Abstract
The activities of two naturally occurring compounds, isobavachalcone and diospyrone, against documented strains and multidrug-resistant (MDR) Gram-negative bacterial isolates were evaluated. The results indicated that the two compounds exhibited intrinsic antibacterial activity against several Gram-negative bacteria, and their activities were significantly improved in the presence of an efflux pump inhibitor (MIC values decreased to below 10 μg/ml). In addition, the activities of isobavachalcone and diospyrone against various strains exhibiting deletions of the major efflux pump components (AcrAB, TolC) were significantly increased. The overall results indicate that isobavachalcone and diospyrone could be candidates for the development of new drugs against MDR strains and that their use in combination with efflux pump inhibitors reinforces their activity.
The continuous emergence of multidrug-resistant (MDR) bacteria drastically reduces the efficacy of our antibiotic armory and, consequently, increases the frequency of therapeutic failure (10, 27). Drug resistance is a consequence of the worldwide use of antibiotics, and the acute challenge for health care is to find measures that efficiently combat resistant organisms (10, 27, 31). This includes improved early infection control, the use of appropriate therapies, and the use of hospital measures to prevent the dissemination of MDR strains, as well as the development of new antibiotics (31). The resistance of bacteria to chemically unrelated antimicrobial agents (or MDR) may be associated with the overexpression of efflux pumps (15, 25). In Gram-negative bacteria, many of these efflux pumps belong to the resistance-nodulation-cell division (RND) family of tripartite efflux pumps. Among those efflux pumps, pumps belonging to the AcrAB-TolC family are detected in many clinical enterobacterial isolates and are reported to be a key factor in the expression of the MDR phenotype (16, 19, 28). Several RND efflux pumps have been identified in clinical isolates of Pseudomonas aeruginosa, another important nosocomial pathogen highly resistant to the commonly used antibiotics (2, 9, 15). This efflux pump mechanism can be blocked by various efflux pump inhibitors that restore the intracellular concentration as well as the activities of the antibiotics (23).
The scarcity of original synthetic antibiotics has stimulated the search for new antibacterial agents from medicinal plants (6, 12, 17). Isobavachalcone has been isolated from several medicinal plants (20, 21, 24). Isobavachalcone was reported to have very interesting activities against Candida albicans and Cryptococcus neoformans (9), and some preliminary results have indicated that this compound has activity against susceptible microorganisms (20). However, the activity of this compound against resistant bacteria and its mode of action were not elucidated. At present, Diospyros canaliculata is the only reported source of diospyrone (29). We have recently described the activity of diospyrone against Neisseria gonorrhoeae and Mycobacterium tuberculosis (14), but its activity against resistant bacteria and its target have not been reported.
In the study described here, we evaluated the activities of isobavachalcone and diospyrone against various Gram-negative bacteria, including MDR hospital isolates. The spectrum of action of these molecules regarding the role of efflux pumps in their activity was also investigated by using various documented strains and a previously described efflux pump inhibitor.
MATERIALS AND METHODS
Chemicals for antimicrobial assays.
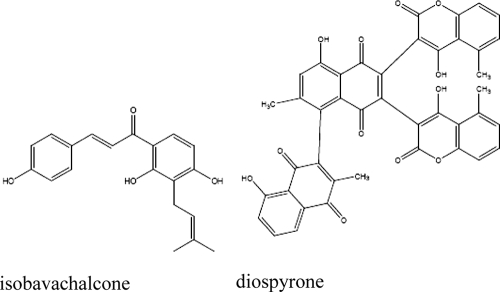
Isobavachalcone and diospyrone (Fig. 1) were obtained from the chemical stock bank of the Laboratory of Organic Chemistry, University of Yaoundé I, Yaoundé, Cameroon. We recently reported on the isolation and identification of isobavachalcone from Dorstenia barteri (20) and diospyrone from Diospyros canaliculata (29). Chloramphenicol and norfloxacin (Sigma-Aldrich, St. Quentin Fallavier, France), tetracycline hydrochloride (Merck KGaA, Darmstadt, Germany), imipenem-cilastatin (500/500 mg; Merck, Paris, France), and cefepime (Bristol-Myers, Reuil-Malmaison, France) were used as selected or reference antibiotics. p-Iodonitrotetrazolium chloride (INT), phenylalanine arginine ß-naphthylamide (PAßN), and 1,3,5-triphenyltetrazolium chloride (TTC) (Sigma-Aldrich) were also used in this study.
FIG. 1.
Chemical structures of isobavachalcone and diospyrone.
Bacterial strains and culture media.
The microbial species used included MDR and reference strains of Escherichia coli, Enterobacter aerogenes, Enterobacter cloacae, Pseudomonas aeruginosa, and Klebsiella pneumoniae. Their features are summarized in Table 1. E. cloacae strains Ec0769 and Ec1194 were from the laboratory collection (UMR-MD1, Université de la Méditerranée, Marseille, France). All strains were precultured overnight on Mueller-Hinton agar, prior to any assay. Mueller-Hinton broth (MHB) was used as the liquid culture medium for susceptibility tests (13, 20).
TABLE 1.
Bacterial strains and features
| Bacterial strain | Relevant feature(s)a | Reference(s) |
|---|---|---|
| E. coli | ||
| ATCC 8739 and ATCC 10536 | Reference strains | |
| AG100 | Wild-type E. coli K-12 | 30 |
| AG100A | AG100 acrAB::Kanr | 22, 30 |
| AG100ATet | Tetr derivative of AG100A in which the acrF gene is markedly overexpressed | 30 |
| AG102 | AG100 overexpressing the AcrAB pump | 7 |
| E. aerogenes | ||
| ATCC 13048 | Reference strain | |
| EA-CM64 | Chlr variant obtained from ATCC 13048 overexpressing the AcrAB pump | 11 |
| EA3 | Clinical MDR isolate; Chlr Norr Ofxr Spxr Moxr Cftr Atmr Fepr | 18, 19 |
| EA5 | Clinical MDR isolate exhibiting energy-dependent norfloxacin and chloramphenicol efflux; Moxr Cftr Atmr Fepr | 18, 19 |
| EA27 | Clinical MDR isolate exhibiting energy-dependent norfloxacin and chloramphenicol efflux with Kanr Ampr Nalr Strr Tetr | 18, 19 |
| EA289 | KAN-sensitive derivative of EA27 | 26 |
| EA294 | EA289 acrA::Kanr | 26 |
| EA298 | EA289 tolC::Kanr | 26 |
| E. cloacae | ||
| Ec0769 and Ec1194 | Clinical isolates | This study |
| K. pneumoniae | ||
| ATCC 12296 | Reference strain | |
| KP55 | Clinical MDR isolate; Tetr Ampr Atmr Cefr | 3 |
| KP63 | Clinical MDR isolate; Tetr Chlr Ampr Atmr | 3 |
| P. aeruginosa | ||
| PA01 | Reference strain | |
| PA124 | Clinical MDR isolate | 17 |
Smpr, Atmr, Cef, Cftr, Chlr, Fepr, Kanr, Moxr, Strr, and Tetr, resistance to ampicillin, aztreonam, cephalothin, cefadroxil, chloramphenicol, cefepime, kanamycin, moxalactam, streptomycin, and tetracycline, respectively.
Bacterial susceptibility determinations.
The MICs of the two compounds and antibiotics were determined by a rapid INT colorimetric assay (8, 13). Briefly, the test sample and selected antibiotics were first dissolved in dimethyl sulfoxide (DMSO)-MHB. The solution obtained was then added to MHB and serially diluted twofold (in a 96-well microplate). One hundred microliters of inoculum (1.5 × 106 CFU/ml) prepared in MHB was then added. The plates were covered with a sterile plate sealer and then agitated with a shaker to mix the contents of the wells and incubated at 37°C for 18 h. The final concentration of DMSO was less than 2.5%, and DMSO did not affect the microbial growth. Wells containing MHB, 100 μl of inoculum, and DMSO at a final concentration of 2.5% served as the negative control (this internal control was systematically added). The MICs of samples were detected after 18 h of incubation at 37°C, following addition (40 μl) of 0.2 mg/ml INT and incubation at 37°C for 30 min. Viable bacteria reduced the yellow dye to pink. The MIC was defined as the lowest sample concentration that prevented this change and that resulted in the complete inhibition of microbial growth. The samples were tested alone and in the presence of PAßN at a final concentration of 20 μg/ml, as described previously (11). The MICs of PAßN were 64 μg/ml for E. coli AG100A, 256 μg/ml for K. pneumoniae ATCC 11296, and >256 μg/ml for all other strains and organisms. Each assay was repeated three times independently.
RESULTS AND DISCUSSION
Activities of isobavachalcone and diospyrone and role of efflux pumps in susceptibility of Gram-negative bacteria.
The various strains and MDR isolates were tested for their susceptibilities to isobavachalcone, diospyrone, and reference antibiotics (norfloxacin, chloramphenicol) alone and then in the presence of PAßN, a well-known efflux pump inhibitor (4, 16, 19, 23). The results presented in Table 2 indicate that the two natural products exhibited activities against all strains. Interestingly, the activities of the two compounds against MDR isolates, e.g., strains EA5 and KP63, were better than those of the commonly used antibiotics (Table 2). The lowest MIC values for diospyrone (4 μg/ml) and isobavachalcone (8 μg/ml) were recorded for E. coli AG100A and E. aerogenes EA298, respectively. This result may indicate that the mechanisms involved in resistance to usual antibiotics are less efficient against these two compounds.
TABLE 2.
MICs of the two natural compounds for reference and documented strains and clinical MDR isolates
| Bacterium and strain | MIC (μg/ml)b |
|||||||
|---|---|---|---|---|---|---|---|---|
| Isobavachalcone |
Diospyrone |
Chloramphenicol |
Norfloxacin |
|||||
| − | + | − | + | − | + | − | + | |
| E. coli | ||||||||
| ATCC 10536 | 128 | 2 | 32 | 2 | 1 | 0.5 | 0.06 | 0.03 |
| ATCC 8739 | 256 | 8 | 128 | 4 | 4 | 1 | 0.12 | 0.12 |
| AG100 | 64 | 0.5 | 64 | 1 | 4 | 0.25 | 0.12 | 0.12 |
| AG100A | 16 | 0.25 | 4 | 0.12 | 0.5 | 0.25 | 0.03 | 0.007 |
| AG100ATet | 64 | 8 | 16 | 0.24 | 32 | 2 | 1 | 0.25 |
| AG102 | 64 | 8 | 64 | 2 | 32 | 2 | 1 | 0.25 |
| E. aerogenes | ||||||||
| ATCC 13048 | 256 | 16 | 128 | 32 | 4 | 1 | 0.25 | 0.25 |
| EA-CM64 | >256 | 256 | 128 | 16 | 256 | 8 | 4 | 2 |
| EA289 | 256 | 16 | 128 | 8 | >256 | 128 | 128 | 128 |
| EA294 | 32 | 0.5 | 128 | 8 | 64 | 16 | 64 | 32 |
| EA298 | 8 | 0.5 | 32 | 16 | 64 | 16 | 8 | 8 |
| EA27 | 256 | 8 | 128 | 16 | >256 | 128 | 256 | 128 |
| EA3 | 128 | 32 | 128 | 64 | >256 | 128 | 128 | 64 |
| EA5 | 64 | 16 | 128 | 64 | >256 | 32 | 256 | 128 |
| K. pneumoniae | ||||||||
| ATCC 11296 | 32 | 4 | 32 | 2 | 2 | 2 | 1 | 0.5 |
| KP55 | 32 | 4 | 64 | 8 | 32 | 4 | 16 | 8 |
| KP63 | 16 | 0.5 | 32 | 4 | >256 | 128 | 16 | 4 |
| P. aeruginosa | ||||||||
| PAO1 | 64 | 16 | 64 | 4 | 128 | 8 | 2 | 1 |
| PA124 | 64 | 4 | 64 | 1 | 256 | 8 | 64 | 32 |
| E. cloacae | ||||||||
| Ec07769 | 128 | 8 | 128 | 8 | >256 | 256 | >256 | >256 |
| Ec1194 | 64 | 1 | 32 | 2 | 2 | 1 | 32 | 32 |
The drugs and compounds were tested in the absence (−) or in the presence (+) of PAßN at a final concentration of 20 μg/ml, as described previously (11). At this concentration, no intrinsic effect against the various bacterial strains (included as internal controls in each assay without antibiotic) was observed.
The antibacterial activities of the two compounds were significantly increased in the presence of the efflux pump inhibitor PAßN, with all MICs obtained decreasing to below 10 μg/ml for the E. coli, K. pneumoniae, and E. cloacae strains (Table 2). In addition, this enhanced activity was observed against various strains of E. coli, E. aerogenes, K. pneumoniae, P. aeruginosa, and E. cloacae. When norfloxacin was included with chloramphenicol and they were used as reference antibiotics, the presence of an additional resistance mechanism, such as the target mutation previously described in the clinical isolates tested, strongly modulates the level of restoration of susceptibility (19). It is important to mention that the susceptibilities of strains AG100A and EA294, with both strains being devoid of AcrA and with EA298 being devoid of TolC, were noticeably increased by PAßN (Table 2). A similar effect concerning the activities of macrolides-ketolides and chloramphenicol against the same strains has previously been reported, suggesting the presence of an additional efflux system (4, 11).
All E. aerogenes strains except EA298 were resistant to isobavachalcone and diospyrone (MICs, 8 and 32 μg/ml, respectively). Nevertheless, the activity of isobavachalcone was better than that of chloramphenicol against six of the eight E. aerogenes strains studied. Diospyrone was also more active than chloramphenicol against all E. aerogenes strains except strains ATCC 13048 and EA294. In the presence of PAßN, the activities of the two compounds against all E. aerogenes strains increased, with the isobavachalcone MIC values being below 1 μg/ml for strains EA294 and EA298 (Table 2).
In this study, the antimicrobial activities of isobavachalcone and diospyrone were significantly improved in the presence of an efflux pump inhibitor (Table 2), suggesting that efflux is likely one of the mechanisms that modulates the susceptibilities of E. coli, E. aerogenes, K. pneumoniae, P. aeruginosa, and E. cloacae. The significant increase in the levels of susceptibility to these compounds observed in strains with acrB and tolC deletions demonstrates the role of the major AcrAB-TolC efflux pump. However, the increase in the susceptibilities of strains EA298 and EA294 (tolC and acrA strains respectively) caused by PAßN indicated that an additional efflux system(s) is active in these strains, as reported previously (4, 11). This PAßN-susceptible efflux system also contributes to resistance to isobavachalcone and diospyrone. The efflux mechanisms clearly appear to be the first line of bacterial defense against these molecules, as has been demonstrated for other natural compounds (1, 5). This study supports the evidence of the antimicrobial potencies of these compounds and also highlights the activities of the compounds against MDR strains. The results obtained in the present study should be taken into consideration given the clinical relevance of the resistant bacteria studied. Both strain KP55 and strain KP63 were reported to be resistant to most of the commonly used antibiotics, showing high levels of resistance to ampicillin, ceftazidime, and aztreonam (MIC values, up to 512 μg/ml) (3). In the present work, we observed that all those MDR bacteria were susceptible to the two compounds studied, especially in the presence of the efflux pump inhibitor. It appeared that isobavachalcone and diospyrone exhibited more significant effects against resistant strains compared with the activities of the reference drugs. In addition, Nishimura et al. (21) showed that isobavachalcone is nontoxic to healthy eukaryotic cells, supporting its possible clinical use.
The overall results of this investigation indicate that isobavachalcone and diospyrone may be interesting candidates for development as new antimicrobials with activity against MDR bacteria. The study also demonstrates that the drug efflux mechanism is one of the primary active defense mechanisms of bacterial cells against these molecules. This therefore indicates that the use of these types of natural products in combination with efflux pump inhibitors in future drug formulations should be considered.
Acknowledgments
We are grateful to C. A. Elkins and L. Amaral for the generous gifts of the microbial strains. We also thank A. Davin, J. Chevalier, A. Lieutaud, E. Goemaere, A. Monitor, and P. Lunga for their helpful advice and fruitful discussions.
Victor Kuete thanks the Agence Universitaire de la Francophonie (AUF) for the travel-stay grant at the Université de la Méditerranée. This work was supported by the Service de Santé des Armées and the Université de la Méditerranée.
Footnotes
Published ahead of print on 16 February 2010.
REFERENCES
- 1.Ball, A. R., G. Casadei, S. Samosorn, J. B. Bremner, F. M. Ausubel, T. I. Moy, and K. Lewis. 2006. Conjugating berberine to a multidrug efflux pump inhibitor creates an effective antimicrobial. ACS Chem. Biol. 1:594-600. [DOI] [PubMed] [Google Scholar]
- 2.Cardoso, O., A. F. Alves, and R. M. Leitao. 2008. Antimicrobial susceptibility among Pseudomonas aeruginosa isolates from a central hospital in the centre of Portugal during four years. Int. J. Infect. Dis. 12:e111. doi: 10.1016/j.ijid.2008.05.277. [DOI] [Google Scholar]
- 3.Chevalier, J., J.-M. Pagès, A. Eyraud, and M. Malléa. 2000. Membrane permeability modifications are involved in antibiotic resistance in Klebsiella pneumoniae. Biochem. Biophys. Res. Commun. 274:496-499. [DOI] [PubMed] [Google Scholar]
- 4.Chollet, R., J. Chevalier, A. Bryskier, and J.-M. Pagès. 2004. The AcrAB-TolC pump is involved in macrolide resistance but not in telithromycin efflux in Enterobacter aerogenes and Escherichia coli. Antimicrob. Agents Chemother. 48:3621-3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cordell, G. A. 1993. New roots for an old science. In Atta-ur-Rahman and F. Z. Basha (ed.), Studies in natural products chemistry. Pharmacognosy 13: Bioactive natural products part A. Elsevier, Amsterdam, Netherlands.
- 6.Cox, S. D., and J. L. Markham. 2007. Susceptibility and intrinsic tolerance of Pseudomonas aeruginosa to selected plant volatile compounds. J. Appl. Microbiol. 103:930-936. [DOI] [PubMed] [Google Scholar]
- 7.Elkins, C. A., and L. B. Mullis. 2007. Substrate competition studies using whole-cell accumulation assays with the major tripartite multidrug efflux pumps of Escherichia coli. Antimicrob. Agents Chemother. 51:923-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eloff, J. N. 1998. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 64:711-713. [DOI] [PubMed] [Google Scholar]
- 9.ElSohly, H. N., A. S. Joshi, A. C. Nimrod, L. A. Walker, and A. M. Clark. 2001. Antifungal chalcones from Maclura tinctoria. Planta Med. 67:87-89. [DOI] [PubMed] [Google Scholar]
- 10.Falagas, M. E., and I. A. Bliziotis. 2007. Pandrug-resistant Gram-negative bacteria: the dawn of the post-antibiotic era? Int. J. Antimicrob. Agents 29:630-636. [DOI] [PubMed] [Google Scholar]
- 11.Ghisalberti, D., M. Masi, J.-M. Pagès, and J. Chevalier. 2005. Chloramphenicol and expression of multidrug efflux pump in Enterobacter aerogenes. Biochem. Biophys. Res. Commun. 328:1113-1118. [DOI] [PubMed] [Google Scholar]
- 12.Gordon, M. C., and J. N. David. 2001. Natural product drug discovery in the next millennium. Pharm. Biol. 39(suppl.): 8-17. [DOI] [PubMed] [Google Scholar]
- 13.Kuete, V., T. A. Mbaveng, M. Tsafack, P. V. Beng, F. X. Etoa, A. E. Nkengfack, J. J. Marion Meyer, and N. Lall. 2008. Antitumor, antioxidant and antimicrobial activities of Bersama engleriana (Melianthaceae). J. Ethnopharmacol. 115:494-501. [DOI] [PubMed] [Google Scholar]
- 14.Kuete, V., J. G. Tangmouo, J. J. Meyer, and N. Lall. 2009. Diospyrone, crassiflorone, and plumbagin: three antimycobacterial and antigonorrheal naphthoquinones from two Diospyros species. Int. J. Antimicrob. Agents 34:322-325. [DOI] [PubMed] [Google Scholar]
- 15.Li, X. Z., and H. Nikaido. 2009. Efflux-mediated drug resistance in bacteria: an update. Drugs 69:1555-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lomovskaya, O. M. S., A. Warren, J. Lee, R. Galazzo, M. Fronko, J. Lee, J. Blais, D. Cho, S. Chamberland, T. Renau, R. Leger, S. Hecker, W. Watkins, K. Hoshino, H. Ishida, and V. J. Lee. 2004. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob. Agents Chemother. 45:105-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorenzi, V., A. Muselli, A. F. Bernardini, L. Berti, J.-M. Pagès, L. Amaral, and J. M. Bolla. 2009. Geraniol restores antibiotic activities against multidrug resistant isolate from gram-negative species. Antimicrob. Agents Chemother. 53:2209-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malléa, M., J. Chevalier, C. Bornet, A. Eyraud, J.-M. Pagès, and A. Davin-Régli. 1998. Porin alteration and active efflux: two in vivo drug resistance strategies used by Enterobacter aerogenes. Microbiology 144:3003-3009. [DOI] [PubMed] [Google Scholar]
- 19.Malléa, M., A. Mahamoud, J. Chevalier, S. Alibert-Franco, P. Brouant, J. Barbe, and J.-M. Pagès. 2003. Alkylaminoquinolines inhibit the bacterial antibiotic efflux pump in multidrug-resistant clinical isolates. Biochem. J. 376:801-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mbaveng, T. A., B. Ngameni, V. Kuete, K. S. Konga, P. Ambassa, R. Roy M. Bezabih, F. X. Etoa, B. T. Ngadjui, B. M. Abegaz, J. J. Marion Meyer, N. Lall, and V. P. Beng. 2008. Antimicrobial activity of the crude extracts and five flavonoids from the twigs of Dorstenia barteri (Moraceae). J. Ethnopharmacol. 116:483-489. [DOI] [PubMed] [Google Scholar]
- 21.Nishimura, R., K. Tabata, M. Arakawa, Y. Ito, Y. Kimura, T. Akihisa, N. Hisashi, S. Atsuko, K. Hideki, and S. Takashi. 2007. Isobavachalcone, a chalcone constituent of Angelica keiskei, induces apoptosis in neuroblastoma. Biol. Pharm. Bull. 30:1878-1883. [DOI] [PubMed] [Google Scholar]
- 22.Okusu, H., D. Ma, and H. Nikaido. 1996. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance Mar mutants. J. Bacteriol. 178:306-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pagès, J.-M., and L. Amaral. 2009. Mechanisms of drug efflux and strategies to combat them: challenging the efflux pump of Gram-negative bacteria. Biochim. Biophys. Acta 1794:826-833. [DOI] [PubMed] [Google Scholar]
- 24.Pistelli, L., K. Spera, G. Flamini, S. Mele, and I. Morelli. 1996. Isoflavonoids and chalcones from Anthyllis hermanniae. Phytochemistry 42:1455-1458. [Google Scholar]
- 25.Poole, K. 2004. Efflux-mediated multiresistance in Gram-negative bacteria. Clin. Microbiol. Infect. 10:12-26. [DOI] [PubMed] [Google Scholar]
- 26.Pradel, E., and J.-M. Pagès. 2002. The AcrAB-TolC efflux pump contributes to multidrug resistance in the nosocomial pathogen Enterobacter aerogenes. Antimicrob. Agents Chemother. 46:2640-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rice, L. B. 2006. Unmet medical needs in antibacterial therapy. Biochem. Pharmacol. 71:991-995. [DOI] [PubMed] [Google Scholar]
- 28.Sulavik, M. C., C. Houseweart, C. Cramer, J. N. Murgolo, B. Greene, D. Beth, S. K. Joy, M. H. George, H. Roberta, and G. Shimer. 2001. Antibiotic susceptibility profiles of Escherichia coli strains lacking multidrug efflux pump genes. Antimicrob. Agents Chemother. 45:1126-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tangmouo, J. G., D. Lontsi, F. N. Ngounou, V. Kuete, A. L. Meli, R. N. Manfouo, H. W. Kamdem, P. Tane, V. P. Beng, B. L. Sondengam, and J. D. Connolly. 2005. Diospyrone, a new coumarinylbinaphthoquinone from Diospyros canaliculata Ebenaceae: structure and antimicrobial activity. Bull. Chem. Soc. Ethiop. 19:81-88. [Google Scholar]
- 30.Viveiros, M., A. Jesus, M. Brito, C. Leandro, M. Martins, D. Ordway, M. Molnar, J. Molnar, and L. Amaral. 2005. Inducement and reversal of tetracycline resistance in Escherichia coli K-12 and expression of proton gradient-dependent multidrug efflux pump genes. Antimicrob. Agents Chemother. 49:3578-3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. 2001. Infection control programmes to contain antimicrobial resistance. World Health Organization, Geneva, Switzerland. http://whqlibdoc.who.int/hq/2001/WHO_CDS_CSR_DRS_2001.7.pdf. Accessed January 2009.