Abstract
The multidrug efflux transporter AcrAB-TolC is known to pump out a diverse range of antibiotics, including β-lactams. However, the kinetic constants of the efflux process, needed for the quantitative understanding of resistance, were not available until those accompanying the efflux of some cephalosporins were recently determined by combining efflux with the hydrolysis of drugs by the periplasmic β-lactamase. In the present study we extended this approach to the study of a wide range of penicillins, from ampicillin and penicillin V to ureidopenicillins and isoxazolylpenicillins, by combining efflux with hydrolysis with the OXA-7 penicillinase. We found that the penicillins had a much stronger apparent affinity to AcrB and higher maximum rates of efflux than the cephalosporins. All penicillins showed strong positive cooperativity kinetics for export. The kinetic constants obtained were validated, as the MICs theoretically predicted on the basis of efflux and hydrolysis kinetics were remarkably similar to the observed MICs (except for the isoxazolylpenicillins). Surprisingly, however, the efflux kinetics of cloxacillin, for example, whose MIC decreased 512-fold in Escherichia coli upon the genetic deletion of the acrB gene, were quite similar to those of ampicillin, whose MIC decreased only 2-fold with the same treatment. Analysis of this phenomenon showed that the extensive decrease in the MIC for the acrB mutant is primarily due to the low permeation of the drug and that comparison of the MICs between the parent and the acrB strains is a very poor measure of the ability of AcrB to pump a drug out.
Resistance to multiple drugs in pathogenic bacteria is currently a topic of major concern (19). Among the Gram-negative bacteria, there are already strains for which almost no effective antimicrobial agent is available (10). In many cases, multidrug efflux pumps of the resistance-nodulation-division (RND) family play a major role in the creation of such a phenotype (8, 9, 27). Among the RND pumps, AcrB of Escherichia coli has been studied the most intensively as the prototype. AcrB pumps out practically all kinds of antibiotics (except aminoglycosides), as well as dyes, detergents, biocides, and even some solvents (24).
In order to understand the function of this transporter, as well as its role in creating drug resistance, knowledge of its kinetic behavior is essential. Nevertheless, it has been very difficult to obtain kinetic constants for AcrB and its relatives, primarily because such pumps exist as a tripartite complex spanning two membranes, which, in the case of AcrB, comprises AcrB together with the outer membrane channel TolC and the periplasmic adaptor protein AcrA (2, 33, 37). Thus, measurement of meaningful kinetic constants must be performed with intact cells, in which the tripartite structure of the pumping apparatus is maintained. In intact cells, however, it is difficult to measure the drug concentration in the periplasm, a location where much of the drug capture takes place (24). We have recently been able to solve this problem by using cephalosporins as substrates for the AcrB-AcrA-TolC complex (16). When cephalosporins are added to intact E. coli cells, they traverse the outer membrane barrier and enter the periplasm, and some of the molecules are pumped out by the AcrB complex, but others are hydrolyzed by the ubiquitous periplasmic β-lactamases. We followed the course of hydrolysis spectrophotometrically, and from the kinetic constants of the β-lactamase, we could determine the periplasmic concentration (Cp) of the drug. Once we knew the value of Cp, we could calculate the drug influx across the outer membrane, as this is a simple diffusion process that is proportional to the permeability coefficient as well as the difference in the concentration of the drug across the membrane, Co − Cp, where Co is the external concentration of the drug. The efflux rate can then be obtained as the difference between this influx and the hydrolysis rate, which was determined experimentally. It was also observed that the plot of the efflux versus Cp is often sigmoidal, showing clear signs for the cooperative binding of the substrate molecules. This was consistent with the idea, initially proposed by X-ray crystallography (15, 29, 30) and then confirmed by biochemical studies (34, 35), that each protomer of the AcrB trimer undergoes a sequence of conformational changes during drug binding and extrusion, the “functionally rotating” mechanism, because under such conditions, more than one molecule of substrate may be interacting with the AcrB trimer at any given moment.
In the study described here, we extended this approach to the efflux of penicillins. Penicillins were especially interesting because the MIC values in AcrB deletion strains suggested that multidrug efflux makes a much stronger contribution to the intrinsic resistance to some penicillins, for example, the acrB deletion decreases the MIC of cloxacillin by 256-fold (12, 20), in contrast to the undetectable or barely detectable changes in the MICs of most cephalosporins (12). So that isoxazolylpenicillins would be rapidly hydrolyzed, we introduced a plasmid containing the OXA-7 enzyme (13). The results indeed showed that penicillins, in general, are much better substrates of the AcrB pump, and the kinetic parameters were validated by theoretically predicting the MIC values that were close to the observed MICs. The use of these constants in a wild-type E. coli strain produced unexpected predictions that emphasized the importance of the outer membrane barrier in intrinsic resistance to more lipophilic penicillins.
MATERIALS AND METHODS
Bacterial strains and plasmid construction.
Strain HN1157 (16) is a derivative of strain RAM121 (14), which produces no OmpF porin and a mutant OmpC porin with a large channel and which contains a deletion of the AcrR repressor gene. Strain HN1159, a ΔacrAB derivative of HN1157, was described by Nagano and Nikaido (16). Since most of the penicillins used in this study are not rapidly hydrolyzed by the chromosomal β-lactamase AmpC (1, 3, 4), we introduced an oxacillinase, OXA-7 (13, 28), on a plasmid. The oxa-7 gene was amplified from plasmid pMG202 (a gift from G. A. Jacoby) (28) by PCR, together with about 130 and 170 bases of upstream and downstream sequences, respectively, and the amplicon was cloned into the PCR BluntII Topo vector (Invitrogen). The cloned sequence was excised from the recombinant plasmid with EcoRI and was inserted into the EcoRI site of a low-copy-number vector, pHSG576 (36), behind the Plac promoter, producing pHSG576oxa7. This was then transformed into both HN1157 and HN1159.
Growth conditions.
HN1157/pHSG576oxa7 was grown in modified LB broth (1% tryptone, 0.5% yeast extract, 0.29% NaCl, 5 mM MgSO4) supplemented with chloramphenicol at 10 μg/ml for maintenance of the plasmid. One loopful of a colony was inoculated into 25 ml of broth, which was incubated at 30°C for 14 h with shaking. At harvest, the cells were in the stationary phase and had an optical density at 600 nm (OD600) of about 3. For oxacillin, cloxacillin, dicloxacillin, ampicillin, and penicillin V, 0.1% glucose was added to the medium to decrease the uninduced expression of oxa-7 from the Plac promoter, in order to get a desirable ratio between the activities of the efflux pump AcrAB-TolC and OXA-7.
Kinetic analysis by microiodometric assay.
The cells described above were harvested by centrifugation at room temperature, and after they were washed twice with 50 mM potassium phosphate buffer, pH 7.0, containing 5 mM MgCl2, they were resuspended in 10 ml of the same buffer. For the efflux assay, 2 ml of intact cells with an OD600 of 0.2 (which is assumed to correspond to 75 μg cells [dry weight]/ml) was mixed with the β-lactam antibiotics (oxacillin, cloxacillin, dicloxacillin, piperacillin, azlocillin, mezlocillin, penicillin V, and ampicillin) at a desired final concentration, and the reaction was stopped after 5 min at room temperature by adding 0.15 ml of 10% (wt/vol) trichloroacetic acid (TCA). Sonicated extracts (30-s rest + 30-s pulse, 16 times) of cells with an original OD600 of 0.025 (corresponding to 9.38 μg cells [dry weight]/ml) were used for the β-lactamase assay in a similar manner, but the reaction was stopped after 2 min by addition of TCA. The product of hydrolysis was then assayed by microiodometry by immediately adding 1 ml of starch-iodine solution (32), and the OD592 was read after exactly 30 min with a Uvikon 860 spectrophotometer by using a 10-mm-light-path cuvette. In order to correct for the acid-catalyzed slow hydrolysis of penicillins, TCA was also added at 0 min to a duplicate reaction mixture, and the OD592 of this control was subtracted from that of the reaction mixture incubated with cells or enzymes for 5 or 2 min to determine the change in the OD592 (ΔOD592).
Since the microiodometric assay does not quantitate the hydrolysis product directly, it was necessary to obtain a conversion factor, E, that relates the ΔOD592 to the molar amounts of penicilloic acids produced. These values were obtained by allowing the enzymatic hydrolysis of penicillins to proceed to completion (25). The values of E (ΔOD592/penicilloic acid concentration [μM]/length of light path [in cm]) were 0.13, 0.11, 0.09, 0.16, 0.12, 0.10, 0.11, and 0.12 for dicloxacillin, cloxacillin, oxacillin, piperacillin, azlocillin, mezlocillin, penicillin V, and ampicillin, respectively; and these values were close to the value of 0.128 reported by Novick (25) for ampicillin. Although these slightly different values probably reflected the weighing errors and the different water contents of the drug samples, we used the experimentally determined values for calculation for each drug.
The principle of the kinetic assay is described in Results. Kinetic constants were derived by the curve fitting of the efflux rate, Ve, against the periplasmic concentration, Cp, by the program CurveExpert, version 1.3. Since these curves were obviously sigmoidal, we used the Michaelis-Menten equation modified for cooperativity, Ve = 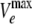 ·(Cp)h/[(K0.5)h + (Cp)h], where
·(Cp)h/[(K0.5)h + (Cp)h], where  is the maximum rate of efflux, h is the Hill coefficient, and K0.5 (in μM) is the drug concentration giving the half-maximum velocity. The Michaelis-Menten constant, Km (in μM), was also derived by curve fitting by using the standard Michaelis-Menten equation.
is the maximum rate of efflux, h is the Hill coefficient, and K0.5 (in μM) is the drug concentration giving the half-maximum velocity. The Michaelis-Menten constant, Km (in μM), was also derived by curve fitting by using the standard Michaelis-Menten equation.
Theoretical prediction of MIC.
We modified the procedure used earlier for prediction of the MIC, which considered only the hydrolysis of the β-lactams (21). We first obtained the minimal drug concentration needed for the inhibition of the target, or Cinh. The most convenient way of obtaining this parameter was to determine the MIC of a strain lacking both the β-lactamase and efflux, that is, HN1159. When Cp reaches Cinh in strain HN1157/pHSG576oxa7, the sum of degradation and efflux, Ve + Vh, should be precisely balanced by the influx, Vin. Thus, we expect that P·A·(Co − Cinh) = Ve + Vh, where P is the permeability coefficient and A is the surface area of the cells. Thus, the MIC or Co at this point can be calculated by inserting the values of Ve and Vh calculated for Cp equal to Cinh by the formula MIC = Co = [(Ve + Vh)/(P·A)] + Cinh.
Determination of measured MIC.
The inoculum was cultivated overnight in the modified LB broth supplemented with 10 μg/ml chloramphenicol for strains containing pHSG576 and its derivatives. Linear-gradient plates containing various β-lactam antibiotics in the bottom layer were made with the modified LB agar in square petri dishes, and the bacterial suspensions diluted to an OD660 of 0.1 were streaked across the plates (34). The plates were observed after an overnight incubation at 30°C.
Calculation of log P values.
The lipophilicities of the 6 substituents in various penicillins was calculated at the website http://www.molinspiration.com/cgi-bin/properties. The substituent structure was modified by changing the carboxyl group to a methyl group, because we wanted to avoid the effect of potential ionization in the calculation.
Reagents.
All antibiotics except piperacillin and mezlocillin were obtained from Sigma-Aldrich. Piperacillin was obtained from Lederle Laboratories Division (Pearl River, NY), and mezlocillin was obtained from Bayer (Leverkusen, Germany). All other reagents were of analytical grade.
RESULTS AND DISCUSSION
OXA-7 β-lactamase.
OXA-7 is known to readily hydrolyze benzylpenicillin, ampicillin, oxacillin, and cloxacillin (13). We introduced an oxa-7-containing low-copy-number plasmid, pHSG576oxa7, into an E. coli K-12 strain, strain HN1157, and found that the strain produced enough enzyme activity without the induction of the Plac promoter. In fact, in order to have an optimal balance between the hydrolysis rates and efflux rates, for some drugs it was necessary to repress the background transcription from the Plac promoter by adding glucose to the medium (Table 1).
TABLE 1.
Kinetic parameters of OXA-7 enzyme
| Substrate | % glucose used for growth |
 (nmol/mg/s)a (nmol/mg/s)a
|
Km (μM)a |
|---|---|---|---|
| Dicloxacillin | 0.1 | 5.9 | 34 |
| Cloxacillin | 0.1 | 6.3 | 31 |
| Oxacillin | 0.1 | 9.3 | 52 |
| Azlocillin | 0 | 4.3 | 18 |
| Mezlocillin | 0 | 5.0 | 29 |
| Piperacillin | 0 | 3.5 | 34 |
| Penicillin V | 0.1 | 6.7 | 29 |
| Ampicillin | 0.1 | 4.1 | 21.5 |
The values shown are the averages of three independent experiments.  is the maximal rate of hydrolysis.
is the maximal rate of hydrolysis.
Another advantage of OXA-7 was that the Km values were reasonably high, in the range of 10's of μM (Table 1), so that over the range of Cp values used (usually less than 2 μM), the hydrolysis rates were sensitive to Cp, allowing the precise determination of Cp values.
Host strain HN1157 contained the chromosomal ampC gene, which codes for a class C β-lactamase. Thus, we were initially concerned that the coexistence of two different β-lactamases might produce complex kinetics for drug hydrolysis. However, the AmpC enzyme is essentially inert to the isoxazolylpenicillins (oxacillin, cloxacillin, and dicloxacillin) (1, 3, 4). Even for a readily hydrolyzed compound, ampicillin, the maximum rate of hydrolysis (Vmax) of AmpC was very low thanks to the very weak promoter (5). Its Vmax of 0.16 nmol/mg/s (21) was less than 5% of the Vmax of OXA-7 (Table 1), and we never saw any situation in which the curve fitting had to be done on the basis of the presence of two different enzymes. We therefore neglected the contribution of the host chromosomal AmpC in the study of efflux kinetics.
Kinetics of efflux of penicillins.
The efflux kinetics of the penicillins were determined by using the principle described for cephalosporins by Nagano and Nikaido (16). Briefly, the rate of hydrolysis of the penicillins (Vh) in intact cells was determined by using the microiodometric assay. Since we also determined the kinetic parameters of the periplasmic OXA-7 enzyme by using crude sonicates of the cells, this allowed us to calculate the periplasmic concentration of the substrate (Cp). The rate of diffusion of the drugs across the outer membrane (Vin) was then calculated by using the permeability coefficient, P, determined by using intact cells in which the AcrB pump was inactivated by deenergization with the proton uncoupler carbonyl cyanide m-chlorophenylhydrazone (CCCP). According to Fick's first law of diffusion, Vin = P·A·(Co − Cp), where A and Co stand for the surface area of the cells (taken to be 132 cm2/mg [dry weight] [23]) and the external concentration of the drug, respectively, and therefore, measurement of Vh allowed us to derive values of P from this equation, as Ve is equal to 0 and Vin is equal to Vh. Ve was then calculated as the difference between the influx rate and the hydrolysis rate, i.e., Vin − Vh, in unpoisoned cells. Although penicillins bind to penicillin-binding proteins, this does not affect the kinetics of their hydrolysis, once the steady state becomes established.
Since we stopped the reaction by adding 0.5% (wt/vol, final concentration) TCA and since we read the final OD592 of the iodine-starch mixture after 30 min at room temperature, it was necessary to correct for the acid-induced, nonenzymatic hydrolysis of penicillins. This was achieved by adding TCA at 0 min and reading the OD592 of the mixture after 30 min. The correction was not insignificant but did not in any experiment exceed 30% of the total ΔOD592 caused by hydrolysis by the cells or the cell extracts.
The results of these experiments (Fig. 1 to 3; Table 2) clearly showed that each drug was pumped out at significant rates (in comparison with the rate of enzymatic hydrolysis). Furthermore, the efflux kinetics were clearly sigmoidal in each case, implying positive cooperativity (17). The positive cooperativity is consistent with the functionally rotating mechanism of the AcrB trimer, originally proposed on the basis of the asymmetric crystal structure of the trimer (15, 29, 30), in which each protomer undergoes a cyclic conformational change that accommodates the conformational changes of its neighboring protomers. In such a scheme, which was also confirmed by biochemical-genetic studies (34, 35), at any given moment the trimer may be interacting with more than one molecule of substrates, and thus, the positive cooperativity kinetics fit with this mechanism.
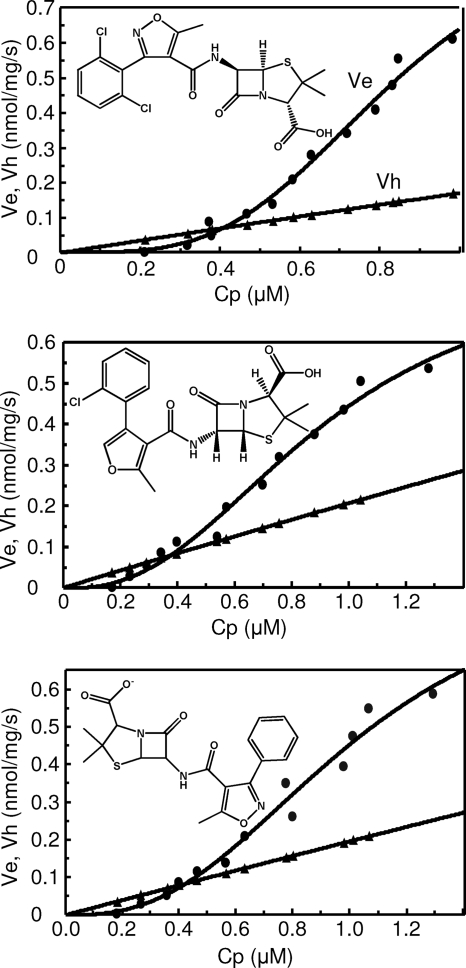
FIG. 1.
Ves (circles) and Vhs (triangles) of isoxazolylpenicillins in strain HN1157/pHSGoxa7. The rates were plotted against Cp, determined from the rates of hydrolysis in intact cells, as described in the text. Top, dicloxacillin; middle, cloxacillin; bottom, oxacillin.
FIG. 3.
Ves (circles) and Vhs (triangles) of penicillin V and ampicillin in strain HN1157/pHSGoxa7. The rates were plotted against Cp, determined from the hydrolysis rates in intact cells, as described in the text. Top, penicillin V; bottom, ampicillin.
TABLE 2.
Kinetic parameters of the efflux process
| Substrate |
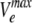 (nmol/mg/s) (nmol/mg/s) |
Hill coefficient | K0.5 (μM) | Permeability coefficient (μm/s) | Substituent log Pa |
|---|---|---|---|---|---|
| Dicloxacillin | 0.98, 0.95, 0.94 | 3.6, 4.7, 4.3 | 0.53, 0.83, 0.82 | 0.6, 0.6, 0.6 | 3.4 |
| Cloxacillin | 0.89, 0.99, 0.80 | 2.3, 2.7, 2.4 | 0.95, 1.03, 0.91 | 0.6, 0.6, 0.6 | 2.8 |
| Oxacillin | 0.94, 1.1, 0.94 | 2.6, 2.7, 2.6 | 1.04, 0.97, 1.03 | 0.6, 0.6, 0.6 | 2.1 |
| Azlocillin | 0.42, 0.41, 0.35 | 4.0, 4.0, 4.6 | 1.08, 1.06, 1.09 | 0.4, 0.4, 0.4 | 0.71 |
| Mezlocillin | 0.51, 0.47, 0.50 | 4.4, 4.5, 4.5 | 1.09, 1.08, 1.11 | 0.5, 0.5, 0.5 | 0.67 |
| Piperacillin | 0.39, 0.35, 0.39 | 4.1, 3.7, 4.1 | 1.06, 1.08, 1.08 | 0.4, 0.4, 0.3 | 0.45 |
| Penicillin V | 0.72, 0.85, 0.72 | 4.7, 4.7, 4.6 | 0.82, 0.84, 0.83 | 0.8, 0.7, 0.7 | 1.54 |
| Ampicillin | 0.38, 0.37, 0.37 | 1.9, 2.3, 2.5 | 0.88, 0.92, 1.03 | 0.8, 0.8, 0.8 | −1.03 |
The log P values of the 6-substituent were calculated after the carboxyl group was changed to a methyl group at the website http://www.molinspiration.com/services/lopP.html.
The rather high Hill coefficients observed (Table 2), however, were surprising because there are only three protomers of AcrB. One possible (partial) explanation could be the underestimation of the value of P, which was determined from the hydrolysis rates in cells in which the AcrB pump was deenergized by the proton conductor CCCP. In our previous study (16), we observed that CCCP apparently partitions also into the outer membrane bilayer, decreasing its passive permeability. Thus, with penicillins, which are generally more lipophilic than cephalosporins and which are more likely to permeate the outer membrane bilayer, the determination of P by using CCCP could result in the underestimation of P. For piperacillin, for example, the Hill coefficient of 3.7, calculated by using the original value of P, would decrease to 3.0 if P was assumed to be twice the value obtained by using the CCCP assay. (This does not change the K0.5 values significantly, although the value of  increases substantially.)
increases substantially.)
Because the isoxazolylpenicillins had very similar structures and yet their 6-substituents covered a significant range of lipophilicities (Table 2, last column), we may be justified in comparing the behaviors of these drugs. As seen in Table 2, the K0.5 values decreased significantly as the lipophilicity increased, suggesting a higher affinity to the pump. This is consistent with the knowledge that the crystallographically elucidated binding site (15) is bordered by lipophilic and, especially, aromatic side chains (24, 29). Interestingly, the Hill coefficient also increased in the most lipophilic member, dicloxacillin (Table 2). A similar tendency might exist in the pair penicillin V and ampicillin (Table 2), but it is unclear whether the structural comparison is valid because the latter exists at least partially as a zwitterionic compound. It is also difficult to compare convincingly the members of the ureidopenicillin group (azlocillin, mezlocillin, and piperacillin), because the differences in the lipophilicities of the 6-substituents were not large. In any case, the effect of lipophilicity is consistent with an earlier conclusion that lipophilic side chains are needed for the efficient efflux of β-lactams, obtained by using a crude and very indirect criterion of the MIC change upon deletion of the acrAB genes (20) (but see the section “Situations in wild-type cells of E. coli K-12” below).
Diffusion through the narrow, wild-type OmpF and OmpC porin channels is strongly retarded by the lipophilicities of the solutes (22, 23, 38). However, it was difficult to show this effect convincingly in this study, although the average values of P decreased very slightly, from 0.634 μm/s for oxacillin to 0.565 μm/s for cloxacillin. This lack of sensitivity to the lipophilicities of solutes is likely to be due to the fact that we used, in an effort to increase the value of Vin for the purpose of increasing the precision of the assay, a strain producing a mutant porin with a much larger channel (14), which is not likely to be affected much by the properties of the solute. Also, a fraction of these lipophilic molecules can possibly diffuse across the bilayer region of the outer membrane. Although penicillins, which have strongly acidic groups, may be thought to be essentially unable to permeate the bilayers, the rare protonated species is apparently sufficient to produce a rather rapid mass diffusion across the bilayer of the cytoplasmic membrane (7, 20).
Comparison of efflux of penicillins with that of cephalosporins.
It was suspected that penicillins in general may be better substrates for the AcrB pump than cephalosporins in general, because the deletion of AcrAB often produces large decreases in the MICs of penicillins (in an extreme case, 256-fold for cloxacillin [12, 20]), whereas the MICs of cephalosporins usually do not undergo large changes (12). Indeed, the K0.5 values for the penicillins tested (Table 2) were uniformly about 1 μM or even lower, whereas those for the cephalosporins were between 5 and nearly 300 μM (16), consistent with the hypothesis that penicillins probably bind much better to the binding site of AcrB, as the penicillin nucleus is significantly more lipophilic than the cephalosporin nucleus. In terms of the Vmax values of efflux, the penicillins had rates between 0.4 and 1 nmol/mg/s (Table 2), whereas for the cephalosporins, they were between 0.023 (nitrocefin) and 0.37 (cefamandole) with the sole exception of the rate for cephaloridine, which was 1.8 nmol/mg/s (16). Thus, these kinetic constants seem to suggest that penicillins are indeed significantly better substrates for the AcrB pump.
Among the cephalosporins, only nitrocefin did not show detectable positive cooperativity (16). We speculated at that time that this lack of cooperativity might be related to the low Km (K0.5) value for this substrate. However, all the penicillins studied here had even lower K0.5 values yet and showed very strong cooperativity, so the previous explanation is not valid, and currently it is not known why nitrocefin behaves in this exceptional manner.
Another consideration is the functional state of the efflux machinery at the relevant values of Cp, at which the cells begin to be killed by the drugs. When we consider this periplasmic concentration, Cinh (see Materials and Methods), that is achieved when drugs are present in the medium at the MICs, we see by comparison of the data in Table 3 (for Cinh) and Table 2 (for K0.5) that the former is way over the latter with all penicillins. Thus, with clinically relevant concentrations of penicillins, the efflux system is working at almost full force. In contrast, with cephalosporins, at Cinh the AcrB pump is usually way below the level of saturation (16).
TABLE 3.
Predicted and measured MICs for HN1157/pHSGoxa7
| Penicillin | Cinh (μg/ml) | MIC (μg/ml) |
|
|---|---|---|---|
| Predicted | Measured | ||
| Dicloxacillin | 18.4 (39)a | 285 | 560 |
| Cloxacillin | 4.9 (11) | 147 | 295 |
| Oxacillin | 4.8 (12) | 135 | 282 |
| Piperacillin | 0.6 (1.2) | 30 | 46 |
| Azlocillin | 0.6 (1.3) | 40 | 44 |
| Mezlocillin | 0.6 (1.1) | 33 | 30 |
| Penicillin V | 11.5 (33) | 181 | 178 |
| Ampicillin | 1.0 (2.9) | 26 | 39 |
The numbers in parentheses are in μM.
Can the observed efflux parameters correctly predict MIC values?
If the constants obtained are correct, then the efflux according to these constants, added to the periplasmic hydrolysis by OXA-7, again following its kinetic constants, should exactly balance the rate of inflow of drugs (Vin) across the outer membrane when the drug concentration in the external medium (Co) reaches the MIC and produces a periplasmic concentration (Cp = Cinh) that just inhibits the growth of cells. We estimated Cinh as the MIC of HN1159, which lacks the constitutive AcrB efflux pump as well as the OXA-7 β-lactamase (Table 3). We then calculated the Co that would generate exactly this value of Cp, as described in Materials and Methods. The values of Co or the theoretically predicted values of MIC were remarkably close to the experimentally observed MIC values for most compounds (Table 3). Isoxazolylpenicillins, however, showed some discrepancy, with the observed MIC values being about 2-fold higher than the predicted ones. The cause for this behavior is not known. However, these are compounds with the most lipophilic side chains, and indeed, their molecules are known to aggregate in aqueous solutions (31). Although the aggregation required higher concentrations, in the range of 20 to 30 mM, this was done in distilled water in which the repulsion between the anionic carboxylate groups would be maximal, and in our Mg2+-containing 50 mM buffer, such a repulsion would be minimized. Thus, over the range of MICs used (nearly 1 mM), we cannot rule out that the aggregation is taking place, decreasing the diffusion across the outer membrane and making the bacteria more resistant.
Situations in wild-type cells of E. coli K-12.
As was emphasized in our earlier study (16), this assay was technically difficult to carry out with the required precision. Therefore, we had to optimize the system by introducing several conditions that do not exist in unmodified cells of E. coli K-12. (i) To ensure rapid hydrolysis and the rapid efflux of the drugs, we used an E. coli mutant producing the OmpC porin with an expanded channel. The strain was indeed hypersusceptible to penicillin G, ampicillin, and chloramphenicol (14); and the isolated porin showed higher ionic conductance in the planar bilayer assay (6). The permeability coefficients for various penicillins (Table 2) were indeed much higher than those previously determined for wild-type K-12 cells (21). (ii) In order to produce the hydrolysis of various penicillins at a reasonable rate and with relatively high Km values, the oxa-7 gene was introduced on a plasmid. (iii) The synthesis of the AcrB pump was enhanced (about 3-fold [11]) by the inactivation of the acrR repressor gene. Thus, although the kinetic parameters reported for efflux are believed to be correct (except that the Vemax values are somewhat inflated), other parameters such as penicillinase activity and porin permeability are very strongly distorted in our intentionally artificial construct. Thus, the MIC values shown in Table 3 have only a very remote relationship with what will happen in the wild-type cells.
It is therefore desirable to examine the potential contribution of the pump and the endogenous, chromosomally coded AmpC enzyme to the MIC values of the wild-type K-12 strain. For cloxacillin, we measured Cinh to be 4.9 μg/ml, or roughly 10 μM. Neglecting the hydrolysis by AmpC, which is in the undetectable range (1, 4), the Ve at this concentration is calculated to be about 0.3 nmol/mg/s by use of the parameters shown in Table 2. The MICs of the wild-type strains are 512 μg/ml (20) or 256 μg/ml (12). At 512 μg/ml, the rate of inflow across the outer membrane is estimated to be 0.3 nmol/mg/s, precisely balancing the efflux rate, if the P value previously determined for benzylpenicillin (0.2 × 10−5 cm/s) (21) is used.
In contrast, for ampicillin, the MIC of the acrB mutant was 1 μg/ml (this study) and that of the ampC mutant was 0.7 μg/ml (21). However, these mutants still contain the AmpC enzyme and AcrB-directed efflux, respectively, so these values cannot be taken directly as Cinh. We assumed here that the true value of Cinh is one-half of 0.7 μg/ml, or 1 μM; this is close to the MIC of mutants with the ampC acrB double mutations (0.5 μg/ml) reported by Mazzariol et al. (12). At this periplasmic concentration, Ve is calculated to be 0.05 nmol/mg/s. The rate of hydrolysis by the AmpC enzyme is also about 0.05 nmol/mg/s, on the basis of the values of the parameters reported earlier (21). At an external concentration of 2.5 μg/ml, the rate of diffusion across the outer membrane is thus P·A·(Co − Cinh), which equals 0.08 nmol/mg/s, as determined by using the previously determined value of P (9.8 × 10−5 cm/s) (21), and it is close to the sum of the rates of hydrolysis and efflux. The MIC of the wild-type K-12 strain was reported to be 2.5 μg/ml (26) or 2 μg/ml (12), close to the value used in the calculation presented above.
This rather crude analysis, however, gives us important conclusions. Since the discovery of their multidrug efflux function more than a decade ago, the kinetic parameters of RND-pump-mediated drug efflux have never been elucidated, until our recent study of cephalosporins (16) and this study. Thus, the only indirect measure for the efficiency of efflux of any drug was to compare the MIC of the wild-type strain with that of the pump-deficient mutant. With this criterion, isoxazolylpenicillins such as cloxacillin appeared to be very strong substrates of the AcrB pump, because genetic deletion of the pump decreased the MIC 256-fold or even more (12, 20). In contrast, compounds like ampicillin were always thought to be very poor substrates of the pump, because AcrB deletion lowered the MIC only 2-fold. However, this quantitative study of efflux showed that both cloxacillin and ampicillin are similarly good substrates of the pump, the K0.5 values are identical, and there is only a slightly more than 2-fold difference in the Vemax values. Thus, it is clear that the ratios of MICs do not serve even as a crude index of the efflux efficiency of any given drug. So if ampicillin and cloxacillin are both pumped out well by AcrB, why does the deletion of the pump produce such a spectacular change in the MIC only of cloxacillin? Scrutiny of the calculations given above shows that the major factor in play is the poor permeations of the lipophilic isoxazolylpenicillins, modeled after the permeation of penicillin G, although an additional factor is the contribution of the AmpC-catalyzed hydrolysis of ampicillin but not cloxacillin. Thus, as was proposed earlier (18), what makes RND multidrug efflux pumps so effective is the synergy with the outer membrane permeability barrier. This point is also confirmed by the observation that with the large-channel mutant porin, the cloxacillin MIC of the strain containing pHSGoxa7 goes down only 2.5-fold upon deletion of the acrAB genes (results not shown).
FIG. 2.
Ves (circles) and Vhs (triangles) of ureidopenicillins in strain HN1157/pHSGoxa7. The rates were plotted against Cp, determined from the hydrolysis rates in intact cells, as described in the text. Top, azlocillin; middle, mezlocillin; bottom, piperacillin.
Acknowledgments
This study was supported by a grant from U.S. Public Health Service (grant AI-09644).
We thank George Jacoby for a plasmid coding for the OXA-7 enzyme and Keiji Nagano for his advice during the early phase of this work.
Footnotes
Published ahead of print on 16 February 2010.
REFERENCES
- 1.Bush, K., G. A. Jacoby, and A. A. Medeiros. 1995. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eswaran, J., E. Koronakis, M. K. Higgins, C. Hughes, and V. Koronakis. 2004. Three's company: component structures bring a closer view of tripartite drug efflux pumps. Curr. Opin. Struct. Biol. 14:741-747. [DOI] [PubMed] [Google Scholar]
- 3.Galleni, M., and J.-M. Frère. 1988. A survey of the kinetic parameters of class C β-lactamases. Penicillins. Biochem. J. 255:119-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacoby, G. A. 2009. AmpC β-lactamases. Clin. Microbiol. Rev. 22:161-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaurin, B., T. Grundstrom, and S. Normark. 1982. Sequence elements determining ampC promoter strength in E.coli. EMBO J. 1:875-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lakey, J. H., E. J. Lea, and F. Pattus. 1991. ompC mutants with allow growth on maltodextrins show increased channel size and greater voltage sensitivity. FEBS Lett. 278:31-34. [DOI] [PubMed] [Google Scholar]
- 7.Li, X.-Z., D. Ma, D. Livermore, and H. Nikaido. 1994. Role of intrinsic pump(s) in intrinsic resistance of Pseudomonas aeruginosa: active efflux as a contributing factor to β-lactam resistance. Antimicrob. Agents Chemother. 38:1742-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li, X.-Z., and H. Nikaido. 2004. Efflux-mediated resistance in bacteria. Drugs 64:159-204. [DOI] [PubMed] [Google Scholar]
- 9.Li, X.-Z., and H. Nikaido. 2009. Efflux-mediated resistance in bacteria: an update. Drugs 69:1555-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livermore, D. M. 2004. The need for new antibiotics. Clin. Microbiol. Infect. 10(Suppl. 4):1-9. [DOI] [PubMed] [Google Scholar]
- 11.Ma, D., M. Alberti, C. Lynch, H. Nikaido, and J. E. Hearst. 1996. The local repressor AcrR plays a modulating role in the regulation of acrAB genes of Escherichia coli by global stress signals. Mol. Microbiol. 19:101-112. [DOI] [PubMed] [Google Scholar]
- 12.Mazzariol, A., G. Cornaglia, and H. Nikaido. 2000. Contributions of the AmpC β-lactamase and the AcrAB multidrug efflux system in intrinsic resistance of Escherichia coli K-12 to β-lactams. Antimicrob. Agents Chemother. 44:1378-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medeiros, A. A., M. Cohenford, and G. A. Jacoby. 1985. Five novel plasmid-determined β-lactamases. Antimicrob. Agents Chemother. 27:715-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Misra, R., and S. A. Benson. 1988. Isolation and characterization of OmpC porin mutants with altered pore properties. J. Bacteriol. 170:528-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murakami, S., R. Nakashima, E. Yamashita, T. Matsumoto, and A. Yamaguchi. 2006. Crystal structures of a multidrug transporter reveal a functionally rotating mechanism. Nature 443:173-179. [DOI] [PubMed] [Google Scholar]
- 16.Nagano, K., and H. Nikaido. 2009. Kinetic behavior of the major multidrug efflux pump AcrB of Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 106:5854-5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neet, K. E. 1995. Cooperative enzyme function: equilibrium and kinetic aspects. Methods Enzymol. 249:519-567. [DOI] [PubMed] [Google Scholar]
- 18.Nikaido, H. 2001. Preventing drug access to targets: cell surface permeability barriers and active efflux in bacteria. Semin. Cell Dev. Biol. 12:215-223. [DOI] [PubMed] [Google Scholar]
- 19.Nikaido, H. 2009. Multidrug resistance in bacteria. Annu. Rev. Biochem. 78:119-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikaido, H., M. Basina, V. Nguyen, and E. Y. Rosenberg. 1998. Multidrug efflux pump AcrAB of Salmonella typhimurium excretes only those beta-lactam antibiotics containing lipophilic side chains. J. Bacteriol. 180:4686-4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikaido, H., and S. Normark. 1987. Sensitivity of Escherichia coli to various β-lactams is determined by the interplay of outer membrane permeability and degradation by periplasmic β-lactamases. Mol. Microbiol. 1:29-36. [DOI] [PubMed] [Google Scholar]
- 22.Nikaido, H., and E. Y. Rosenberg. 1983. Porin channels in Escherichia coli: studies with liposomes reconstituted from purified protein. J. Bacteriol. 153:241-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikaido, H., E. Y. Rosenberg, and J. Foulds. 1983. Porin channels in Escherichia coli: studies with β-lactams in intact cells. J. Bacteriol. 153:232-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikaido, H., and Y. Takatsuka. 2009. Mechanisms of RND multidrug efflux pumps. Biochim. Biophys. Acta 1794:769-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novick, R. P. 1962. Micro-iodometric assay for penicillinase. Biochem. J. 83:236-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okusu, H., D. Ma, and H. Nikaido. 1996. AcrB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J. Bacteriol. 178:306-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poole, K. 2005. Efflux-mediated antimicrobial resistance. J. Antimicrob. Chemother. 56:20-51. [DOI] [PubMed] [Google Scholar]
- 28.Scoulica, E., A. Aransay, and Y. Tselentis. 1995. Molecular characterization of the OXA-7 β-lactamase gene. Antimicrob. Agents Chemother. 39:1379-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seeger, M. A., A. Schiefner, T. Eicher, F. Verrey, K. Diedrichs, and K. M. Pos. 2006. Structural asymmetry of AcrB trimer suggests a peristaltic pump mechanism. Science 313:1295-1298. [DOI] [PubMed] [Google Scholar]
- 30.Sennhauser, G., P. Amstutz, C. Briand, O. Storchenegger, and M. G. Grütter. 2006. Drug export pathway of multidrug exporter AcrB revealed by DARPin inhibitors. PLoS Biol. 5:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seoane, L., P. Martinez-Landeira, L. Besada, J. M. Ruso, F. Sarmiento, and G. Prieto. 2002. A thermodynamic study of the aggregation process of oxacillin sodium salt in aqueous solution. Colloid Polym. Sci. 280:624-629. [Google Scholar]
- 32.Sykes, R. B., and K. Nordstrom. 1972. Microiodometric determination of β-lactamase activity. Antimicrob. Agents Chemother. 1:94-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Symmons, M. F., E. Bokma, E. Koronakis, C. Hughes, and V. Koronakis. 2009. The assembled structure of a complete tripartite bacterial multidrug efflux pump. Proc. Natl. Acad. Sci. U. S. A. 106:7173-7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takatsuka, Y., and H. Nikaido. 2007. Site-directed disulfide cross-linking shows that cleft flexibility in the periplasmic domain is needed for the multidrug efflux pump AcrB of Escherichia coli. J. Bacteriol. 189:8677-8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takatsuka, Y., and H. Nikaido. 2009. Covalently linked trimer of the AcrB multidrug efflux pump provides support for the functional rotating mechanism. J. Bacteriol. 191:1729-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeshita, S., M. Sato, M. Toba, W. Masahashi, and T. Hashimoto-Gotoh. 1987. High-copy-number and low-copy-number plasmid vectors for lacZα-complementation and chloramphenicol- or kanamycin-resistance selection. Gene 61:63-74. [DOI] [PubMed] [Google Scholar]
- 37.Tikhonova, E. B., and H. I. Zgurskaya. 2004. AcrA, AcrB, and TolC of Escherichia coli form a stable intermembrane multidrug efflux complex. J. Biol. Chem. 279:32116-32124. [DOI] [PubMed] [Google Scholar]
- 38.Yoshimura, F., and H. Nikaido. 1985. Diffusion of β-lactam antibiotics through the porin channels of Escherichia coli K-12. Antimicrob. Agents Chemother. 27:84-92. [DOI] [PMC free article] [PubMed] [Google Scholar]