Abstract
Extracorporeal membrane oxygenation (ECMO) is used to temporarily sustain cardiac and respiratory function in critically ill infants but can cause pharmacokinetic changes necessitating dose modifications. Cefotaxime (CTX) is used to prevent and treat infections during ECMO, but the current dose regimen is based on pharmacokinetic data obtained for non-ECMO patients. The objective of this study was to validate the standard dose regimen of 50 mg/kg of body weight twice a day (postnatal age [PNA], <1 week), 50 mg/kg three times a day (PNA, 1 to 4 weeks), or 37.5 mg/kg four times a day (PNA, >4 weeks). We included 37 neonates on ECMO, with a median (range) PNA of 3.3 (0.67 to 199) days and a median (range) body weight of 3.5 (2.0 to 6.2) kg at the onset of ECMO. Median (range) ECMO duration was 108 (16 to 374) h. Plasma samples were taken during routine care, and pharmacokinetic analysis of CTX and its active metabolite, desacetylcefotaxime (DACT), was done using nonlinear mixed-effects modeling (NONMEM). A one-compartment pharmacokinetic model for CTX and DACT adequately described the data. During ECMO, CTX clearance (CLCTX) was 0.36 liter/h (range, 0.19 to 0.75 liter/h), the volume of distribution of CTX (VCTX) was 1.82 liters (0.73 to 3.02 liters), CLDACT was 1.46 liters/h (0.48 to 5.93 liters/h), and VDACT was 11.0 liters (2.32 to 28.0 liters). Elimination half-lives for CTX and DACT were 3.5 h (1.6 to 6.8 h) and 5.4 h (0.8 to 14 h). Peak CTX concentration was 98.0 mg/liter (33.2 to 286 mg/liter). DACT concentration varied between 0 and 38.2 mg/liter, with a median of 10 mg/liter in the first 12 h postdose. Overall, CTX concentrations were above the MIC of 8 mg/liter over the entire dose interval. Only 1 of the 37 patients had a sub-MIC concentration for over 50% of the dose interval. In conclusion, the standard cefotaxime dose regimen provides sufficiently long periods of supra-MIC concentrations to provide adequate treatment of infants on ECMO.
Extracorporeal membrane oxygenation (ECMO) is used as a standardized last resort to support critically ill infants who can no longer maintain sufficient cardiac and respiratory function with conventional life support techniques (5, 8). Over a period of up to a maximum of 3 weeks, blood flow is continuously diverted via a venous cannula into an extracorporeal circuit, oxygenated via a membrane, and returned to the general circulation via a venous or arterial cannula. A hemofiltration unit can be added to the circuit to supplement insufficient renal function. Standard pharmacological treatment includes high doses of antibiotics for the treatment of preexisting or nosocomial infections, which are facilitated by the direct microbial access to the patient's general circulation via cannulae and circuit components (15). One of the antibiotics commonly used in neonates on ECMO is cefotaxime (CTX), which possesses antimicrobial activity against many of the pathogens commonly involved in neonatal and ECMO-related infections, such as Escherichia coli, Klebsiella pneumoniae, Enterobacter, and Staphylococcus spp. (27). In adults, cefotaxime can be excreted unchanged via the renal system, but also after hepatic conversion into its active metabolite, desacetylcefotaxime (DACT) (for 15 to 25% of a dose) (33). There appears to be an inverse correlation between renal function and elimination half-life, particularly for DACT (32).
In the absence of specific pharmacokinetic (PK) data, our current cefotaxime dose regimen is the same for both ECMO and non-ECMO patients. In general, however, ECMO is associated with altered pharmacokinetics for a variety of drugs, probably due to an increase in circulatory volume, a disease-related clearance reduction, or adsorption of drugs to membranes and other circuit components (7). We designed this study to evaluate the pharmacokinetics of cefotaxime and desacetylcefotaxime during ECMO and to validate our dose regimen.
MATERIALS AND METHODS
All neonates about to receive ECMO treatment at the Sophia Children's Hospital (Erasmus University Medical Center) from December 2006 to June 2009 were eligible. The local institutional ethics review board approved this study. Parental informed consent was obtained for blood sampling and use of clinical data. Criteria for ECMO treatment were as follows: gestational age of >34 weeks, birth weight of >2.0 kg, mechanical ventilation for <7 days, an alveolar arterial oxygen difference of <500 mm Hg, and an oxygenation index of >25. Concomitant drugs were given in accordance with the departmental treatment protocol, and doses were adapted to each neonate's clinical condition. The most recent weight available prior to ECMO was used for dose calculation and pharmacokinetic analysis. Drug administrations, laboratory results, and real-time parameters such as ECMO flow were recorded in a patient data management system.
ECMO.
The ECMO circuit consisted of extracorporeal cannulae (Medtronic, Kerkrade, Netherlands), polyvinyl chloride (PVC) tubing (Bentley Bypass 70 tubing; Baxter, Netherlands), a silicone rubber membrane oxygenator (pediatric extended-membrane oxygenator; Medtronic), and a heat exchanger (heat exchanger monitoring adapter and Luer-lock; Medtronic). Priming volume ranged between 300 and 350 ml. A continuous venovenous hemofiltration (CVVH) filter (Multiflow 60; Hospal, Lyon, France) was placed parallel to the ECMO circuit, distal to the ECMO roller pump. Pressure was measured proximal and distal to the filter; the difference was kept constant at 40 mm Hg.
Cefotaxime administration.
Cefotaxime was given intravenously as a bolus injection (maximum period, 3 min). Dose regimens were standardized hospitalwide to vary with postnatal age, from 50 mg/kg twice a day (b.i.d.) (postnatal age [PNA], <1 week) and 50 mg/kg three times a day (t.i.d.) (PNA, 1 to 4 weeks) to 37.5 mg/kg four times a day (q.i.d.) (PNA, >4 weeks) (13), for ECMO and non-ECMO patients alike, but doctors could deviate from protocol at their own discretion. Doses were rounded off to the nearest 5 mg to allow reliable administration of prescribed CTX doses. Nurses validated physician-prescribed medication orders and recorded actual injection times in the data management system as part of their standard care routine. CTX was administered via an extracorporeal line after the oxygenator, just before blood was returned to the patient's circulation.
Blood sampling and assay.
Blood was collected during routine laboratory rounds three times daily. When possible, additional samples were taken 1 h before and 0, 1, and 3 h after cannulation to characterize early pharmacokinetic changes. Sampling continued for a maximum of 24 h after decannulation. Blood (maximum of 1 ml) was taken from a venous preoxygenator access point dedicated to sample withdrawal on the ECMO circuit and collected in EDTA-decoagulation vials, which were stored at 4 to 7°C until further processing. After centrifugation (5 min, 4,000 × g), the supernatant serum was stored at −80°C until assay. Sampling times and duration of storage at 4 to 7°C were recorded. CTX and DACT concentrations were quantified via liquid chromatography-mass spectrometry (LC-MS) as previously described (2). The limit of quantification was 0.2 mg/liter for both CTX and DACT. Intra- and interassay coefficients of variation were <15%.
Blood culture.
Blood cultures are performed daily at our institution. Samples were taken from a venous access port and sent in for microbiological surveillance.
PK model development.
CTX and DACT models were developed sequentially, using nonlinear mixed-effects modeling software (NONMEM VI 2.0; Globomax LLC, Ellicott City, MD). NONMEM allows the estimation of typical population pharmacokinetic parameters and their respective inter- and intraindividual variability in combination with the estimation of residual random variability. The first-order conditional estimation (FOCE) method, with interaction between the interindividual and random effects, was used throughout method development. Differential equations were used with NONMEM's ADVAN 6 subroutine to describe the population PK of CTX and DACT. After selection of an appropriate base model, interindividual random effects were evaluated on clearance (CL) and volumes of distribution (V) by use of an exponential model. Covariance between CL and V was modeled using an omega block function. Residual variability was described with a proportional error model; the proportional variance coefficient was separately estimated for samples taken within 1 h postdose to account for expected variable discrepancies between the actual and recorded dose times. Postsampling degradation was incorporated into the error model by calculating the concentration at the time of sampling, using the degradation rate constant for EDTA-decoagulated whole blood from the literature (kdeg = 0.0132; half-life [t1/2] = 52 h) (2); the median correction of observed CTX concentrations was +15.7%. Covariate effects on CL or V were incorporated into the model as previously described (1), and their statistical significance was assessed in a stepwise inclusion and exclusion procedure (22). The tested covariates included gestational age (GA), PNA, body weight (WT), time after dose (tdose), time after start or end of extracorporeal circulation (tEC or tEND), ECMO on or off, ECMO flow (QECMO), CVVH flow (QCVVH), indication, the number of ECMO runs, ECMO modality (venovenous or venoarterial), sex, body temperature, urine output, fluid balance, serum albumin, serum creatinine, and concomitant use of vasopressive medication (norepinephrine, dopamine, dobutamine, or epinephine). After selection of appropriate covariates, remaining interoccasion variability was tested on CL and V for CTX and DACT, where occasions were defined as tEC periods of 48 h; pre- and post-ECMO observations were considered separate occasions.
PK model performance.
Evaluation of models was based on improvements in the minimum value of objective function (OFV), estimates of the standard errors of parameters, and goodness-of-fit plots generated via the Xpose software package (v 4.0.4; M. Karlsson, University of Uppsala, Sweden) (18) within R (v 2.8.1; The R Foundation for Statistical Computing [www.R-project.org]). Additional plots were prepared using GraphPad Prism 4.03 (GraphPad Software Inc., La Jolla, CA). Goodness-of-fit plots included, among others, plots of measured drug concentrations versus population (PRED) or individual (IPRED) predictions, conditional weighted residuals (CWRES) (14) versus time or other covariates, and plots of observed concentrations (dependent variable [DV]), PRED, and IPRED versus time. Bayesian IPRED concentrations were obtained via NONMEM's post hoc option. Statistical significance of a potential model improvement was determined via the log-likelihood ratio test for nested models, using the OFV produced by NONMEM. A decrease in OFV of 3.84 (P = 0.05, χ2 distribution, 1 degree of freedom) was considered statistically significant. A stricter criterion (P = 0.01, ΔOFV = 6.63) was used in the backward elimination procedure for covariate effects: if deletion of a covariate did not result in a significant worsening of the objective function, then the covariate was removed from the model. The resulting model was considered the final model. Shrinkage was calculated to assess whether the estimated η and ɛ parameter distributions matched those of the original data, assuming a normal distribution (30). Stability and performance of the final model were checked using an internal validation procedure via the bootstrap resampling technique, in which 1,200 bootstrap data sets were generated by random sampling with replacement (10). We used the Wings for NONMEM software package (v6.12 March 2007; N. Holford, Auckland, New Zealand). Model validity was assessed by calculating median values and the 2.5th and 97.5th percentiles of parameter distribution generated by the bootstrap set and comparing them with the original estimates. The bootstrap set was also used to calculate the standard error for each estimate.
Dose regimen evaluation.
The fraction of a dose interval during which the cefotaxime concentration exceeds the MIC for susceptible microorganisms (t>MIC; percentage of the dose interval over 24 h) is considered an appropriate measure of efficacy (9, 24). Based on bacteriological screening results for our ECMO patients and literature on pathogens involved in pediatric meningitis (27), the main pathogens include Escherichia, Staphylococcus, Klebsiella, Serratia, and Enterobacter species. Reported MIC values (MIC distributions of wild-type microorganisms [www.Eucast.org]) are at or below 4 μg/ml (S. aureus). Assuming a worst-case scenario of up to 40% protein binding (29), the maximal MIC value in plasma is around 8 μg/ml. Using the individual parameter estimates derived from the final PK model, concentration-time curves were constructed for each individual by simulating the predicted concentration over intervals of 0.2 h. We calculated t>MIC over 24 h for each individual patient and compared the median values for each dose regimen; we considered the antimicrobial effect to be optimal at a t>MIC of at least 50% (24).
RESULTS
Data.
We included 37 patients with a total of 392 samples (median per patient, 10; range, 1 to 17). Pre-ECMO samples were available for 8 individuals (1 each); post-ECMO samples were available for 13 individuals (on average, 2.1 each). See Table 1 for patient characteristics. CTX and DACT were successfully quantified in all samples, with 4 (CTX, 1.0%) and 3 (DACT, 0.8%) concentrations falling below the quantification limit (BQL). DACT concentrations were converted to CTX equivalents by using a molecular weight ratio of 455.5/413.4 (Mr CTX/Mr DACT).
TABLE 1.
Patient characteristics
| Characteristic | Description or valuea |
|---|---|
| General characteristics | |
| Sex (no. of males/no. of females) | 18/19 |
| Primary diagnosis (n [% of patients | |
| with diagnosis]) | |
| Meconium aspiration syndrome | 17 (46) |
| Congenital diaphragmatic hernia | 8 (22) |
| Pulmonary hypertension (other causes) | 5 (14) |
| Congenital heart defects | 4 (11) |
| Other (sepsis, viral infections, etc.) | 3 (7) |
| Body wt (kg) | 3.5 (2.0-6.2) |
| Gestation (wk) | 37 (3442) |
| Postnatal age at start of ECMO (days) | 3.3 (0.67-199) |
| Survival (no. of survivors/no. who did | |
| not survive) | 25/12 |
| Cefotaxime dose (intravenous) (n [%]) | |
| 50 mg/kg b.i.d. | 24 (65) |
| 50 mg/kg t.i.d. | 7 (19) |
| 37.5 mg/kg q.i.d. | 3 (8) |
| 25 mg/kg b.i.d. | 2 (5) |
| 37.5 mg/kg t.i.d. | 1 (3) |
| Serum chemistry | |
| Albumin (g/liter) | 31 (21-40) |
| Serum creatinine (μmol/liter) | 32 (19-69) |
| Aspartate amino transferase (IU/liter) | 44 (14-369) |
| Alanine aminotransferase (IU/liter) | 10 (0.5-40) |
| ECMO characteristics | |
| ECMO modality (n [%])b | |
| Venovenous (VV) | 22 (54) |
| Venoarterial (VA) | 19 (46) |
| Median ECMO flow (ml/kg/min) | 308 (50-530) |
| Duration of ECMO (h) | 108 (16-374) |
| No. of patients undergoing CVVH/no. of | |
| patients not undergoing CVVH | 30/7 |
| CVVH flow (ml/min) | 193 (100-350) |
| Body temp (no. of patients) | |
| Hypothermic (24°C) | 2 |
| Normothermic (36°C) | 35 |
Unless indicated otherwise, parameters are expressed as medians (ranges).
Four patients had 2 ECMO runs each: 3 VV + VA and 1 VA + VV.
Blood culture.
Thirty-four patients had negative blood cultures throughout their ECMO runs during CTX administration. Two patients had one positive culture, on days 8 and 10 of ECMO, but both had negative cultures beforehand and at least 2 days thereafter; it is unclear whether these were false-positive cultures or transient infections. One patient had positive cultures on days 11 and 13, in which an enterococcus could be isolated.
PK model development.
A one-compartment model with first-order elimination for both CTX and DACT best fit the data; additional compartments improved neither goodness-of-fit plots nor the OFV. BQL concentrations were removed from the data set; deletion did not change CL and V parameter estimates for the base model. Proportional residual error terms improved the model, whereas an additional error did not. There was a structural deviation in plots of CWRES versus tdose, indicating lower-than-expected concentrations in the first hour after CTX infusion. A separate proportional residual error for samples with tdose values of <1 h reduced this deviation. Alternatively, first-order absorption and lag-time models were tested, but they did not significantly improve fit, probably because only a fraction of the concentrations were overpredicted. No other covariates were correlated with this deviation. Interindividual variability was successfully estimated for CL and V for both compounds. Covariance between CL and V significantly improved minimization and stability; correlation varied from 70.6% (CLDACT-VDACT) to 90.8% (VCTX-VDACT). Interoccasion variability (occasions of 48 h) was tested only after trends with tEC or other time-varying covariates proved nonsignificant, and this improved fit, with a significant (P < 0.001) reduction in OFV. An increase in CLCTX and CLDACT upon cannulation, which could be seen in 8 patients, based on one pre-ECMO sample each, could not be modeled with statistical significance. Allometric scaling (4) was tested before other covariates, but this did not reduce the OFV. The covariate inclusion procedure suggested that the following covariates might be correlated to V or CL and improve the OFV upon inclusion (P < 0.05): for CLCTX, GA, QCVVH, WT, PNA, vasopressor use, and tEND; for VCTX, fluid balance and serum creatinine; for CLDACT, sex, duration of pregnancy, WT, QECMO, tEND, and QCVVH; and for VDACT, tEND. After stepwise exclusion, the only significant remaining effects were WT (CLCTX), QCVVH (CLDACT), and tEND (CLCTX and CLDACT), but drops in unexplained interindividual variability were small, with values of −2.7% (WT-CLCTX), −8.1% (QCVVH-CLDACT), −0.5% (tEND-CLCTX), and −4.2% (tEND-CLDACT). None of the covariates reduced interindividual variability for VCTX or VDACT. See Table 2 for parameter estimates of the final model. See Appendix for the differential equations used in the final model, including covariate effects.
TABLE 2.
Parameter estimatesa
| Population parameter | CTX |
DACT |
Remarks | ||
|---|---|---|---|---|---|
| Estimate (CV %) | Bootstrap median (95% CI) | Estimate (CV %) | Bootstrap median (95% CI) | ||
| V (liter) | 1.82 (8.2) | 1.86 (1.60-2.20) | 11.0 (14.0) | 11.0 (7.90-14.0) | |
| CL (liters/h) | 0.36 (7.9) | 0.36 (0.30-0.41) | 1.46 (11.5) | 1.42 (1.10-1.77) | |
| Covariate effects | |||||
| θWT | 0.56 (43.7) | 0.55 (0.02-1.00) | CL = CLpop × 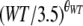
|
||
| θCVVH | 0.72 (35.8) | 0.69 (0.10-1.10) | CL = CLpop × 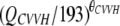 ; without CVVH, CL = CLpop ; without CVVH, CL = CLpop
|
||
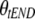
|
0.16 (80.8) | 0.16 (0.002-0.48) | 0.53 (53.7) | 0.51 (0.18-1.20) | CL = CLpop × 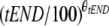 ; when tEND = 0, CL = CLpop ; when tEND = 0, CL = CLpop
|
| Interindividual variability | |||||
| V (%) | 35.4 (24.2) | 35.9 (16.7-51.5) | 59.8 (19.7) | 60.8 (39.4-84.2) | |
| CL (%) | 36.1 (21.5) | 34.8 (24.1-53.1) | 51.4 (18.6) | 53.3 (39.7-76.1) | |
| Interoccasion variability | |||||
| V (%) | 25.0 (20.7) | 24.5 (15.7-35.8) | 25.0 (20.7) | 24.5 (15.7-35.8) | Calculated over periods of 48 h on ECMO |
| CL (%) | 25.0 (20.7) | 24.5 (15.7-35.8) | 25.0 (20.7) | 24.5 (15.7-35.8) | Calculated over periods of 48 h on ECMO |
| Residual variability | |||||
| Proportional (tdose < 1 h) (%) | 69.4 (25.4) | 68.3 (44.9-90.7) | 69.4 (25.4) | 68.3 (44.9-90.7) | |
| Proportional (tdose > 1 h) (%) | 32.7 (8.2) | 32.3 (27.4-37.6) | 32.7 (8.2) | 32.3 (27.4-37.6) | |
CTX, cefotaxime; DACT, desacetylcefotaxime; CV, coefficient of variation; 95% CI, 95% confidence interval; V, volume of distribution; CL, clearance; WT, body weight, in kg; QCVVH CVVH flow; tEND, time after decannulation, in h; tdose, time after last dose. CL and V estimates for DACT were calculated assuming a conversion fraction (FDACT/CTX) of 1.
PK model performance.
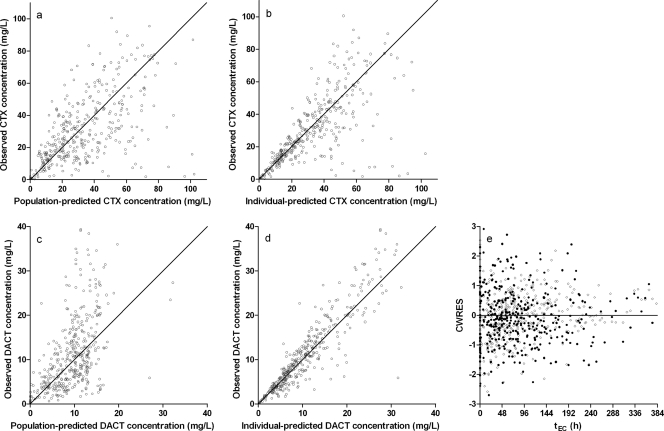
See Fig. 1 for the goodness-of-fit plots. For certain individuals, DACT was structurally underestimated (Fig. 1c), but there was no significant trend with any covariate; interindividual variability on PK parameters corrected this pattern (Fig. 1d). There was no trend in CWRES versus tEC. All parameter estimates were within the 95% confidence interval calculated using bootstrap data (Table 2). The higher coefficients of variation for the covariate effects show that their estimation was difficult with this data set, probably due to the small sample size and high residual variability. Shrinkage was calculated for interindividual variability (η) on CLCTX (5.2%), VCTX (4.7%), CLDACT (6.4%), and VDACT (4.4%) and for the residual variability (ɛ; 2.2%), using Perl-speaks-NONMEM (21).
FIG. 1.
Goodness-of-fit plots for the final model. Plots are shown for observed CTX concentration versus population-predicted (a) and individual-predicted (b) concentrations. (c and d) Similar plots are displayed for DACT. (e) There is no apparent pattern in CWRES versus tEC for CTX (closed circles) or DACT (open circles).
CTX and DACT pharmacokinetics.
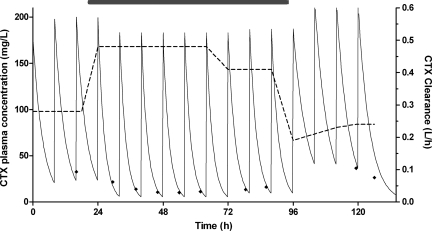
See Table 2 for parameter estimates. During ECMO, the median CLCTX = 0.36 liter/h (0.19 to 0.75 liter/h), VCTX = 1.82 liters (0.73 to 3.02 liters), CLDACT = 1.46 liters/h (0.48 to 5.93 liters/h), and VDACT = 11.0 liters (2.32 to 28.0 liters). Over the weight range of 2 to 6.2 kg, median CLCTX varied from 0.26 to 0.50 liter/h. The elimination half-life was 3.5 h for CTX (1.6 to 6.8 h) and 5.4 h for DACT (0.8 to 14 h). In the individuals for which pre- or post-ECMO samples were available, CTX and DACT clearance appeared to increase upon cannulation (median CLCTX = 0.30 to 0.36 liter/h; CLDACT = 1.37 to 1.46 liters/h). After decannulation, CLCTX and CLDACT dropped almost instantaneously but recovered steadily over the following 72 h (from 0.22 to 0.40 liter/h and from 0.18 to 1.38 liters/h). See Fig. 2 for plasma concentrations and clearance estimates for one of the studied individuals.
FIG. 2.
Characteristic concentration-time curve for one of the subjects (with a dose of 50 mg/kg t.i.d.) with a number of samples pre- and post-ECMO. Displayed are the Bayesian estimated CTX plasma concentration profile (continuous curve; left axis), observed concentrations (diamonds; left axis), and CTX clearance (dashed curve; right axis). The duration of ECMO treatment is indicated by the gray box.
Dose regimen.
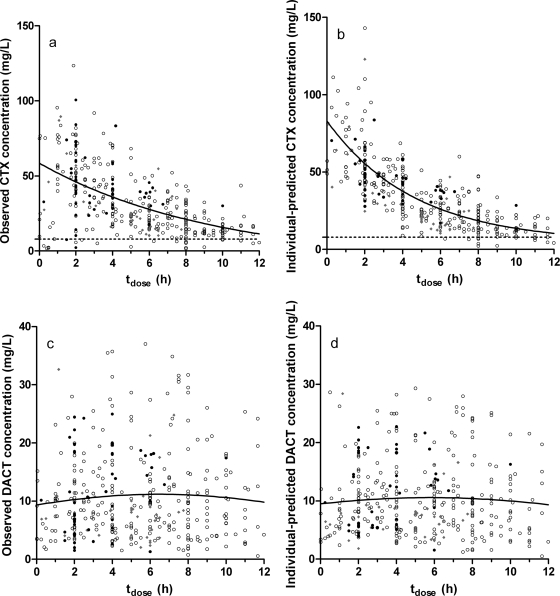
Individual post hoc estimates of CTX plasma concentration at intervals of 0.2 h over the entire observation period were used to calculate the t>MIC for each patient. The median peak CTX concentration was 98.0 mg/liter (33.2 to 286 mg/liter). DACT concentrations varied between 0 and 38.2 mg/liter, with a median of 10 mg/liter in the first 12 h postdose. The median t>MIC (calculated for CTX only) was 100%. Thirty-six of 37 patients had a t>MIC of >50% for all their CTX doses. The remaining patient (PNA, <1 week) had declining plasma concentrations even after a new dose; it is possible that one or more doses were skipped due to medical procedures at dose time, inadvertent dose registration without actually having given the dose, or other unknown reasons. This caused this individual's t>MIC to drop to 49%. See Fig. 3 for the individual predicted CTX and DACT concentrations over a dose interval of 12 h. With the exception of the aforementioned patient, concentrations in all three age categories (PNA of <1 wk, n = 26; PNA of 1 to 4 weeks, n = 7; and PNA of >4 weeks, n = 4) were above the MIC over a period of at least 6 h. In general, the patients with a PNA of 1 to 4 weeks were at the bottom of the concentration-time curve, but their dose interval was only 8 h.
FIG. 3.
Observed and individual-predicted concentrations versus dose-time for cefotaxime (CTX) (a and b) and desacetylcefotaxime (DACT) (c and d). In plots a and b, the target MIC is indicated by a dashed line. Data points are marked to stratify data by PNA: open circles, <1 week; gray diamonds, 1 to 4 weeks; and closed circles, >4 weeks. The solid lines represent naive pooling fits of all data for CTX (nonlinear first-order decline curve) and DACT (course LOWESS curve).
DISCUSSION
In the present study, the standard dose regimens provided sufficient t>MIC values for antibiotic efficacy during ECMO, which was reflected in the small number of positive blood cultures. The patient with the lowest t>MIC (49%) had negative cultures throughout his ECMO run, while the patients with positive cultures had t>MIC values of 90% or higher, but this could have been caused by resistance or lack of efficacy of other concomitant antibiotics. The CTX clearance estimate we found for ECMO patients (0.36 liter/h) was similar to those for non-ECMO-treated full-term neonates, which vary from 0.20 to 0.55 liter/h (6, 19, 23). The distribution volume, however, was larger than those for non-ECMO patients (1.82 liters versus 0.68 to 1.14 liters) (19, 23), which could be caused by hemodilution or capillary leakage of protein-bound drug into the extravascular compartment, especially in the early phase of ECMO (24 to 36 h after cannulation). This increase is consistent with studies on the pharmacokinetics of vancomycin (3) and theophylline (26) during ECMO. There were no signs of the rapid increase of V following cannulation that has been described for midazolam (1, 25). Unfortunately, we had only a few samples from both before and after ECMO, but for patients for whom we did have some samples there was an interesting clearance pattern, upon which we might formulate a hypothesis on the physiological processes involved. It would seem that these critically ill patients have a reduced clearance before cannulation. Many of them have received vasopressor drugs, with prolonged periods of circulatory shock and profound effects on renal function. As soon as ECMO is initiated, clearance rises to that of a non-ECMO-treated patient, possibly due to the continuous hemofiltration and improved organ perfusion that extracorporeal circulation provides. After decannulation, clearance drops again (as the patient is still critically ill) but slowly increases due to maturation or improved disease state. This pattern is visible for both CTX and DACT.
The t>MIC was sufficiently high despite the increased distribution volume, which suggests that cefotaxime is dosed higher than strictly necessary in non-ECMO patients. This need not be a problem with drugs that are as safe as cephalosporins are considered to be (11, 16). Our standard dose regimen is based on studies of neonatal and pediatric patients that have identified the influence of gestational age (20), body weight (20), postnatal age (6), and renal function (28) on CTX pharmacokinetics. Although creatinine clearance is a clinically relevant predictor of renal CTX clearance in non-ECMO patients (28), we had no measure of creatinine clearance due to the young age of most patients and the underlying disease state (12). Serum creatinine was measured, but there was no correlation with CTX clearance after body weight had been added to the model. Interestingly, gestational age and postnatal age did not predict CL or V; other factors, such as disease state, protein binding, organ perfusion, etc., might be responsible. A study of 107 neonates (6) showed that clearance increased dramatically with PNA during the first week after birth, but there was no sign of this development in our data set. It is possible that critical illness in our ECMO patients, with the use of drugs influencing renal perfusion (i.e., high doses of norepinephrine and dopamine), led to a low baseline renal clearance that was artificially supplemented by CVVH; the median QCVVH per individual did not vary much. Although we were able to identify several variables with statistically significant effects on CTX and DACT pharmacokinetics, the percentage of variability explained is 8.1% at maximum, which illustrates our limited understanding of ECMO-related sources of PK variability. Considering the sufficiently high t>MIC values in all patients, we probably do not need to adjust the dosage based on these covariates.
DACT concentrations are highly variable, as indicated in Fig. 3c and d. The contribution to the antibacterial effect varies with the microbial species involved, which makes it difficult to make a general assessment of efficacy (17). DACT concentrations are similar to those in other studies (6, 31); there does not seem to be an increased risk of DACT accumulation, as has been suggested for hydrophilic metabolites during ECMO (1). The concentrations may have been overestimated slightly because of the increased CTX hydrolysis that can occur following hemolysis, caused by contact with circuit surfaces or storage in plasma tubes (17).
Since most samples were taken during routine care, the data set contained a large number of samples for each patient, spread out over the full duration of ECMO. This allows a reliable characterization of time effects on PK parameters. A potential drawback of this method, as opposed to dose and sample registration by dedicated researchers or their assistants, is additional variability due to interobserver differences in registration. We expected a maximum discrepancy of 30 min between actual and recorded dose times, based on a comparison of observed work routines of individual nurses. A high residual variability in the first hour postdose was probably caused by internurse variability in the time between CTX injection and medication order validation. Since this phenomenon appeared to be distributed randomly over individuals, doses, tEC, etc., we estimated a separate residual variability, which in effect entails less influence on the final model than the samples taken at later dose times. This also affects the median curve of individual predictions compared to the same curve for the original observations (Fig. 3a versus b). Data that were recorded during standard clinical practice should therefore be used with caution, but a balanced data set without blood withdrawal at nonroutine sampling times offers important advantages.
Conclusions.
The standard cefotaxime dose regimen provides a sufficiently high t>MIC in ECMO infants. The CTX distribution volume is higher in ECMO patients than in non-ECMO patients (1.82 versus 0.68 to 1.14 liters), whereas CTX clearances are similar. A dose regimen of 50 mg/kg b.i.d. (PNA, <1 week), 50 mg/kg t.i.d. (PNA, 1 to 4 weeks), or 37.5 mg/kg q.i.d. (PNA, > 4 weeks) can be used to effectively treat these patients.
APPENDIX
The following equations were used to develop the final PK model for cefotaxime and desacetylcefotaxime.
Cefotaxime (CTX) equations were as follows.
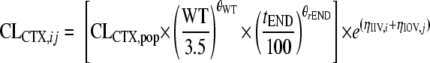 |
(A1) |
where CLCTX,ij is the CTX clearance for individual i at the jth occasion, CLCTX,pop is the population average CL for patients with a median weight (3.5 kg), WT is body weight, tEND is time after ECMO decannulation, ηIIV,i is the interindividual variability for individual i, and ηIOV,j is the accompanying interoccasion variability (in periods of 48 h during ECMO). When tEND = 0 (i.e., before and during ECMO), the accompanying covariate effect is removed from the equation.
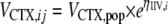 |
(A2) |
where VCTX,ij is the CTX distribution volume for individual i at the jth occasion, VCTX,pop is the population average, and ηIIV,i is the interindividual variability for individual i.
Desacetylcefotaxime (DACT) equations were as follows.
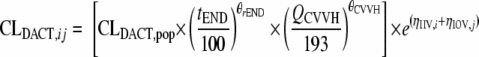 |
(A3) |
where CLDACT,ij is the DACT clearance for individual i at the jth occasion, CLDACT,pop is the population average, tEND is time after ECMO decannulation, QCVVH is the CVVH flow, ηIIV,i is the interindividual variability for individual i, and ηIOV,j is the accompanying interoccasion variability (in periods of 48 h during ECMO). When tEND = 0 or QCVVH = 0, the accompanying covariate effects are removed from the equation.
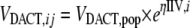 |
(A4) |
where VDACT,ij is the DACT distribution volume for individual i at the jth occasion, VDACT,pop is the population average, and ηIIV,i is the interindividual variability for individual i.
Differential equations used were as follows.
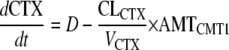 |
(A5) |
where dCTX/dt is the rate of CTX transit, D is the administered dose, CLCTX is CTX clearance, VCTX is the apparent volume of distribution, and AMTCMT1 is the amount of CTX present in compartment 1 at any one time.
 |
(A6) |
where dDACT/dt is the rate of DACT transit, CLCTX is CTX clearance, VCTX is the apparent volume of distribution of CTX, CLDACT is DACT clearance, VDACT is the apparent volume of distribution of DACT, AMTCMT1 is the amount of CTX present in compartment 1, and AMTCMT2 is the amount of DACT present in compartment 2 at any one time, assuming that all CTX is converted to DACT.
Footnotes
Published ahead of print on 22 February 2010.
REFERENCES
- 1.Ahsman, M. J., M. Hanekamp, E. D. Wildschut, D. Tibboel, and R. A. Mathot. Population pharmacokinetics of midazolam and metabolites during venoarterial extracorporeal membrane oxygenation in neonates. Clin. Pharmacokinet., in press. [DOI] [PubMed]
- 2.Ahsman, M. J., E. D. Wildschut, D. Tibboel, and R. A. Mathot. 2009. Microanalysis of beta-lactam antibiotics and vancomycin in plasma for pharmacokinetic studies in neonates. Antimicrob. Agents Chemother. 53:75-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amaker, R. D., J. T. DiPiro, and J. Bhatia. 1996. Pharmacokinetics of vancomycin in critically ill infants undergoing extracorporeal membrane oxygenation. Antimicrob. Agents Chemother. 40:1139-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson, B. J., K. Allegaert, and N. H. Holford. 2006. Population clinical pharmacology of children: modelling covariate effects. Eur. J. Pediatr. 165:819-829. [DOI] [PubMed] [Google Scholar]
- 5.Bennett, C. C., and C. F. Davis. 2003. Evidence-based use of neonatal extracorporeal membrane oxygenation (ECMO). Curr. Paediatr. 2003:146-150. [Google Scholar]
- 6.Bertels, R. A., B. A. Semmekrot, G. P. Gerrits, and J. W. Mouton. 2008. Serum concentrations of cefotaxime and its metabolite desacetyl-cefotaxime in infants and children during continuous infusion. Infection 36:415-420. [DOI] [PubMed] [Google Scholar]
- 7.Buck, M. L. 2003. Pharmacokinetic changes during extracorporeal membrane oxygenation: implications for drug therapy of neonates. Clin. Pharmacokinet. 42:403-417. [DOI] [PubMed] [Google Scholar]
- 8.Cook, L. N. 2004. Update on extracorporeal membrane oxygenation. Paediatr. Respir. Rev. 5(Suppl. A):S329-S337. [DOI] [PubMed] [Google Scholar]
- 9.de Hoog, M., J. W. Mouton, and J. N. van den Anker. 2005. New dosing strategies for antibacterial agents in the neonate. Semin. Fetal Neonatal Med. 10:185-194. [DOI] [PubMed] [Google Scholar]
- 10.Ette, E. I. 1997. Stability and performance of a population pharmacokinetic model. J. Clin. Pharmacol. 37:486-495. [DOI] [PubMed] [Google Scholar]
- 11.Fanos, V., and A. Dall'Agnola. 1999. Antibiotics in neonatal infections: a review. Drugs 58:405-427. [DOI] [PubMed] [Google Scholar]
- 12.Harrison, A. M., S. Davis, S. Eggleston, R. Cunningham, R. B. Mee, and P. M. Bokesch. 2003. Serum creatinine and estimated creatinine clearance do not predict perioperatively measured creatinine clearance in neonates undergoing congenital heart surgery. Pediatr. Crit. Care Med. 4:55-59. [DOI] [PubMed] [Google Scholar]
- 13.Hartwig, N. G., P. C. J. De Laat, and L. M. Hanff (ed.). 2005. Vademecum pediatrische antimicrobiële therapie (handbook of pediatric antimicrobial therapy), 3rd ed. Erasmus University Medical Center, Rotterdam, Netherlands.
- 14.Hooker, A. C., C. E. Staatz, and M. O. Karlsson. 2007. Conditional weighted residuals (CWRES): a model diagnostic for the FOCE method. Pharm. Res. 24:2187-2197. [DOI] [PubMed] [Google Scholar]
- 15.Hsu, M. S., K. M. Chiu, Y. T. Huang, K. L. Kao, S. H. Chu, and C. H. Liao. 2009. Risk factors for nosocomial infection during extracorporeal membrane oxygenation. J. Hosp. Infect. 73:210-216. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs, R. F. 1991. Efficacy and safety of cefotaxime in the management of pediatric infections. Infection 19(Suppl. 6):S330-S336. [DOI] [PubMed] [Google Scholar]
- 17.Jones, R. N. 1995. Cefotaxime and desacetylcefotaxime antimicrobial interactions. The clinical relevance of enhanced activity: a review. Diagn. Microbiol. Infect. Dis. 22:19-33. [DOI] [PubMed] [Google Scholar]
- 18.Jonsson, E. N., and M. O. Karlsson. 1999. Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput. Methods Programs Biomed. 58:51-64. [DOI] [PubMed] [Google Scholar]
- 19.Kafetzis, D. A., D. C. Brater, A. N. Kapiki, C. V. Papas, H. Dellagrammaticas, and C. J. Papadatos. 1982. Treatment of severe neonatal infections with cefotaxime. Efficacy and pharmacokinetics. J. Pediatr. 100:483-489. [DOI] [PubMed] [Google Scholar]
- 20.Kearns, G. L., R. F. Jacobs, B. R. Thomas, T. L. Darville, and J. M. Trang. 1989. Cefotaxime and desacetylcefotaxime pharmacokinetics in very low birth weight neonates. J. Pediatr. 114:461-467. [DOI] [PubMed] [Google Scholar]
- 21.Lindbom, L., P. Pihlgren, and E. N. Jonsson. 2005. PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput. Methods Programs Biomed. 79:241-257. [DOI] [PubMed] [Google Scholar]
- 22.Mandema, J. W., D. Verotta, and L. B. Sheiner. 1992. Building population pharmacokinetic-pharmacodynamic models. I. Models for covariate effects. J. Pharmacokinet. Biopharm. 20:511-528. [DOI] [PubMed] [Google Scholar]
- 23.McCracken, G. H., Jr., N. E. Threlkeld, and M. L. Thomas. 1982. Pharmacokinetics of cefotaxime in newborn infants. Antimicrob. Agents Chemother. 21:683-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mueller, M., A. de la Pena, and H. Derendorf. 2004. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: kill curves versus MIC. Antimicrob. Agents Chemother. 48:369-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulla, H., P. McCormack, G. Lawson, R. K. Firmin, and D. R. Upton. 2003. Pharmacokinetics of midazolam in neonates undergoing extracorporeal membrane oxygenation. Anesthesiology 99:275-282. [DOI] [PubMed] [Google Scholar]
- 26.Mulla, H., F. Nabi, S. Nichani, G. Lawson, R. K. Firmin, and D. R. Upton. 2003. Population pharmacokinetics of theophylline during paediatric extracorporeal membrane oxygenation. Br. J. Clin. Pharmacol. 55:23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Odio, C. M. 1995. Cefotaxime for treatment of neonatal sepsis and meningitis. Diagn. Microbiol. Infect. Dis. 22:111-117. [DOI] [PubMed] [Google Scholar]
- 28.Paap, C. M., M. C. Nahata, M. A. Mentser, J. D. Mahan, S. K. Puri, and J. W. Hubbard. 1991. Pharmacokinetics of cefotaxime and its active metabolite in children with renal dysfunction. Antimicrob. Agents Chemother. 35:1879-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel, K. B., D. P. Nicolau, C. H. Nightingale, and R. Quintiliani. 1995. Pharmacokinetics of cefotaxime in healthy volunteers and patients. Diagn. Microbiol. Infect. Dis. 22:49-55. [DOI] [PubMed] [Google Scholar]
- 30.Savic, R. M., and M. O. Karlsson. 2007. Shrinkage in empirical Bayes estimates for diagnostics and estimation: problems and solutions. Population Approach Group Europe, Copenhagen, Denmark.
- 31.Trang, J. M., R. F. Jacobs, G. L. Kearns, A. L. Brown, T. G. Wells, F. L. Underwood, and R. B. Kluza. 1985. Cefotaxime and desacetylcefotaxime pharmacokinetics in infants and children with meningitis. Antimicrob. Agents Chemother. 28:791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wise, R., and N. Wright. 1985. The pharmacokinetics of cefotaxime and ceftriaxone in renal and hepatic dysfunction. Infection 13(Suppl. 1):S145-S150. [DOI] [PubMed] [Google Scholar]
- 33.Wishart, D. S., C. Knox, A. C. Guo, D. Cheng, S. Shrivastava, D. Tzur, B. Gautam, and M. Hassanali. 2008. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 36:D901-D906. [DOI] [PMC free article] [PubMed] [Google Scholar]