Abstract
Cutaneous models have proven useful in studies of the pathogenesis and treatment of Gram-positive bacterial infections. Because cutaneous invasive aspergillosis (IA) occurs in the clinical setting, we sought to develop a nonlethal murine cutaneous model of IA. We induced cutaneous IA in cyclophosphamide-treated nude BALB/c mice by subcutaneous injection of Aspergillus fumigatus conidia. Skin lesion areas correlated well with tissue fungal burdens, allowing dynamic visual monitoring of cutaneous infections. The cutaneous model accurately reflected alterations in A. fumigatus pathogenicity resulting from deletions of recognized virulence genes (pabaA, sidA, and pksP). Moreover, analysis of the roles of conidial and mycelial catalases revealed that the former is required for the initiation of cutaneous aspergillosis, whereas the latter contributes to its propagation. Finally, posaconazole treatment reduced skin lesion areas relative to those of untreated and fluconazole-treated controls. This novel cutaneous model system should be applicable to comparative studies of the pathogenesis, treatment, and tissue specificity of IA.
Studies of microbial pathogenicity and antimicrobial drug efficacy rely on the use of animal model hosts. For invasive aspergillosis (IA), current animal models are based on the introduction of a large inoculum of asexual spores (conidia) into an immunosuppressed rodent via the respiratory or intravenous route, resulting in a rapidly fatal pneumonic or disseminated infection (1, 12, 18, 22, 24). Pulmonary models that are not rapidly fatal have also been developed (5, 22, 23). Cutaneous infection models have been used extensively to study the pathogenesis and in vivo response to antibacterial agents of Gram-positive bacteria (8, 10). The potential benefits of these models include their technical simplicity and reproducibility and the ability to visually monitor the infected lesion over time. Moreover, because cutaneous models are nonlethal, they allow prolonged monitoring and repeated manipulations of infected animals.
Cutaneous aspergillosis occurs in 5 to 10% of IA cases, making the skin the second most frequently involved site after the lungs (9, 19). Cutaneous infection develops either as a result of systemic dissemination of pulmonary aspergillosis or, less commonly, because of cutaneous inoculation of conidia (primary cutaneous aspergillosis). The latter instance invariably involves trauma to the skin, such as an intravascular access site or extensive burns, usually in patients with significant underlying immunosuppression; outbreaks of cutaneous aspergillosis in neonates have been associated with contaminated dressings (27, 28). However, the efficacy of antifungal drugs and the expression of Aspergillus virulence factors in the setting of soft tissue infections have not been studied. We hypothesized that experimental soft tissue IA in murine thighs could serve as the basis for a simple and nonlethal model of IA. Here, we describe the optimization of this model and report on its performance as a platform for studies of virulence and antifungal drug efficacy. Importantly, the cutaneous model of IA is subacute, nonlethal, and highly reproducible and allows dynamic monitoring of fungal burden by determination of skin lesion dimensions.
(This study was presented in part at the 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy-Infectious Diseases Society of America 46th Annual Meeting, Washington, DC, 25 to 28 October 2008 [4].)
MATERIALS AND METHODS
Aspergillus strains.
The Aspergillus fumigatus strains used in this study are summarized in Table 1. A. fumigatus strain 293 (Af293) was used in preliminary experiments to establish and optimize the cutaneous model and in antifungal drug treatment studies. A. fumigatus strains with deletion mutations of known virulence-associated genes and their parental wild-type strains were used in virulence studies. The ΔsidA mutant (deficient in the enzyme catalyzing the first committed step in hydroxamate-type siderophore biosynthesis [21]) was a gift from H. Haas, Innsbruck Biocenter, Austria; the ΔpabaA mutant (para amino benzoic acid auxotroph [7]) was a gift from E. M. Bignell, Imperial College School of Medicine, United Kingdom; and the ΔpksP mutant (deficient in polyketide synthase [25]) was a gift from K. A. Marr, Johns Hopkins University, Baltimore, MD. The production of A. fumigatus strains deficient in conidial catalase (catA) and mycelial catalases (cat1 and cat2) was described previously (16).
TABLE 1.
A. fumigatus strains used in this study
| Strain | Description | Source |
|---|---|---|
| Af293 | Clinical isolate | Nierman et al. (14) |
| B-5233 | Wild type | Tsai et al. (25) |
| ΔpksP mutant | B-5233 ΔpksP::Hygr | Tsai et al. (25) |
| CEA17 | Wild type | Schrettl et al. (21) |
| ΔsidA mutant | CEA17 ΔsidA::Hygr | Schrettl et al. (21) |
| Af237 | Wild type | Brown et al. (7) |
| ΔpabaA mutant | Af237 ΔpabaA::Hygr | Brown et al. (7) |
| G10 | Wild type | Monod et al. (13) |
| ΔcatA mutant | G10 ΔcatA::Phleor | Paris et al. (16) |
| Δcat1 Δcat2 mutant | G10 Δcat1::Hygr Δcat2::Phleor | Paris et al. (16) |
All strains were grown on yeast extract-agar-glucose (YAG; yeast extract, 5 g/liter; glucose, 10 g/liter; agar, 15 g/liter; 1 M MgCl2, 10 ml/liter; trace elements, 1 ml/liter) plates for 72 h in a humidified 37°C incubator. On the day of inoculation, conidia were collected in sterile saline with 0.08% Tween 20, washed twice, and filtered through 40-μm nylon filters (Bioscience Discovery Labware, Bedford, MA). Conidia were counted in a hemacytometer and resuspended in sterile saline at the desired concentrations (see below). The viability of conidia was determined by quantitative culture to be >95%.
Cutaneous model optimization.
Eight-week-old female nude (nu/nu) BALB/c mice (National Cancer Institute, Bethesda, MD) weighing 18 to 20 g were used in all experiments. All procedures were performed according to the highest standards for humane handling, care, and treatment of research animals and were approved by the M. D. Anderson Cancer Center Institutional Animal Care and Use Committee. Mice were housed in presterilized, filter-topped cages and provided with sterile food and drinking water containing tetracycline (1 g/liter).
Mice were anesthetized by inhalation of 2% isoflurane, and 100 μl of conidial suspension was injected subcutaneously (s.c.) with a 27-gauge needle into the right thigh. To determine the optimal parameters for this model, different combinations of immunosuppressive regimens and inoculum concentrations were tested for their ability to induce reproducible skin lesions.
The immunosuppressive regimens studied were as follows: 100 or 150 mg/kg of body weight cyclophosphamide, injected intraperitoneally (i.p.) 4 days and 1 day prior to inoculation and every 2 days thereafter starting on day 2 after inoculation; 250 mg/kg i.p. cyclophosphamide 1 day before inoculation, 200 mg/kg 2 days after inoculation, and 150 mg/kg 5 days after inoculation; and 250 mg/kg cortisone acetate injected s.c. 1 day before inoculation, alone or in combination with a cyclophosphamide regimen. The neutrophil count was determined to be less than 200 cells/μl in a subgroup of 5 mice who received 3 doses of cyclophosphamide at 100 mg/kg. Inoculums of 5 × 105, 5 × 106, and 5 × 107 conidia were tested with each of these drug regimens. Sham inoculation with 100 μl of sterile normal saline was performed as a negative control.
Skin lesions were measured daily using digital calipers (Fisher Scientific, Rochester, NY), and the longest and shortest diameters were recorded. The skin lesion area was approximated using the following ellipse area formula: lesion area = π × 0.5w × 0.5l, where w and l equal the width and length (in millimeters) of the lesion, respectively.
Tissue fungal burden.
We assessed 3 different methods for the determination of the tissue fungal burden: quantitative cultures, real-time quantitative PCR (RT-qPCR), and tissue galactomannan content. Thigh tissue (including skin, subcutaneous tissue, and lateral thigh muscles) was excised on day 5 after inoculation and stored in sterile 2-ml collection tubes at −20°C until testing. Tissue was weighed and homogenized in 1 ml of sterile 0.9% saline with a bead beater (Biospec, Bartlesville, OK), and homogenates were centrifuged for 10 min at 20,800 × g. Quantitative cultures were performed by plating serial 10-fold dilutions of the tissue homogenates on YAG and counting CFU after 18 h of incubation at 37°C. The results were expressed as numbers of CFU per gram of tissue. DNA was extracted using the DNeasy tissue kit (Qiagen, Valencia, CA), and RT-qPCR was performed using the primers and probe previously described (6). For galactomannan assays, supernatants were diluted and assayed with the Platelia galactomannan enzyme immunoassay kit (Bio-Rad Laboratories, Redmond, WA) according to the manufacturer's instructions. Because of the galactomannan assay's nonlinear characteristics, tissue homogenates had to be diluted to achieve linear correlation with skin lesion areas. The dilution factor that yielded the best correlation with skin lesion areas (1:100) was determined in preliminary experiments using serial 10-fold dilutions of thigh homogenates. The assay's negative, cutoff, and positive controls, as well as uninfected thigh tissue, served as controls. Optical density at 450 nm was determined using a spectrophotometer (PowerWave HT; BioTek Instruments, Winooski, VT) and divided by the cutoff control reading to produce the galactomannan index (GMI). To correct for variations in tissue weight, the galactomannan index was normalized using the formula cGMI = (200 × GMI)/W, where cGMI is the tissue weight-corrected GMI and W is the sample weight in grams.
Histopathologic analysis.
Infected thigh tissue was excised, fixed in 10% formalin, and embedded in paraffin. Tissue sections were stained with hematoxylin-eosin or Grocott-Gomori methenamine silver (GMS).
Virulence studies.
To assess the sensitivity of the thigh infection model to alterations in the pathogenicity of A. fumigatus, we analyzed its response to infection with defined hypovirulent A. fumigatus deletion mutants and their parental wild-type strains. Isolates were inoculated in parallel in separate groups of immunosuppressed nude BALB/c mice (5 mice per group), as described above. Lesion sizes (monitored daily), tissue fungal burdens, and histopathologic findings on day 5 after inoculation were compared between the two groups. Experiments were repeated three times.
To determine if conidial or mycelial catalase production by A. fumigatus is required for cutaneous infection, we infected mice with a ΔcatA or Δcat1 Δcat2 A. fumigatus strain and simultaneously injected an identical inoculum of parental wild-type spores (A. fumigatus strain G10) into the contralateral thigh (Table 1). We then compared the skin lesion areas and fungal burdens of thighs infected with catalase-deficient strains to those of thighs infected with wild-type strains.
Antifungal drug treatment.
We evaluated the ability of treatment with posaconazole (Schering Plough, Kenilworth, NJ) to attenuate cutaneous lesion areas in our thigh infection model. The treatment group received 40 mg/kg posaconazole in an oral suspension daily. Two groups of mice served as negative controls: a nontreatment group and a group treated with the triazole fluconazole (Pfizer, New York, NY; oral suspension, 40 mg/kg daily), which is not active against mold. Fluconazole and posaconazole were diluted in 200 μl of normal saline and administered by oral gavage. Twenty mice were included in each treatment and control group.
Statistical analysis.
Skin lesion areas and fungal burdens were compared between groups of mice using the unpaired Student t test. For mice inoculated simultaneously with catalase-negative and parental wild-type strains, the paired Student t test was used to compare lesion areas and fungal burdens. Because both skin lesion area and tissue galactomannan content values were normally distributed, we used Pearson's coefficient to calculate the linear correlation between these parameters. Graphs were plotted using Prism 4.0 software (Graph Pad Software, San Diego, CA). All statistical analyses were performed in SPSS 15.0 (SPSS, Inc., Chicago, IL). A two-tailed P value of <0.05 was considered statistically significant.
RESULTS
Cutaneous-model optimization.
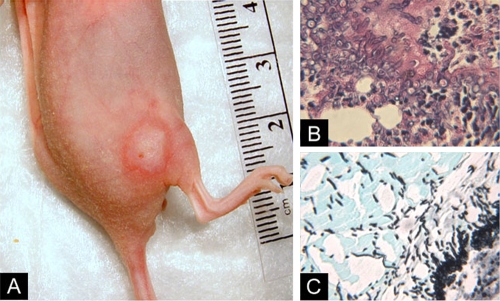
Our initial aim was to establish experimental parameters that yielded highly reproducible skin lesions while minimizing immunosuppressive-drug-related toxicity. Inoculation of nonimmunosuppressed BALB/c mice failed to induce thigh infections. Of the various immunosuppressive regimens evaluated, optimal results were obtained with 100 mg/kg of cyclophosphamide injected i.p. 4 days and 1 day prior to inoculation and 2, 4, and 6 days after inoculation. Larger doses of cyclophosphamide or the addition of cortisone acetate to this regimen resulted in greater toxicity (accelerated weight loss and mortality) without enhancing the performance of the model. Of the different inocula studied, the most consistent results were obtained by s.c. injection of 5 × 106 conidia in 100 μl saline. With the use of these parameters, all mice developed skin lesions at the site of infection within 48 h after inoculation. Lesions were typically erythematous macules with peripheral enhancement (Fig. 1A). Central eschars were apparent in about 20% of lesions. Skin lesions progressed slowly over 7 days of follow-up and were associated with minimal systemic morbidity and no mortality. Mice generally lost up to 10% of their body weight during the initial 4 days of immunosuppression; weight subsequently stabilized or increased. Histopathologic analysis revealed extension of fungal hyphae from subcutaneous tissue into the underlying thigh muscles and tissue infiltration by polymorphonuclear leukocytes (PMNLs) (Fig. 1B). Sham inoculations did not induce visible skin lesions.
FIG. 1.
Cutaneous model of IA. (A) A circumscribed skin lesion with peripheral enhancement appeared 48 h after subcutaneous injection of 5 × 106 A. fumigatus conidia in cyclophosphamide-treated nude BALB/c mice. (B, C) Histopathologic examination of thigh tissue sections revealed hyphal proliferation in the dermis and invasion of muscle tissue (hematoxylin and eosin, ×200 magnification [B]; GMS, ×200 magnification [C]).
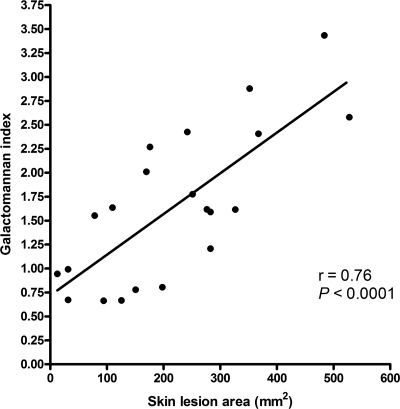
Skin lesion area correlates with tissue galactomannan content.
Of the 3 methods that we evaluated for the determination of fungal burden, only the tissue galactomannan content performed well in our model. Quantitative cultures yielded a mean fungal burden of 7.4 × 105 CFU/g tissue (95% confidence interval, 5.0 × 105 to 9.9 × 105 CFU/g); control mice inoculated with sterile saline had a mean fungal burden of 0 CFU/g tissue. However, numbers of CFU/g correlated poorly with skin lesion size (Pearson's coefficient, 0.14; P = 0.5). RT-qPCR was affected by frequent false-negative results, which we attributed to the known PCR-inhibitory effect of myoglobin (2). In contrast, we found a linear correlation between skin lesion areas and the corresponding fungal burdens in mouse thighs as determined by the tissue galactomannan content (Pearson's coefficient, 0.76; P < 0.0001) (Fig. 2).
FIG. 2.
Correlation between skin lesion area and tissue galactomannan content. A significant linear correlation between skin lesion area and thigh tissue galactomannan content is described by a Pearson's coefficient of 0.76 (P < 0.0001).
Hypovirulent Aspergillus fumigatus mutants produce attenuated skin lesions.
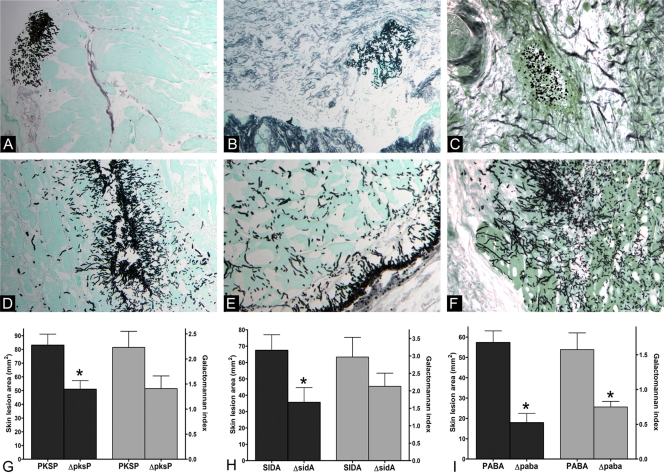
To determine the sensitivity of the cutaneous model to alterations in A. fumigatus virulence, we compared skin lesions and fungal burdens between A. fumigatus strains with deletion mutations in genes encoding well-described virulence factors (pksP, pabaA, and sidA) and their respective parental wild-type strains (Table 1). All three A. fumigatus deletion mutants (ΔpksP, ΔpabaA, and ΔsidA mutants) were associated with skin lesion areas significantly smaller than those of mice infected with the wild-type strains (Fig. 3). Specifically, the mean skin lesion area on day 3 after inoculation was 39% smaller in ΔpksP mutant-infected mice (P = 0.007), 69% smaller in ΔpabaA mutant-infected mice (P = 0.001), and 47% smaller in ΔsidA mutant-infected mice (P = 0.03) than in wild-type-infected mice. On GMS-stained tissue sections from thighs infected with hypovirulent strains, fungal elements were few and clustered within subcutaneous tissue, with only limited penetration into the underlying muscle tissue. In the case of ΔpabaA mutant-infected mice, almost no germination of conidia was observed (Fig. 3). In contrast, widespread distribution and extension of hyphal elements into muscle tissue was observed in mice infected with the parental wild-type strains. The tissue galactomannan content was lower in mice infected with the ΔpabaA mutant than that in mice infected with the parental wild type (P = 0.01). The difference in galactomannan content bordered on statistical significance for the mice infected with the ΔpksP mutant (P = 0.07) and was nonsignificant for the mice infected with the ΔsidA mutant (P = 0.3) (Fig. 3).
FIG. 3.
Features of murine thigh infection with hypovirulent A. fumigatus mutants and their parental wild-type strains. (A to F) GMS-stained thigh tissue sections of mice infected with A. fumigatus isolates with deletion mutations in known virulence-associated genes and parental wild-type strains are shown (original magnification, ×200). (A to C) In tissue infected with the ΔpksP, ΔsidA, and ΔpabaA mutants, fungal elements were few, clustered, and limited to subcutaneous tissue. (C) ΔpabaA conidia failed to germinate. (D to F) In contrast, the parental wild types produced widespread infection with deep invasion of muscle tissue. (G to I) The corresponding differences in skin lesion areas (black bars) and tissue galactomannan content (gray bars) are shown. Bars and error bars represent means and standard errors, respectively (15 mice per A. fumigatus strain). *, P < 0.05.
Catalase production by A. fumigatus spores and hyphae is required for initiation and propagation of cutaneous IA.
Some genes of potential importance in the pathogenicity of A. fumigatus appear to be dispensable for experimental invasive pulmonary aspergillosis. For example, conidial catalase (encoded by catA) and mycelial catalases (encoded by cat1 and cat2) were hypothesized to facilitate IA by enhancing resistance to oxidative stress. However, deletion of catA, while increasing the susceptibility of conidia to H2O2, has not been clearly shown to impair the pathogenicity of A. fumigatus in an inhalational model system (16). Conversely, deletion of both mycelial catalases had a minor effect on hyphal H2O2 susceptibility yet impaired A. fumigatus virulence in the inhalational model (16). We sought to determine whether the cutaneous IA model could be used to elucidate the pathogenic roles of A. fumigatus catalases.
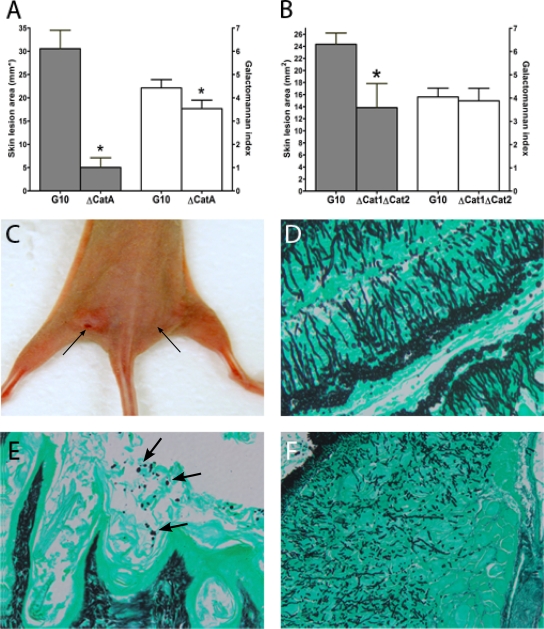
Deletion of the spore-specific catalase gene (catA) was associated with a 77% reduction in the mean skin lesion area (P = 0.002) and significantly lower tissue galactomannan content than that associated with the parental wild-type strain. Examination of GMS-stained tissue sections 5 days after inoculation with the ΔcatA mutant revealed ungerminated spores and no hyphal elements (Fig. 4). A reduction in the mean skin lesion area (57% smaller than that seen in parental wild-type-strain-infected mice) was observed in thighs infected with the mycelial-catalase-deficient Δcat1 Δcat2 double mutant (P = 0.01); however, the galactomannan content in these thighs was not significantly lower than that in wild-type-strain-infected thighs. Compared with the wild-type strain, the Δcat1 Δcat2 mutant formed shorter, more stunted hyphae (Fig. 4), indicating that hyphal catalase production is required for filamentous growth in tissue.
FIG. 4.
Importance of catalase production by A. fumigatus spores and hyphae for cutaneous IA. Nude BALB/c mice were inoculated simultaneously with catalase-deficient mutants and their parental wild-type strains in opposite thighs. (A and C) Deletion of the spore-specific catA gene was associated with significant reductions in the mean skin lesion area and galactomannan content compared with those after infection with the wild-type strain. (C) Left arrow, site of strain G10 inoculation; right arrow, site of ΔcatA mutant inoculation. (E) Failure of ΔcatA spores (arrows) to germinate and produce invasive hyphae can be seen in GMS-stained tissue. (B) Deletion of the mycelium-specific catalase genes cat1 and cat2 was associated with a small reduction in skin lesion area and a nonsignificant reduction in tissue galactomannan content compared with those of mice infected with the parental wild-type strain. (D and F) Histopathologic analysis revealed that Δcat1 Δcat2 conidia germinated but formed shorter hyphal elements (F) than the wild-type strain (D) (original magnification, ×200). Bars and error bars represent means and standard errors, respectively (10 mice per A. fumigatus strain). *, P < 0.05.
Posaconazole treatment reduces skin lesion size.
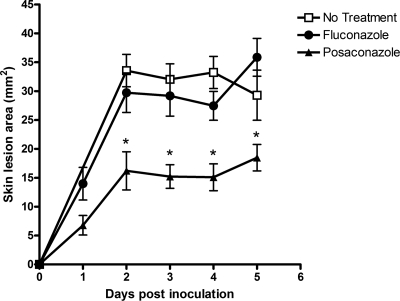
A unique feature of the cutaneous model is that it enables measurement of changes in skin lesion dimensions over time, reflecting the extent of A. fumigatus growth and tissue penetration. This characteristic offers the potential to dynamically monitor the in vivo activities of antifungal drugs. We compared the evolution of skin lesions in mice treated orally with posaconazole (40 mg/kg daily) with those of untreated mice and mice treated with the drug fluconazole, which is not active against mold. A significant reduction in the mean skin lesion area was observed in posaconazole-treated mice compared with those of mice in both control groups (Fig. 5). On days 2 through 5 after inoculation, the mean skin lesion area was 47% to 49% smaller in posaconazole-treated mice than in the control mice (P < 0.001).
FIG. 5.
Posaconazole treatment reduces skin lesion areas. Compared with no drug treatment or fluconazole treatment (40 mg/kg/day), treatment with posaconazole (40 mg/kg/day) resulted in a 47% to 49% reduction in the mean skin lesion area on days 2 through 5 after inoculation with strain Af293 (P < 0.001). Each datum point represents the mean skin lesion area (±standard error); 20 mice were used for each treatment group. *, P < 0.001.
DISCUSSION
We developed and optimized a murine model of cutaneous IA. This model has several characteristics that qualify it as a potentially useful addition to currently available IA animal models (18): it is highly reproducible, owing at least in part to the precise delivery of a predetermined inoculum by subcutaneous injection; it is technically simple to perform; and it does not require excessively expensive equipment or materials. Moreover, some unique features of this cutaneous model have not been available in previous IA models; specifically, the ability to infer the tissue fungal burden from the dimensions of skin lesions allows visual assessment of the infection process. As we have shown, this feature can be exploited to dynamically monitor the course of infection and response to antifungal treatment. In addition, because this is a subacute, nonlethal model, it may facilitate the study of Aspergillus virulence factors and responses to pharmacotherapy with greater resolution than that of models in which an overwhelming, rapidly fatal infection is induced.
A further advantage of the cutaneous model is that, because the site of infection is localized and easily accessible, it is amenable to specialized assays and manipulations. For example, we recently utilized this model to assess angiogenesis in A. fumigatus-infected tissue. To that end, a proangiogenic extract of basement membrane (Matrigel) was injected subcutaneously at the site of invasive aspergillosis, where it polymerized and formed solid plugs. Seven days after infection, the plugs were excised and the extent of endothelial cell migration and network formation was determined (3). Thus, the accessibility and subacute nature of the cutaneous model allowed us to monitor angiogenesis in situ and to describe the antiangiogenic activity of A. fumigatus in vivo.
In most clinical cases, IA occurs after inhalation of airborne conidia (11). Once they traverse the upper airways, most conidia are phagocytosed by resident alveolar macrophages and destroyed by oxidative mechanisms (20). Subcutaneously injected conidia likely first encounter different subsets of immune cells, such as Langerhans cells and dendritic cells. One indication of the importance of these differences is that corticosteroids were not required in our cutaneous model, whereas their use is needed to downregulate alveolar macrophage function in inhalational-IA models. Nevertheless, we found concordance between the expression of the virulence-associated A. fumigatus genes sidA, pabaA, and pksP in the cutaneous model and that in previously described inhalational (7, 21) and disseminated (25) models. Thus, although the cutaneous model does not mimic invasive pulmonary aspergillosis, our results indicate that it provides useful information on the pathogenicity of Aspergillus strains.
Cutaneous aspergillosis occurs in immunocompromised patients either as a primary infection, often following localized trauma, or as a secondary event arising from spread from adjacent infected tissue or hematogenous dissemination from a distant site (27). Interestingly, the Aspergillus species that cause primary cutaneous aspergillosis differ significantly from those encountered in pulmonary aspergillosis. For example, Aspergillus flavus, an uncommon cause of invasive pulmonary aspergillosis, has been associated with 44% of primary cutaneous aspergillosis cases in non-HIV-infected patients (17, 27). Similarly, of the 21 cases of invasive Aspergillus ustus infection reported in the medical literature since 1971, 10 occurred in patients with primary cutaneous aspergillosis (15). In contrast, A. fumigatus was recovered in only 26% of primary cutaneous aspergillosis cases (17, 27). These associations suggest the existence of tissue-specific Aspergillus virulence factors that have thus far not been characterized.
Extracellular catalase production by A. fumigatus has been hypothesized to facilitate invasive infection by detoxifying H2O2 produced by PMNLs. ΔcatA conidia are hypersensitive to H2O2 but not more susceptible than the parental wild-type strain to attack by macrophages (16). Thus, the nonattenuated virulence of the ΔcatA mutant in an inhalational model (16) can be explained by the fact that catA does not appear to protect conidia from the oxidative burst of alveolar macrophages. In contrast, we found that deletion of catA prevented A. fumigatus conidia from germinating in subcutaneous tissue and initiating invasive infection. This finding is consistent with the predominance of PMNLs in A. fumigatus skin lesions, suggesting that conidial catalase plays a central role in allowing conidia to evade oxidative damage by PMNLs. Deletion of the mycelial catalase genes cat1 and cat2 was previously shown to result in a slight increase in mycelial susceptibility to H2O2 and restricted hyphal growth in lung tissue (16). Similarly, Δcat1 Δcat2 A. fumigatus was associated with smaller skin lesions and limited hyphal elongation in our cutaneous model. Together, these observations suggest that conidial catalase is an organ-specific A. fumigatus virulence factor whose pathogenic role is evident in the presence of a PMNL-biased immune response. More generally, organ-specific model systems such as our cutaneous model may be used to dissect the relative importance of A. fumigatus virulence factors in different organs.
The optimal method for determining tissue fungal burden in animal models of IA is the subject of debate. Quantitative cultures of hyphal organisms may have limited reproducibility, because hyphal fragmentation secondary to tissue grinding can produce spuriously high colony counts (18). Specifically, the poor correlation that we observed between the number of CFU/g tissue and skin lesion size suggests that the vigorous homogenization necessary to process thigh tissue affected the reproducibility of quantitative cultures. Surrogate biomarkers, such as RT-qPCR and a galactomannan immunoassay, have been successfully utilized to measure fungal burden in tissues (6, 26). Measurement of thigh galactomannan content as described here correlated well with the skin lesion area and appeared to be an adequate method of validating tissue fungal burden.
Our study has certain limitations. Chiefly, the applicability of any virulence factor identified using the cutaneous model to the pathogenesis of invasive pulmonary aspergillosis will need to be verified using an inhalational model. However, because the endpoint of the cutaneous model is the area of skin lesions rather than mortality, it is well suited for the rapid screening of A. fumigatus mutant libraries to detect phenotypic changes using a relatively small number of animals. Further testing could then be performed with a pulmonary model. Second, we did not study the non-fumigatus Aspergillus species commonly associated with cutaneous aspergillosis in our model. Nevertheless, our results show that cutaneous aspergillosis with A. fumigatus can be easily induced in cyclophosphamide-treated BALB/c mice and that virulence data obtained with the cutaneous model parallels that of pulmonary models. Lastly, because local immunological defenses and pharmacokinetics may differ significantly between soft tissues and the lungs, the activities of antifungal drugs observed in the cutaneous model should be validated in a pulmonary model before conclusions are made about efficacy against invasive pulmonary aspergillosis.
We conclude that the cutaneous model of IA is a useful addition to current animal models of inhalational and disseminated IA. This subacute, localized model is robust, its results are reproducible, and it allows dynamic monitoring of the infection site.
Acknowledgments
We thank Gregory S. May, Hubertus Haas, K. J. Kwon-Chung, and Kieren Marr for kindly providing A. fumigatus deletion mutants.
This work was supported in part by the University of Texas M. D. Anderson Faculty E. N. Cobb Scholar Award Research Endowment and an M. D. Anderson Cancer Center Core Grant (CA16672) from the University of Texas (to D.P.K.).
Footnotes
Published ahead of print on 9 February 2010.
REFERENCES
- 1.Andriole, V. T., P. Miniter, D. George, D. Kordick, and T. F. Patterson. 1992. Animal models: usefulness for studies of fungal pathogenesis and drug efficacy in aspergillosis. Clin. Infect. Dis. 14(Suppl. 1):S134-S138. [DOI] [PubMed] [Google Scholar]
- 2.Bélec, L., J. Authier, M. C. Eliezer-Vanerot, C. Piedouillet, A. S. Mohamed, and R. K. Gherardi. 1998. Myoglobin as a polymerase chain reaction (PCR) inhibitor: a limitation for PCR from skeletal muscle tissue avoided by the use of Thermus thermophilus polymerase. Muscle Nerve 21:1064-1067. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Ami, R., R. E. Lewis, K. Leventakos, and D. P. Kontoyiannis. 2009. Aspergillus fumigatus inhibits angiogenesis through the production of gliotoxin and other secondary metabolites. Blood 114:5393-5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Ami, R., R. E. Lewis, K. Leventakos, and D. P. Kontoyiannis. 2008. A murine model of myocutaneous invasive aspergillosis, abstr. M-1548. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (ISDA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 5.Berenguer, J., M. C. Allende, J. W. Lee, K. Garrett, C. Lyman, N. M. Ali, J. Bacher, P. A. Pizzo, and T. J. Walsh. 1995. Pathogenesis of pulmonary aspergillosis. Granulocytopenia versus cyclosporine and methylprednisolone-induced immunosuppression. Am. J. Respir. Crit. Care Med. 152:1079-1086. [DOI] [PubMed] [Google Scholar]
- 6.Bowman, J. C., G. K. Abruzzo, J. W. Anderson, A. M. Flattery, C. J. Gill, V. B. Pikounis, D. M. Schmatz, P. A. Liberator, and C. M. Douglas. 2001. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrob. Agents Chemother. 45:3474-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, J. S., A. Aufauvre-Brown, J. Brown, J. M. Jennings, H. Arst, Jr., and D. W. Holden. 2000. Signature-tagged and directed mutagenesis identify PABA synthetase as essential for Aspergillus fumigatus pathogenicity. Mol. Microbiol. 36:1371-1380. [DOI] [PubMed] [Google Scholar]
- 8.Bunce, C., L. Wheeler, G. Reed, J. Musser, and N. Barg. 1992. Murine model of cutaneous infection with gram-positive cocci. Infect. Immun. 60:2636-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgos, A., T. E. Zaoutis, C. C. Dvorak, J. A. Hoffman, K. M. Knapp, J. J. Nania, P. Prasad, and W. J. Steinbach. 2008. Pediatric invasive aspergillosis: a multicenter retrospective analysis of 139 contemporary cases. Pediatrics 121:e1286-e1294. [DOI] [PubMed] [Google Scholar]
- 10.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. [DOI] [PubMed] [Google Scholar]
- 11.Latgé, J.-P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis, R. E., and N. P. Wiederhold. 2005. Murine model of invasive aspergillosis. Methods Mol. Med. 118:129-142. [DOI] [PubMed] [Google Scholar]
- 13.Monod, M., S. Paris, J. Sarfati, K. Jaton-Ogay, P. Ave, and J.-P. Latgé. 1993. Virulence of alkaline protease-deficient mutants of Aspergillus fumigatus. FEMS Microbiol. Lett. 106:39-46. [DOI] [PubMed] [Google Scholar]
- 14.Nierman, W. C., A. Pain, M. J. Anderson, J. R. Wortman, H. S. Kim, J. Arroyo, M. Berriman, K. Abe, D. B. Archer, C. Bermejo, J. Bennett, P. Bowyer, D. Chen, M. Collins, R. Coulsen, R. Davies, P. S. Dyer, M. Farman, N. Fedorova, N. Fedorova, T. V. Feldblyum, R. Fischer, N. Fosker, A. Fraser, J. L. Garcia, M. J. Garcia, A. Goble, G. H. Goldman, K. Gomi, S. Griffith-Jones, R. Gwilliam, B. Haas, H. Haas, D. Harris, H. Horiuchi, J. Huang, S. Humphray, J. Jimenez, N. Keller, H. Khouri, K. Kitamoto, T. Kobayashi, S. Konzack, R. Kulkarni, T. Kumagai, A. Lafon, J.-P. Latgé, W. Li, A. Lord, C. Lu, W. H. Majoros, G. S. May, B. L. Miller, Y. Mohamoud, M. Molina, M. Monod, I. Mouyna, S. Mulligan, L. Murphy, S. O'Neil, I. Paulsen, M. A. Penalva, M. Pertea, C. Price, B. L. Pritchard, M. A. Quail, E. Rabbinowitsch, N. Rawlins, M. A. Rajandream, U. Reichard, H. Renauld, G. D. Robson, S. Rodriguez de Cordoba, J. M. Rodriguez-Pena, C. M. Ronning, S. Rutter, S. L. Salzberg, M. Sanchez, J. C. Sanchez-Ferrero, D. Saunders, K. Seeger, R. Squares, S. Squares, M. Takeuchi, F. Tekaia, G. Turner, C. R. Vazquez de Aldana, J. Weidman, O. White, J. Woodward, J. H. Yu, C. Fraser, J. E. Galagan, K. Asai, M. Machida, N. Hall, B. Barrell, and D. W. Denning. 2005. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 438:1151-1156. [DOI] [PubMed] [Google Scholar]
- 15.Panackal, A. A., A. Imhof, E. W. Hanley, and K. A. Marr. 2006. Aspergillus ustus infections among transplant recipients. Emerg. Infect. Dis. 12:403-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paris, S., D. Wysong, J.-P. Debeaupuis, K. Shibuya, B. Philippe, R. D. Diamond, and J.-P. Latgé. 2003. Catalases of Aspergillus fumigatus. Infect. Immun. 71:3551-3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasqualotto, A. C. 2009. Differences in pathogenicity and clinical syndromes due to Aspergillus fumigatus and Aspergillus flavus. Med. Mycol. 47(Suppl. 1):S261-S270. [DOI] [PubMed] [Google Scholar]
- 18.Patterson, T. F. 2005. The future of animal models of invasive aspergillosis. Med. Mycol. 43(Suppl. 1):S115-S119. [DOI] [PubMed] [Google Scholar]
- 19.Patterson, T. F., W. R. Kirkpatrick, M. White, J. W. Hiemenz, J. R. Wingard, B. Dupont, M. G. Rinaldi, D. A. Stevens, and J. R. Graybill. 2000. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. I3 Aspergillus Study Group. Medicine (Baltimore) 79:250-260. [DOI] [PubMed] [Google Scholar]
- 20.Philippe, B., O. Ibrahim-Granet, M. C. Prévost, M. A. Gougerot-Pocidalo, M. Sanchez Perez, A. Van der Meeren, and J.-P. Latgé. 2003. Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect. Immun. 71:3034-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schrettl, M., E. Bignell, C. Kragl, C. Joechl, T. Rogers, H. N. Arst, Jr., K. Haynes, and H. Haas. 2004. Siderophore biosynthesis but not reductive iron assimilation is essential for Aspergillus fumigatus virulence. J. Exp. Med. 200:1213-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheppard, D. C., J. R. Graybill, L. K. Najvar, L. Y. Chiang, T. Doedt, W. R. Kirkpatrick, R. Bocanegra, A. C. Vallor, T. F. Patterson, and S. G. Filler. 2006. Standardization of an experimental murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 50:3501-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheppard, D. C., G. Rieg, L. Y. Chiang, S. G. Filler, J. E. Edwards, Jr., and A. S. Ibrahim. 2004. Novel inhalational murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 48:1908-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinbach, W. J., D. K. Benjamin, Jr., S. A. Trasi, J. L. Miller, W. A. Schell, A. K. Zaas, W. M. Foster, and J. R. Perfect. 2004. Value of an inhalational model of invasive aspergillosis. Med. Mycol. 42:417-425. [DOI] [PubMed] [Google Scholar]
- 25.Tsai, H.-F., Y. C. Chang, R. G. Washburn, M. H. Wheeler, and K. J. Kwon-Chung. 1998. The developmentally regulated alb1 gene of Aspergillus fumigatus: its role in modulation of conidial morphology and virulence. J. Bacteriol. 180:3031-3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vallor, A. C., W. R. Kirkpatrick, L. K. Najvar, R. Bocanegra, M. C. Kinney, A. W. Fothergill, M. L. Herrera, B. L. Wickes, J. R. Graybill, and T. F. Patterson. 2008. Assessment of Aspergillus fumigatus burden in pulmonary tissue of guinea pigs by quantitative PCR, galactomannan enzyme immunoassay, and quantitative culture. Antimicrob. Agents Chemother. 52:2593-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Burik, J.-A. H., R. Colven, and D. H. Spach. 1998. Cutaneous aspergillosis. J. Clin. Microbiol. 36:3115-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh, T. J. 1998. Primary cutaneous aspergillosis—an emerging infection among immunocompromised patients. Clin. Infect. Dis. 27:453-457. [DOI] [PubMed] [Google Scholar]