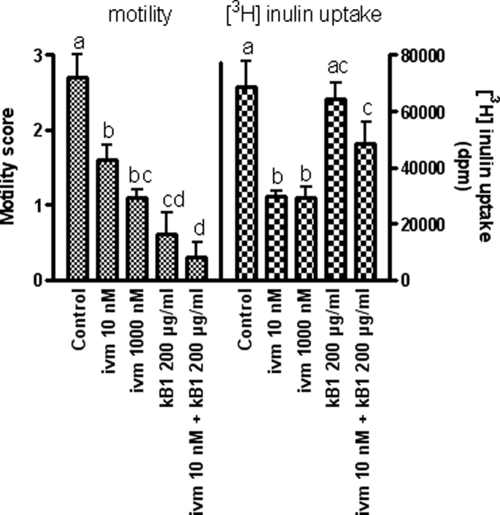
The effects of kB1 on worms in the presence or absence of ivermectin is shown in Fig. 4. Motility remained high in controls during the experimental period (mean score, 2.7) while it decreased in the presence of ivermectin at 10 nm and 1,000 nM, as expected. Compared to controls, motility was significantly decreased in worms treated with kB1 alone or in combination with ivermectin. Motility in these two treatments was not significantly different. Motility in the presence of ivermectin and kB1 was significantly less than with ivermectin alone, indicating that the peptide was toxic to the worms in the absence of ingestion. Inulin recovery levels were equivalent at 10 and 1,000 nM ivermectin. The presence of kB1 alone did not affect the level of inulin recovered from the worms relative to controls, despite these worms showing very little motility, suggesting that the pharynx continued to function normally in these kB1-affected worms along with significant inhibition of motility. The recovery of inulin from worms treated with both kB1 and ivermectin was significantly greater than for worms treated with ivermectin alone. Because this increased uptake could not be due to ingestion (in the presence of the ivermectin ligature), its occurrence suggests that recovery of inulin with the worm was increased in the presence of the cyclotide by means other than pharyngeal uptake.
FIG. 4.
Adult worm motility and [3H]inulin uptake in the presence of a pharyngeal ligature. Effects of kalata B1 alone, ivermectin alone, or the combination on adult worm motility and [3H]inulin uptake over a 24-h period (following a 6-h ivermectin or control pretreatment period) were determined. The columns labeled with identical letters were not significantly different at P = 0.05.
Larval migration assays.
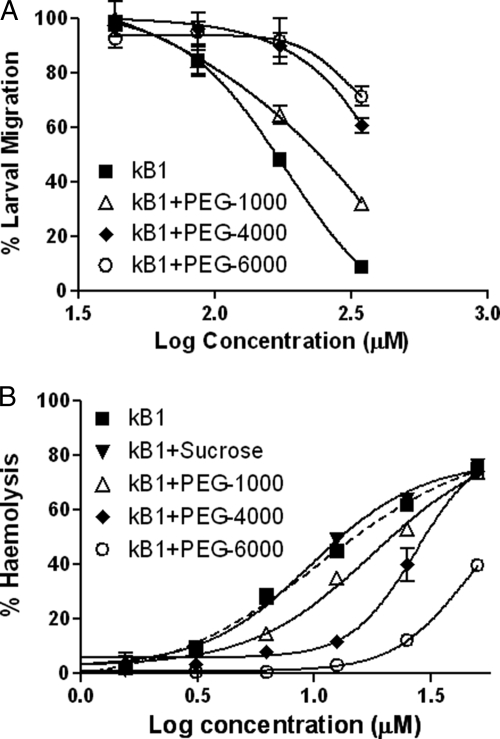
The effects of kB1 on the migration of L3-stage larvae were assessed over a range of concentrations of kB1 alone or in the presence of osmotic stabilizing agents (PEGs) of different sizes. Figure 5A shows that kB1 reduced the ability of L3-stage larvae to migrate through a filter matrix in a dose-dependent manner. Inclusion of the smallest PEG used (PEG 1000) increased migration rates slightly, whereas the medium (PEG 4000) and large (PEG 6000) PEGs enabled L3 larvae to migrate at rates comparable to control worms in up to 173 μM kB1. The larger PEGs also displayed a substantial level of protection at the highest kB1 concentration (346 μM), with approximately 60 to 70% migration in the presence of the PEGs, compared to less than 10% with kB1 alone.
FIG. 5.
Osmotic protection by PEGs of different sizes. Dose-response curves for the percent migration of L3-stage larvae of H. contortus (A) and the percent hemolysis of human erythrocytes (B) in various concentrations of the cyclotide kalata B1.
Osmotic protection in hemolytic activity assays.
To confirm the osmotic protective effect observed in the nematode migration assay, hemolysis reactions were performed in the presence of PEGs. Figure 5B shows a plot of hemolysis versus kB1 concentration. Sucrose was used as a negative control and exerted no protective effect, as would be expected. As the size of the PEGs increased, so did their observed osmotic protection. The smallest PEG (1000) showed little or no protection, and PEG 4000 showed an intermediate level of protection with no protection at the highest kB1 concentration tested (50 μM), whereas PEG 6000 completely inhibited hemolysis up to 12.5 μM kB1 and was able to inhibit hemolysis by 50% at the highest kB1 concentration tested. RBCs that had been incubated with the PEGs were washed in PBS to remove the PEGs, and rapid hemolysis was observed (data not shown), indicating that the PEGs acted solely as osmotic protection agents and did not inhibit the action of kB1.
DISCUSSION
We were interested in the site of action of cyclotides in nematodes for several reasons. First, it was uncertain whether molecules the size of cyclotides would be able to permeate the nematode cuticle, and hence whether a cyclotide required ingestion to reach its target site or if interaction with the nematode's external surfaces was sufficient. The molecular masses of currently used anthelminthics are significantly lower than that of kB1 (thiabendazole, 201 Da; levamisole, 241 Da; ivermectin, 873 Da; kB1, 2,890 Da). Second, evidence that kB1 results in damage to the gut lining of Helicoverpa armigera (2) demonstrated an interaction with the gut membranes in this species, suggesting that ingestion of the peptide might be important for it to reach this site of action in nematodes. The incubation of adult nematodes with a biotinylated form of kB1 enabled localization of the peptide by probing cross-sections with a fluorescent conjugate. The labeled cyclotide was demonstrated to localize throughout the internal areas of the worm as well as in a thin layer at its surface. The ability to ligate adult worms with ivermectin to prevent the functioning of the pharynx, and hence prevent their feeding, and the availability of a nonfeeding larval life stage provided means to specifically examine the interaction with external surfaces in isolation from any effects of the molecule following ingestion by the worm. The distribution of the fluorescently labeled peptide in ivermectin-ligated worms will be examined in future studies aimed at delineating the actions occurring at the external surface versus those observed at internal surfaces. The approach of focusing on external surface interactions was subsequently supported by evidence from adult and larval experiments that indicated that the peptide was toxic to nonfeeding worms. The observed decreased motility of adult worms in the presence of kB1 and ivermectin compared to ivermectin alone and the toxicity toward L3-stage larvae indicate the importance of the transcuticular pathway for the cyclotide to exert its anthelminthic effects.
Although the data indicated a toxic effect mediated through the cuticle, the question remained as to whether kB1 penetrated the cuticle to exert its effects or was acting at the cuticle surface. The absence of fluorescent staining from the cuticle layer itself, while a thin band of stain was observable at the surface of the worm, suggested that uptake of the peptide through the cuticle might not occur. Ho et al. (18) examined the transport of a number of radiolabeled permeants through the cuticle of adult Ascaris suum worms. They found that the aqueous pores of the collagen matrix of the cuticle formed the initial discriminating barrier governing the passage of molecules based on their physical size relative to the pores. The aqueous pores had an average radius of 1.5 nm, although this estimate varied between 1.18 and 1.80 nm, depending on which permeant was examined. The molecular radius of ivermectin (0.6 nm) is considered to approach the limit of the cuticle permeability (15). Thompson et al. (51) suggested that drugs with molecular masses greater than 3,000 Da would not be absorbed by the transcuticular route, while Ho et al. (19) suggested that drugs with molecular masses approaching 2,000 Da would not be absorbed across the cuticle due to the size restrictions imposed by the collagen matrix. Given these estimates, the dimensions of kB1 (radii of 1.2 by 0.9 by 0.75 nm) and its molecular mass (2,890 Da) suggest that its passage would be severely limited. An indication of the likelihood of this is the fact that inulin, with a molecular mass of approximately 5,000 Da and a radius of approximately 1.4 nm (38), is too large to pass through the pores in nematode cuticle (18).
Given that passage of kB1 through the cuticle is unlikely due to these size constraints, along with its toxicity in the absence of ingestion, our data suggest that it can be toxic toward nonfeeding nematode stages while still external to the cuticle collagen matrix. The nematode surface consists of (from the external surface inwards) (4, 29) the following: (i) the surface coat, a glycan/mucin-rich layer, approximately 5 to 20 nm in thickness; (ii) the epicuticle, a lipid-rich layer (described in more detail below), 6 to 40 nm thick; (iii) the cuticle, a collagen-rich extracellular matrix, generally 300 to 500 nm thick, consisting of cortical, medial, and basal zones; and (iv) epidermis or hypodermis.
An interaction external to the worm's cuticle collagen matrix, along with the known effects of cyclotides in disrupting cell membranes through interactions with the membrane lipid components in a number of systems (21, 28), suggests that the cyclotide may be interacting with the lipid-rich epicuticle layer of worms.
The question as to whether the nematode epicuticle represents a classical plasma membrane has been the subject of some debate (4, 29, 34, 53). The epicuticle has the appearance of a trilaminate plasma membrane (two electron-dense layers separated by an electron-lucent layer) and is known to contain lipid. It can, however, be significantly wider than a normal lipid bilayer plasma membrane: 6 to 40 nm compared to 6 to 10 nm. Support for its membrane nature has, however, come from freeze-fracture studies, which showed it to possess some characteristics of cellular membranes, including the presence of fracture faces containing particles resembling the intramembranous particles observed in biological membranes (32, 33, 48).
We utilized [3H]inulin as a marker for measuring ingestion levels in adult worms in the presence or absence of the ivermectin ligature. An interesting observation was that in the presence of kB1 the amount of inulin recovered with the worms increased significantly (Fig. 4). As mentioned above, inulin is considered too large to pass through the nematode cuticle, and hence [3H]inulin has been used as a marker for nematode feeding in numerous studies (16, 36, 45). A disadvantage with its use is that some inulin is recovered along with ligated worms, as shown in Fig. 3, suggesting that some of the carbohydrate is able to bind to the outside surface of the worms. This issue is overcome by careful measurement of “background” inulin levels recovered from ligated worms to allow noningestion recovery to be viewed along with actual ingestion levels. Worms treated with kB1 and ivermectin showed increased uptake of [3H]inulin compared to those treated with ivermectin alone (Fig. 4). That is, uptake of the radiolabel was increased in nonfeeding worms in the presence of kB1 compared to nonfeeding worms in the absence of kB1. The pharynx is not functioning in these ligated worms, and as described above, the inulin would not be expected to pass through the collagen region of the cuticle. Hence, given this limitation in its potential movement into the worm, the increased uptake suggests that inulin may permeate only through the epicuticle (exterior to the collagen-rich layer), possibly through pores formed in this layer by kB1. This suggestion is consistent with the known pore-forming action of the cyclotides. The dimensions of inulin (molecular radius, 1.4 nm) are consistent with it being able to pass through the 4.1- to 4.7-nm-diameter pores formed by kalata in lipid bilayers (21).
The question arises as to whether disruption of the epicuticle would be expected to be lethal to the worm. There is little information available on the consequences of manipulations of the epicuticle for worm survival. However, it is expected that its disruption through insertion of pores might compromise the ability of the worm to survive osmotic stress, hence reducing the viability of kB1-affected worms. The osmotic protection assays with whole larvae provide some evidence for this; PEGs were able to protect the worms from the effects of kB1, presumably due to their ability to prevent osmotic imbalances arising in the presence of the peptide. Searcy et al. (41) showed that deterioration of the epicuticle with age in Caenorhabditis briggsae was related to an increase in permeability and a reduced ability to survive osmotic stress.
The results of the osmotic protection assays are consistent with the hypothesis that pores of a discrete size or range of sizes are formed by the cyclotide interaction with both the nematode cuticle and the RBC membrane. Cyclotides are known to have hemolytic activity (3), and this activity might result from either the gross disruption of the RBC membrane or the formation of pores, leading to an influx of water down the osmotic gradient and consequent cell swelling and lysis. Inhibition of RBC lysis by PEGs is a widely used method for studying pore formation in membranes (5, 27). The ability of a specific PEG to prevent RBC lysis indicates that macromolecules of a certain size are unable to move though the pores formed in the cell, thus indicating that the size of the particular PEG exceeds the size of the pores. We observed a similar pattern of inhibition of RBC lysis and kB1 toxicity toward whole worm larvae in the presence of PEGs. This observation suggests a similar mechanism of protection is occurring with the worm larvae as is normally observed in these assays with RBCs. It has been postulated that as the cyclotide concentration increases, the size of the pores formed in membranes increases, a hypothesis based on the observation that cyclotides self-associate and oligomerize (21, 30). Our data correlate with this hypothesis, as the smaller PEGs showed a slight protective effect at low kB1 concentrations but little or no effect at high kB1 concentrations. At the highest kB1 concentration tested against worm larvae, PEG 4000 and PEG 6000, with diameters of 4 nm and 5 nm, respectively, provided significant protection, suggesting that the pores were mostly smaller than these diameters, consistent with the size estimations of 4.1 to 4.7 nm from Huang et al. (21), as described above.
In conclusion, our investigations on the effects of the cyclotide kB1 on the external surface of nematodes have demonstrated that kB1 does not need to be ingested to exert its toxic effects but that an interaction with the external surface alone is toxic to worms. Evidence for this was the toxicity toward adult worms in the presence of a chemically induced pharyngeal ligature and the toxicity toward the nonfeeding larval life stage. Uptake of tritiated inulin in ligated adult worms was increased in the presence of cyclotide, suggesting that the cyclotides increase the permeability of the external membrane of adult nematodes. Additionally, the protective effects of PEG suggest that pores are formed in the nematode by kB1. However, although it is clear that interaction with external surfaces is toxic to adult and larval nematodes, it remains possible that the cyclotide also interacts with internal membranes following ingestion by actively feeding worms. Further study will be required to address the relative importance of cyclotides acting within the nematode following ingestion, compared to those acting at the worm's external surface.
Acknowledgments
This work was supported by an ARC-CSIRO Linkage grant (M.L.C.), an IMB-CSIRO Ph.D. scholarship (Y.H.H.), and an Australian Research Council Professorial Fellowship (D.J.C.).
We thank Rekha Bharathi for extraction of cyclotides from plant material.
Footnotes
Published ahead of print on 8 March 2010.
REFERENCES
- 1.Albers, G. A. A., and S. K. Burgess. 1988. Serial passage of Haemonchus contortus in resistant and susceptible sheep. Vet. Parasitol. 28:303-306. [DOI] [PubMed] [Google Scholar]
- 2.Barbeta, B. L., A. Marshall, A. D. Gillon, D. J. Craik, and M. A. Anderson. 2008. Plant cyclotides disrupt epithelial cells in the midgut of lepidopteran larvae. Proc. Natl. Acad. Sci. U. S. A. 105:1221-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barry, D. G., N. L. Daly, R. J. Clark, L. Sando, and D. J. Craik. 2003. Linearization of a naturally occurring circular protein maintains structure but eliminates hemolytic activity. Biochemistry 42:6688-6695. [DOI] [PubMed] [Google Scholar]
- 4.Bird, A. F., and J. Bird (ed.). 1991. The structure of nematodes, 2nd ed., p. 44-74. Academic Press, San Diego, CA.
- 5.Chen, D., R. M. Kini, R. Yuen, and H. E. Khoo. 1997. Haemolytic activity of stonustoxin from stonefish (Synanceja horrida) venom: pore formation and the role of cationic amino acid residues. Biochem. J. 325:685-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark, R. J., N. L. Daly, and D. J. Craik. 2006. Structural plasticity of the cyclic-cystine knot framework: implications for biological activity and drug design. Biochem. J. 394:85-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colgrave, M. L., and D. J. Craik. 2004. Thermal, chemical, and enzymatic stability of the cyclotide kalata B1: the importance of the cyclic cystine knot. Biochemistry 43:5965-5975. [DOI] [PubMed] [Google Scholar]
- 8.Colgrave, M. L., A. C. Kotze, J. O'Grady, S. Simonsen, and D. J. Craik. 2008. Cyclotides: natural, circular plant peptides that possess significant activity against gastrointestinal nematode parasites of sheep. Biochemistry 47:5581-5589. [DOI] [PubMed] [Google Scholar]
- 9.Colgrave, M. L., A. C. Kotze, D. C. Ireland, C. K. Wang, and D. J. Craik. 2008. The anthelmintic activity of the cyclotides: natural variants with enhanced activity. ChemBioChem 9:1939-1945. [DOI] [PubMed] [Google Scholar]
- 10.Colgrave, M. L., A. C. Kotze, S. Kopp, J. S. McCarthy, G. T. Coleman, and D. J. Craik. 2009. Anthelmintic activity of cyclotides: In vitro studies with canine and human hookworms. Acta Trop. 109:163-166. [DOI] [PubMed] [Google Scholar]
- 11.Craik, D. J., N. L. Daly, T. Bond, and C. Waine. 1999. Plant cyclotides: a unique family of cyclic and knotted proteins that defines the cyclic cystine knot structural motif. J. Mol. Biol. 294:1327-1336. [DOI] [PubMed] [Google Scholar]
- 12.Craik, D. J., N. L. Daly, and C. Waine. 2001. The cystine knot motif in toxins and implications for drug design. Toxicon 39:43-60. [DOI] [PubMed] [Google Scholar]
- 13.Daly, N. L., R. J. Clark, and D. J. Craik. 2003. Disulfide folding pathways of cysteine knot proteins. Tying the knot within the circular backbone of the cyclotides. J. Biol. Chem. 278:6314-6322. [DOI] [PubMed] [Google Scholar]
- 14.Daly, N. L., S. Love, P. F. Alewood, and D. J. Craik. 1999. Chemical synthesis and folding pathways of large cyclic polypeptides: studies of the cystine knot polypeptide kalata B1. Biochemistry 38:10606-10614. [DOI] [PubMed] [Google Scholar]
- 15.Geary, T. G., K. L. Blair, N. F. Ho, S. M. Sims, and D. P. Thompson. 1995. Biological functions of nematode surfaces, p. 57-76. In J. C. Boothroyd and R. Komuniecki (ed.), Molecular approaches to parasitology. Wiley-Liss, New York, NY.
- 16.Geary, T. G., S. M. Sims, E. M. Thomas, I. Vanover, J. P. Davis, A. Winterrowd, R. D. Klein, N. F. Ho, and D. P. Thompson. 1993. Haemonchus contortus: ivermectin-induced paralysis of the pharynx. Exp. Parasitol. 77:88-96. [DOI] [PubMed] [Google Scholar]
- 17.Gustafson, K. R., R. C. Sowder II, L. E. Henderson, I. C. Parsons, Y. Kashman, J. H. Cardellina II, J. B. McMahon, R. W. Buckheit Jr., L. K. Pannell, and M. R. Boyd. 1994. Circulins A and B. Novel human immunodeficiency virus (HIV)-inhibitory macrocyclic peptides from the tropical tree Chassalia parvifolia. J. Am. Chem. Soc. 116:9337-9338. [Google Scholar]
- 18.Ho, N. F., T. G. Geary, T. J. Raub, C. L. Barsuhn, and D. P. Thompson. 1990. Biophysical transport properties of the cuticle of Ascaris suum. Mol. Biochem. Parasitol. 41:153-165. [DOI] [PubMed] [Google Scholar]
- 19.Ho, N. F., S. M. Sims, T. J. Vidmar, J. S. Day, C. L. Barsuhn, E. M. Thomas, T. G. Geary, and D. P. Thompson. 1994. Theoretical perspectives on anthelmintic drug discovery: interplay of transport kinetics, physicochemical properties, and in vitro activity of anthelmintic drugs. J. Pharm. Sci. 83:1052-1059. [DOI] [PubMed] [Google Scholar]
- 20.Huang, Y.-H., M. L. Colgrave, R. J. Clark, A. C. Kotze, and D. J. Craik. 26 January 2010, posting date. Lysine-scanning mutagenesis reveals an amendable face of the cyclotide kalata B1 for optimisation of nematocidal activity. J. Biol. Chem. [Epub ahead of print.] doi: 10.1074/jbc.M109.089854. [DOI] [PMC free article] [PubMed]
- 21.Huang, Y.-H., M. L. Colgrave, N. L. Daly, A. Keleshian, B. Martinac, and D. J. Craik. 2009. The biological activity of the prototypic cyclotide kalata B1 is modulated by the formation of multimeric pores. J. Biol. Chem. 284:20699-20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ireland, D. C., C. K. Wang, J. A. Wilson, K. R. Gustafson, and D. J. Craik. 2007. Cyclotides as natural anti-HIV agents. Biopolymers 90:51-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jennings, C., J. West, C. Waine, D. J. Craik, and M. A. Anderson. 2001. Biosynthesis and insecticidal properties of plant cyclotides: the cyclic knotted proteins from Oldenlandia affinis. Proc. Nat. Acad. Sci. U. S. A. 98:10614-10619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jennings, C. V., K. J. Rosengren, N. L. Daly, M. Plan, J. Stevens, M. J. Scanlon, C. Waine, D. G. Norman, M. A. Anderson, and D. J. Craik. 2005. Isolation, solution structure, and insecticidal activity of kalata B2, a circular protein with a twist: do Möbius strips exist in nature? Biochemistry 44:851-860. [DOI] [PubMed] [Google Scholar]
- 25.Kotze, A. C., and S. J. McClure. 2001. Haemonchus contortus utilizes catalase in defence against exogenous hydrogen peroxide in vitro. Int. J. Parasitol. 31:1563-1571. [DOI] [PubMed] [Google Scholar]
- 26.Kotze, A. C., L. F. Le Jambre, and J. O'Grady. 2006. A modified larval migration assay for detection of resistance to macrocyclic lactones in Haemonchus contortus, and drug screening with Trichostrongylidae parasites. Vet. Parasitol. 137:294-305. [DOI] [PubMed] [Google Scholar]
- 27.Lange, S., E. Kauschke, W. Mohrig, and E. L. Cooper. 1999. Biochemical characteristics of eiseniapore, a pore-forming protein in the coelomic fluid of earthworms. Eur. J. Biochem. 262:547-556. [DOI] [PubMed] [Google Scholar]
- 28.Lindholm, P., U. Goransson, S. Johansson, P. Claeson, J. Gullbo, R. Larsson, L. Bohlin, and A. Backlund. 2002. Cyclotides: a novel type of cytotoxic agents. Mol. Cancer Ther. 1:365-369. [PubMed] [Google Scholar]
- 29.Maizels, R. M., M. L. Blaxter, and M. E. Selkirk. 1993. Forms and functions of nematode surfaces. Exp. Parasitol. 77:380-384. [DOI] [PubMed] [Google Scholar]
- 30.Nourse, A., M. Trabi, N. L. Daly, and D. J. Craik. 2004. A comparison of the self-association behavior of the plant cyclotides kalata B1 and kalata B2 via analytical ultracentrifugation J. Biol. Chem. 279:562-570. [DOI] [PubMed] [Google Scholar]
- 31.O'Grady, J., and A. Kotze. 2004. Haemonchus contortus: in vitro drug screening assays with the adult life stage. Exp. Parasitol. 106:164-172. [DOI] [PubMed] [Google Scholar]
- 32.Peixoto, C. A., and W. De Souza. 1994. Freeze-fracture characterization of the cuticle of adult and dauer forms of Caenorhabditis elegans. Parasitol. Res. 80:53-57. [DOI] [PubMed] [Google Scholar]
- 33.Peixoto, C. A., and W. De Souza. 1995. Freeze-fracture and deep-etched view of the cuticle of Caenorhabditis elegans. Tissue Cell 27:561-568. [DOI] [PubMed] [Google Scholar]
- 34.Proudfoot, L., J. R. Kusel, H. V. Smith, and M. W. Kennedy. 1991. Biophysical properties of the nematode surface, p. 1-26. In M. W. Kennedy (ed.), Parasitic nematodes: membranes, antigens and genes. Taylor and Francis, London, United Kingdom.
- 35.Redmond, D. L., D. P. Knox, G. Newlands, and W. D. Smith. 1997. The nature and prospects for gut membrane proteins as vaccine candidates for Haemonchus contortus and other ruminant trichostrongyloids. Mol. Biochem. Parasitol. 85:77-87. [DOI] [PubMed] [Google Scholar]
- 36.Richards, J. C., J. M. Behnke, and I. R. Duce. 1995. In vitro studies on the relative sensitivity to ivermectin of Necator americanus and Ancylostoma ceylanicum. Int. J. Parasitol. 25:1185-1191. [DOI] [PubMed] [Google Scholar]
- 37.Saether, O., D. J. Craik, I. D. Campbell, K. Sletten, J. Juul, and D. G. Norman. 1995. Elucidation of the primary and three-dimensional structure of the uterotonic polypeptide kalata B1. Biochemistry 34:4147-4158. [DOI] [PubMed] [Google Scholar]
- 38.Scherrer, R., and P. Gerhardt. 1971. Molecular sieving by the Bacillus megaterium cell wall and protoplast. J. Bacteriol. 107:718-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schnolzer, M., P. Alewood, A. Jones, D. Alewood, and S. B. Kent. 1992. In situ neutralization in Boc-chemistry solid phase peptide synthesis. Int. J. Pept. Protein Res. 40:180-193. [DOI] [PubMed] [Google Scholar]
- 40.Schöpke, T., M. I. Hasan Agha, R. Kraft, A. Otto, and K. Hiller. 1993. Hamolytisch aktive komponenten aus Viola tricolor L. und Viola arvensis Murray. Sci. Pharm. 61:145-153. [Google Scholar]
- 41.Searcy, D. G., M. J. Kisiel, and B. M. Zuckerman. 1976. Age-related increase of cuticle permeability in the nematode Caenorhabditis briggsae. Exp. Aging Res. 2:293-301. [DOI] [PubMed] [Google Scholar]
- 42.Sélo, I., L. Negroni, C. Creminon, J. Grassi, and J. M. Wal. 1996. Preferential labelling of alpha-amino N-terminal groups in peptides by biotin: application to the detection of specific anti-peptide antibodies by enzyme immunoassays. J. Immunol. Methods 199:127-138. [DOI] [PubMed] [Google Scholar]
- 43.Shenkarev, Z. O., K. D. Nadezhdin, E. N. Lyukmanova, V. A. Sobol, L. Skjeldal, and A. S. Arseniev. 2008. Divalent cation coordination and mode of membrane interaction in cyclotides: NMR spatial structure of ternary complex Kalata B7/Mn2+/DPC micelle. J. Inorg. Biochem. 102:1246-1256. [DOI] [PubMed] [Google Scholar]
- 44.Shenkarev, Z. O., K. D. Nadezhdin, V. A. Sobol, A. G. Sobol, L. Skjeldal, and A. S. Arseniev. 2006. Conformation and mode of membrane interaction in cyclotides. Spatial structure of kalata B1 bound to a dodecylphosphocholine micelle. FEBS J. 273:2658-2672. [DOI] [PubMed] [Google Scholar]
- 45.Sheriff, J. C., A. C. Kotze, N. C. Sangster, and D. R. Hennessy. 2005. Effect of ivermectin on feeding by Haemonchus contortus in vivo. Vet. Parasitol. 128:341-346. [DOI] [PubMed] [Google Scholar]
- 46.Simonsen, S. M., L. Sando, D. C. Ireland, M. L. Colgrave, R. Bharathi, U. Goransson, and D. J. Craik. 2005. A continent of plant defense peptide diversity: cyclotides in Australian Hybanthus (Violaceae). Plant Cell 17:3176-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simonsen, S. M., L. Sando, K. J. Rosengren, C. K. Wang, M. L. Colgrave, N. L. Daly, and D. J. Craik. 2008. Alanine scanning mutagenesis of the prototypic cyclotide reveals a cluster of residues essential for bioactivity. J. Biol. Chem. 283:9805-9813. [DOI] [PubMed] [Google Scholar]
- 48.Smith, V. P., M. E. Selkirk, and K. Gounaris. 1996. Brugia malayi: ultrastructural morphology of the cuticular surface membranes of adult parasites. Exp. Parasitol. 83:304-313. [DOI] [PubMed] [Google Scholar]
- 49.Svangard, E., R. Burman, S. Gunasekera, H. Lovborg, J. Gullbo, and U. Goransson. 2007. Mechanism of action of cytotoxic cyclotides: cycloviolacin O2 disrupts lipid membranes. J. Nat. Prod. 70:643-647. [DOI] [PubMed] [Google Scholar]
- 50.Tam, J. P.,Y. A. Lu, J. L. Yang, and K. W. Chiu. 1999. An unusual structural motif of antimicrobial peptides containing end-to-end macrocycle and cystine-knot disulfides. Proc. Natl. Acad. Sci. U. S. A. 96:8913-8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thompson, D. P., N. F. Ho, S. M. Sims, and T. G. Geary. 1993. Mechanistic approaches to quantitate anthelmintic absorption by gastrointestinal nematodes. Parasitol. Today 9:31-35. [DOI] [PubMed] [Google Scholar]
- 52.Witherup, K. M., M. J. Bogusky, P. S. Anderson, H. Ramjit, R. W. Ransom, T. Wood, and M. Sardana. 1994. Cyclopsychotride A, a biologically active, 31-residue cyclic peptide isolated from Psychotria longipes. J. Nat. Prod. 57:1619-1625. [DOI] [PubMed] [Google Scholar]
- 53.Wright, D. J., P. S. Grewal, and M. Stolinski. 1997. Relative importance of neutral lipids and glycogen as energy stores in dauer larvae of two entomopathogenic nematodes, Steinernema carpocapsae and Steinernema feltiae. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 118:269-273. [DOI] [PubMed] [Google Scholar]