Abstract
The study presented here was performed to determine the pharmacokinetics of intravenously administered clindamycin in pregnant women. Seven pregnant women treated with clindamycin were recruited. Maternal blood and arterial and venous umbilical cord blood samples were obtained. Maternal clindamycin concentrations were analyzed by nonlinear mixed-effects modeling with the NONMEM program. The data were best described by a linear three-compartment model. The clearance and the volume of distribution at steady state were 10.0 liters/h and 6.32 × 103 liters, respectively. Monte Carlo simulations were performed to determine the area under the concentration curve (AUC) for the free (unbound) drug (f) in maternal serum for 24 h divided by the MIC (fAUC0-24/MIC). At a MIC of 0.5 mg/liter, which is the EUCAST breakpoint, the attainment at the lower 95% confidence interval (CI) was 24.6 if the level of protein binding was 65%, and this value concurred well with the target value of 27. However, for higher degrees of protein binding, as has been described in the literature, the attainment was lower, down to 10.2 for a protein binding level of 85% (lower 95% CI). The concentrations in umbilical cord blood were lower than those in maternal blood. The concentration-time profiles in maternal serum indicate that the level of exposure to clindamycin may be too low in these patients. Together with the lower concentrations in umbilical cord blood, this finding suggests that the current dosing regimen may not be adequate to protect all neonates from group B streptococcal disease.
Group B streptococci (GBS) are still a major cause of infectious morbidity and mortality in neonates (29). To prevent neonatal GBS disease, antibiotics are administered during delivery to pregnant women at increased risk. Beta-lactam antibiotics are the antibiotics of first choice. Unfortunately, approximately 8 to 10% of people report allergies to penicillins (17, 20). Most of these patients are treated with clindamycin (20).
In nonpregnant individuals, it has been shown that clindamycin distributes widely throughout the body. It is metabolized and subsequently excreted into the urine and bile. The level of protein binding in nonpregnant humans ranges from 62% to 94% (9, 13, 16, 18, 32). During pregnancy and labor, physiological changes that may modify the pharmacokinetics (PKs) of drugs occur. The antibiotics administered to prevent neonatal GBS disease reach the fetus indirectly via the mother, and therefore, it is essential that data on the pharmacokinetics of clindamycin during labor in the mother and on the transfer of drugs over the placental barrier are available.
Studies of the pharmacokinetics of drugs during labor face considerable ethical and logistical difficulties, limiting the possibility of collection of blood samples and thereby hampering noncompartmental data analysis. Fortunately, these limitations may be overcome by application of powerful approaches to the analysis of sparse data. Specifically, nonlinear mixed-effects modeling (NONMEM) allows weighted analysis of data sets, including large, complete data sets as well as small or incomplete data sets (3, 31). A more detailed background on population modeling can be found elsewhere (4, 30). Monte Carlo simulation (MCS) is used to evaluate the probability that different dosing schedules will achieve therapeutic concentrations. MCS is performed by using PK parameter values and data on the dose-effect relationship and interindividual variability (2, 6, 7, 22-24). The relationship between pharmacokinetic properties, the susceptibility of the microorganism (as reflected in the MIC), and clinical effects is increasingly well understood. Specifically, the efficacy of clindamycin is primarily correlated to the area under the concentration curve (AUC) for the free (unbound) drug (f) in maternal serum for 24 h divided by the MIC (fAUC0-24/MIC). The objective of the study presented here was to describe the pharmacokinetics of intravenously administered clindamycin in pregnant women in the perinatal period and the transfer of clindamycin over the placental barrier. Furthermore, the probability that therapeutic concentrations will be achieved was evaluated by using the dosing regimen currently prescribed for this special population.
MATERIALS AND METHODS
Patients.
Between 7 February 2005 and 28 February 2007, all women from 26 weeks of gestation who needed antibiotic treatment with clindamycin were eligible for this study. Following local guidelines, all women with proven or unknown GBS carriage status were treated with antibiotics when the pregnancy was complicated by one or more of the following six factors: preterm premature rupture of the membranes, rupture of the membranes for >18 h, prematurity, fever (temperature, >37.8°C), bacteriuria in the current pregnancy, or a previous delivery of a child with invasive GBS disease. From November 2005 on, all drugs were electronically prescribed by the treating physicians, and from the resulting data from the pharmacy, the numbers of patients who received clindamycin intravenously in the delivery room or the maternity ward were recorded. The choice of the antibiotic used in this study was dictated by local guidelines, which recommend the use of clindamycin in the case of penicillin allergy for the prevention of GBS disease. In addition, patients without risk factors for neonatal GBS disease but with an increased risk of endocarditis received clindamycin approximately 1 h before delivery.
The study was approved by the Medical Ethics Committee of the Medical Center Haaglanden, The Hague, Netherlands. Written informed consent was obtained from all patients. Women were excluded from the study if they had been treated with oral or intramuscular antibiotics within 2 days before the start of therapy. All patients were at least 18 years of age.
All patients received a standard examination that included a medical history (operations, illnesses, allergies) and biochemical and hematological examinations (complete blood count, renal and liver function tests) at the onset of the study. Furthermore, blood pressure, pulse, oral temperature, and body weight were recorded before antibiotic administration.
Drug administration and blood sampling.
Before the administration of clindamycin, two intravenous catheters were placed, one in each arm. Clindamycin was administered through the first catheter, according to the local guidelines of the hospital. The dose of 600 mg, as prescribed for the prevention of endocarditis, was administered over 20 min (12 mg/ml 0.9% NaCl) every 6 h. The dose of 900 mg, which is used for the prevention of GBS disease, was administered over 30 min (9 mg/ml 0.9% NaCl) every 8 h. The exact duration of infusion was recorded.
Blood samples of 2 ml were collected from the second catheter in the contralateral arm at timed intervals beginning at 1 min after the start of the infusion and at 10 and 20 min (for the 600-mg infusion) or 15 and 30 min (for the 900-mg infusion). After completion of the infusion, sampling was scheduled at 3, 6, 10, 16, and 36 min and then every 30 to 45 min thereafter until the next antibiotic dosage. The need to prevent physical and emotional inconvenience to the woman sometimes precluded sampling at the desired time. Consequently, the time of blood sampling occasionally deviated from the scheduled time or blood sampling at the scheduled time had to be skipped altogether. Therefore, the exact sampling times were always recorded. Immediately after birth, both arterial and venous umbilical cord blood samples were obtained.
The blood samples were placed immediately on ice, allowed to clot for a minimum of 15 min, and processed within 1 h after collection. The samples were centrifuged at 1,200 × g for approximately 10 min at room temperature. The supernatants were transferred into plastic storage tubes and frozen at −70°C until analysis.
Clindamycin high-pressure liquid chromatography (HPLC).
The samples were extracted by adding 0.05 ml sodium hydroxide (1.2 M) containing 40 mg/liter propranolol as the internal standard. The sample was vortexed, and 2.6 ml dichloromethane (Sigma-Aldrich, Netherlands) was then added. After the samples were thoroughly vortexed, the solution was centrifuged at 1,500 × g for 5 min. The supernatant was then removed and 2 ml was pipetted into a new vial. Subsequently, the contents were dried at 40°C under an airflow of 5 liters/min and finally resolved in 0.2 ml potassium dihydrophosphate (pH 4.6).
Clindamycin concentrations were determined by HPLC (Shimadzu, Den Bosch, Netherlands) with a column heater set at 40°C. A reverse-phase method (0.066 M potassium dihydrophosphate, pH 4.6, with 20% acetonitrile) and a C18 column (Bester, Amstelveen, Netherlands) with a UV-visible detector and a wavelength of 200 nm were used. The run time was 10 min, the injection volume was 0.05 ml, and the flow was set at 1 ml/min. A standard calibration curve was determined for clindamycin (Sigma-Aldrich) during each run by using the peak height as a correlate. The lower limit of detection and quantification was 0.1 mg/liter, and the assay was linear up to 50 mg/liter. The between-sample between-day coefficient of variation (CV) was <5%.
Pharmacokinetic analysis.
A pharmacokinetic model of the maternal venous serum concentrations was determined. Pharmacokinetic parameters were estimated by the use of NONMEM. The model was implemented in the NONMEM ADVAN11 subroutine, and the analysis was performed by the first-order conditional estimation (FOCE) method with the INTERACTION option. All fitting procedures were performed with Visual FORTRAN (standard edition 6.6; Compaq Computer Cooperation, Euston, TX) and the NONMEM software package (version VI, release 2; ICON Development Solutions, Ellicott City, MD).
To determine the basic structural pharmacokinetic parameters, various two- and three-compartment models were tested. Model selection and identification of variability were based on the evaluation of the mean objective function value (MOFV), pharmacokinetic parameter point estimates and their respective confidence intervals, and goodness-of-fit plots. To detect significant differences between two structural models, MOFV with a prespecified level of significance (P < 0.001) was used (this level of significance corresponds to a difference in MOFVs of at least 10.8 points). NONMEM minimizes an objective function when nonlinear regression analysis is performed. To detect systematic deviations in the model fits, the goodness-of-fit plots were visually inspected. Data for individual observations versus individual or population predictions should be randomly distributed around the line of identity. The weighted residuals versus time or population predictions should be randomly distributed around zero. Population values were estimated for the parameters clearance (CL), volume of distribution (V), and intercompartmental clearance (Q). The volume of distribution at steady state (Vss) was calculated by standard procedures (11). The uncertainty in the population parameters (CV) was estimated in NONMEM by implementation of the $covariance step.
Individual estimates of the pharmacokinetic parameters were assumed to follow a log-normal distribution. Therefore, an exponential distribution model was used to account for interindividual variability. A possible correlation between interindividual variability coefficients on parameters was estimated, and if they were present, they were accounted for in the stochastic model (NONMEM Omega block option).
The residual error term contains all the remaining error terms which cannot be explained by the structural model or interindividual variability and refers to, for example, measurement and experimental errors and structural model misspecification. Selection of an appropriate residual error model was based on evaluation of the MOFVs and inspection of the goodness-of-fit plots. A proportional error model, an additive error model, and a combined proportional-additive error model were tested to describe the residual variability between the observed concentrations and those predicted by the model. Furthermore, an exploratory covariate analysis was performed (significance level, 0.05).
The precision of the final population model for the entire population was established by using the bootstrap option in the NONMEM program, which consists of repeated random sampling with replacement from the original data (1,000 times). The parameters estimated from the bootstrap analysis were compared to the estimates from the original data. A visual predictive check was also performed.
The mean pharmacokinetic estimates of the final model derived from the PK analysis in NONMEM were used in the Berkeley Madonna software program (version 8.3.5; Berkeley Madonna Inc., University of California) to construct the mean concentration-time profile after administration of the 900-mg clindamycin dose in pregnant women during labor. These maternal concentrations were used to calculate the ratios of the umbilical cord concentrations and the corresponding maternal concentrations. For clindamycin, the ratio for the total drug concentration should be at least 147, whereas for free drug concentrations, this ratio should be at least 27 (5).
Monte Carlo simulation.
The estimates of the pharmacokinetic parameters and measures of dispersion were used to simulate various dosing regimens and obtain fAUC0-24/MIC ranges as a function of the MIC (24). In the literature, the values for the level of protein binding of clindamycin vary from 62% to 94% (9, 13, 16, 18, 32). MCS was performed with the MICLAB (version 3.07) program (Medimatics, Maastricht, Netherlands) and by performing a simulation with 5,000 subjects for each regimen. The program allows inclusion of the covariance matrix (or correlation matrix) of the parameter estimates used in the simulations. The output consisted of a probability distribution, a cumulative probability distribution, and specific confidence intervals over user-defined MIC and fAUC0-24/MIC ranges.
RESULTS
Between November 2005 and March 2007, intravenously administered clindamycin was prescribed to 10 patients, according to the pharmacy data. In total, 7 patients were included over the entire study period. Of these patients, four received clindamycin for the prevention of neonatal GBS disease and two received clindamycin to prevent endocarditis in the mother. None of the patients was clinically ill. One patient needed antibiotics to prevent both neonatal GBS disease and endocarditis. Six patients with singleton pregnancies were in labor. One patient was treated because of preterm premature rupture of the membranes and had a twin pregnancy. The characteristics of the study population are presented in Table 1. Of the three patients receiving clindamycin for endocarditis prophylaxis, one had a minor stenosis of the mitral valve. The second patient had a prosthetic aortic valve and a prosthetic mitral valve, and her tricuspidal valve had been operated on in the past. The third patient had a dysfunction of the aortic valve and autoimmune thrombocytopenic purpura, which had resulted in a splenectomy.
TABLE 1.
Population characteristics
| Dataa | Units | Mean | SD | Range | No. of patients |
|---|---|---|---|---|---|
| Maternal age | yr | 36.1 | 4.24 | 31.3-41.8 | 7 |
| Gestational age | wk | 38.3 | 3.01 | 34-42.3 | 7 |
| Body mass index | kg/m2 | 32.1 | 5.36 | 22.1-39.1 | 7 |
| Wt | kg | 86.1 | 14.2 | 59.5-104.8 | 7 |
| Edema (none/around the ankle/up to knee) | No. of patients | 5/0/2 | |||
| Temp | °C | 36.9 | 0.23 | 36.7-37.4 | 7 |
| Creatinine concn | μmol/liter | 55.9 | 20.3 | 38-100 | 7 |
| ALP | U/liter | 495 | 601 | 168-1,794 | 7 |
| AST | U/liter | 22.6 | 6.78 | 15-34 | 7 |
| ALT | U/liter | 10.9 | 5.27 | 5-18 | 7 |
| Vaginal/secondary cesarean mode of delivery | No. of patients | 4/3 |
ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
A total of 177 samples were included in the study. Two samples were excluded because the concentrations were <0.1 mg/liter. For one patient, it was not possible to place each of the two catheters in a separate arm. Both catheters were therefore placed in the right arm. The catheter used for the clindamycin infusion was flushed after clindamycin administration and occluded. The data obtained with the samples collected from this patient during the clindamycin infusion were excluded from the analysis. In three patients, a dose of clindamycin was also administered in the postpartum period. These data were also included in the analysis. Samples were obtained from each patient for periods that ranged from 0.95 h to 29.53 h.
Both arterial and venous umbilical cord blood samples were taken from all six patients in labor. The data are shown in Table 2. The ratios of the venous umbilical cord blood concentrations and simultaneous maternal blood concentrations ranged from 0.22 to 0.89, and there was one extreme value of 1.59. Ratios of arterial umbilical cord blood concentrations and simultaneous maternal blood concentrations ranged from 0.48 to 0.67, and there was one extreme value of 1.59.
TABLE 2.
Clindamycin concentrations in arterial and venous umbilical cord blood and maternal blood
| Patient | Clindamycin dose (mg) | Time between start of infusion and sampling (h) | Time between maternal peak concn and sampling (h) | Concn (mg/liter) |
||
|---|---|---|---|---|---|---|
| Arterial cord blood | Venous cord blood | Maternal blooda | ||||
| 1 | 900 | 1.85 | 1.38 | 2.5 | 1.1 | 5.1 |
| 2 | 900 | 4.27 | 3.67 | 0.9 | 1.0 | 1.9 |
| 3 | 900 | 1.45 | 0.85 | 4.0 | 3.3 | 6.0 |
| 4 | 600 | 4.53 | 4.15 | 1.1 | 1.1 | 0.69 |
| 5 | 900 | 10.5 | 10.0 | 0.1 | 0.1 | 0.19 |
| 6 | 600 | 0.95 | 0.65 | 1.7 | 2.7 | 3.0 |
Maternal blood concentrations were determined with the Berkeley Madonna software program by using the mean parameter estimates from the final PK model.
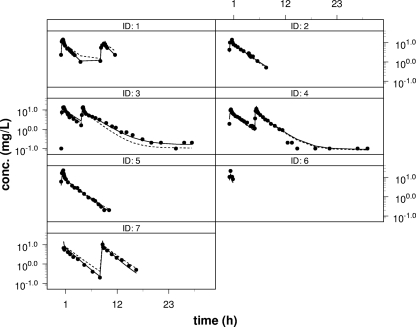
A three-compartment open model best described the maternal data. Due to the limited number of samples obtained from the umbilical cord, the incorporation of umbilical cord concentrations into the maternal pharmacokinetic model was not possible. Eventually, a proportional error model with interindividual variability (omega-sigma interaction option under FOCE in the NONMEM program) was found to be the most adequate for describing the data. The interindividual variability of the PK parameters was explained by variations in CL and volume of distribution of the second peripheral compartment (V3) (Table 3). None of the covariates could improve the model fit. Vss was calculated to be 6.32 × 103 liters, and the gamma-phase half-life (t1/2) was 2.6 h. The final estimates of the pharmacokinetic parameters and their respective CVs and 95% confidence intervals are presented in Table 3. Due to the limited number of patients, the parameter estimates for the interindividual variability for CL and V3 were not statistically significant. The individual plots and the plots of the observed concentrations versus the predicted concentrations are shown in Fig. 1 and 2, respectively.
TABLE 3.
Final estimates of the pharmacokinetic parameters and the respective CVs obtained with the three-compartment model
| Model and parametera | Units | Estimates for all patients |
||
|---|---|---|---|---|
| Mean | % CV | 95% CI | ||
| Structural model | ||||
| CL | liters/h | 10.0 | 37.5 | 2.65-17.35 |
| V1 | liters | 12.4 | 10.6 | 9.83-14.9 |
| V2 | liters | 52.2 | 6.25 | 45.8-58.6 |
| V3 | liters | 6,250 | 22.9 | 3,447-9,052 |
| Q1 | liters/h | 137 | 6.32 | 120-154 |
| Q2 | liters/h | 21.1 | 9.95 | 17.0-25.2 |
| Variance model | ||||
| IIV in CL | 0.292 | 56.2 | −0.0294-0.613 | |
| IIV in V3 | 0.00185 | 156 | −0.00379-0.00749 | |
| IIV in error | 0.306 | 50.0 | 0.00612-0.606 | |
| Residual variability | 0.0424 | 42.5 | 0.00712-0.0777 | |
CL, clearance; V, volume of distribution; V1, volume of distribution of the central compartment; V2, volume of distribution of the first peripheral compartment; V3, volume of distribution of the second peripheral compartment; Q1, intercompartmental clearance from V1 to V2; Q2, intercompartmental clearance from V1 to V3; IIV, interindividual variability.
FIG. 1.
Individual plots with the final three-compartment model. Each black dot represents a measured concentration. The solid line represents the individual estimate, and the dotted line represents the population estimate. ID, patient identifier.
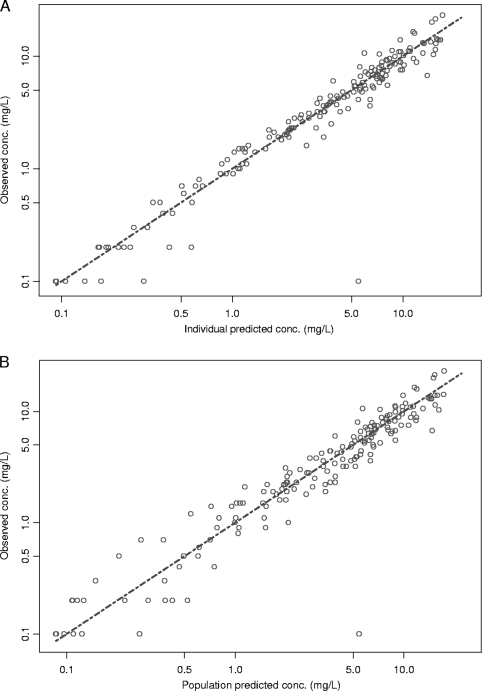
FIG. 2.
Diagnostic plot of the observed versus the individual predicted concentrations (A) and the observed versus the population predicted concentrations (B) determined by use of the final three-compartment model.
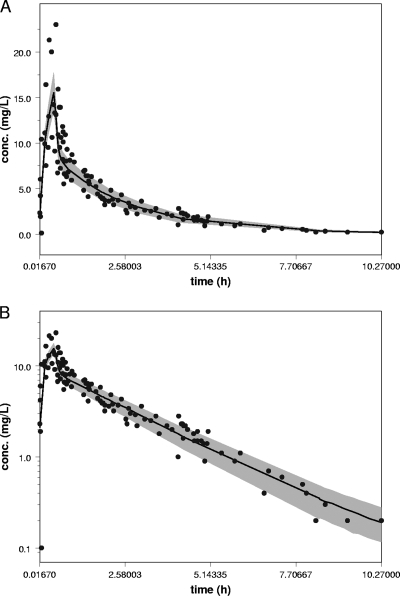
The bootstrap validation of the model was performed with 1,000 runs and was successful for 598 runs. From the mean parameter estimates of the runs obtained from the bootstrap analysis, only the estimate for V3 deviated significantly from the predicted values from the NONMEM PK analysis. The estimates for V3 could not be determined with good precision, because only two patients with prolonged concentration-time profiles were included in the study population. A visual predictive check (VPC) was performed by simulating the 50th percentile for plasma concentrations after the first dose only. Simulations were applied by using the average values for the amount (814 mg) and the infusion rate (1,743 mg/h) for the patient population. The patient data observed after the administration of the first dose only were plotted in the VPC (Fig. 3). The results indicated that the precision of the final model was good.
FIG. 3.
VPC performed by simulating the 50th percentile for plasma concentrations after administration of the first dose only. Simulations were applied by using the average value for the dose (814 mg) and the infusion rate (1,743 mg/h) for the patient population. (A) VPC in linear scale; (B) VPC in semilogarithmic scale.
Table 4 shows the results of the MCS. The data show that at a MIC of 0.5 mg/liter, which is the EUCAST breakpoint (8), the attainment at the lower 95% confidence interval (CI) was 24.6 if the level of protein binding is 65%, and this value concurred well with the target value of 27 (3). However, for higher values of protein binding, as has been described in the literature, the attainment was lower, down to 10.2 for a protein binding level of 85% (lower 95% CI).
TABLE 4.
Results of Monte Carlo simulations obtained by using the population model developed and a dosing regimen of 900 mg every 8 h
| % protein binding |
fAUC0-24/MIC ratio for MIC (μg/ml) ofa: |
|||||||
|---|---|---|---|---|---|---|---|---|
| 0.125 |
0.25 |
0.5 |
1 |
|||||
| Lower 95% CI | Avg | Lower 95% CI | Avg | Lower 95% CI | Avg | Lower 95% CI | Avg | |
| 65 | 102 | 223 | 50.3 | 116 | 24.6 | 55.8 | 11.8 | 27.9 |
| 75 | 73.1 | 159 | 35.8 | 79.4 | 17.3 | 39.7 | 7.94 | 19.8 |
| 85 | 44.2 | 95.4 | 21.6 | 47.7 | 10.2 | 23.9 | 4.44 | 11.9 |
DISCUSSION
The pharmacokinetics of clindamycin in pregnant patients are best described by a three-compartment model. CL and gamma-phase t1/2 were 10.0 liters/h and 2.6 h, respectively. Clindamycin has time-dependent action in vitro, but its clinical efficacy is more closely related to the fAUC0-24/MIC ratio (1, 5). The therapeutic goal required to achieve a static effect is a ratio of at least 27, taking into account the level of clindamycin protein binding. The efficacy of clindamycin might be increased with higher ratios. At the lower degrees of protein binding, the target value for the fAUC0-24/MIC ratio was reached for nearly the entire population; however, at higher degrees of protein binding, the target attainment was too low. The level of exposure to clindamycin may thus be too low in these patients. The distribution of the MICs for the wild type ends at 0.25 mg/liter (www.eucast.org), and wild-type strains therefore seem to be covered by this regimen.
A limitation of the study was the small number of patients studied. Despite the limited number of patients, all structural parameters were estimated with adequate precision (i.e., CV < 51%). The pharmacokinetic parameter estimate for CL for the mothers resulted in a value less than the published values (10.0 liter/h for our study versus values of 19.8 to 26.4 liter/h in the literature [10, 12, 28, 33, 34]). Two previous studies determined the ratio of umbilical cord blood and maternal blood (27, 34). Weinstein et al. found a ratio of 0.46 after intravenous administration during delivery by cesarean section (34). In contrast, Philipson et al. (27) found ratios of 0.18 and 0.25 after administration of an oral dose of clindamycin in women with gestational ages of 10 to 22 weeks during therapeutic abortions. Our values are comparable to the ratios reported by Weinstein et al. (34). The low values reported by Philipson et al. (27) might be explained by the low gestational age.
Clindamycin is mainly bound to alpha1-acid glycoprotein, an acute-phase protein. The level of protein binding is dependent on the concentration of both the alpha1-acid glycoprotein and clindamycin (16). High concentrations of alpha1-acid glycoprotein result in high levels of protein binding (up to 92% at an alpha1-acid glycoprotein concentration of at least 201 mg/dl), whereas due to the nonlinearity in the level of protein binding, an increase in the clindamycin concentration leads to an increase in the level of protein binding (up to 88% at a concentration of 4 mg/liter) (16). In our patients, the percentage of protein binding is unknown, but compared to that found in nonpregnant healthy volunteers, it is likely to be reduced due to the state of pregnancy; but it is possibly increased during labor, stress, or the presence of infection (26). Since only the free unbound fraction of drug is active and the level of plasma protein binding of clindamycin is relatively high, even a minor increase in the level of protein binding might influence its efficacy.
To prevent neonatal GBS disease, both the concentration in maternal serum and the concentration in fetal serum must be adequate. The concentration of alpha1-acid glycoprotein in neonates increases with gestational age (26). As has been shown for alprenolol, the affinity of alpha1-acid glycoprotein might be decreased in the first 7 days of life, partly due to displacement by bilirubin (26). Peak concentrations in the fetus are likely to be lower than the peak concentrations in the mother, as has previously been shown for amoxicillin (25). Arterial umbilical cord blood directly originates from the fetus; therefore, the concentration in arterial umbilical cord blood represents the concentration in the fetus. The number of clindamycin concentrations in the arterial umbilical cord samples measured in our study was relatively low. When the level of protein binding and the interindividual variability are taken into account, it is doubtful whether adequate concentration-time profiles are reached in the fetus. However, the fAUC0-24/MIC ratio used to determine efficacy is based on infection in an animal model rather than prophylaxis. Therefore, values for efficacy in prophylaxis could possibly be lower.
Compared to penicillins, the antibiotics of first choice for the prevention of GBS early-onset disease (EOD), the use of clindamycin has several considerable concerns. Penicillins are bactericidal, whereas clindamycin is bacteriostatic. The resistance of GBS to penicillins has not been reported, but for clindamycin, the proportion of resistant strains varies from 1% to 26% (14, 19, 20). Clindamycin does not reach the cerebrospinal fluid, whereas penicillins achieve concentrations in the cerebrospinal fluid of up to 20% of the concentration in serum in patients with meningitis. For the penicillins, the level of protein binding is low and does not influence their efficacy. The high level of protein binding of clindamycin might well affect its efficacy. Finally, the (repeated) use of clindamycin might be limited because of the benzyl alcohol used in preparations for intravenous administration. The use of benzyl alcohol in neonates is associated with increased rates of mortality and morbidity (15, 21). Safety is crucial, especially when drug treatments are used as prophylactic measures. Since the amount of benzyl alcohol at which toxicity may occur is unknown and benzyl alcohol may cross the placenta, the (repeated) use of intravenously administered clindamycin in pregnant women should be limited to avoid exposure of the fetus to benzyl alcohol.
On the basis of the current data on the PKs of clindamycin and these considerations, we conclude that the level of exposure to clindamycin in this patient population might be too low. In pregnant women, allergy skin testing is a safe procedure. Penicillins can safely be administered to patients with negative skin tests to prevent GBS EOD. In this way, the use of clindamycin for this purpose can be minimized.
Acknowledgments
This work was supported by grant SNO-T-06-31 from the Stichting Nuts Ohra.
Footnotes
Published ahead of print on 22 February 2010.
REFERENCES
- 1.Ambrose, P. G., S. M. Bhavnani, C. M. Rubino, A. Louie, T. Gumbo, A. Forrest, and G. L. Drusano. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79-86. [DOI] [PubMed] [Google Scholar]
- 2.Ambrose, P. G., and D. M. Grasela. 2000. The use of Monte Carlo simulation to examine pharmacodynamic variance of drugs: fluoroquinolone pharmacodynamics against Streptococcus pneumoniae. Diagn. Microbiol. Infect. Dis. 38:151-157. [DOI] [PubMed] [Google Scholar]
- 3.Beal, S. L., L. B. Sheiner, and A. J. Boeckmann. 1989-;-2006. NONMEM user's guides. Icon Development Solutions, Ellicott City, MD.
- 4.Bonate, P. L. 2005. Recommended reading in population pharmacokinetic pharmacodynamics. AAPS J. 7:E363-E373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craig, W. A., S. Kiem, and D. R. Andes. 2002. Free-drug AUC/MIC is the PK-PD target that correlates with in vivo efficacy of macrolides, azilides, ketolides and clindamycin, abstr. A-1264. Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 6.Drusano, G. L., D. Z. D'Argenio, S. L. Preston, C. Barone, W. Symonds, S. LaFon, M. Rogers, W. Prince, A. Bye, and J. A. Bilello. 2000. Use of drug effect interaction modeling with Monte Carlo simulation to examine the impact of dosing interval on the projected antiviral activity of the combination of abacavir and amprenavir. Antimicrob. Agents Chemother. 44:1655-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drusano, G. L., S. L. Preston, C. Hardalo, R. Hare, C. Banfield, D. Andes, O. Vesga, and W. A. Craig. 2001. Use of preclinical data for selection of a phase II/III dose for evernimicin and identification of a preclinical MIC breakpoint. Antimicrob. Agents Chemother. 45:13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Committee on Antimicrobial Susceptibility Testing. 2009. Antimicrobial wild type distributions of microorganisms. http://217.70.33.99/Eucast2/. Accessed 1 July 2009.
- 9.Flaherty, J. F., Jr., G. Gatti, J. White, J. Bubp, M. Borin, and J. G. Gambertoglio. 1996. Protein binding of clindamycin in sera of patients with AIDS. Antimicrob. Agents Chemother. 40:1134-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flaherty, J. F., L. C. Rodondi, B. J. Guglielmo, J. C. Fleishaker, R. J. Townsend, and J. G. Gambertoglio. 1988. Comparative pharmacokinetics and serum inhibitory activity of clindamycin in different dosing regimens. Antimicrob. Agents Chemother. 32:1825-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabrielsson, J., and D. Weimer. 2000. Pharmacokinetic concepts, p. 45-174. In Pharmacokinetic and pharmacodynamic data analysis: concepts & applications, 3rd ed. Apothekarsocieteten (Pharmaceutical Society), Stockholm, Sweden.
- 12.Gatti, G., J. Flaherty, J. Bubp, J. White, M. Borin, and J. Gambertoglio. 1993. Comparative study of bioavailabilities and pharmacokinetics of clindamycin in healthy volunteers and patients with AIDS. Antimicrob. Agents Chemother. 37:1137-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon, R. C., C. Regamey, and W. M. Kirby. 1973. Serum protein binding of erythromycin, lincomycin, and clindamycin. J. Pharm. Sci. 62:1074-1077. [DOI] [PubMed] [Google Scholar]
- 14.Gygax, S. E., J. A. Schuyler, L. E. Kimmel, J. P. Trama, E. Mordechai, and M. E. Adelson. 2006. Erythromycin and clindamycin resistance in group B streptococcal clinical isolates. Antimicrob. Agents Chemother. 50:1875-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall, C. M., D. W. Milligan, and J. Berrington. 2004. Probable adverse reaction to a pharmaceutical excipient. Arch. Dis. Child Fetal Neonatal Ed. 89:F184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kays, M. B., R. L. White, G. Gatti, and J. G. Gambertoglio. 1992. Ex vivo protein binding of clindamycin in sera with normal and elevated alpha 1-acid glycoprotein concentrations. Pharmacotherapy 12:50-55. [PubMed] [Google Scholar]
- 17.Kerr, J. R. 1994. Penicillin allergy: a study of incidence as reported by patients. Br. J. Clin. Pract. 48:5-7. [PubMed] [Google Scholar]
- 18.Kremer, J. M., J. Wilting, and L. H. Janssen. 1988. Drug binding to human alpha-1-acid glycoprotein in health and disease. Pharmacol. Rev. 40:1-47. [PubMed] [Google Scholar]
- 19.Matsubara, K., Y. Nishiyama, K. Katayama, G. Yamamoto, M. Sugiyama, T. Murai, and K. Baba. 2001. Change of antimicrobial susceptibility of group B streptococci over 15 years in Japan. J. Antimicrob. Chemother. 48:579-582. [DOI] [PubMed] [Google Scholar]
- 20.Matteson, K. A., S. P. Lievense, B. Catanzaro, and M. G. Phipps. 2008. Intrapartum group B streptococci prophylaxis in patients reporting a penicillin allergy. Obstet. Gynecol. 111:356-364. [DOI] [PubMed] [Google Scholar]
- 21.Menon, P. A., B. T. Thach, C. H. Smith, M. Landt, J. L. Roberts, R. E. Hillman, and L. S. Hillman. 1984. Benzyl alcohol toxicity in a neonatal intensive care unit. Incidence, symptomatology, and mortality. Am. J. Perinatol. 1:288-292. [DOI] [PubMed] [Google Scholar]
- 22.Mouton, J. W. 2002. Breakpoints: current practice and future perspectives. Int. J. Antimicrob. Agents 19:323-331. [DOI] [PubMed] [Google Scholar]
- 23.Mouton, J. W., N. Punt, and A. A. Vinks. 2005. A retrospective analysis using Monte Carlo simulation to evaluate recommended ceftazidime dosing regimens in healthy volunteers, patients with cystic fibrosis, and patients in the intensive care unit. Clin. Ther. 27:762-772. [DOI] [PubMed] [Google Scholar]
- 24.Mouton, J. W., A. Schmitt-Hoffmann, S. Shapiro, N. Nashed, and N. C. Punt. 2004. Use of Monte Carlo simulations to select therapeutic doses and provisional breakpoints of BAL9141. Antimicrob. Agents Chemother. 48:1713-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller, A. E., P. M. Oostvogel, J. DeJongh, J. W. Mouton, E. A. Steegers, P. J. Dorr, M. Danhof, and R. A. Voskuyl. 2009. Pharmacokinetics of amoxicillin in maternal, umbilical cord and neonatal sera. Antimicrob. Agents Chemother. 53:1574-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Notarianni, L. J. 1990. Plasma protein binding of drugs in pregnancy and in neonates. Clin. Pharmacokinet. 18:20-36. [DOI] [PubMed] [Google Scholar]
- 27.Philipson, A., L. D. Sabath, and D. Charles. 1973. Transplacental passage of erythromycin and clindamycin. N. Engl. J. Med. 288:1219-1221. [DOI] [PubMed] [Google Scholar]
- 28.Plaisance, K. I., G. L. Drusano, A. Forrest, R. J. Townsend, and H. C. Standiford. 1989. Pharmacokinetic evaluation of two dosage regimens of clindamycin phosphate. Antimicrob. Agents Chemother. 33:618-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schrag, S., R. Gorwitz, K. Fultz-Butts, and A. Schuchat. 2002. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recommend. Rep. 51(RR-11):1-22. [PubMed] [Google Scholar]
- 30.Sheiner, B. L., and S. L. Beal. 1981. Evaluation of methods for estimating population pharmacokinetic parameters. II. Biexponential model and experimental pharmacokinetic data. J. Pharmacokinet. Biopharm. 9:635-651. [DOI] [PubMed] [Google Scholar]
- 31.Sheiner, B. L., and T. H. Grasela. 1991. An introduction to mixed effect modeling: concepts, definitions, and justification. J. Pharmacokinet. Biopharm. 19:11S-24S. [Google Scholar]
- 32.Suh, B., W. A. Craig, A. C. England, and R. L. Elliott. 1981. Effect of free fatty acids on protein binding of antimicrobial agents. J. Infect. Dis. 143:609-616. [DOI] [PubMed] [Google Scholar]
- 33.Townsend, R. J., and R. P. Baker. 1987. Pharmacokinetic comparison of three clindamycin phosphate dosing schedules. Drug Intell. Clin. Pharm. 21:279-281. [DOI] [PubMed] [Google Scholar]
- 34.Weinstein, A. J., R. S. Gibbs, and M. Gallagher. 1976. Placental transfer of clindamycin and gentamicin in term pregnancy. Am. J. Obstet. Gynecol. 124:688-691. [DOI] [PubMed] [Google Scholar]