Abstract
We have evaluated the in vitro activity of posaconazole (PSC) against 50 clinical strains of Rhizopus oryzae using a broth microdilution method, the Neo-Sensitabs tablet diffusion method, and minimal fungicidal concentration (MFC) determination. In general, PSC showed low MICs against this fungus, and the MICs correlated with the inhibition zone diameters. Most of the MFCs, however, were from 1 to 4 dilutions higher than their corresponding MICs. We then investigated the efficacies of several different doses of PSC in a murine model. All treatments began 24 h after challenge and lasted for 7 days. The drug was administered twice a day to mice infected with three strains that showed intermediate PSC susceptibility (MIC = 2 μg/ml) and three PSC-susceptible strains (MIC = 0.25 μg/ml). A dose of 10 mg/kg of body weight was ineffective, while doses of 20 and 30 mg/kg prolonged the survival of the mice. The 50 strains tested were segregated into two groups on the basis of the in vitro data. For the group with the most strains (85%), the strains had low PSC MICs, mice infected with the strains showed higher rates of survival (30 to 40%), and PSC was able to reduce the fungal load in the kidney and less regularly in the brain. For the second group (15% of the strains), the strains had intermediate PSC MICs, mice infected with the strains had lower survival rates (10 to 20%), and PSC treatment resulted in variable and no reductions in the fungal loads in the kidneys and brains, respectively.
Infections caused by some fungi of the order Mucorales are highly invasive and potentially fatal, and they mainly affect patients with diabetes mellitus or hematological malignances (20). In a recent survey of the occurrence of Mucorales in clinical samples in the United States, approximately half of the isolates identified belonged to the species Rhizopus oryzae (2). Treatment of Mucorales infections is based on four critical factors: rapid diagnosis, removal of predisposing factors, surgical debridement, and appropriate antifungal therapy, with liposomal amphotericin B (LAMB) being the drug of choice (21). Although posaconazole (PSC) has been demonstrated to be less effective than LAMB for the treatment of human R. oryzae infections and its use is not recommended as the primary treatment, this drug is considered a reasonable option for patients who are refractory to or intolerant of polyenes (8, 22). PSC has been tested as salvage therapy in several clinical trials, 14 to 37% of the patients showed a complete response (8, 22). However, even in such cases the actual role of this drug is difficult to assess, as most of these patients had previously been treated with AMB or their lipid formulations. It is also unknown whether these success rates were related to the patients' immune status and/or other conditions or to the susceptibility of the strains involved in these infections. Furthermore, in clinical trials in which PSC has been evaluated, the fungi involved were not identified to the species level, thereby making it impossible to know which species of Mucorales responded better to treatment. On the basis of the findings of a recent study of Alvarez et al. (2) demonstrating that R. oryzae is the most common organism involved in various forms of zygomycosis, albeit the data were restricted to the United States, it seems likely that this may have been the fungus tested. Considering the low frequency of infection with this organism and the difficulty in obtaining statistically significant data regarding the efficacies of antifungal agents, animal models have proved very useful for this purpose. Previous animal studies evaluating the efficacy of PSC against zygomycetes have included only one or two strains of R. oryzae (3, 4, 9, 19). In the study described here, we have tested a panel of 50 clinical isolates of R. oryzae and, after determining their in vitro susceptibilities to PSC by different methods, have selected a subset of strains with various degrees of susceptibility to evaluate the efficacy of PSC in a murine model of infection.
MATERIALS AND METHODS
The strains used in the testing for this study were those that had been well characterized in our previous study (2). The in vitro antifungal susceptibilities of clinical strains of R. oryzae to PSC were determined by evaluating their MICs (in μg/ml), their inhibition zone diameters (IZDs; in mm), and their minimum fungicidal concentrations (MFCs; in μg/ml). MICs were determined by a broth microdilution method, according to the guidelines of the Clinical and Laboratory Standards Institute (formerly the National Committee for Clinical Laboratory Standards) for molds (13). For the Neo-Sensitabs diffusion method, the isolates were tested on Mueller-Hinton agar plates and by following the guidelines for molds. Tablets 9 mm in diameter containing 5 μg of PSC were provided by Rosco Laboratory (6). To determine the MFCs, 20 μl of each well that showed complete inhibition (100% inhibition or an optically clear well) was plated onto potato dextrose agar (PDA) plates. The plates were incubated at 35°C for 24 to 48 h. The MFC was the lowest drug concentration that resulted in no growth (7). The suggested MIC (μg/ml) and IZD (mm) breakpoints at 24 h for PSC against zygomycetes are as follows: susceptible, ≤1 μg/ml and ≥17 mm, respectively; intermediate, 2 μg/ml and 14 to 16 mm, respectively; and resistant, ≥4 μg/ml and ≤13 mm, respectively (5, 13).
For the in vivo studies, we chose six of the strains previously tested in vitro: three strains with intermediate susceptibility to PSC (MIC = 2 μg/ml), i.e., strains UTHSC 07-365, UTHSC 05-3032, and UTHSC 04-3109, and three PSC-susceptible strains (MIC = 0.25 μg/ml), i.e., strains UTHSC 02-2882, UTHSC 03-511, and UTHSC 06-89. The isolates were stored at −80°C, and before they were tested, they were subcultured on PDA at 35°C. On the day of infection, the cultures from the PDA plates were suspended in sterile saline and filtered through sterile gauze to remove clumps of spores or hyphae. The resulting suspensions were adjusted to the desired inoculum, on the basis of hemocytometer counts and by serial plating on PDA to confirm the viability of the fungi. Male OF1 mice weighing 30 g (Charles River, Criffa S.A., Barcelona, Spain) were used in this study. The animals were housed under standard conditions. All animal care procedures were supervised and approved by the Universitat Rovira i Virgili Animal Welfare Committee. The animals were immunosuppressed 1 day prior to infection by administering a single dose of 200 mg of cyclophosphamide per kg of body weight intraperitoneally (i.p.) plus a single dose of 150 mg of 5-fluorouracil per kg intravenously (i.v.). We had previously demonstrated with this immunosuppressive regimen that the peripheral blood polymorphonuclear leukocyte counts were <100/μl from day 3 to day 9 or later (15). The mice were challenged with 2 × 104 CFU in 0.2 ml of sterile normal saline injected via a lateral tail vein. Preliminary experiments demonstrated that these concentrations are optimal for producing an acute infection, with 100% of the animals dying within 6 to 8 days of infection (data not shown).
We tested PSC (Noxafil; Schering Plough Ltd., Hertfordshire, United Kingdom) administered at 10, 20, or 30 mg/kg of body weight/dose twice a day (BID) orally (p.o.) by gavage. The control animals received no treatment.
The efficacies of the drug treatments were evaluated by evaluation of the length of prolongation of survival and the reduction in the fungal burden in the tissues of infected mice. All treatments began 24 h after challenge and lasted for 7 days. To prevent bacterial infection, the mice received ceftazidime (5 mg/day subcutaneously) from days 1 to 7 after infection.
For the survival studies, groups of 10 mice infected with each of the selected R. oryzae strains for each treatment group were randomly established and checked daily for 20 days after challenge. For the tissue burden studies, groups of 10 mice were also established, and the animals were killed on day 4 after infection. The kidneys and brains were removed aseptically and gently homogenized in 2 ml sterile saline; care was taken to minimize tissue trauma. Serial 10-fold dilutions of the homogenates were plated on PDA and incubated for 24 h at 35°C.
The mean survival time was estimated by the Kaplan-Meier method, and the survival times among the groups were compared by the log rank test. The colony counts in the tissue burden studies were analyzed by the Kruskal-Wallis test. When the results of this test were significant, we used the Mann-Whitney U test to compare treatment pairs. The Bonferroni correction was used to avoid an increase in the type I error due to multiple comparisons. When P was <0.05, the observed differences were considered statistically significant.
RESULTS
Table 1 summarizes the in vitro results for the 50 strains of R. oryzae tested. PSC demonstrated significant activity against this fungus, with the majority of the strains showing MICs and IZDs close to the suggested breakpoints indicative of susceptibility, i.e., ≤1 μg/ml (86% of the strains) and ≥17 mm (78% of the strains), respectively. The remainder of the strains showed intermediate susceptibility, with the MICs being 2 μg/ml (14%) and the IZDs ranging from 14 to 16 mm (22%). MICs higher than 2 μg/ml or zone diameters ≤13 mm were not seen. In general, the MICs did not correlate with the MFCs, since most of the values of the latter parameter were 1 to 4 dilutions higher than the corresponding MICs.
TABLE 1.
In vitro activity of posaconazolea against 50 clinical strains of Rhizopus oryzae determined by broth microdilution, disk diffusion, and minimal fungicidal concentration studies
| R. oryzae PSC susceptibility and isolate | MIC (μg/ml) | IZD (mm) | MFC (μg/ml) |
|---|---|---|---|
| Susceptible | |||
| UTHSC 03-228 | 0.25 | 25 | 1 |
| UTHSC 06-3913 | 0.50 | 20 | 4 |
| UTHSC 04-278 | 1 | 18 | >16 |
| UTHSC 06-4133 | 0.5 | 23 | 1 |
| UTHSC 02-2882 | 0.25 | 22 | 0.5 |
| UTHSC 04-443 | 0.5 | 22 | 1 |
| UTHSC 04-1241 | 0.5 | 22 | 0.5 |
| UTHSC 06-89 | 0.25 | 23 | 1 |
| UTHSC 04-1613 | 0.5 | 22 | 0.5 |
| UTHSC 04-2459 | 1 | 18 | 0.5 |
| UTHSC 06-731 | 0.5 | 17 | 0.5 |
| UTHSC 04-2159 | 0.5 | 20 | 1 |
| UTHSC 2868 | 1 | 19 | 8 |
| UTHSC 06-1080 | 1 | 18 | >16 |
| UTHSC 06-4152 | 0.25 | 22 | 1 |
| UTHSC 06-329 | 0.5 | 20 | 0.5 |
| UTHSC 05-2975 | 1 | 20 | 2 |
| UTHSC 03-1826 | 0.5 | 18 | 0.5 |
| UTHSC 05-1502 | 0.5 | 21 | 1 |
| UTHSC 06-1351 | 0.25 | 25 | 0.5 |
| UTHSC 06-4035 | 0.5 | 17 | 0.5 |
| UTHSC 05-3132 | 0.5 | 20 | 0.5 |
| UTHSC 03-547 | 0.25 | 25 | 0.5 |
| UTHSC 05-197 | 0.25 | 21 | 0.5 |
| UTHSC 02-439 | 0.25 | 22 | 0.5 |
| UTHSC 06-2457 | 1 | 14 | >16 |
| UTHSC 03-511 | 0.25 | 22 | 1 |
| UTHSC 03-3110 | 1 | 17 | 1 |
| UTHSC 03-2970 | 1 | 20 | 4 |
| UTHSC 05-1817 | 0.25 | 25 | 2 |
| UTHSC 412-546 | 1 | 15 | 8 |
| UTHSC 06-434 | 1 | 15 | 1 |
| UTHSC 06-1301 | 0.5 | 25 | 1 |
| UTHSC 05-3580 | 0.5 | 25 | 1 |
| UTHSC 05-2447 | 1 | 17 | 1 |
| UTHSC 04-1220 | 0.5 | 20 | 1 |
| UTHSC 06-2374 | 0.5 | 23 | 1 |
| UTHSC 03-112 | 0.5 | 20 | 1 |
| UTHSC 06-4336 | 0.5 | 20 | 1 |
| UTHSC 06-3827 | 1 | 18 | 16 |
| UTHSC 03-299 | 1 | 15 | >16 |
| UTHSC 05-3111 | 0.25 | 20 | 2 |
| UTHSC 05-1679 | 0.5 | 18 | 0.5 |
| Intermediate | |||
| UTHSC 03-285 | 2 | 14 | >16 |
| UTHSC 07-365 | 2 | 15 | 16 |
| UTHSC 03-285 | 2 | 15 | >16 |
| UTHSC 05-3032 | 2 | 14 | >16 |
| UTHSC 04-3109 | 2 | 14 | >16 |
| UTHSC 05-114 | 2 | 15 | 16 |
| UTHSC 03-3165 | 2 | 16 | 16 |
Posaconazole was administered 24 h after challenge of the mice with the fungal strains.
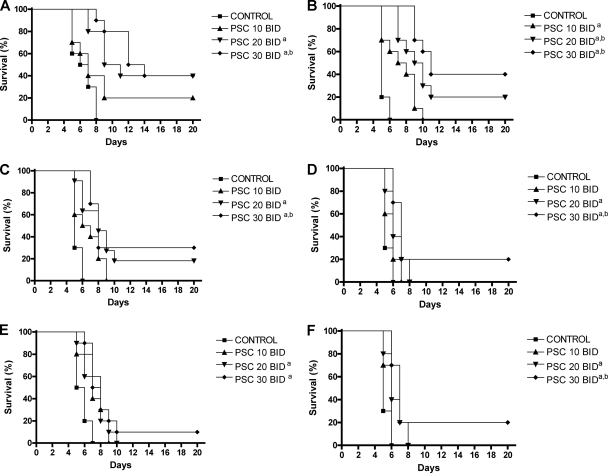
The in vivo results are shown in Fig. 1. For all strains, PSC doses of 20 or 30 mg/kg BID significantly prolonged survival compared with that for the control group. Moreover, PSC at 30 mg/kg also significantly prolonged survival compared with that achieved with the lower dose (10 mg/kg BID) for most of the strains tested. The rates of survival (30% to 40%) of the mice infected with PSC-susceptible strains and treated with PSC at 30 mg/kg were significantly (P < 0.001) better than those (10% to 20%) of the mice infected with PSC-intermediate strains.
FIG. 1.
Cumulative mortality of mice infected with Rhizopus oryzae UTHSC 02-2882 (A), UTHSC 06-89 (B), UTHSC 03-511 (C), UTHSC 07-365 (D), UTHSC 05-3032 (E), and UTHSC 04-3109 (F) and treated with PSC. a, P < 0.05 versus the results for the control; b, P < 0.05 versus the results for PSC administered at 10 mg/kg p.o. twice a day. PSC 10, posaconazole administered at 10 mg/kg p.o. twice a day; PSC 20, posaconazole administered at 20 mg/kg p.o. twice a day; PSC 30, posaconazole administered at 30 mg/kg p.o. twice a day.
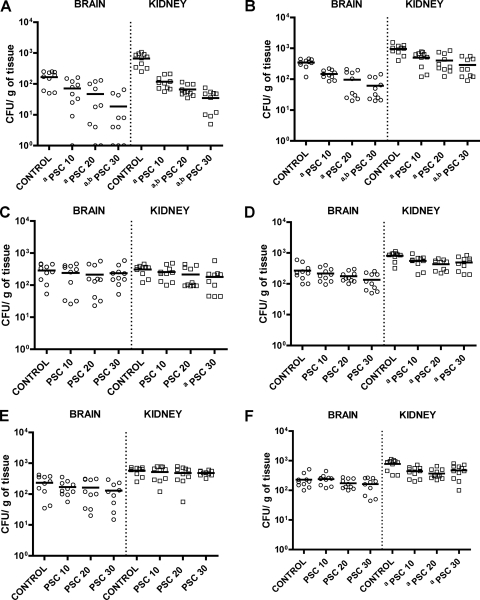
For two of the PSC-susceptible strains, strains UTHSC 02-2882 and UTHSC 06-89, all doses of the drug reduced the fungal loads in the brains and kidneys compared with those in the brains and kidneys of the control group; moreover, the highest dose (30 mg/kg) significantly (P < 0.08) reduced the fungal loads in both organs compared with those achieved with the lowest dose (10 mg/kg) (Fig. 2). For the third PSC-susceptible strain, strain UTHSC 03-511, only PSC at 30 mg/kg BID significantly reduced the fungal load in the kidneys compared with that in the kidneys of the control group.
FIG. 2.
Effects of antifungal treatment on colony counts of R. oryzae UTHSC 02-2882 (A), UTHSC 06-89 (B), UTHSC 03-511(C), UTHSC 07-365 (D), UTHSC 05-3032 (E), and UTHSC 04-3109 (F) in brain and kidney tissues of mice. a, P < 0.05 versus the results for the control; b, P < 0.05 versus the results for PSC administered at 10 mg/kg p.o. twice a day. PSC 10, posaconazole administered at 10 mg/kg p.o. twice a day; PSC 20, posaconazole administered at 20 mg/kg p.o. twice a day; PSC 30, posaconazole administered at 30 mg/kg p.o. twice a day; horizontal lines, mean values.
For two of the PSC-intermediate strains, strains UTHSC 07-365 and UTHSC 04-3109, the three doses of PSC tested significantly reduced the fungal load in kidney tissue but not in brain tissue. For the third PSC-intermediate strain, strain UTHSC 05-3032, no dose of PSC was able to reduce the fungal load either in the brain or in the kidney.
DISCUSSION
In previous animal studies with PSC and R. oryzae, several investigators reported that PSC either had a low degree of efficacy in treating mice infected with R. oryzae (4, 9, 19) or demonstrated only prophylactic activity (3). In a previous experimental study with the same murine model, we also observed a lack of efficacy of PSC at 40 mg/kg given once a day (19). However, we later demonstrated that the administration of PSC twice a day was more effective than administration once a day for the treatment of infections caused by a closely related fungus of the same genus, R. microsporus (18). In that study, with the administration of PSC at 20 mg/kg BID, we obtained higher serum PSC levels (>7.5 μg/ml at 3 h and >2 μg/ml at 24 h after the last dose) than those achieved with the daily administration of a single dose of 40 mg/kg (>5 μg/ml at 3 h and ≤1 μg/ml at 24 h after the last dose). Similarly, in this study, we observed a significant correlation between the in vitro and in vivo results using the twice-a-day dosing regimen. This study demonstrated that clinical isolates of R. oryzae are segregated into two groups on the basis of their in vitro susceptibilities. The strains in the group with the most strains (85% of the strains) had low MICs and high IZDs. Mice infected with representative strains from this group and treated with PSC showed higher survival rates (30 to 40%), and in general, the drug was able to reduce the fungal load in the kidney and variably in the brain. In addition, we observed a dose-response for PSC in vivo for two drug-susceptible strains but not for the third drug-susceptible strain. These different responses could probably be due to different behaviors of the strains of this species in vivo. The strains in the group with fewer strains (15% of the strains) had intermediate MICs and lower IZDs. When mice were infected with representative strains from this group, treatment with PSC showed no efficacy in the brain and variable efficacy in the kidney and resulted in lower survival rates (10 to 20%). It was also demonstrated that the results obtained by the tablet diffusion method correlated closely with those obtained by the broth microdilution method. This can be especially useful for clinical laboratories, since the simplicity and low cost of the latter method allow the test to be easily implemented as part of the routine workload. While the PSC MIC range was very narrow (0.25 to 2 μg/ml), the MFC range was considerably broader (0.5 to >16 μg/ml). This phenomenon has previously been noted by other investigators when they tested other genera (14). Although the role of the MFCs in determining clinical efficacy needs to be elucidated, some authors suggest that in severely immunosuppressed patients, the value of this parameter is more predictive of the clinical outcome (12, 14, 17). In our study, the low degree of efficacy of PSC against isolates with high MFCs (≥16 μg/ml) also suggests that high MFCs may be predictors of poorer outcomes. It should also be noted that the tentative breakpoints suggested previously (5) are indeed only tentative and that the interpretive criteria for MICs of 2 μg/ml (now considered intermediate) may change as more clinical correlates become available.
Several studies with febrile patients with neutropenia and/or invasive fungal infections have shown that when PSC is administered at the recommended dosage of 400 mg orally twice daily, the resulting human serum PSC levels are generally <1 μg/ml (10, 11, 21). In a previous study with mice infected with R. microsporus (18), the serum PSC levels at 40 mg/kg were similar (<1 to 2 μg/ml) to those obtained with the drug administered at the recommended dosage in humans. This would suggest that PSC at this concentration is therapeutic only against those strains which show low MICs (<1 μg/ml). These data agree with our in vivo results, as PSC was the most efficacious against those strains with the lowest MICs.
Several different in vitro studies performed with strains of R. oryzae have shown low MICs (1, 12, 16), corroborating the findings of our work, in which 60% of the isolates had PSC MICs of <1 μg/ml. This would suggest the potential utility of PSC in treating zygomycosis due to R. oryzae infection, pending the availability of in vitro susceptibility data for the infecting isolate. It should also be noted, however, that the susceptibility to PSC is strain dependent, with a significant number of isolates displaying elevated MICs and MFCs.
Footnotes
Published ahead of print on 9 February 2010.
REFERENCES
- 1.Almyroudis, N. G., D. A. Sutton, A. W. Fothergill, M. G. Rinaldi, and S. Kusne. 2007. In vitro susceptibilities of 217 clinical isolates of zygomycetes to conventional and new antifungal agents. Antimicrob. Agents Chemother. 51:2587-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez, E., D. A. Sutton, J. Cano, A. W. Fothergill, A. Stchigel, M. G. Rinaldi, and J. Guarro. 2009. Spectrum of zygomycete species identified in clinically significant specimens in the United States. J. Clin. Microbiol. 47:1650-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barchiesi, F., E. Spreghini, A. Santinelli, A. W. Fothergill, E. Pisa, D. Giannini, M. G. Rinaldi, and G. Scalise. 2007. Posaconazole prophylaxis in experimental systemic zygomycosis. Antimicrob. Agents Chemother. 51:73-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dannaoui, E., J. F. G. M. Meis, D. Loebenberg, and P. E. Verweij. 2003. Activity of posaconazole in treatment of experimental disseminated zygomycosis. Antimicrob. Agents Chemother. 47:3647-3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espinel-Ingroff, A., B. Arthington-Skaggs, N. Iqbal, D. Ellis, M. A. Pfaller, S. Messer, M. Rinaldi, A. Fothergill, D. L. Gibbs, and A. Wang. 2007. Multicenter evaluation of a new disk agar diffusion method for susceptibility testing of filamentous fungi with voriconazole, posaconazole, itraconazole, amphotericin B, and caspofungin. J. Clin. Microbiol. 45:1811-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espinel-Ingroff, A., and E. Canton. 2008. Comparison of Neo-Sensitabs tablet diffusion assay with CLSI broth microdilution M38-A and disk diffusion methods for testing susceptibility of filamentous fungi with amphotericin B, caspofungin, itraconazole, posaconazole, and voriconazole. J. Clin. Microbiol. 46:1793-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espinel-Ingroff, A., V. Chaturvedi, A. Fothergill, and M. G. Rinaldi. 2002. Optimal testing conditions for determining MICs and minimum fungicidal concentrations of new and established antifungal agents for uncommon molds: NCCLS collaborative study. J. Clin. Microbiol. 40:3776-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenberg, R. N., K. Mullane, J. A. van Burik, I. Raad, M. J. Abzug, G. Anstead, R. Herbrecht, A. Langston, K. A. Marr, G. Schiller, M. Schuster, J. R. Wingard, C. E. Gonzalez, S. G. Revankar, G. Corcoran, R. J. Kryscio, and R. Hare. 2006. Posaconazole as salvage therapy for zygomycosis. Antimicrob. Agents Chemother. 50:126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibrahim, A. S., T. Gebremariam, J. A. Schwartz, J. E. Edwards, Jr., and B. Spellberg. 2009. Posaconazole mono- or combination therapy for treatment of murine zygomycosis. Antimicrob. Agents Chemother. 53:772-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishna, G., M. Martinho, P. Chandrasekar, A. J. Ullman, and H. Patino. 2007. Pharmacokinetics of oral posaconazole in allogenic hematopoietic stem cell transplant recipients with graft-versus-host disease. Pharmacotherapy 27:1627-1636. [DOI] [PubMed] [Google Scholar]
- 11.Krishna, G., A. Sansone-Parsons, M. Martinho, B. Kantesaria, and L. Pedicone. 2007. Posaconazole plasma concentrations in juvenile patients with invasive fungal infection. Antimicrob. Agents Chemother. 51:812-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lass-Flörl, C., A. Mayr, S. Perkhofer, G. Hintenberger, J. Hausdorfer, C. Speth, and M. Fille. 2008. Activities of antifungal agents against yeasts and filamentous fungi: assessment according to the methodology of the European Committee on Antimicrobial Susceptibility Testing. Antimicrob. Agents Chemother. 52:3637-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards/Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi: approved standard, 2nd ed. Document M38-A2. National Committee for Clinical Laboratory Standards/Clinical and Laboratory Standards Institute, Wayne, PA.
- 14.Nguyen, M. H., C. J. Clancy, V. L. Yu, Y. C. Yu, A. J. Morris, D. R. Snydman, D. A. Sutton, and M. G. Rinaldi. 1998. Do in vitro susceptibility data predict the microbiologic response to amphotericin B? Results of a prospective study of patients with Candida fungemia. J. Infect. Dis. 177:425-430. [DOI] [PubMed] [Google Scholar]
- 15.Ortoneda, M., J. Capilla, F. J. Pastor, C. Serena, and J. Guarro. 2004. Interaction of granulocyte colony-stimulating factor and high doses of liposomal amphotericin B in the treatment of systemic murine scedosporiosis. Diagn. Microbiol. Infect. Dis. 50:247-251. [DOI] [PubMed] [Google Scholar]
- 16.Pfaller, M. A., S. A. Messer, R. J. Hollis, and R. N. Jones. 2002. Antifungal activities of posaconazole, ravuconazole, and voriconazole, compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: report from SENTRY Antimicrobial Surveillance Program, 2000. Antimicrob. Agents Chemother. 46:1032-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rex, J., M. Pfaller, T. Walsh, V. Chaturvedi, A. Espinel-Ingroff, M. Ghannoum, L. Gosey, F. C. Odds, M. Rinaldi, D. Sheehan, and D. Warnock. 2001. Antifungal susceptibility testing: practical aspects and current challenges. Clin. Microbiol. Rev. 14:643-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodríguez, M. M., F. J. Pastor, E. Calvo, V. Salas, D. A. Sutton, and J. Guarro. 2009. Correlation of in vitro activity, serum levels, and in vivo efficacy of posaconazole against Rhizopus microsporus in a murine disseminated infection. Antimicrob. Agents Chemother. 53:5022-5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodríguez, M. M., C. Serena, M. Mariné, F. J. Pastor, and J. Guarro. 2008. Posaconazole combined with amphotericin B, an effective therapy for a murine disseminated infection caused by Rhizopus oryzae. Antimicrob. Agents Chemother. 52:3786-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers, T. R. 2008. Treatment of zygomycosis: current and new options. J. Antimicrob. Chemother. 61:35-39. [DOI] [PubMed] [Google Scholar]
- 21.Spellberg, B., T. J. Walsh, D. P. Kontoyiannis, J. E. Edwards, Jr., and A. S. Ibrahim. 2009. Recent advances in the management of mucormycosis: from bench to bedside. Clin. Infect. Dis. 48:1743-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Burik, J. A. H., R. S. Hare, H. F. Solomon, M. L. Corrado, and D. P. Kontoyiannis. 2006. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin. Infect. Dis. 42:61-65. [DOI] [PubMed] [Google Scholar]