Abstract
The in vitro activity of CEM-101, a new fluoroketolide, was determined against Gram-positive organisms with various macrolide susceptibility profiles. Experiments for determination of the MICs and minimum bactericidal concentrations (MBCs), timed killing, single-step and multistep mutation rates, the erythromycin induction of resistance, postantibiotic effect (PAE), and drug interactions were performed for CEM-101; and the results were compared to those obtained with telithromycin, macrolides, and lincosamides. The MBCs of CEM-101 remained lower overall than those of telithromycin, and CEM-101 displayed a 2-fold greater potency than the ketolide. Timed-killing curve testing showed that CEM-101 had greater bactericidal activity than telithromycin (a ≥3-log10-CFU/ml decrease in the initial inoculum at 24 h) against the staphylococcal isolates tested. The propensity of CEM-101 to cause resistance was low, as determined from the rates of resistance determined in single-step mutational studies (<10−8 or 10−9). In multipassaging studies, mutants of two strains (both of which were USA300 isolates) resistant to CEM-101 emerged. That number was comparable to the number resistant to clindamycin but less than the number resistant to telithromycin. Erythromycin induced CEM-101 resistance in Staphylococcus aureus and Streptococcus pneumoniae, similar to telithromycin; however, in seven of eight beta-hemolytic streptococci, CEM-101 resistance induction was not observed. CEM-101 showed a significant concentration- and exposure-dependent PAE against the strains tested, with the values ranging from 2.3 to 6.1 h for Gram-positive organisms (these times were longer than those for telithromycin). No antagonism was found in synergy analyses, with enhanced inhibition being most noted for combinations with CEM-101 and ceftriaxone, gentamicin, and trimethoprim-sulfamethoxazole. Overall, this new antimicrobial agent (CEM-101) showed good antimicrobial characteristics compared with those of the agents in its class and exhibited measured parameter values similar or superior to those of utilized comparators, indicating that CEM-101 warrants further clinical evaluation.
Increased antimicrobial resistance among Gram-positive pathogens is occurring worldwide (1). Infections caused by methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and penicillin-resistant Streptococcus pneumoniae are becoming increasingly difficult to treat. Additionally, emerging cases of macrolide-resistant S. pneumoniae and Streptococcus pyogenes are causing global alarm (1, 14). Therefore, new oral and/or parenteral antimicrobial agents with activities against these Gram-positive pathogens are in demand.
Ketolides are semisynthetic antimicrobial agents derived from erythromycin A and were designed to overcome macrolide-resistant S. pneumoniae (10, 23). Ketolides have a keto group at the C-3 position of the lactone ring rather than l-cladinose, which is found in erythromycin (10). Telithromycin was the first ketolide approved for clinical use, and while this antimicrobial agent performs well in vitro against Gram-positive bacterial strains and some fastidious Gram-negative bacterial strains (Haemophilus influenzae, Moraxella catarrhalis), issues with hepatotoxicity and other adverse events have been documented (2, 8, 22, 23). Telithromycin often lacks activity against strains with constitutive macrolide-lincosamide-streptogramin B (cMLSB) resistance and is capable of inducing erm methylase genes within a narrow concentration range (10).
CEM-101 is a new fluoroketolide that displays activity against many pathogens that cause respiratory tract infections, uncomplicated skin and skin structure infections (SSSIs), and urogenital infections (8, 21). This new compound has potent activity against Gram-positive pathogens, including macrolide-resistant strains and various fastidious Gram-negative strains, including Haemophilus spp., Moraxella spp., and species of Mycoplasma and Ureaplasma (8, 21). Preliminary in vitro studies have shown that CEM-101 demonstrates activity comparable or superior to the activities of telithromycin, erythromycin, azithromycin, and clarithromycin (8).
The purpose of the study described here was to further investigate the potential in vitro activity of CEM-101 against a collection of isolates, including strains with reduced susceptibilities to macrolides that carry distinct resistance mechanisms.
MATERIALS AND METHODS
Bacterial isolates.
Isolates were selected for passaging, single-step mutation, postantibiotic effect (PAE), and timed-killing studies on the basis of their MIC profiles and molecular characteristics, as defined in Table 1. Erythromycin resistance induction testing included 81 clinical isolates from surveillance initiatives displaying an erythromycin-resistant, clindamycin-susceptible phenotype. Selected organisms (D-test positive) were screened by PCR molecular methods for mechanisms of macrolide resistance, as described previously (3). Forty clinical isolates were tested in minimum bactericidal concentration (MBC) studies, including 10 S. pneumoniae isolates; 10 S. aureus isolates; and 5 isolates each of beta-hemolytic streptococci, viridans group streptococci, coagulase-negative staphylococci (CoNS), and enterococci. Additionally, 22 clinical and ATCC strains, including 9 S. aureus strains, 6 beta-hemolytic streptococci, and 7 S. pneumoniae strains, were used for drug interaction (synergy) studies.
TABLE 1.
Bacterial isolates used in this study
| Isolate | Expt(s) performed | CEM-101 MIC range (μg/ml) | Characteristic(s) |
|---|---|---|---|
| S. aureus ATCC 29213 | Passaging,a single-step mutation, postantibiotic effect, timed-killing, MBC, synergy | 0.06-0.12 | Macrolide susceptible |
| S. aureus NRS384 | Passagingb | 0.12 | USA300-0114 |
| S. aureus 004-573D | Passagingb | 0.12 | USA300c |
| S. aureus 117-472D | Passagingb | 0.12 | USA300c |
| S. aureus 024-11490A | Passagingb | 0.12 | USA300c |
| S. aureus 117-453D | Passagingb | 0.12 | USA300c |
| S. haemolyticus 064-4090A | Passaginga | 0.12 | ermA |
| S. epidermidis 095-2777A | Time-kill, MBC | 0.12 | Macrolide susceptible |
| E. faecalis ATCC 29212 | Passaging,a single-step mutation, timed-killing | 0.03 | Macrolide susceptible |
| E. faecalis 061-6556A | Passagingb | 0.06 | Erythromycin susceptible |
| E. faecalis 067-6633A | Passagingb | 2 | ermB |
| Enterococcus faecium 067-1457A | Passagingb | 0.06 | Erythromycin susceptible |
| E. faecium 086-15387A | Passagingb | 1 | ermB |
| S. pneumoniae ATCC 49619 | Passaging,a postantibiotic effect, timed-killing, MBC, synergy | 0.008-0.015 | Macrolide susceptible |
| S. pneumoniae 063-1085A | Passaging,a single-step mutation, MBC, synergy | 0.015 | Wild type |
| S. pneumoniae 075-241B | Passaging,a single-step mutation, timed-killing, MBC | 0.015-0.03 | ermB |
| S. pneumoniae 127-2273B | Passaging,a MBC, synergy | 0.06 | mefA |
| S. pyogenes 117-1612A | Passaging,a postantibiotic effect, timed-killing, MBC | 0.015 | Wild type |
| S. pyogenes 088-11708A | Timed-killing, MBC, synergy | 0.06 | Macrolide resistant, CEM-101 susceptible |
| S. mitis 051-4933A | Passaginga | 0.12 | mefA |
| S. mitis 112-1885A | Timed-killing, MBC | ≤0.008 | Macrolide susceptible |
| H. influenzae ATCC 49247 | Postantibiotic effect | 2 | |
| M. catarrhalis 117-10142A | Postantibiotic effect | 0.12 | Wild type |
Passaging with CEM-101, telithromycin, clarithromycin, and azithromycin.
Passaging with CEM-101 only.
The pulsed-field gel electrophoresis profile was identical to that of S. aureus strain NRS384 (USA300-0114) from the Network on Antimicrobial Resistance in Staphylococcus aureus.
MBC and timed-killing tests.
MIC and MBC determinations used Clinical and Laboratory Standards Institute (CLSI) procedures (MIC and MBC ranges, 0.008 to 16 μg/ml) (17). The lowest concentration of a tested agent that killed ≥99.9% of the initial inoculum was defined as the MBC endpoint. Timed-killing bactericidal activity was performed for CEM-101, telithromycin, clarithromycin, and azithromycin by previously described methods (15-17). The compounds were tested at 2×, 4×, and 8× MIC; and colony count determinations were performed at 0, 2, 4, 8, and 24 h.
Single-step mutation studies.
Fresh colonies from agar plates were emulsified in sterile broth to achieve a 4 McFarland turbidity standard (target concentration, 1.2 × 109 CFU/ml). An aliquot of the suspension was plated on agar plates containing 4×, 8×, and 16× the CEM-101 MIC for the isolate. Serial dilutions of the inoculum suspension were also plated on antimicrobial-free plates to determine the colony count (numbers of CFU/ml).
Passaging studies.
Isolates were tested by reference broth microdilution methods according to CLSI guidelines (4). Azithromycin, clarithromycin, and telithromycin were tested as comparators. Passaging was performed by removing the entire content of the last well with growth in the MIC panel and placing it into broth medium to reach a 0.5 McFarland standard. A suspension (5 × 105 CFU/ml) was transferred for susceptibility testing, and this procedure was repeated through 7 passage days. The reversion of resistance was assessed by three consecutive passages performed on drug-free agar, and the final MIC was determined by a reference broth microdilution method (5). The significant development of resistant mutants was considered a ≥8-fold change in the initial MIC value (12).
Erythromycin resistance induction.
D tests were performed according to the CLSI disk diffusion methodology (5). Erythromycin was used as the inducing agent; and clindamycin, telithromycin, and CEM-101 disks were placed around the erythromycin disk at distances of 12 and 15 mm for Streptococcus spp. and staphylococci, respectively. Quality control (QC) was performed according to CLSI guidelines by using strains S. aureus ATCC 25923, BAA-977, and BAA-976; S. pneumoniae ATCC 49619; S. pyogenes ATCC 19615; and Streptococcus agalactiae ATCC 12386. All QC results were within published limits.
PAE.
The PAE values for CEM-101 and telithromycin were determined by established procedures (11). Both antimicrobial agents were tested against each isolate at 4× MIC. Colony count determinations were performed before antimicrobial exposure (time zero) and 1 or 2 h after antimicrobial exposure. After the antimicrobial agents were diluted (1:1,000), colony count determinations were performed every hour until turbidity was noted (up to 10 h postdilution) to determine the length of the PAE.
Drug interaction (synergy) studies.
The activities of CEM-101 in combination with five agents (ceftriaxone, gentamicin, levofloxacin, trimethoprim-sulfamethoxazole, and vancomycin), each of which represents a distinct antimicrobial class, against the isolates were tested on checkerboard susceptibility panels. The drug interaction categories defined elsewhere (15) were used.
RESULTS AND DISCUSSION
MBC testing.
CEM-101 was very active against the streptococci (MIC50, ≤0.008 to 0.015 μg/ml) and the staphylococci (MIC50, 0.06 to 0.12 μg/ml), being 2-fold more potent than telithromycin. In general, CEM-101 exhibited MBC/MIC ratios of ≤4 when it was tested against macrolide-susceptible streptococci and CoNS, indicating that it had bactericidal activity (Table 2). In contrast, S. aureus and enterococci showed elevated CEM-101 MBCs. All six macrolide-susceptible S. pneumoniae strains and two macrolide-resistant, clindamycin-susceptible S. pneumoniae strains had CEM-101 MBCs at or 2-fold higher than the MIC value. Conversely, two macrolide- and clindamycin-resistant S. pneumoniae strains exhibited elevated CEM-101 MBC/MIC ratios (≥32). The CEM-101 MBC/MIC ratios were generally elevated for S. aureus and were independent of the macrolide susceptibility pattern. The CEM-101 MBCs were not as high as those of telithromycin and remained ≤2 μg/ml for 3 of 10 S. aureus strains processed (Table 2).
TABLE 2.
Distribution of isolates according to MBC/MIC ratios for CEM-101, telithromycin, clarithromycin, and azithromycin
| Organism and antimicrobial agent (no. of isolates tested) | No. of strains with MBC/MIC ratio of: |
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | 16 | ≥32 | |
| S. pneumoniae (10) | ||||||
| CEM-101 | 3 | 5 | 0 | 0 | 0 | 2 |
| Telithromycin | 2 | 6a | 0 | 0 | 0 | 2 |
| Clarithromycin | 2 | 3 | 1 | 0 | 0 | —b |
| Azithromycin | 2 | 4 | 0 | 0 | 0 | —c |
| Beta-hemolytic streptococci (5) | ||||||
| CEM-101 | 0 | 1 | 2 | 0 | 0 | 2 |
| Telithromycin | 0 | 1 | 1 | 1 | 0 | 2 |
| Clarithromycin | 0 | 0 | 1 | 1 | 0 | 2b |
| Azithromycin | 0 | 0 | 0 | 0 | 2 | 2c |
| Viridans group streptococci (5) | ||||||
| CEM-101 | 3 | 0 | 1 | 0 | 0 | 1 |
| Telithromycin | 2 | 1 | 1 | 0 | 0 | 1 |
| Clarithromycin | 0 | 0 | 1 | 0 | 0 | 3b |
| Azithromycin | 0 | 0 | 0 | 0 | 1 | 3c |
| S. aureus (10) | ||||||
| CEM-101 | 1 | 0 | 0 | 0 | 1 | 8 |
| Telithromycin | 0 | 0 | 0 | 0 | 0 | 10 |
| Clarithromycin | 0 | 0 | 0 | 0 | 0 | 6b |
| Azithromycin | 0 | 0 | 0 | 0 | 0 | 6c |
| Coagulase-negative staphylococci (5) | ||||||
| CEM-101 | 1 | 1 | 0 | 3 | 0 | 0 |
| Telithromycin | 0 | 0 | 0 | 0 | 2 | 3 |
| Clarithromycin | 0 | 0 | 0 | 0 | 0 | 4b |
| Azithromycin | 0 | 0 | 0 | 0 | 0 | 4c |
| Enterococcus spp. (5) | ||||||
| CEM-101 | 0 | 0 | 0 | 0 | 0 | 5 |
| Telithromycin | 0 | 0 | 0 | 0 | 0 | 5 |
| Clarithromycin | 0 | 0 | 0 | 0 | 0 | 2c |
| Azithromycin | 0 | 0 | 0 | 0 | 0 | 2b |
Includes six isolates with MICs of ≤0.008 μg/ml and an MBC of 0.015 μg/ml (off-scale comparisons).
The MBC was not evaluated for isolates with resistance-level MIC results of ≥4 μg/ml.
The MBC was not evaluated for isolates with resistance-level MIC results of ≥16 μg/ml.
These observations are in accordance with those from a study by Okamoto et al. (19), which showed that MBC/MIC ratios remained elevated for S. aureus strains, regardless of their macrolide susceptibility pattern, when telithromycin was tested.
Timed-killing tests.
CEM-101 showed bactericidal activity (reduction of the initial inoculum of ≥3 log10 CFU/ml in 24 h) against macrolide-susceptible strains S. aureus ATCC 29213, Staphylococcus epidermidis, S. pneumoniae ATCC 49619, S. pyogenes (8× MIC only), and viridans group streptococci and a macrolide-resistant S. pyogenes strain (Table 3). Overall, CEM-101 produced a higher level of reduction in the numbers of CFU/ml and more rapid killing than telithromycin or clarithromycin and azithromycin. A tendency toward higher levels of killing and more rapid killing was also noted with increased concentrations of CEM-101, indicating that it has concentration-dependent killing activity, similar to other ketolides and unlike the macrolide compounds, which show time-dependent killing activity (25). In previous studies, telithromycin displayed a slower bacteriostatic effect against S. pyogenes than against S. pneumoniae (18). Conversely, in the present study, CEM-101 and telithromycin showed similar killing patterns when the results for the two S. pyogenes isolates and wild-type S. pneumoniae strain evaluated were compared. Previous studies have also concluded that at concentrations of 2× to 10× MIC, telithromycin and cethromycin (also a ketolide) are mainly bacteriostatic against S. aureus (25). Noteworthy in our study was the finding that CEM-101 is bactericidal at 2×, 4×, and 8× the MICs for the S. aureus and S. epidermidis strains tested.
TABLE 3.
Summary of timed-killing curve results
| Strain | Result for: |
|||
|---|---|---|---|---|
| CEM-101 | Telithromycin | Clarithromycin | Erythromycin | |
| S. aureus ATCC 29213 | Cidal at 2×, 4×, 8× MIC | Cidal at 8× MIC only | Cidal at 8× MIC only | Cidal at 8× MIC only |
| S. epidermidis 2777A | Cidal at 2×, 4×, 8× MIC | Static | Static | Static |
| E. faecalis ATCC 29212 | Static | Static | Static | Static |
| S. pneumoniae ATCC 49619 | Cidal at 2×, 4×, 8× MIC | Cidal at 2×, 4×, 8× MIC | Cidal at 2×, 4×, 8× MIC | Cidal at 2×, 4×, 8× MIC |
| S. pneumoniae 241B | Static | Static | NTa | NT |
| S. pyogenes 1612A | Cidal at 8× MIC only | Cidal at 8× MIC only | Cidal at 8× MIC only | Cidal at 8× MIC only |
| S. pyogenes 11708A | Cidal at 2×, 4×, 8× MIC | Cidal at 2×, 4×, 8× MIC | NT | NT |
| S. mitis 1885A | Cidal at 2×, 4×, 8× MIC | Cidal at 2×, 4×, 8× MIC | Cidal at 8× MIC only | Cidal at 4×, 8× MIC |
NT, not tested.
Resistance development studies.
The emergence of resistant mutants was not observed in the single-step mutational studies. The isolates tested included one isolate each of wild-type Enterococcus faecium, S. aureus, and S. pneumoniae as well as one ermB-carrying S. pneumoniae isolate. Strains were exposed to 4×, 8×, and 16× CEM-101 MIC, with no mutant colonies being detected. The mutation rates by organism were as follows: E. faecium, < 4.0 × 10−9; S. aureus, < 6.0 × 10−9; and S. pneumoniae <1.4 × 10−9 and < 6.5 × 10−8 for the wild-type strain and the ermB-harboring strain, respectively.
Telithromycin-resistant single-step mutants were detected in previous studies that tested macrolide-susceptible and -resistant S. pneumoniae isolates, and resistance rates of 1.5 × 10−8 to 2.0 × 10−7 were noted for macrolide-susceptible strains, whereas mutational resistance rates of 1.1 × 10−7 to >1.0 × 10−3 were observed for mefE- and ermB-carrying isolates (12). Noteworthy in our study was the lack of detection of mutants resistant to CEM-101, regardless of the macrolide susceptibility patterns of the strains tested. S. pneumoniae mutants resistant to clarithromycin and erythromycin have occurred in shorter time periods than mutants resistant to the ketolides when the isolates were tested by multistep resistance selection (12).
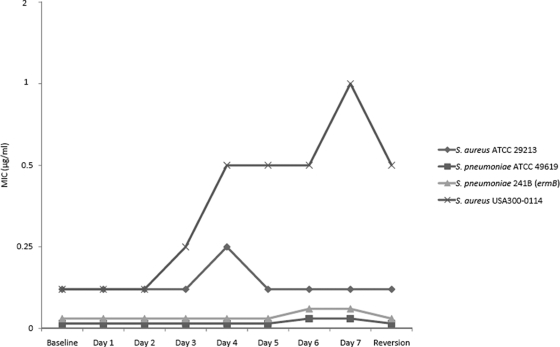
Among the 18 isolates tested by multistep resistance selection, no significant variation (more than 1 log2 dilution) in the CEM-101 MIC values was observed for eight strains (44.4%). The remaining 10 strains exhibited modest increases in CEM-101 MIC values of 4- or 8-fold, with no reversion or only 2-fold decreases in the MIC after three subcultures (Fig. 1). A 4-fold increase in the CEM-101 MIC occurred for an ermA-carrying Staphylococcus haemolyticus strain and S. pneumoniae wild-type strain 1085A, whereas the incidence of mutants of both organisms resistant to telithromycin increased 8-fold. The mefA-carrying Streptococcus mitis strain showed 4-fold increases in the MIC values for CEM-101, telithromycin, and azithromycin, while the only agent to which the mutants were resistant was clarithromycin (8-fold increase). The activity of CEM-101 against five USA300-like strains was also tested, and 4- and 8-fold increases in the MICs were detected (three and two strains, respectively). Three of five USA300 strains displayed a 2-fold reversion. Overall, CEM-101 had resistance selection results less than or equivalent to those for telithromycin.
FIG. 1.
CEM-101 passaging results for selected strains (7 days).
Erythromycin induction of resistance.
Erythromycin induced clindamycin resistance in 21 of 31 (68%) Staphylococcus sp. strains (Table 4). Moreover, telithromycin and CEM-101 resistance was induced in all Staphylococcus spp. evaluated. Among the S. aureus strains with inducible clindamycin resistance (CEM-101 and telithromycin resistance was also inducible for these strains), 11 harbored ermA and 4 carried ermC. The CoNS isolates displayed induced resistance to all three agents and were found to carry ermC. All the staphylococcal isolates showing clindamycin-susceptible patterns (10 strains) harbored mrsA, which encodes an efflux resistance mechanism. Telithromycin inducibility was previously observed among Staphylococcus spp., and isolates harboring ermA and ermC were also clindamycin inducible (6).
TABLE 4.
Patterns of inducible CEM-101, telithromycin, and clindamycin resistance by erythromycin determined by a modified D-test method
| Induced resistance to: |
No. of occurrences |
|||
|---|---|---|---|---|
| Clindamycin | Telithromycin | CEM-101 | Staphylococci (n = 31) | Streptococci (n = 50) |
| + | + | + | 21 | 18 |
| − | + | + | 10 | 0 |
| + | + | − | 0 | 7 |
| − | − | − | 0 | 25 |
Three patterns of resistance were noted among the beta-hemolytic streptococci: erythromycin-inducible resistance to all three agents tested (8 isolates), erythromycin-inducible resistance to clindamycin and telithromycin but not CEM-101 (7 isolates), and no inducible resistance to any of the three antimicrobial agents (5 isolates). Among the S. pneumoniae isolates, two distinct patterns were observed: no induction of resistance to any of the agents (14 isolates) or the complete induction of resistance to clindamycin, telithromycin, and CEM-101 (6 isolates). All S. pneumoniae strains with inducible resistance harbored ermB. A similar pattern of resistance was detected for the viridans group streptococci: four strains exhibited resistance to all three agents, while the remaining six isolates failed to show evidence of inducible resistance.
The clinical usefulness of detecting induced telithromycin resistance is currently under evaluation, since studies analyzing beta-hemolytic streptococci and S. pneumoniae indicate that the results of that phenotypic test showed a poor correlation with the MIC values and/or the presence of the erm and mef genes (9, 20). This evaluation should be applied to CEM-101, since that fluoroketolide displayed resistance induction results similar to those noted for telithromycin.
Postantibiotic effect.
The PAE results obtained with 4× MIC for CEM-101 and telithromycin were 2.3 and 2.6 h, respectively, for S. aureus ATCC 29213; 3.0 and 1.9 h, respectively, for S. pneumoniae ATCC 49619; 6.1 and 3.4 h, respectively, for S. pyogenes 1612A; 3.2 and 1.2 h, respectively, for H. influenzae ATCC 49247; and 6.3 and 4.0 h, respectively, for M. catarrhalis 10142A. All strains except S. pneumoniae and H. influenzae had an exposure time of 2 h; S. pneumoniae and H. influenzae were exposed for only 1 h. Overall, the PAE of CEM-101 was usually more extended (1 to 3 h longer) than that of telithromycin; both ketolide antimicrobial agents presented similar PAEs for S. aureus.
In general, the PAE values for the ketolides are usually improved compared to those for the macrolide comparators, and the ketolides have a theoretical maximum PAE against different isolates as the exposure concentration of ketolide increases (25). However, the results obtained in the present evaluation with 4× MIC were similar to those obtained in the study of Jacobs et al. (7), who reported telithromycin PAE values of 0.3 to 2.4 h for S. aureus and 1.5 to 3.8 h for S. pneumoniae at a higher level of exposure (10× MIC).
Drug interaction (synergy) studies.
The vast majority of the results (76/110) showed indifferent MIC values for the codrugs in the presence of CEM-101 compared with the MIC values for the codrugs tested alone (Table 5). Three test combinations for CEM-101-ceftriaxone were not interpretable due to oxacillin resistance. Additive (22/110) and partial (7/110) synergies were also detected, whereas complete synergy with CEM-101 and gentamicin was observed for only two S. pneumoniae strains. More importantly, antagonistic effects were not detected, and since the majority of combinations displayed indifference, CEM-101 would likely not have a negative therapeutic effect when it is used in combination with these antimicrobial pairings for the treatment of S. pneumoniae, S. aureus, and S. pyogenes infections.
TABLE 5.
CEM-101 drug interaction (synergy) results for five antimicrobial agents tested against S. aureus, S. pyogenes, and S. pneumoniaea
| Agent used in combination with CEM-101 | No. of isolates in the following interactive category: |
|||||
|---|---|---|---|---|---|---|
| Synergy |
Additive | Indifferent | Antagonism | Indeterminate | ||
| Complete | Partial | |||||
| Ceftriaxone | 0 | 2 | 5 | 12 | 0 | 3 |
| Gentamicin | 2 | 2 | 4 | 14 | 0 | 0 |
| Levofloxacin | 0 | 0 | 3 | 19 | 0 | 0 |
| Trimethoprim-sulfamethoxazole | 0 | 2 | 4 | 16 | 0 | 0 |
| Vancomycin | 0 | 1 | 6 | 15 | 0 | 0 |
Nine S. aureus strains, six S. pyogenes strains, and seven S. pneumoniae strains were tested.
Due to the broad spectrum of activity covering Gram-positive cocci, atypical bacteria, intracellular pathogens, H. influenzae, and M. catarrhalis (azithromycin and clarithromycin only), macrolides have been widely used to treat upper and lower respiratory tract infections and as an alternative agent for use by patients allergic to β-lactams (24). However, emerging trends of resistance in Gram-positive pathogens and others organisms have created the need for new antimicrobial agents. Structural changes to macrolides have expanded their spectra of activity and potencies and have given origin to the ketolide group (25). CEM-101, a new fluoroketolide, has shown in vitro activity against macrolide-susceptible and -resistant strains and has proven to be at least 2-fold more potent than telithromycin in our studies. Earlier studies with pneumococci and S. pyogenes found that CEM-101 has a MIC value 4-fold lower than that of telithromycin, regardless of the macrolide susceptibility pattern (13). Additionally, CEM-101 has shown potency 2-fold greater than that of telithromycin against evolving multidrug-resistant serogroup 19A pneumococcal strains (6a).
The results of the timed-killing experiments reported here demonstrated that CEM-101 reduces the colony counts (in CFU/ml) to a greater extent and displays more rapid killing than telithromycin. Single-step mutational studies did not detect mutants resistant to CEM-101, and the rates of resistance to this new ketolide were equivalent to those to telithromycin when isolates were selected for resistance in multistep mutational studies. Similar results were also obtained with CEM-101 and telithromycin when they were used to test for erythromycin resistance induction. Furthermore, CEM-101 displayed superiority over telithromycin, as it had PAEs 1 to 3 h longer and proved to have no negative therapeutic effect with the codrug combinations tested (Table 5) in our synergy studies. With telithromycin proving to be less potent in multiple studies and having safety issues, CEM-101 appears to be a promising option for the treatment of community-acquired bacterial pneumonia (CABP) and complicated SSSIs.
Acknowledgments
We thank Paul R. Rhomberg, Douglas J. Biedenbach, and Gary J. Moet for their excellent technical support during this study.
Footnotes
Published ahead of print on 22 February 2010.
REFERENCES
- 1.Albrich, W. C., D. L. Monnet, and S. Harbarth. 2004. Antibiotic selection pressure and resistance in Streptococcus pneumoniae and Streptococcus pyogenes. Emerg. Infect. Dis. 10:514-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinker, A. D., R. T. Wassel, J. Lyndly, J. Serrano, M. Avigan, W. M. Lee, and L. B. Seeff. 2009. Telithromycin-associated hepatotoxicity: clinical spectrum and causality assessment of 42 cases. Hepatology 49:250-257. [DOI] [PubMed] [Google Scholar]
- 3.Cassone, M., M. M. D'Andrea, F. Iannelli, M. R. Oggioni, G. M. Rossolini, and G. Pozzi. 2006. DNA microarray for detection of macrolide resistance genes. Antimicrob. Agents Chemother. 50:2038-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2009. M07-A8. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 8th ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Clinical and Laboratory Standards Institute. 2009. M100-S19. Performance standards for antimicrobial susceptibility testing, 19th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Davis, K. A., S. A. Crawford, K. R. Fiebelkorn, and J. H. Jorgensen. 2005. Induction of telithromycin resistance by erythromycin in isolates of macrolide-resistant Staphylococcus spp. Antimicrob. Agents Chemother. 49:3059-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Farrell, D. J., H. S. Hader, M. Castanheira, D. J. Biedenbach, P. R. Rhomberg, and R. N. Jones. Antimicrobial characterization of CEM-101 activity against respiratory tract pathogens including multidrug-resistant pneumococcal serogroup 19A isolates. Int. J. Antimicrob. Agents, in press. [DOI] [PubMed]
- 7.Jacobs, M. R., S. Bajaksouzian, and P. C. Appelbaum. 2003. Telithromycin post-antibiotic and post-antibiotic sub-MIC effects for 10 Gram-positive cocci. J. Antimicrob. Chemother. 52:809-812. [DOI] [PubMed] [Google Scholar]
- 8.Jones, R. N., D. J. Biedenbach, P. R. Rhomberg, T. R. Fritsche, and H. S. Sader. 2008. Antimicrobial characterization of CEM-101 activity against 331 respiratory tract pathogens including multidrug-resistant pneumococcal serogroup 19A (MDR-19A) isolates, abstr. F1-3975. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 9.Kaieda, S., N. Okitsu, H. Yano, Y. Hosaka, R. Nakano, R. Okamoto, H. Takahashi, and M. Inoue. 2003. Induction of telithromycin resistance in Streptococcus pneumoniae. J. Antimicrob. Chemother. 52:736-737. [DOI] [PubMed] [Google Scholar]
- 10.Kouvela, E. C., D. L. Kalpaxis, D. N. Wilson, and G. P. Dinos. 2009. Distinct mode of interaction of a novel ketolide antibiotic that displays enhanced antimicrobial activity. Antimicrob. Agents Chemother. 53:1411-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorian, V. 1996. Antibiotics in laboratory medicine. Williams & Wilkins Co., Baltimore, MD.
- 12.Matic, V., K. Kosowska, B. Bozdogan, L. M. Kelly, K. Smith, L. M. Ednie, G. Lin, K. L. Credito, C. L. Clark, P. McGhee, G. A. Pankuch, M. R. Jacobs, and P. C. Appelbaum. 2004. Antipneumococcal activities of two novel macrolides, GW 773546 and GW 708408, compared with those of erythromycin, azithromycin, clarithromycin, clindamycin, and telithromycin. Antimicrob. Agents Chemother. 48:4103-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGhee, P., K. Nagai, and P. C. Appelbaum. 2008. Activity of CEM-101 compared to other agents against macrolide-susceptible and -resistant streptococci, abstr. F1-3974. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 14.Menichetti, F. 2005. Current and emerging serious Gram-positive infections. Clin. Microbiol. Infect. 11(Suppl. 3):22-28. [DOI] [PubMed] [Google Scholar]
- 15.Moody, J. 2004. Susceptibility tests to evaluate synergism, p. 5.12.11-5.12.23. In H. D. Isenberg (ed.), Clinical microbiology procedures handbook, vol. I. ASM Press, Washington, DC. [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. 1999. M21-A3. Methodology for the serum bactericidal test; approved guideline. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 17.National Committee for Clinical Laboratory Standards. 1999. M26-A. Methods for determining bactericidal activity of antibacterial agents; approved guideline. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 18.Odenholt, I., E. Lowdin, and O. Cars. 2001. Pharmacodynamics of telithromycin in vitro against respiratory tract pathogens. Antimicrob. Agents Chemother. 45:23-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okamoto, H., S. Miyazaki, K. Tateda, Y. Ishii, and K. Yamaguchi. 2000. Comparative in vitro activity of telithromycin (HMR 3647), three macrolides, amoxycillin, cefdinir and levofloxacin against gram-positive clinical isolates in Japan. J. Antimicrob. Chemother. 46:797-802. [DOI] [PubMed] [Google Scholar]
- 20.Raney, P. M., F. C. Tenover, R. B. Carey, J. E. McGowan, Jr., and J. B. Patel. 2006. Investigation of inducible clindamycin and telithromycin resistance in isolates of beta-hemolytic streptococci. Diagn. Microbiol. Infect. Dis. 55:213-218. [DOI] [PubMed] [Google Scholar]
- 21.Waites, K. B., D. M. Crabb, and L. B. Duffy. 2009. Comparative in vitro susceptibilities of human mycoplasmas and ureaplasmas to a new investigational ketolide, CEM-101. Antimicrob. Agents Chemother. 53:2139-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh, F., F. Carnegy, J. Willcock, and S. Amyes. 2004. Comparative in vitro activity of telithromycin against macrolide-resistant and-susceptible Streptococcus pneumoniae, Moraxella catarrhalis and Haemophilus influenzae. J. Antimicrob. Chemother. 53:793-796. [DOI] [PubMed] [Google Scholar]
- 23.Wierzbowski, A. K., J. A. Karlowsky, D. J. Hoban, and G. G. Zhanel. 2009. In vitro activity of the investigational ketolide cethromycin against macrolide- and penicillin-resistant Streptococcus pneumoniae: review of the 1998 to 2006 Canadian Respiratory Organism Susceptibility Study (CROSS). J. Antimicrob. Chemother. 63:620-622. [DOI] [PubMed] [Google Scholar]
- 24.Zhanel, G. G., M. Dueck, D. J. Hoban, L. M. Vercaigne, J. M. Embil, A. S. Gin, and J. A. Karlowsky. 2001. Review of macrolides and ketolides: focus on respiratory tract infections. Drugs 61:443-498. [DOI] [PubMed] [Google Scholar]
- 25.Zhanel, G. G., M. Walters, A. Noreddin, L. M. Vercaigne, A. Wierzbowski, J. M. Embil, A. S. Gin, S. Douthwaite, and D. J. Hoban. 2002. The ketolides: a critical review. Drugs 62:1771-1804. [DOI] [PubMed] [Google Scholar]