Abstract
Anidulafungin, micafungin, and caspofungin in vitro activities against Candida metapsilosis, C. orthopsilosis, and C. parapsilosis were evaluated by MICs and time-kill methods. All echinocandins showed lower MICs (mean MICs, 0.05 to 0.71 mg/liter) and the highest killing rates (−0.06 to −0.05 CFU/ml/h) for C. metapsilosis and C. orthopsilosis rather than for C. parapsilosis (mean MICs, 0.59 to 1.68 mg/liter). Micafungin and anidulafungin killing rates were greater than those determined for caspofungin. None of the echinocandins had fungicidal activity against C. parapsilosis.
Until recently, both Candida metapsilosis and C. orthopsilosis were grouped with C. parapsilosis, but a recent survey demonstrated that around 6.1% are C. orthopsilosis isolates and 1.8% are C. metapsilosis isolates (12). In addition, small nosocomial outbreaks caused by C. parapsilosis, but which could have been caused by the two new species, have been reported (11, 17, 20). Although anidulafungin, caspofungin, and micafungin share the same spectrum of activity against Candida spp., their in vitro activities are consistently reduced against the three species in the C. parapsilosis group (8). However, echinocandin MIC distributions have been significantly lower for C. metapsilosis and C. orthopsilosis than for C. parapsilosis (12). The echinocandins' fungicidal activities and killing rates have been evaluated against several Candida spp. (2-4, 7, 10), but only one study has investigated the killing activity of caspofungin against the three species found in the C. parapsilosis group (18). The present study compared the killing activities of anidulafungin, caspofungin, and micafungin against C. metapsilosis, C. orthopsilosis, and C. parapsilosis.
Caspofungin (Merck Sharpe & Dohme, Madrid, Spain) and micafungin (Fujisawa Pharmaceutical Company, Japan) were dissolved in water, and anidulafungin (Pfizer España, Madrid, Spain) was dissolved in dimethyl sulfoxide; further dilutions were prepared in standard RPMI 1640 medium (Sigma-Aldrich, Madrid, Spain). Final drug concentrations ranged from 0.03 to 64 mg/liter. Genotypic identification of C. parapsilosis group isolates was performed using specific primers based on the intron of the RPS0 gene (13, 19).
MICs (minimum concentrations that produce ≥50% growth reduction, determined by following the protocol in the CLSI M27-A3 document) (5) and time-kill study results were obtained for 10 bloodstream and quality control (QC) C. parapsilosis ATCC 22019 isolates, which are listed in Table 1.
TABLE 1.
Echinocandin MICsa
| Species | Isolate | MIC (mg/liter) of indicated agent |
||
|---|---|---|---|---|
| CAS | MCF | AND | ||
| C. orthopsilosis | CP-131 | 2 | 0.25 | 0.25 |
| CP-150 | 0.5 | 0.25 | 0.25 | |
| CP-151 | 0.5 | 0.25 | 0.5 | |
| ATC-194 | 0.5 | 0.5 | 0.12 | |
| GM | 0.71 | 0.3 | 0.25 | |
| C. metapsilosis | CP-132 | 0.25 | 0.5 | 0.06 |
| CP-133 | 0.5 | 0.12 | 0.06 | |
| ATC-193 | 0.12 | 0.25 | 0.03 | |
| GM | 0.25 | 0.25 | 0.05 | |
| C. parapsilosis | CP-300 | 2 | 0.25 | 0.5 |
| CP-301 | 2 | 1 | 1 | |
| CP-302 | 2 | 1 | 1 | |
| ATCC 22019 | 1 | 0.5 | 1 | |
| GM | 1.68 | 0.59 | 0.84 | |
MICs determined by following the protocol in the CLSI M27-A3 document (5). CAS, caspofungin; MCF, micafungin; AND, anidulafungin; GM, geometric mean MIC.
Antifungal carryover effect and time-kill curves were determined as previously described (RPMI 1640 medium, 1 × 106 to 5 × 106 CFU/ml inoculum, and a 5-ml volume) (1-3). Concentrations evaluated ranged between 0.03 and 32 mg/liter, which are within the range achieved clinically (15). Aliquots of 0.1 ml were removed to determine the number of CFU/ml at 0, 2, 4, 6, 24, and 48 h. The lowest limit of accurate and reproducible detectable colony counts was 100. All experiments were performed twice, with three replicates used for every dilution of each time point. Time-kill data were fitted to an exponential equation, as follows: Nt = N0 × e−kt (t, incubation time; Nt, viable cells at time t; N0, starting inoculum; k, killing rate). The goodness of fit for each isolate/drug was assessed by r2 values (>0.8). The time (hours) needed to achieve 50, 90, 99, and 99.9% reductions in growth (T50, T90, T99, and T99.9, respectively) compared with that of the starting inoculum was calculated with the K value as described elsewhere (1).
To our knowledge, this is the first study that has compared head-to-head the killing activities of the three echinocandins using blood isolates from the three species included in the C. parapsilosis group. Against this group, the in vitro fungistatic activities of the three echinocandins were species and agent dependent (Table 1). Our MIC results were similar to those previously reported (6, 9, 12, 18) but lower than those observed by Silva et al. (16). These discrepant results could be due to the different isolation sources: our isolates were obtained from blood cultures, while those used in the latter study were obtained from either urine (C. orthopsilosis) or mucosal surfaces (C. metapsilosis). Anidulafungin had the best fungistatic activity for C. metapsilosis and C. orthopsilosis, and micafungin had the best fungistatic activity for C. parapsilosis.
Figure 1 depicts the means and standard deviations of the results obtained from the killing curves. During the first 6 h, the killing activities of the three drugs were concentration independent, in which the killing rate did not rise substantially by increasing the drug concentrations for the three species. However, after 6 h, little killing and sometimes even a regrowth were observed, depending on the concentration and species tested. Against C. parapsilosis, the three drugs demonstrated killing activities until at 6 h, after which a regrowth was observed. The maximum log decreases in the number of CFU/ml reached at 6 h were 1.22 ± 0.56 log with 1 mg/liter of micafungin, 1.43 ± 0.4 log with 4 mg/liter of caspofungin, and 1.48 ± 0.2 log with 4 mg/liter of anidulafungin. A miniparadoxical effect (minor killing rate) was observed with 16 and/or 32 mg/liter of caspofungin (three isolates) and anidulafungin (two isolates) among the C. orthopsilosis and C. parapsilosis isolates evaluated, unlike for C. metapsilosis (Fig. 1 and 2). Our results are similar to those previously reported for caspofungin against C. parapsilosis and C. orthopsilosis (18). The lack of prior or similar studies of micafungin and anidulafungin precluded comparison with our results obtained using those two agents.
FIG. 1.
Mean time-kill plots for caspofungin (CAS), micafungin (MCF), and anidulafungin (AND) against 4 C. orthopsilosis, 3 C. metapsilosis, and 4 C. parapsilosis clinical isolates. Each data point represents the mean result ± standard deviation (error bars) for the indicated number of isolates. The dotted lines represent a ≥99.9% growth reduction compared with that of the initial inoculum (fungicidal effect). CT, drug-free control.
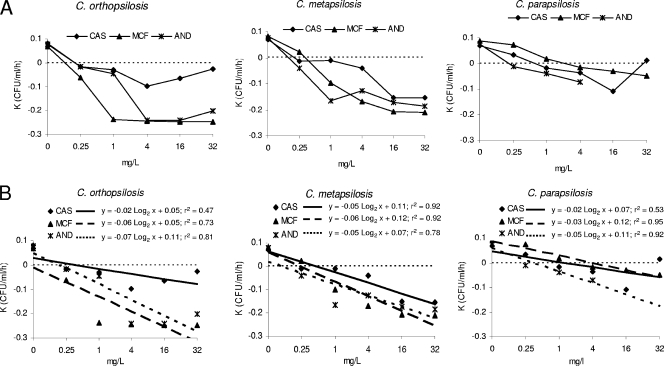
FIG. 2.
Killing rates of caspofungin (CAS), micafungin (MCF), and anidulafungin (AND) against C. orthopsilosis, C. metapsilosis, and C. parapsilosis (A) and the corresponding adjusted regression lines (B). Dotted line at top, growth; dotted line at bottom, killing.
The relationship between killing rate and concentration was linear for the three drugs, and the statistical regression analysis was significant (P < 0.05). Killing rates are shown in Fig. 2A, and the adjusted regression lines are shown in Fig. 2B. The killing activity began at the MIC for each species/echinocandin combination. The maximum killing rates were observed with 4 mg/liter of the three drugs for C. orthopsilosis, with 1 mg/liter of anidulafungin and 16 mg/liter of micafungin and caspofungin for C. metapsilosis, and with 16 mg/liter (caspofungin), 32 mg/liter (micafungin), and 8 mg/liter (anidulafungin) for C. parapsilosis (Fig. 2A).
Table 2 shows the mean times needed to kill 50, 90, 99, and 99.9% of the initial inoculum for each species/concentration and the echinocandin obtained with the regression line of each killing curve. The lowest concentration at which a ≥3-log decrease (killing endpoint) was achieved was 4 mg/liter at 12 to 48 h for the three drugs and the three species.
TABLE 2.
Time needed to achieve 50, 90, 99, and 99.9% growth reduction from that of the starting inoculuma
| Agent | Growth parameter | Mean time (h) at the indicated concn (mg/liter) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
C. orthopsilosis |
C. metapsilosis |
C. parapsilosisb |
||||||||||||||
| 0.25 | 1 | 4 | 16 | 32 | 0.25 | 1 | 4 | 16 | 32 | 0.25 | 1 | 4 | 16 | 32 | ||
| CAS | T50 | 17.50 | 7.45 | 3.42 | 3.86 | 10.53 | >48 | 22.30 | 7.70 | 2.59 | 1.99 | >48 | 22.30 | 7.70 | 2.59 | 1.99 |
| T90 | >48 | 24.75 | 11.35 | 12.82 | 34.97 | >48 | >48 | 25.58 | 8.61 | 6.60 | >48 | >48 | 25.58 | 8.61 | 6.60 | |
| T99 | >48 | >48 | 22.70 | 25.64 | >48 | >48 | >48 | >48 | 17.21 | 13.20 | >48 | >48 | >48 | >48 | >48 | |
| T99.9 | >48 | >48 | 34.05 | 38.46 | >48 | >48 | >48 | >48 | 25.82 | 19.80 | >48 | >48 | >48 | >48 | >48 | |
| MCF | T50 | 15.93 | 3.05 | 1.66 | 1.38 | 1.54 | >48 | 3.06 | 1.77 | 1.45 | 1.43 | NK | NK | 17.60 | 9.50 | 6.16 |
| T90 | >48 | 10.13 | 5.53 | 4.59 | 5.11 | >48 | 10.15 | 5.89 | 4.80 | 4.76 | NK | NK | >48 | 31.55 | 20.45 | |
| T99 | >48 | 20.26 | 11.06 | 9.17 | 10.22 | >48 | 20.30 | 11.79 | 9.61 | 9.53 | NK | NK | >48 | >48 | >48 | |
| T99.9 | >48 | 30.40 | 16.59 | 13.76 | 15.34 | >48 | 30.46 | 17.68 | 14.41 | 14.29 | NK | NK | >48 | >48 | >48 | |
| AND | T50 | 17.40 | 6.36 | 2.73 | 1.88 | 1.48 | 7.74 | 1.57 | 2.39 | 1.51 | 1.41 | NK | NK | 26.41 | 7.84 | 4.20 |
| T90 | >48 | 21.14 | 9.08 | 6.24 | 4.91 | 25.71 | 5.22 | 7.94 | 5.01 | 4.69 | NK | NK | >48 | 26.04 | 13.95 | |
| T99 | >48 | 42.28 | 18.17 | 12.48 | 9.81 | 51.41 | 10.44 | 15.89 | 10.02 | 9.37 | NK | NK | >48 | >48 | 27.89 | |
| T99.9 | >48 | >48 | 27.25 | 18.73 | 14.72 | 77.12 | 15.67 | 23.83 | 15.03 | 14.06 | NK | NK | >48 | >48 | 41.84 | |
CAS, caspofungin; MCF, micafungin; AND, anidulafungin; NK, no killing; T50, time needed to achieve 50% growth reduction from that of the starting inoculum; T90, time needed to achieve 90% growth reduction from that of the starting inoculum; T99, time needed to achieve 99% growth reduction from that of the starting inoculum; T99.9, time needed to achieve 99.9% growth reduction from that of the starting inoculum.
Corresponding concentrations of AND for C. parapsilosis are 0.03, 0.12, 0.5, 2, and 8 mg/liter.
In summary, the highest killing rates were obtained for C. orthopsilosis, followed by C. metapsilosis. Micafungin and anidulafungin had similar killing rates, both being greater than those of caspofungin. Against C. parapsilosis, none of the drugs reached the fungicidal endpoint, and regrowth was observed after 6 h. Since treatment guidelines (14) favored use of fluconazole rather than echinocandins when the infecting isolate is C. parapsilosis, identification at the species level for this group is important, as the echinocandins appear to have selective activities based on the species, and C. parapsilosis, rather than C. orthopsilosis or C. metapsilosis, seems to be the problem.
Footnotes
Published ahead of print on 9 February 2010.
REFERENCES
- 1.Cantón, E., J. Pemán, M. Gobernado, A. Viudes, and A. Espinel-Ingroff. 2004. Patterns of amphotericin B killing kinetics against seven Candida species. Antimicrob. Agents Chemother. 48:2477-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cantón, E., J. Pemán, M. Sastre, M. Romero, and A. Espinel-Ingroff. 2006. Killing kinetics of caspofungin, micafungin, and amphotericin B against Candida guilliermondii. Antimicrob. Agents Chemother. 50:2829-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantón, E., J. Pemán, A. Valentin, A. Espinel-Ingroff, and M. Gobernado. 2009. In vitro activities of echinocandins against Candida krusei determined by three methods: MIC and minimal fungicidal concentration measurements and time-kill studies. Antimicrob. Agents Chemother. 53:3108-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clancy, C. J., H. Huang, S. Cheng, H. Derendorf, and M. H. Nguyen. 2006. Characterizing the effects of caspofungin on Candida albicans, Candida parapsilosis, and Candida glabrata isolates by simultaneous time-kill and post-antifungal-effect experiments. Antimicrob. Agents Chemother. 50:2569-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard. CLSI document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Diekema, D. J., S. A. Messer, L. B. Boyken, R. J. Hollis, J. Kroeger, S. Tendolkar, and M. A. Pfaller. 2009. In vitro activity of seven systemically active antifungal agents against a large global collection of rare Candida species as determined by CLSI broth microdilution methods. J. Clin. Microbiol. 47:3170-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernst, E. J., E. E. Roling, C. R. Petzold, D. J. Keele, and M. E. Klepser. 2002. In vitro activity of micafungin (FK-463) against Candida spp.: microdilution, time-kill, and postantifungal-effect studies. Antimicrob. Agents Chemother. 46:3846-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Effron, G., S. K. Katiyar, S. Park, T. D. Edlind, and D. S. Perlin. 2008. A naturally occurring proline-to-alanine amino acid change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 52:2305-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez-Lopez, A., A. Alastruey-Izquierdo, D. Rodriguez, B. Almirante, A. Pahissa, J. L. Rodriguez-Tudela, M. Cuenca-Estrella, and the Barcelona Candidemia Project Study Group. 2008. Prevalence and susceptibility profile of Candida metapsilosis and Candida orthopsilosis: results from population-based surveillance of candidemia in Spain. Antimicrob. Agents Chemother. 52:1506-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karlowsky, J. A., G. A. Harding, S. A. Zelenitsky, D. J. Hoban, A. Kabani, T. V. Balko, M. Turik, and G. G. Zhanel. 1997. In vitro kill curves of a new semisynthetic echinocandin, LY-303366, against fluconazole-sensitive and-resistant Candida species. Antimicrob. Agents Chemother. 41:2576-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin, D. M., L. C. Wu, M. G. Rinaldi, and P. F. Lehmann. 1995. Three distinct genotypes within Candida parapsilosis from clinical sources. J. Clin. Microbiol. 33:243-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lockhart, S. R., S. A. Messer, M. A. Pfaller, and D. J. Diekema. 2008. Geographic distribution and antifungal susceptibility of the newly described species Candida orthopsilosis and Candida metapsilosis in comparison to the closely related species Candida parapsilosis. J. Clin. Microbiol. 46:2659-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez, J. M., E. V. Gomez, J. Pemán, E. Cantón, M. G. García, and L. del Castillo. Identification of pathogenic yeast species by polymerase chain reaction amplification of the RPS0 gene intron fragment. J. Appl. Microbiol., in press. [DOI] [PubMed]
- 14.Pappas, P. G., C. A. Kauffman, D. Andes, D. K. Benjamin, Jr., T. F. Calandra, J. E. Edwards, Jr., S. G. Filler, J. F. Fisher, B. J. Kullberg, L. Ostrosky-Zeichner, A. C. Reboli, J. H. Rex, T. J. Walsh, J. D. Sobel, and the Infectious Diseases Society of America. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48:503-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaller, M. A., D. J. Diekema, L. Ostrosky-Zeichner, J. H. Rex, B. D. Alexander, E. M. Johnson, D. Andes, S. D. Brown, V. Chaturvedi, M. A. Ghannoum, C. C. Knapp, D. J. Sheehan, and T. J. Walsh. 2008. Correlation of MIC with outcome for Candida species tested against caspofungin, anidulafungin and micafungin: analysis and proposal for interpretive MIC breakpoints. J. Clin. Microbiol. 46:2620-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva, A. P., I. M. Miranda, C. Lisboa, C. Pina-Vaz, and A. G. Rodrigues. 2009. Prevalence, distribution, and antifungal susceptibility profiles of Candida parapsilosis, C. orthopsilosis, and C. metapsilosis in a tertiary care hospital. J. Clin. Microbiol. 47:2392-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tavanti, A., L. A. Hensgens, E. Ghelardi. M. Campo, and S. Senesi. 2007. Genotyping of Candida orthopsilosis clinical isolates by amplification fragment length polymorphism reveals genetic diversity among independent isolates and strain maintenance with patients. J. Clin. Microbiol. 45:1455-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varga, I., G. Sóczó, G. Kardos, A. Borbély, Z. Szabó, A. Kemény-Beke, and L. Majoros. 2008. Comparison of killing activity of caspofungin against Candida parapsilosis, Candida orthopsilosis and Candida metapsilosis. J. Antimicrob. Chemother. 62:1466-1468. [DOI] [PubMed] [Google Scholar]
- 19.Vercher, M. P. 2009. Diagnostico molecular diferencial del grupo *psilosis. Graduation thesis. Universidad de Valencia, Valencia, Spain.
- 20.Zancope-Oliviera, R. M., M. J. James, A. P. Derossi, J. L. Sampaio, M. M. Muniz, R. K. Li, A. S. Nascimento, J. M. Peralta, and E. Reiss. 2000. Strain characterization of Candida parapsilosis fungemia by molecular typing methods. Eur. J. Clin. Microbiol. Infect. 19:514-520. [DOI] [PubMed] [Google Scholar]