Abstract
The in vitro activities of ACH-702 and other antimicrobials against 30 Nocardia brasiliensis isolates were tested. The MIC50 (MIC for 50% of the strains tested) and MIC90 values of ACH-702 were 0.125 and 0.5 μg/ml. The same values for econazole were 2 and 4 μg/ml. The MIC50 and MIC90 values of imipenem and meropenem were 64 and >64 μg/ml and 2 and 8 μg/ml, respectively; the addition of clavulanic acid to the carbapenems had no effect.
Actinomycetoma caused by Nocardia brasiliensis is a localized but progressive infectious disease which affects the skin, subcutaneous tissue, and bones (21). Several antimicrobials, including sulfonamides, aminoglycosides, beta-lactams, etc., have been used in the therapy of actinomycetoma (3). However, in some cases a cure is not obtained, making it important to evaluate the in vitro and in vivo activities of new antimicrobials.
Quinolones have been widely used to treat infections by gram-negative and gram-positive bacteria (14); among them, moxifloxacin and gatifloxacin are active in vitro and in vivo against N. brasiliensis (6, 8, 18). ACH-702, (R)-7-[3-(1-amino-1-methyl-ethyl)-pyrrolidin-1-yl]-9-cyclopropyl-6-fluoro-8-methoxy-9H isothiazolo[5,4-b]quinoline-3,4-dione (Fig. 1), is a novel isothiazoloquinolone compound with good antibacterial activity against Staphylococcus aureus and enterococci, even against methicillin- and vancomycin-resistant isolates (16, 20). Its high activity against these gram-positive microorganisms raises the possibility that Nocardia spp., especially N. brasiliensis isolates, would also be susceptible to this new isothiazoloquinolone.
FIG. 1.
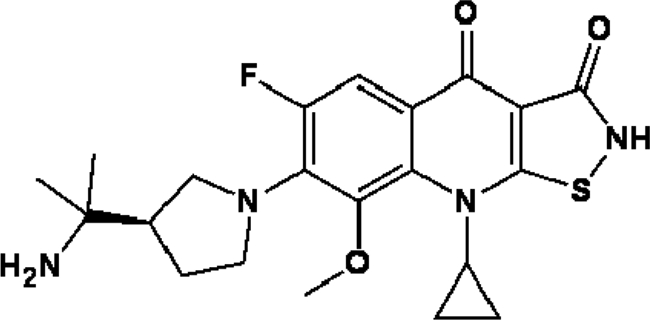
Chemical structure of ACH-702.
Imidazoles have been traditionally used against eukaryotic organisms since they interfere in the synthesis of ergosterol by inhibiting the cytochrome oxidase group of enzymes (CYP proteins); these enzymes were thought not to exist in prokaryotic organisms. However, sequencing in recent years of the genomes of many prokaryotes, including Mycobacterium tuberculosis, has shown the presence of CYP proteins in many of them, prompting testing of the activity of these compounds against species of Mycobacterium and Nocardia. Some of these compounds were active in vitro, particularly econazole (1, 2, 7). However, in one study (7), only one isolate of N. brasiliensis isolate was used, exhibiting an MIC of 1 μg/ml, making it necessary to extend the study using more clinical isolates.
Beta-lactams have been used widely to treat gram-positive infections. Because of the presence of potent beta-lactamases in nocardiae, their use against these microorganisms has been very limited (3, 21); amoxicillin-potassium clavulanate acid has been one of the most successfully used compounds to treat human cases of actinomycetoma (5). Imipenem has been used in clinical cases of N. brasiliensis infection in combination with other drugs, with good results observed (10). More recently, it has been observed that some carbapenems, such as imipenem and meropenem, are active against M. tuberculosis isolates, but only when combined with a beta-lactamase inhibitor, clavulanic acid (12).
In this work, we report the susceptibility of N. brasiliensis isolates to ACH-702, as well as other potentially active compounds, including econazole and carbapenems (imipenem and meropenem), alone or combined with clavulanic acid.
We studied 30 isolates from the collection of the Laboratorio Interdisciplinario de Investigación Dermatológica of the Servicio de Dermatología, Hospital Universitario, Universitario de Nuevo León, including N. brasiliensis HUJEG-1, which was utilized previously in other in vitro and in vivo assays (11, 17). All of the isolates tested came from human cases of actinomycetoma and were identified as N. brasiliensis by biochemical methods and by nucleotide sequence analysis of a fragment of the 16S RNA gene of the small ribosomal unit as previously described (17).
ACH-702 was kindly donated by Achillion Pharmaceuticals, Inc., New Haven, CT. Econazole and clavulanic acid were purchased from Sigma Chemical Co. (St. Louis, Mo.). Imipenem and meropenem were obtained from commercial sources.
The broth microdilution method used was based on the CLSI M24-A document (15) and has been previously described (18). Briefly, ground colonies were suspended in 1 ml of saline solution and diluted with cation-adjusted Mueller-Hinton broth until the turbidity matched that of a 0.5 McFarland standard. This suspension was diluted to obtain a solution with a final concentration of 1 × 104 to 5 × 104 CFU per well in 0.1 ml. This solution then was added to microplate wells (Microtest Primaria; Becton Dickinson and Co., Franklin Lakes, NJ) containing an equal volume of broth with serial dilutions of the drugs to be tested. As a growth control, we similarly inoculated a well containing cation-adjusted Mueller-Hinton broth without drug. After 3 days of incubation at 35°C, the plates were read and the MIC was determined as the lowest concentration of drug that totally inhibited nocardial growth. As external controls, we used Escherichia coli ATCC 25922 and S. aureus ATCC 29213. Econazole, imipenem, and meropenem were tested at concentrations of 64 to 0.25 μg/ml. The lowest concentration of ACH-702 used was 0.03 μg/ml. To test the effect of the beta-lactamase inhibitor on carbapenem activity, 0.25 μg/ml clavulanic acid was added to all of the carbapenems (12).
The MICs of ACH-702 and the other antimicrobial agents tested for the 30 clinical isolates of N. brasiliensis tested are summarized in Table 1. The MIC50 (MIC for 50% of the strains tested) and MIC90 values for ACH-702 were 0.125 and 0.5 μg/ml. The same values for econazole were 2 and 4 μg/ml. The MIC50 and MIC90 values for imipenem were 64 and >64 μg/ml, respectively. Only seven isolates had an MIC of <2 μg/ml. For meropenem, the values were 2 and 8 μg/ml. For this compound, 16 out of 30 isolates tested exhibited an MIC value of ≤2 μg/ml. The addition of clavulanic acid to the carbapenems did not change the MIC values significantly.
TABLE 1.
In vitro activities of ACH-702 and other antimicrobials against N. brasiliensis isolated from human cases of actinomycetoma
| Antimicrobial agent(s) | MIC (μg/ml) |
||
|---|---|---|---|
| Range | 50% of strains tested | 90% of strains tested | |
| ACH-702 | 0.03-2 | 0.125 | 0.5 |
| Econazole | 1-8 | 2 | 4 |
| Imipenem | 1->64 | 64 | >64 |
| Imipenem + clavulanic acid | 1->64 | 64 | >64 |
| Meropenem | 1->64 | 2 | 8 |
| Meropenem + clavulanic acid | 1->64 | 2 | 8 |
Although imidazoles have been traditionally used against eukaryotic organisms, recently it has been observed that actinobacteria, including mycobacteria and nocardiae, are also susceptible to this class of antimicrobials (2, 7). In our work, we observed that most (90%) of the isolates exhibited an MIC of ≤2 μg/ml for econazole. In mice, econazole given orally at 3.3 mg/kg, is rapidly metabolized and achieves plasma concentrations (maximum concentration of drug in plasma, 0.23 μg/ml; time to maximum concentration of drug in plasma, 1 h) below this MIC value (2). Given these parameters, we believe that econazole will not work effectively in the experimental mouse model. However, new imidazole compounds are being launched in the market and it is possible that in the future more potent drugs or other delivery methods would open up the possibility of therapy of actinomycetoma caused by N. brasiliensis using this class of antimicrobials.
Beta-lactams have been of limited use in the treatment of N. brasiliensis infections. Recently, it has been reported that carbapenems plus clavulanic acid have shown some activity against M. tuberculosis isolates (12). In our work, we did not find any difference when clavulanic acid was added, with MIC50 and MIC90 values remaining the same. At best, imipenem or meropenem can be effective in human patients with isolates exhibiting MIC values of ≤2 μg/ml, since the levels of these drugs remain over 2 μg/ml for 3 to 4 h in humans after the intravenous administration of 1,000 mg (9). Given the observed differences in susceptibility among clinical isolates, it would be important to perform susceptibility tests prior to the initiation of therapy with these agents. Also, it will be important to assess the antibacterial activity of future beta-lactams that have the desirable advantage of oral administration for the treatment of Nocardia infections.
Quinolones, including ofloxacin, ciprofloxacin, and moxifloxacin, have been used in the therapy in infections caused by Nocardia spp. (4, 13, 22). However N. brasiliensis is a more resistant species and only newer compounds such as gatifloxacin, garenoxacin, and moxifloxacin have shown excellent in vitro and in vivo effects against this microorganism (3, 6, 8, 18). Of these quinolones, only moxifloxacin is available on the market, but its use has not been reported for actinomycetoma patients. ACH-702 showed MIC50 and MIC90 values (0.125 and 0.5 μg/ml) lower than those of gatifloxacin (0.5 and 2 μg/ml) and moxifloxacin (0.5 and 2 μg/ml) (18), which suggests that this may be a promising compound to treat N. brasiliensis infections, although it will be important to test its intracellular (19) and in vivo activities against this bacterium.
Footnotes
Published ahead of print on 22 March 2010.
REFERENCES
- 1.Ahmad, Z., S. Sharma, G. K. Khuller, P. Singh, J. Faujdar, and V. M. Katoch. 2006. Antimycobacterial activity of econazole against multidrug-resistant strains of Mycobacterium tuberculosis. Int. J. Antimicrob. Agents 28:543-544. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad, Z., S. Sharma, and G. K. Khuller. 2006. Azole antifungals as novel chemotherapeutic agents against murine tuberculosis. FEMS Microbiol. Lett. 261:181-186. [DOI] [PubMed] [Google Scholar]
- 3.Ameen, M., and R. Arenas. 2009. Developments in the management of mycetomas. Clin. Exp. Dermatol. 34:1-7. [DOI] [PubMed] [Google Scholar]
- 4.Bath, P. M., K. W. Pettingale, and J. Wade. 1989. Treatment of multiple subcutaneous Nocardia asteroides abscesses with ciprofloxacin and doxycycline. Postgrad. Med. J. 65:190-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonifaz, A., P. Flores, A. Saúl, E. Carrasco-Gerard, and R. M. Ponce. 2007. Treatment of actinomycetoma due to Nocardia spp. with amoxicillin-clavulanate. Br. J. Dermatol. 156:308-311. [DOI] [PubMed] [Google Scholar]
- 6.Chacon-Moreno, B. E., O. Welsh, N. Cavazos-Rocha, M. de la Luz Salazar-Cavazos, H. G. Garza-Lozano, S. Said-Fernandez, J. Ocampo-Candiani, and L. Vera-Cabrera. 2009. Efficacy of ciprofloxacin and moxifloxacin against Nocardia brasiliensis in vitro and in an experimental model of actinomycetoma in BALB/c mice. Antimicrob. Agents Chemother. 53:295-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dabbs, E. R., S. Naidoo, C. Lephoto, and N. Nikitina. 2003. Pathogenic Nocardia, Rhodococcus, and related organisms are highly susceptible to imidazole antifungals. Antimicrob. Agents Chemother. 47:1476-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daw-Garza, A., O. Welsh, S. Said-Fernández, H. G. Lozano-Garza, N. Waksman de Torres, N. C. Rocha, J. Ocampo-Candiani, and L. Vera-Cabrera. 2008. In vivo therapeutic effect of gatifloxacin on BALB/c mice infected with Nocardia brasiliensis. Antimicrob. Agents Chemother. 52:1549-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dreetz, M., J. Hamacher, J. Eller, K. Borner, P. Koeppe, T. Schaberg, and H. Lode. 1996. Serum bactericidal activities and comparative pharmacokinetics of meropenem and imipenem-cilastatin. Antimicrob. Agents Chemother. 40:105-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuentes, A., R. Arenas, M. Reyes, R. F. Fernández, and R. Zacarías. 2006. Actinomycetoma and Nocardia sp. Report of five cases treated with imipenem or imipenem plus amikacin. Gac. Med. Mex. 142:247-252. [PubMed] [Google Scholar]
- 11.Gonzalez-Suarez, M. L., M. C. Salinas-Carmona, and I. Pérez-Rivera. 2009. IgM but not IgG monoclonal anti-Nocardia brasiliensis antibodies confer protection against experimental actinomycetoma in BALB/c mice. FEMS Immunol. Med. Microbiol. 57:17-24. [DOI] [PubMed] [Google Scholar]
- 12.Hugonnet, J. E., L. W. Tremblay, H. I. Boshoff, C. E. Barry III, and J. S. Blanchard. 2009. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science 323:1215-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kandasamy, J., H. J. Iqbal, R. P. Cooke, and P. R. Eldridge. 2008. Primary Nocardia farcinica brain abscess with secondary meningitis and ventriculitis in an immunocompetent patient, successfully treated with moxifloxacin. Acta Neurochir. (Wien) 150:505-506. [DOI] [PubMed] [Google Scholar]
- 14.King, D. E., R. Malone, and S. H. Lilley. 2000. New classification and update on the quinolone antibiotics. Am. Fam. Physician 61:2741-2748. [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. 2003. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved standard. NCCLS document M24-A. National Committee for Clinical Laboratory Standards, Wayne, PA. [PubMed]
- 16.Pucci, M. J., J. Cheng, S. D. Podos, C. L. Thoma, J. A. Thanassi, D. D. Buechter, G. Mushtaq, G. A. Vigliotti, B. J. Bradbury, and M. S. Deshpande. 2007. In vitro and in vivo antibacterial activities of heteroaryl isothiazolones against resistant gram-positive pathogens. Antimicrob. Agents Chemother. 51:1259-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vera-Cabrera, L., W. M. Johnson, O. Welsh, F. L. Resendiz-Uresti, and M. C. Salinas-Carmona. 1999. Distribution of a Nocardia brasiliensis catalase gene fragment in members of the genera Nocardia, Gordona, and Rhodococcus. J. Clin. Microbiol. 37:1971-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vera-Cabrera, L., E. Gonzalez, S. H. Choi, and O. Welsh. 2004. In vitro activities of new antimicrobials against Nocardia brasiliensis. Antimicrob. Agents Chemother. 48:602-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vera-Cabrera, L., N. A. Espinoza-González, O. Welsh, J. Ocampo-Candiani, and J. Castro-Garza. 2009. Activity of novel oxazolidinones against Nocardia brasiliensis growing within THP-1 macrophages. J. Antimicrob. Chemother. 64:1013-1017. [DOI] [PubMed] [Google Scholar]
- 20.Wang, Q., E. Lucien, A. Hashimoto, G. C. Pais, D. M. Nelson, Y. Song, J. A. Thanassi, C. W. Marlor, C. L. Thoma, J. Cheng, S. D. Podos, Y. Ou, M. Deshpande, M. J. Pucci, D. D. Buechter, B. J. Bradbury, and J. A. Wiles. 2007. Isothiazoloquinolones with enhanced antistaphylococcal activities against multidrug-resistant strains: effects of structural modifications at the 6-, 7-, and 8-positions. J. Med. Chem. 50:199-210. [DOI] [PubMed] [Google Scholar]
- 21.Welsh, O., L. Vera-Cabrera, and M. C. Salinas-Carmona. 2007. Mycetoma. Clin. Dermatol. 25:195-202. [DOI] [PubMed] [Google Scholar]
- 22.Yew, W. W., P. C. Wong, S. Y. Kwan, C. Y. Chan, and M. S. Li. 1991. Two cases of Nocardia asteroides sternotomy infection treated with ofloxacin and a review of other active antimicrobial agents. J. Infect. 23:297-302. [DOI] [PubMed] [Google Scholar]


