Abstract
The optimal approach for empirical antibiotic therapy in patients with severe sepsis and septic shock remains controversial. A retrospective cohort study was conducted in the intensive care units of a university hospital. The data from 760 patients with severe sepsis or septic shock associated with Gram-negative bacteremia was analyzed. Among this cohort, 238 (31.3%) patients received inappropriate initial antimicrobial therapy (IIAT). The hospital mortality rate was statistically greater among patients receiving IIAT compared to those initially treated with an appropriate antibiotic regimen (51.7% versus 36.4%; P < 0.001). Patients treated with an empirical combination antibiotic regimen directed against Gram-negative bacteria (i.e., β-lactam plus aminoglycoside or fluoroquinolone) were less likely to receive IIAT compared to monotherapy (22.2% versus 36.0%; P < 0.001). The addition of an aminoglycoside to a carbapenem would have increased appropriate initial therapy from 89.7 to 94.2%. Similarly, the addition of an aminoglycoside would have increased the appropriate initial therapy for cefepime (83.4 to 89.9%) and piperacillin-tazobactam (79.6 to 91.4%). Logistic regression analysis identified IIAT (adjusted odds ratio [AOR], 2.30; 95% confidence interval [CI] = 1.89 to 2.80) and increasing Apache II scores (1-point increments) (AOR, 1.11; 95% CI = 1.09 to 1.13) as independent predictors for hospital mortality. In conclusion, combination empirical antimicrobial therapy directed against Gram-negative bacteria was associated with greater initial appropriate therapy compared to monotherapy in patients with severe sepsis and septic shock. Our experience suggests that aminoglycosides offer broader coverage than fluoroquinolones as combination agents for patients with this serious infection.
Bacterial resistance to antibiotics creates a therapeutic challenge for clinicians when treating patients with a known or suspected infection. Increasing rates of resistance lead many clinicians to empirically treat patients with multiple broad-spectrum antibiotics, which can perpetuate the cycle of increasing resistance and create an economic burden to society (4, 7). Conversely, inappropriate initial antimicrobial therapy (IIAT), defined as an antimicrobial regimen that lacks in vitro activity against the isolated organism(s) responsible for the infection, can lead to treatment failures and adverse patient outcomes (21). IIAT is a potentially modifiable factor that has also been linked to increased mortality in patients with serious infections (11, 16, 20, 25). Individuals with severe sepsis and septic shock appear to be at particularly high risk of excess mortality when IIAT is administered (10, 13, 14, 24). The most recent Surviving Sepsis Guidelines recommend empirical combination therapy targeting Gram-negative bacteria, particularly for patients with known or suspected Pseudomonas infections, as a means to decrease the likelihood of administering IIAT (9). However, the authors of this guideline acknowledge that “no study or meta-analysis has convincingly demonstrated that combination therapy produces a superior clinical outcome for individual pathogens in a particular patient group.”
The de-escalation approach to antimicrobial therapy for serious infections is a treatment strategy that attempts to provide appropriate initial antimicrobial therapy to reduce the risk of negative patient outcomes while also avoiding the consequences of excessive or unnecessary antibiotic administration (22). Appropriate initial antimicrobial selection is usually based on an individual patient's risk profile for infection with potentially antibiotic-resistant bacteria, fungi, or molds and other opportunistic microorganisms. Avoiding unnecessary use of antibiotics occurs by narrowing the spectrum or number of antimicrobial agents once the etiologic cause of the infection and the patient's response to the initial treatment are evaluated, while also using the shortest course of antibiotic therapy that is clinically indicated. The initial use of combination therapy for Gram-negative bacteria is usually recommended in de-escalation strategies for serious infections (2). Then again, there is limited published data supporting such a strategy, especially for patients with severe sepsis or septic shock. Therefore, we performed a study with the main goal of determining whether combination antimicrobial therapy directed against Gram-negative bacteria was associated with lower hospital mortality in patients with severe sepsis and septic shock.
MATERIALS AND METHODS
Study location and patients.
This study was conducted at a university-affiliated, urban teaching hospital: Barnes-Jewish Hospital (1200 beds). During a 6-year period (January 2002 to December 2007), all hospitalized patients with a positive blood culture for Gram-negative bacteria were eligible for this investigation. This study was approved by the Washington University School of Medicine Human Studies Committee.
Study design and data collection.
A retrospective cohort study design was used. Two investigators (J.A.D. and R.M.R.) identified potential study patients by the presence of a positive blood culture for Gram-negative bacteria combined with primary or secondary ICD-9-CM codes indicative of acute organ dysfunction. Based on the initial study database construction, three investigators (E.C.W., J.K., and M.P.) merged patient-specific data from the automated hospital medical records, microbiology database, and pharmacy database of Barnes-Jewish Hospital to complete the clinical database under the auspices of the definitions described below.
The baseline characteristics collected by the study investigators included: age, gender, race, the presence of congestive heart failure, chronic obstructive pulmonary disease, diabetes mellitus, chronic liver disease, underlying malignancy, and end-stage renal disease requiring renal replacement therapy. All cause hospital mortality was evaluated as the primary outcome variable. Secondary outcomes evaluated included the occurrence of renal toxicity and acquisition of Clostridium difficile-associated diarrhea (CDAD). Acute Physiology and Chronic Health Evaluation II (APACHE II) (19) and Charlson comorbidity scores were calculated to evaluate severity of illness based on clinical data present during the 24 h after the positive blood cultures were drawn. This was done since we included patients with community-acquired infections who only had clinical data available after blood cultures were drawn.
Definitions.
All definitions were selected prospectively as part of the original study design. Cases of Gram-negative bacteremia were classified into mutually exclusive groups comprised of either community-acquired or health care-associated infection. Patients with health care-associated bacteremia were categorized as community-onset or hospital-onset as previously described (18). In brief, patients with health care-associated community-onset bacteremia had the positive culture obtained within the first 48 h of hospital admission in combination with one or more of the following risk factors: (i) residence in a nursing home, rehabilitation hospital, or other long-term nursing facility; (ii) previous hospitalization within the immediately preceding 12 months; (iii) receiving outpatient hemodialysis, peritoneal dialysis, wound care, or infusion therapy necessitating regular visits to a hospital-based clinic; and (iv) having an immunocompromised state. Patients were classified as having health care-associated hospital-onset bacteremia when the culture was obtained 48 h or more after admission. Community-acquired bacteremia occurred in patients without healthcare risk factors and a positive blood culture within the first 48 h of admission. Prior antibiotic exposure was defined as having occurred within the previous 30 days from the onset of severe sepsis or septic shock. Renal toxicity was defined as a 0.5-mg/dl increase in the serum creatinine in conjunction with a 50% increase in the serum creatinine from the day therapy was initiated for Gram-negative bacteremia. In addition, the need for renal replacement therapy stemming from the episode of renal toxicity was collected. CDAD was defined by the presence of diarrhea or pseudomembranous colitis and a positive assay for Clostridium difficile toxin A, toxin B, or both toxins A and B occurring after the index case of Gram-negative bacteremia.
To be included in the analysis, patients had to meet criteria for severe sepsis based on discharge ICD-9-CM codes for acute organ dysfunction as previously described (3). The organs of interest included the heart, lungs, kidneys, bone marrow (hematologic), brain, and liver. Patients were classified as having septic shock if vasopressors (norepinephrine, dopamine, epinephrine, phenylephrine, or vasopressin) were initiated within 24 h of the blood culture collection date and time. Antimicrobial treatment was classified as being appropriate if the initially prescribed antibiotic regimen was active against the identified pathogen based on in vitro susceptibility testing and administered within 24 h of blood culture collection. For patients with polymicrobial infection the initial antimicrobial regimen had to be active against all identified pathogens in order to be classified as appropriate. Appropriate antimicrobial treatment also had to be prescribed for at least 24 h. However, the total duration of antimicrobial therapy was at the discretion of the treating physicians.
Antimicrobial monitoring.
From January 2002 through the present Barnes-Jewish Hospital utilized an antibiotic control program to help guide antimicrobial therapy. During this time the use of cefepime and gentamicin was unrestricted. However, the initiation of intravenous ciprofloxacin, imipenem/cilastatin, meropenem, or piperacillin-tazobactam was restricted and required preauthorization from either a clinical pharmacist or infectious diseases physician. Each intensive care unit had a clinical pharmacist who reviewed all antibiotic orders to ensure that dosing and interval of antibiotic administration was adequate for individual patients based on body size, renal function, and the resuscitation status of the patient. After daytime hours the on-call clinical pharmacist reviewed and approved the antibiotic orders. Starting in June 2005, a sepsis order set was implemented in the Emergency Department, general medical wards, and the intensive care units with the intent of standardizing empirical antibiotic selection for patients with sepsis based on the infection type (i.e., community-acquired pneumonia, health care-associated pneumonia, intra-abdominal infection, etc.) and the local antibiogram (26, 30). However, antimicrobial selection, dosing, and de-escalation of therapy were still optimized by clinical pharmacists in these clinical areas.
Antimicrobial susceptibility testing.
The microbiology laboratory performed antimicrobial susceptibility of the Gram-negative bacterial isolates by the Kirby-Bauer disk diffusion method according to guidelines and breakpoints established by the Clinical Laboratory and Standards Institute (CLSI), using 150-mm round plates of Mueller-Hinton agar (BBL/Becton Dickinson, Cockeysville, MD). A technologist experienced in reading zones of inhibition with a ruler against a black background measured zone diameters manually.
Data analysis.
Continuous variables were reported as mean ± the standard deviation. The Student t test was used when we compared normally distributed data, and the Mann-Whitney U test was used to analyze non-normally distributed data. Categorical data was expressed as frequency distributions, and the chi-square test was used to determine whether differences existed between groups. We performed multiple logistic regression analysis to identify clinical risk factors that were associated with hospital mortality (SPSS, Inc., Chicago, IL). All risk factors that were significant at 0.2 in the univariate analysis were included in the multivariable analyses. All tests were two-tailed, and a P value of <0.05 was determined to represent statistical significance.
RESULTS
Patient characteristics.
A total of 760 patients were included in the study, of whom 522 (68.7%) received initially appropriate antimicrobial treatment, and 238 (31.3%) received IIAT for severe sepsis or septic shock associated with Gram-negative bacteremia. The mean age was 59.3 ± 16.3 (range, 18 to 99), with 399 (52.5%) males and 361 (47.5%) females. The infection sources included community-acquired (n = 72, 9.5%), healthcare-associated community-onset (n = 269, 35.4%) and healthcare-associated hospital-onset (n = 419, 55.1%). Patients receiving IIAT were statistically less likely to have either community-acquired or healthcare-associated community-onset sources of infection and were more likely to have healthcare-associated hospital-onset sources of infection compared to patients receiving appropriate initial treatment (Table 1) . Patients treated with IIAT were also statistically more likely to have chronic kidney disease, diabetes mellitus, respiratory organ dysfunction, the lungs as the source of infection, mechanical ventilation, and prior antibiotic exposure and were less likely to have active malignancy and the urinary tract as the source of their infection (Table 1).
TABLE 1.
Baseline characteristics
| Variablea | Appropriate antibiotic therapy | Inappropriate antibiotic therapy | P |
|---|---|---|---|
| No. of subjects | 522 | 238 | |
| Mean age (yr) ± SD | 59.9 ± 16.5 | 57.7 ± 15.8 | 0.082 |
| No. of males (%) | 283 (54.2) | 116 (48.7) | 0.161 |
| Infection onset source | |||
| Community acquired | 58 (11.1) | 14 (5.9) | 0.023 |
| Healthcare-associated community onset | 210 (40.2) | 59 (24.8) | <0.001 |
| Healthcare-associated hospital onset | 254 (48.7) | 165 (69.3) | <0.001 |
| Underlying comorbidities | |||
| CHF | 94 (18.0) | 51 (21.4) | 0.275 |
| COPD | 89 (17.0) | 48 (20.2) | 0.310 |
| Chronic kidney disease | 60 (11.5) | 49 (20.6) | 0.001 |
| Hemodialysis | 48 (9.2) | 30 (12.6) | 0.158 |
| Liver disease | 57 (10.9) | 38 (16.0) | 0.058 |
| Active malignancy | 179 (34.3) | 64 (26.9) | 0.042 |
| Neutropeniab | 44 (8.4) | 14 (5.9) | 0.220 |
| Diabetes | 104 (19.9) | 65 (27.3) | 0.024 |
| Mean Charlson comorbidity score ± SD | 4.8 ± 3.7 | 4.8 ± 3.6 | 0.994 |
| Mean APACHE II score ± SD | 23.9 ± 6.7 | 23.2 ± 6.6 | 0.203 |
| ICU admission | 403 (77.2) | 197 (82.8) | 0.081 |
| Vasopressors | 303 (58.0) | 141 (59.2) | 0.756 |
| Mechanical ventilation | 269 (51.5) | 149 (62.6) | 0.004 |
| Dysfunctional organ systems | |||
| Cardiovascular | 323 (61.9) | 147 (61.8) | 0.976 |
| Respiratory | 304 (58.2) | 164 (68.9) | 0.005 |
| Renal | 285 (54.6) | 123 (51.7) | 0.454 |
| Hepatic | 40 (7.7) | 15 (6.3) | 0.502 |
| Hematologic | 152 (29.1) | 79 (33.2) | 0.257 |
| Neurologic | 33 (6.3) | 14 (5.9) | 0.872 |
| ≥2 dysfunctional organ systems | 352 (67.4) | 175 (73.5) | 0.091 |
| Source of bloodstream infection | |||
| Lungs | 186 (35.6) | 114 (47.9) | 0.001 |
| Urinary tract | 169 (32.4) | 60 (25.2) | 0.046 |
| Central venous catheter | 45 (8.6) | 12 (5.0) | 0.102 |
| Intra-abdominal | 94 (18.0) | 47 (19.7) | 0.567 |
| Unknown | 38 (7.3) | 13 (5.5) | 0.435 |
| Prior antibioticsc | 172 (33.0) | 144 (60.5) | <0.001 |
The values represent the “number of subjects (%)” except as noted. CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; APACHE, acute physiologic and chronic health evaluation; ICU, intensive care unit.
Fewer than 500 neutrophils per mm3 of blood.
That is, in the preceding 30 days.
Microbiology.
Among the 825 Gram-negative bacteria isolated from blood, the most common included Escherichia coli (28.1%), Klebsiella species (22.8%), Pseudomonas aeruginosa (16.0%), Enterobacter species (9.2%), and Acinetobacter species (7.6%) (Table 2). Patients receiving IIAT were statistically more likely to be infected with extended-spectrum β-lactamase (ESBL)-producing Klebsiella pneumonia, Achromobacter species, Acinetobacter species, and Stenotrophomonas maltophilia and less likely to be infected with Escherichia coli, non-ESBL-producing Klebsiella pneumoniae and to have polymicrobial bacteremia compared to patients receiving initial appropriate therapy. The pathogen-specific hospital mortality rate was statistically greater for patients with bacteremia attributed to Acinetobacter species, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia who received IIAT (Table 2).
TABLE 2.
Microbiology
| Bacteria | Appropriate antibiotic therapy (n = 522) |
Inappropriate antibiotic therapy (n = 238) |
Pa | ||
|---|---|---|---|---|---|
| No. of subjects (%) | % Hospital mortality | No. of subjects (%) | % Hospital mortality | ||
| Enterobacteriaceae | |||||
| Citrobacter freundii | 8 (1.5) | 12.5 | 2 (0.8) | 50.0 | 0.733 (0.378) |
| Other Citrobacter species | 1 (0.2) | 0.0 | 1 (0.4) | 0.0 | 0.529 (—) |
| Enterobacter cloacae | 40 (7.7) | 32.5 | 16 (6.7) | 37.5 | 0.765 (0.721) |
| Enterobacter aerogenes | 10 (1.9) | 10.0 | 4 (1.7) | 50.0 | 1.0 (0.176) |
| Other Enterobacter species | 3 (0.6) | 0.0 | 3 (1.3) | 66.7 | 0.384 (0.400) |
| Escherichia coli | 188 (36.0) | 31.9 | 37 (15.5) | 29.7 | <0.001 (0.794) |
| ESBL Escherichia coli | 3 (0.6) | 66.7 | 4 (1.7) | 25.0 | 0.214 (0.486) |
| Klebsiella oxytoca | 10 (1.9) | 30.0 | 3 (1.3) | 66.7 | 0.764 (0.510) |
| Klebsiella pneumoniae | 129 (24.7) | 36.4 | 30 (12.6) | 50.0 | <0.001 (0.170) |
| ESBL Klebsiella species | 5 (1.0) | 60.0 | 11 (4.6) | 45.5 | 0.002 (1.0) |
| Morganella morganii | 9 (1.7) | 55.6 | 0 (0) | 0.0 | 0.064 (—) |
| Proteus mirabilus | 30 (5.7) | 40.0 | 7 (2.9) | 42.9 | 0.104 (1.0) |
| Providencia species | 4 (0.8) | 0.0 | 0 (0) | 0.0 | 0.315 (—) |
| Salmonella species | 2 (0.4) | 0.0 | 4 (1.7) | 0.0 | 0.081 (—) |
| Serratia marcescens | 16 (3.1) | 37.5 | 14 (5.9) | 28.6 | 0.072 (0.709) |
| Nonfermenting Gram-negative rods | |||||
| Achromobacter species | 3 (0.6) | 100.0 | 9 (3.8) | 77.8 | 0.002 (1.0) |
| Acinetobacter species | 19 (3.6) | 26.3 | 44 (18.5) | 61.4 | <0.001 (0.011) |
| Burkholderia species | 1 (0.2) | 0.0 | 1 (0.4) | 0.0 | 0.529 (—) |
| Pseudomonas aeruginosa | 91 (17.4) | 47.3 | 41 (17.2) | 68.3 | 1.0 (0.025) |
| Other Pseudomonas species | 0 (0) | 0.0 | 2 (0.8) | 0.0 | 0.098 (—) |
| Stenotrophomonas maltophilia | 4 (0.8) | 0.0 | 16 (6.7) | 87.5 | <0.001 (0.003) |
| Polymicrobial Gram negative | 45 (8.6) | 24.4 | 10 (4.2) | 40.0 | 0.034 (0.434) |
P values in parentheses represent the comparison of hospital mortality for the two groups. “(—)” indicates analysis not performed.
Antimicrobial susceptibility.
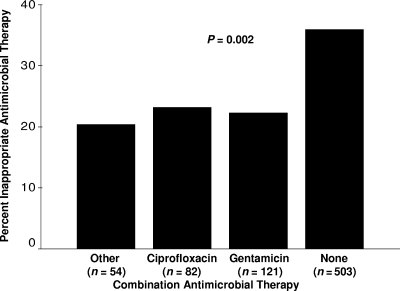
The antimicrobial susceptibilities of the Gram-negative bacterial isolates are shown in Table 3 with overall susceptibility being greatest for imipenem/meropenem, followed by, in descending order, gentamicin, cefepime, piperacillin-tazobactam, and ciprofloxacin. For individual bacterial species Acinetobacter species and Stenotrophomonas maltophilia had the lowest overall susceptibility to all antimicrobial agents tested. Overall, 247 (30.0%) of the isolates were treated with IIAT. IIAT was most common for S. maltophilia, followed by Achromobacter species, Acinetobacter species, and Salmonella species. Among the 359 (43.5%) bacterial isolates resistant to cefepime, imipenem/meropenem, or piperacillin-tazobactam, 99 (27.6%) were susceptible to ciprofloxacin, and 173 (48.2) were susceptible to gentamicin (Table 4). The incremental increases in the appropriateness of initial antimicrobial therapy for patients treated with cefepime, imipenem or meropenem, and piperacillin-tazobactam if ciprofloxacin or gentamicin were added to their antibiotic regimens are shown in Table 5. IIAT was statistically greatest for patients receiving monotherapy compared to combination antimicrobial therapy directed against Gram-negative bacteria (36.0% versus 22.2%; P < 0.001) (Fig. 1). Among the 238 patients initially receiving IIAT, 174 (73.1%) were switched to definitive therapy within 48 h of having their cultures drawn. Among the 257 patients receiving initial combination therapy, 198 (77.0%) were switched to a single agent for definitive therapy.
TABLE 3.
Antibiogram for Gram-negative bacterial isolatesa
| Gram-negative bacterium | Total no. of isolates | No. of isolates (%) susceptible to: |
|||||
|---|---|---|---|---|---|---|---|
| Cefepime | Ciprofloxacin | Gentamicin | Imipenem or meropenem | Piperacillin-tazobactam | IIAT | ||
| Achromobacter spp. | 12 | 3 (25) | 6 (50) | 0 (0) | 12 (100) | 9 (75) | 9 (75) |
| Acinetobacter spp. | 63 | 19 (30.2) | 15 (23.8) | 28 (44.4) | 31 (49.2) | 13 (20.6) | 44 (69.8) |
| Burkholderia spp. | 2 | 1 (50) | 1 (50) | 0 (0) | 1 (50) | 2 (100) | 1 (50) |
| Citrobacter spp. | 12 | 12 (100) | 9 (75) | 11 (91.7) | 12 (100) | 9 (75) | 3 (25) |
| Enterobacter spp. | 76 | 63 (82.9) | 57 (75) | 72 (94.7) | 70 (92.1) | 50 (65.8) | 23 (30.3) |
| Escherichia coli | 232 | 222 (95.7) | 182 (78.4) | 209 (90.1) | 230 (99.1) | 210 (90.5) | 41 (17.7) |
| Klebsiella spp. | 188 | 165 (87.8) | 158 (84.0) | 178 (94.7) | 183 (97.3) | 154 (81.9) | 44 (23.4) |
| Morganella morganii | 9 | 9 (100) | 8 (88.9) | 8 (88.9) | 9 (100) | 9 (100) | 0 (0) |
| Proteus spp. | 37 | 34 (91.9) | 25 (67.6) | 36 (97.3) | 36 (97.3) | 34 (91.9) | 7 (18.9) |
| Providencia spp. | 4 | 4 (100) | 3 (75) | 4 (100) | 3 (75) | 3 (75) | 0 (0) |
| Pseudomonas aeruginosa | 132 | 120 (90.9) | 95 (72) | 110 (83.3) | 116 (87.9) | 120 (90.9) | 41 (31.1) |
| Salmonella spp. | 6 | 5 (83.3) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 4 (66.7) |
| Serratia marcescens | 30 | 27 (90) | 26 (86.7) | 28 (93.3) | 28 (93.3) | 27 (90) | 14 (46.7) |
| Stenotrophomonas maltophilia | 20 | 6 (30) | 11 (55) | 8 (40) | 0 (0) | 7 (35) | 16 (80) |
| Cumulative | 823 | 690 (83.8) | 602 (73.1) | 698 (84.8) | 737 (89.6) | 653 (79.3) | 247 (30) |
Excludes two isolates of other Pseudomonas species isolated from blood cultures. For Escherichia coli, there were 7 ESBL producers; for Klebsiella spp., there were 16 ESBL producers. EBSL, extended-spectrum β-lactamase.
TABLE 4.
Activity of ciprofloxacin and gentamicin against cefepime-, imipenem- or meropenem-, and piperacillin-tazobactam-resistant Gram-negative bacteria associated with infectiona
| Antibiotic with resistance | No. of resistant isolates | No. of isolates (%) susceptible to: |
|
|---|---|---|---|
| Ciprofloxacin | Gentamicin | ||
| Cefepime | 126 | 23 (18.3) | 49 (38.9) |
| Imipenem or meropenem | 78 | 20 (25.6) | 34 (43.6) |
| Piperacillin-tazobactam | 155 | 56 (36.1) | 90 (58.1) |
Comparison of ciprofloxacin and gentamicin susceptibility for the resistant isolates.
TABLE 5.
Appropriateness of various antibiotic combinations against Gram-negative pathogens in the study cohorta
| Antibiotic | % Susceptible to at least one antibiotic plus: |
||
|---|---|---|---|
| None | Ciprofloxacin | Gentamicin | |
| Cefepime | 83.4 | 86.4 | 89.9 |
| Imipenem or meropenem | 89.7 | 92.4 | 94.2 |
| Piperacillin-tazobactam | 79.6 | 87.0 | 91.4 |
FIG. 1.
Percent of patients receiving inappropriate initial antimicrobial therapy (IIAT) according to combination antimicrobial treatment. Other combination antimicrobial therapy included double β-lactam (non-carbapenem) combinations (n = 33), β-lactam carbapenem combinations (n = 16), and combinations including either tigecycline or colistin (n = 5).
Specific individual pathogens. (i) Pseudomonas aeruginosa.
Among the 132 isolates, 41 (31.1%) received IIAT. Seventeen of these isolates were initially treated with ciprofloxacin (n = 8), gentamicin (n = 5), or another antibiotic (n = 4) as the combination agent. For the 36 isolates not treated with gentamicin, 28 (77.8%) were susceptible to gentamicin and would have received appropriate therapy if gentamicin were part of the empirical regimen.
(ii) Acinetobacter species.
Among the 63 isolates, 44 (69.8%) received IIAT. Sixteen of these isolates were initially treated with a combination regimen that included ciprofloxacin (n = 4), gentamicin (n = 4), or another antibiotic (n = 8) as the combination agent. For the 40 isolates not treated with gentamicin, 9 (22.5%) were susceptible to gentamicin and would have received appropriate therapy if gentamicin were part of the empirical regimen.
(iii) Escherichia coli.
Among the 232 isolates, 41 (17.7%) received IIAT. Nine of these isolates were initially treated with a combination regimen that included ciprofloxacin (n = 3), gentamicin (n = 4), or another antibiotic (n = 2) as the combination agent. For the 37 isolates not treated with gentamicin, 29 (78.4%) were susceptible to gentamicin and would have received appropriate therapy if gentamicin were part of the empirical regimen.
Outcomes and multivariate analysis.
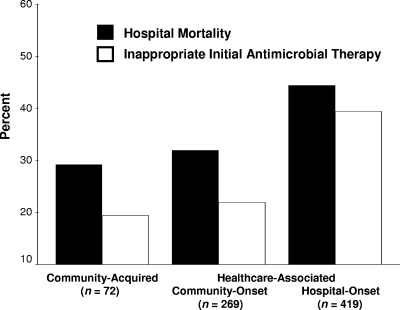
A total of 313 (41.2%) patients died during hospitalization. Hospital mortality was statistically greater for patients receiving IIAT compared to those treated with appropriate initial therapy (51.7% versus 36.4%; P < 0.001). Among the 238 patients receiving IIAT, those switched to definitive therapy within 48 h of having their cultures drawn had a statistically lower risk of hospital mortality compared to those whose therapy was not switched (44.8% versus 70.3%; P < 0.001). Hospital mortality and IIAT were statistically greatest for patients with healthcare-associated hospital-onset infections (Fig. 2). Patients with health care-associated community-onset bacteremia had statistically greater APACHE II scores compared to patients with healthcare-associated hospital-onset bacteremia (24.7 ± 6.5 versus 23.4 ± 6.6; P = 0.012). Patients with healthcare-associated community-onset bacteremia had statistically lower rates of infection with Acinetobacter species (5.9% versus 10.5%; P = 0.039), Achromobacter species (0.4% versus 2.6%; P = 0.034), Serratia marcescens (1.9% versus 6.0%; P = 0.010) and statistically higher rates of infection with non-ESBL-producing Escherichia coli (37.2% versus 19.6%; P < 0.001) and Proteus mirabilis (8.2% versus 2.9%; P = 0.002) compared to patients with healthcare-associated hospital-onset bacteremia. Logistic regression analysis identified IIAT (AOR, 2.30; 95% confidence interval [CI] = 1.89 to 2.80) and increasing APACHE II scores (1-point increments) (adjusted odds ratio [AOR], 1.11; 95% CI = 1.09 to 1.13) as independent predictors for hospital mortality (Hosmer-Lemeshow goodness-of-fit test: 0.655).
FIG. 2.
Hospital mortality and inappropriate initial antimicrobial therapy (IIAT) according to classification of infection source. (P < 0.001 for differences in hospital mortality and IIAT).
The overall occurrence of renal toxicity was 14.5% (n = 110). The renal toxicity was similar for patients receiving combination therapy and monotherapy (17.5% versus 12.7%; P = 0.075). There was a significant increase in renal toxicity for patients receiving combination therapy with an aminoglycoside compared to those not receiving an aminoglycoside (22.3% versus 13.6%; P = 0.014), although the need for renal replacement therapy between these groups was not significantly different (2.5% versus 3.1%; P = 1.0). The overall occurrence of CDAD was 8.3%. CDAD developed in 8.2% of patients receiving combination therapy and 8.3% of patients receiving monotherapy (P = 0.933).
DISCUSSION
Our study demonstrated that IIAT is common among patients with Gram-negative bacteremia complicated by severe sepsis or septic shock, especially for healthcare-associated hospital-onset infections. Patients receiving IIAT had a statistically greater risk for hospital mortality presumably due, at least in part, to the delay in initiating appropriate antimicrobial treatment. We also showed that the addition of an antipseudomonal fluoroquinolone (ciprofloxacin) or an aminoglycoside (gentamicin) to either imipenem or meropenem, piperacillin-tazobactam, or cefepime increased overall susceptibility of the antimicrobial regimens for the Gram-negative bacteria associated with severe sepsis or septic shock. Furthermore, combination therapy with an aminoglycoside resulted in greater overall appropriateness of the antibiotic regimens compared to combination therapy that included an antipseudomonal fluoroquinolone.
Other investigators have attempted to evaluate the role of combination antimicrobial treatment on the outcomes of patients with serious infections attributed to Gram-negative bacteria. The Canadian Trials Group compared a strategy of combination antimicrobial therapy to a strategy of monotherapy with broad-spectrum antibiotics for suspected late-onset ventilator-associated pneumonia (15). Patients were allocated to receive meropenem and ciprofloxacin or meropenem alone. There was no difference in 28-day mortality between the combination and monotherapy groups. The duration of intensive care unit and hospital stay, clinical and microbiological treatment response, emergence of antibiotic-resistant bacteria, isolation of Clostridium difficile in stool, and fungal colonization were also similar in the two groups. However, in a subgroup of patients who had infection due to Pseudomonas species, Acinetobacter species, and multidrug-resistant Gram-negative bacteria at enrollment, the appropriateness of initial antibiotics (84.2% versus 18.8%, P < 0.001) and microbiological eradication of infecting organisms (64.1% versus 29.4%, P = 0.05) were statistically higher in the combination group compared to the monotherapy group.
Beardsley et al. also evaluated patients with hospital-acquired pneumonia in the intensive care units of a teaching hospital (6). These investigators found that the addition of an antipseudomonal fluoroquinolone did not increase the cumulative susceptibility of cefepime, piperacillin-tazobactam, or meropenem compared to using those agents alone (cumulative susceptibility of 81 to 83% compared to 80 to 82% with the addition of a fluoroquinolone). However, the addition of amikacin to either cefepime, piperacillin-tazobactam, or meropenem increased the cumulative susceptibility of Gram-negative bacteria to 96%. A similar finding was made by Trouillet et al., who showed that specific combinations of antimicrobials were more likely to provide coverage of Gram-negative bacteria causing ventilator-associated pneumonia compared to other combinations (31). Specifically, a combination of a carbapenem with an aminoglycoside was most likely to provide appropriate treatment.
Paul et al. performed a meta-analysis of 64 clinical studies with 7,586 patients comparing monotherapy to combination antibiotic treatment for sepsis attributed to Gram-negative bacteria (27). These investigators found no difference in hospital mortality or the development of antibiotic resistance. The addition of an aminoglycoside was associated with a greater risk of nephrotoxicity. However, most studies in their review used multiple-day administration of aminoglycosides for the complete duration of antibiotic therapy as opposed to once-daily administration which has been associated with less nephrotoxicity (5). Moreover, the same investigators found no advantage in febrile neutropenia when combination therapy was used (28). A major limitation of the studies in these two meta-analyses is that they typically had small numbers of patients infected with potentially antibiotic-resistant bacteria, thereby limiting any potential benefit from combination antimicrobial therapy.
More recent studies have also attempted to evaluate the potential benefit of combination therapy with an aminoglycoside for patients infected with Gram-negative bacteria. Two large observational studies found no survival advantage of combination therapy over monotherapy with a β-lactam for Gram-negative bacteremia (12, 23). However, Al-Hasan et al. showed that combination therapy that included a β-lactam and fluoroquinolone was associated with a survival advantage in less severely ill patients with Gram-negative bacteremia but not in critically ill patients with Gram-negative bacteremia (1). Micek et al. studied 305 patients with Pseudomonas aeruginosa bloodstream infection and found that combination antimicrobial therapy was more often appropriate compared to monotherapy (25). In addition, use of an aminoglycoside as the combination agent was more often associated with initially appropriate treatment compared to using a fluoroquinolone. Similar findings were demonstrated in patients with Pseudomonas aeruginosa bacteremia where the use of combination antimicrobial therapy as empirical treatment was associated with better 30-day survival compared to empirical monotherapy (8).
Although the findings in the medical literature are mixed, there is a strong suggestion that combination antimicrobial therapy may improve clinical outcomes for patients with serious Gram-negative bacterial infections if combination therapy is associated with more appropriate initial antibiotic administration. This hypothesis is further supported by a recent clinical study showing improvements in the clinical outcomes of patients with severe sepsis and septic shock when combination antibiotic regimens were used as empirical treatment in the emergency department setting (26). The challenge for clinicians is to identify which combination regimens would be most effective locally. This requires local Gram-negative bacterial susceptibility data to identify whether the addition of a fluoroquinolone or aminoglycoside will significantly improve coverage over the use of β-lactam or carbapenem monotherapy (6).
Our study has several important limitations. First, the retrospective nature of the study precludes any definitive assessment of causality between combination antimicrobial therapy and improved survival. Second, the study was performed at a single center and the results may not be applicable at other hospitals. Indeed, several studies suggest that it is important to assess local antibiotic susceptibility patterns of Gram-negative bacteria in order to identify optimal empirical antibiotic regimens (1, 6, 12, 25). In addition, the local presence of highly resistant strains of Gram-negative bacteria may require the empirical use of alternative combination regimens, including tigecycline or colistin, to maximize appropriate treatment (29). Third, we limited our study to antibiotics routinely used at our hospital. Therefore, our study does not provide information on other aminoglycosides (amikacin and tobramycin) and how they would influence the administration of appropriate therapy. Finally, the availability of several new antibiotics with Gram-negative activity (doripenem and tigecycline) were not in general use during the study period.
Another important limitation of our study was the definition of appropriate antimicrobial treatment that we used. This definition was based on in vitro susceptibility testing alone. It is not clear that aminoglycoside monotherapy should be considered appropriate treatment for patients with bacteremic sepsis despite the presence of in vitro sensitivity. This is due to the lack of clinical data supporting such treatment for serious bacterial infections, with the one exclusion possibly being urinary tract infections (32). Similarly, the lack of clinical data supporting the use of aminoglycoside monotherapy in the setting of neutropenia is another limitation of this definition (28). Another shortcoming of our definition for appropriate antimicrobial treatment is that aminoglycosides do not kill intracellular bacteria such as Salmonella despite the presence of in vitro susceptibility (17). Nevertheless, this definition has been shown to correlate with patient outcomes in studies examining a variety of infections (21).
In conclusion, our study suggests that the use of combination antimicrobial therapy, especially when an aminoglycoside is used as the combination agent, is associated with more appropriate initial therapy of patients with severe sepsis and septic shock due to Gram-negative bacteremia. It would appear reasonable to consider empirical combination antimicrobial therapy directed against Gram-negative bacteria for critically ill patients with severe sepsis or septic shock. This recommendation would be strongest for patients with recent antibiotic exposure or the local presence of antibiotic resistance among Gram-negative bacteria commonly associated with severe sepsis and septic shock. The selection of empirical antibiotic regimens, including combination therapy directed against Gram-negative bacteria, should be based on local patterns of antimicrobial susceptibility.
Acknowledgments
This work was funded in part by the Barnes-Jewish Hospital Foundation.
Footnotes
Published ahead of print on 16 February 2010.
REFERENCES
- 1.Al-Hasan, M. N., J. W. Wilson, B. D. Lahr, K. M. Thomsen, J. E. Eckel-Passow, E. A. Vetter, I. M. Tleyjeh, and L. M. Baddour. 2009. Beta-lactam and fluoroquinolone combination antibiotic therapy for bacteremia caused by gram-negative bacilli. Antimicrob. Agents Chemother. 53:1386-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Thoracic Society and Infectious Diseases Society of America. 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171:388-416. [DOI] [PubMed] [Google Scholar]
- 3.Angus, D. C., W. T. Linde-Zwirble, J. Lidicker, G. Clermont, J. Carcillo, and M. R. Pinsky. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29:1303-1310. [DOI] [PubMed] [Google Scholar]
- 4.Arias, C. A., and B. E. Murray. 2009. Antibiotic-resistant bugs in the 21st century: a clinical super-challenge. N. Engl. J. Med. 360:439-443. [DOI] [PubMed] [Google Scholar]
- 5.Barza, M., J. P. Ioannidis, J. C. Cappelleri, and J. Lau. 1996. Single or multiple daily doses of aminoglycosides: a meta-analysis. BMJ. 312:338-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beardsley, J. R., J. C. Williamson, J. W. Johnson, C. A. Ohl, T. B. Karchmer, and D. L. Bowton. 2006. Using local microbiologic data to develop institution-specific guidelines for the treatment of hospital-acquired pneumonia. Chest 130:787-793. [DOI] [PubMed] [Google Scholar]
- 7.Boucher, H. W., G. H. Talbot, J. S. Bradley, J. E. Edwards, D. Gilbert, L. B. Rice, M. Scheld, B. Spellberg, and J. Bartlett. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1-12. [DOI] [PubMed] [Google Scholar]
- 8.Chamot, E., E. Boffi El Amari, P. Rohner, and C. Van Delden. 2003. Effectiveness of combination antimicrobial therapy for Pseudomonas aeruginosa bacteremia. Antimicrob. Agents Chemother. 47:2756-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dellinger, R. P., M. M. Levy, J. M. Carlet, J. Bion, M. M. Parker, R. Jaeschke, K. Reinhart, D. C. Angus, C. Brun-Buisson, R. Beale, T. Calandra, J. F. Dhainaut, H. Gerlach, M. Harvey, J. J. Marini, J. Marshall, M. Ranieri, G. Ramsay, J. Sevransky, B. T. Thompson, S. Townsend, J. S. Vender, J. L. Zimmerman, and J. L. Vincent. 2008. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit. Care Med. 36:296-327. [DOI] [PubMed] [Google Scholar]
- 10.Dhainaut, J. F., P. F. Laterre, S. P. LaRosa, H. Levy, G. E. Garber, D. Heiselman, G. T. Kinasewitz, R. B. Light, P. Morris, R. Schein, J. B. Sollet, B. M. Bates, B. G. Utterback, and D. Maki. 2003. The clinical evaluation committee in a large multicenter phase 3 trial of drotrecogin alfa (activated) in patients with severe sepsis (PROWESS): role, methodology, and results. Crit. Care Med. 31:2291-2301. [DOI] [PubMed] [Google Scholar]
- 11.Dupont, H., H. Mentec, J. P. Sollet, and G. Bleichner. 2001. Impact of appropriateness of initial antibiotic therapy on the outcome of ventilator-associated pneumonia. Intensive Care Med. 27:355-362. [DOI] [PubMed] [Google Scholar]
- 12.Freundlich, M., R. W. Thomsen, L. Pedersen, H. West, and H. C. Schønheyder. 2007. Aminoglycoside treatment and mortality after bacteraemia in patients given appropriate empirical therapy: a Danish hospital-based cohort study. J. Antimicrob. Chemother. 60:1115-1123. [DOI] [PubMed] [Google Scholar]
- 13.Garnacho-Montero, J., J. L. Garcia-Garmendia, A. Barrero-Almodovar, F. J. Jimenez-Jimenez, C. Perez-Paredes, and C. Ortiz-Leyba. 2003. Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Crit. Care Med. 31:2742-2751. [DOI] [PubMed] [Google Scholar]
- 14.Harbarth, S., J. Garbino, J. Pugin, J. A. Romand, D. Lew, and D. Pittet. 2003. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am. J. Med. 115:529-535. [DOI] [PubMed] [Google Scholar]
- 15.Heyland, D. K., P. Dodek, J. Muscedere, A. Day, D. Cook, et al. 2008. Randomized trial of combination versus monotherapy for the empiric treatment of suspected ventilator-associated pneumonia. Crit. Care Med. 36:737-744. [DOI] [PubMed] [Google Scholar]
- 16.Ibrahim, E. H., G. Sherman, S. Ward, S. V. J. Fraser, and M. H. Kollef. 2000. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118:146-155. [DOI] [PubMed] [Google Scholar]
- 17.Kihlstrom, E., and L. Andaker. 1985. Inability of gentamicin and fosfomycin to eliminate intracellular Enterobacteriaceae. J. Antimicrob. Chemother. 15:723-728. [DOI] [PubMed] [Google Scholar]
- 18.Klevens, R. M., M. A. Morrison, J. Nadle, S. Petit, K. Gershman, S. Ray, L. H. Harrison, R. Lynfield, G. Dumyati, J. M. Townes, A. S. Craig, E. R. Zell, G. E. Fosheim, L. K. McDougal, R. B. Carey, and S. K. Fridkin. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763-1771. [DOI] [PubMed] [Google Scholar]
- 19.Knaus, W. A., E. A. Draper, D. P. Wagner, and J. E. Zimmerman. 1985. APACHE II: a severity of disease classification system. Crit. Care Med. 13:818-829. [PubMed] [Google Scholar]
- 20.Kollef, M. H., G. Sherman, S. Ward, and V. J. Fraser. 1999. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 115:462-474. [DOI] [PubMed] [Google Scholar]
- 21.Kollef, M. H. 2008. Broad-spectrum antimicrobials and the treatment of serious bacterial infections: getting it right up front. Clin. Infect. Dis. 47:S3-S13. [DOI] [PubMed] [Google Scholar]
- 22.Kollef, M. H. 2006. Providing appropriate antimicrobial therapy in the intensive care unit: surveillance versus de-escalation. Crit. Care Med. 34:903-905. [DOI] [PubMed] [Google Scholar]
- 23.Leibovici, L., M. Paul, O. Poznanski, M. Drucker, Z. Samra, H. Konigsberger, and S. D. Pitlik. 1997. Monotherapy versus beta-lactam-aminoglycoside combination treatment for gram-negative bacteremia: a prospective, observational study. Antimicrob. Agents Chemother. 41:1127-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Micek, S. T., W. Isakow, W. Shannon, and M. H. Kollef. 2005. Predictors of hospital mortality for patients with severe sepsis treated with drotrecogin alfa (activated). Pharmacotherapy 25:26-34. [DOI] [PubMed] [Google Scholar]
- 25.Micek, S. T., A. E. Lloyd, D. J. Ritchie, R. M. Reichley, V. J. Fraser, and M. H. Kollef. 2005. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob. Agents Chemother. 49:1306-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Micek, S. T., N. Roubinian, T. Heuring, M. Bode, J. Williams, C. Harrison, T. Murphy, D. Prentice, B. E. Ruoff, and M. H. Kollef. 2006. Before-after study of a standardized hospital order set for the management of septic shock. Crit. Care Med. 34:2707-2713. [DOI] [PubMed] [Google Scholar]
- 27.Paul, M., I. Silbiger, S. Grozinsky, K. Soares-Weiser, and K. Leibovici. 2006. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst. Rev. 1:CD003344. [DOI] [PubMed] [Google Scholar]
- 28.Paul, M., K. Soares-Weiser, and L. Leibovici. 2003. Beta lactam monotherapy versus beta lactam-aminoglycoside combination therapy for fever with neutropenia: systematic review and meta-analysis. BMJ 326:1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrosillo, N., E. Ioannidou, and M. E. Falagas. 2008. Colistin monotherapy versus combination therapy: evidence from microbiological, animal and clinical studies. Clin. Microbiol. Infect. 14:816-827. [DOI] [PubMed] [Google Scholar]
- 30.Thiel, S. W., M. F. Asghar, S. T. Micek, R. M. Reichley, J. A. Doherty, and M. H. Kollef. 2009. Hospital-wide impact of a standardized order set for the management of bacteremic severe sepsis. Crit. Care Med. 37:819-824. [DOI] [PubMed] [Google Scholar]
- 31.Trouillet, J. L., J. Chastre, A. Vuagnat, M. L. Joly-Guillou, D. Combaux, M. C. Dombret, and C. Gibert. 1998. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am. J. Respir. Crit. Care Med. 157:531-539. [DOI] [PubMed] [Google Scholar]
- 32.Vidal, L., A. Gafter-Gvili, S. Borok, A. Fraser, and M. Paul. 2007. Efficacy and safety of aminoglycoside monotherapy: systematic review and meta-analysis of randomized controlled trials. J. Antimicrob. Chemother. 60:247-257. [DOI] [PubMed] [Google Scholar]