Abstract
A rapid turnaround is a prerequisite of therapeutic drug monitoring (TDM). For antifungals, this need is still unmet, since hardly any method has been established to simultaneously quantitate concentrations of different antifungal classes. A liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was developed allowing quantitation of anidulafungin (ANF), caspofungin (CSF), isavuconazole (ISC), micafungin (MCF), posaconazole (PSC), and voriconazole (VRC). Quantitation was successful with diluted plasma samples, peripheral blood mononuclear cells (PBMC), polymorphonuclear leukocytes (PMN), and erythrocytes (RBC). A triple quadrupole mass spectrometer in selected reaction monitoring mode was used with positive electrospray ionization. Cells and calibration standards were extracted with acetonitrile containing internal standard. Internal standards were a CSF derivate for echinocandins and itraconazole for triazoles. Chromatographic separation of the supernatant was achieved by a gradient method facilitating a BetaBasic C4 column. Analytes were quantified in a single 8-min run. Calibration curves were linear and fitted using least squares with a weighting factor of the reciprocal concentration. Limits of detection (ng/ml) were ANF, 8.3; CSF, 31.5; ISC, 1.5; MCF, 97.7; PSC, 3.3; and VRC, 1.4. The lower limits of quantitation (ng/ml) were ANF, 64; CSF, 108; ISC, 4.5; MCF, 160; PSC, 10; and VRC, 4.2. Intraday precisions ranged from 6.3% to 8.8% for azoles and 8.8% to 15.4% for echinocandins. Intraday and interday accuracies (percent bias) of all analytes were within 13.8%. The method was established as standard practice for the quantitation of intracellular antifungal concentrations and optimizes TDM by applying a rapid single method for 6 antifungals.
Therapeutic drug monitoring (TDM) aims at optimizing the benefits and risks of pharmacotherapy, specifically for drugs exhibiting significant pharmacokinetic variability (18, 34).
TDM of antifungals is increasingly recommended to tailor therapy for individual patients (25, 28). Itraconazole (ITC) plasma concentration correlates with prophylactic efficacy (15). Voriconazole (VRC) exhibits nonlinear pharmacokinetics likely caused by saturation of its P450-dependent clearance (19). Recently, correlations of efficacy and toxicity with seemingly difficult-to-predict VRC plasma levels have been described (4, 21, 25, 35, 41). Posaconazole (PSC) demonstrates linear pharmacokinetics, but with a saturation of absorption effect above a daily dose of 800 mg (10). There is an exposure-dependent response rate for invasive aspergillosis (38), and while FDA reports suggest a possible correlation of lower plasma levels and failure of antifungal prophylaxis (30), no cutoff PSC plasma concentration could be defined for breakthrough fungal infections (8). For isavuconazole (ISC), phase III clinical trials are currently ongoing, and it might be too early to assess the role of TDM for this drug.
Relative to the azoles, echinocandins are larger and structurally different molecules; hence, we expect different patterns of distribution. Currently, three antifungals of the echinocandin class, i.e., anidulafungin (ANF), caspofungin (CSF), and micafungin (MCF), are commercially available (1, 22, 27). Common to all echinocandins is their low oral bioavailability, high protein binding, and relatively low urinary excretion of the parent drug. The relation between echinocandin blood levels and treatment outcome is currently undefined.
Some drugs may achieve different concentrations in different compartments and tissues. Recently, a 33-fold increased PSC level, i.e., for the maximum concentration of drug (Cmax) and the area under the curve (AUC), was found for alveolar cells compared to plasma concentrations (7). Peripheral blood mononuclear cells (PBMC) and polymorphonuclear leukocytes (PMN) are important pillars of host defense against invasive fungal infections (IFI). After phagocytosis, intracellular antifungal concentrations may well influence their lytic capability. For example, it is known that intracellular voriconazole in monocyte-derived macrophages augments the killing of intracellular Candida glabrata, Candida krusei, and Candida parapsilosis (3), and synergy of itraconazole cocultured with macrophages on the killing of Blastomyces dermatitidis has been described (5). However, little is known about the interaction of antifungal drugs with peripheral blood cells. The only data published refer to fluconazole (FLC) and VRC interacting with PMN, both undergoing rapid intracellular uptake and elution. While FLC concentrations were twice as high as those in the surrounding medium (26), VRC concentrations were 8.5-fold (2). ISC and PSC are expected to behave likewise, but this has not been proven so far. The same holds true for the echinocandins.
Since plasma concentrations, especially of PSC and VRC, show substantial variability (16, 36, 37), their intracellular concentrations might more accurately correlate with their efficacy. We therefore developed a feasible method of quantitating most clinically relevant antifungal drugs in different compartments of the peripheral blood.
MATERIALS AND METHODS
Chemicals and reagents.
ANF was kindly provided by the Central Research Division, Pfizer Inc. (Groton, CT). CSF and CSF internal standard (CIS) were kindly provided by Merck Research Laboratories (Rahway, NJ). CIS, which is a derivate of CSF, was used as an internal standard for all echinocandins. ISC was kindly provided by Basilea Pharmaceutical AG (Basel, Switzerland). MCF was kindly provided by Astellas Pharma Inc. (Osaka, Japan). PSC was kindly provided by the Chemical Research Division, Schering-Plough Research Institute (Kenilworth, NJ). VRC was kindly provided by the Central Research Division, Pfizer Inc. (Sandwich, United Kingdom). The internal standard ITC for the azole antifungals (AIS) was purchased from Jannsen Research Foundation (Beerse, Belgium). Methanol and acetonitrile were purchased from Roth (Karlsruhe, Germany). Histopaque 1077 and Histopaque 1119 were both purchased from Sigma-Aldrich Chemie GmbH (Munich, Germany). Formic acid, sodium chloride, potassium chloride, disodium hydrogen phosphate, and potassium dihydrogen phosphate were purchased from Merck (Darmstadt, Germany). All reagents were of analytical or high-performance liquid chromatography (HPLC) grade. Deionized water, further purified with a Milli-Q water purifying system (Millipore Corporation, Bedford, MA), was used. HPLC eluent A was water with 0.1% formic acid. Acetonitrile was used as HPLC eluent B.
Materials and equipment.
Samples were analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) using a triple-stage quadruple mass spectrometer (TSQ Quantum; Thermo Finnigan, San Jose, CA), working in selected reaction monitoring (SRM) mode with positive electrospray ionization (ESI). The system was equipped with a Surveyor quaternary narrow-bore LC pump, a Surveyor autosampler, fitted with a temperated tray and column oven. The Finnigan Xcalibur software (version 1.3), installed on a personal computer, was used for instrument control and data acquisition. The MS/MS conditions were optimized using the Quantum Tune Master software (Thermo Finnigan). The HPLC column used was a BetaBasic C4 (100 by 3.0 mm; Thermo Hypersil-Keystone, Dreieich, Germany). Attached to the HPLC column was a guard column (10 by 3.0 mm; Thermo Hypersil-Keystone, Dreieich, Germany).
Standards.
Stock standard solutions of all analytes and the internal standards were prepared in either methanol-water (50:50 [vol/vol]) for the echinocandins or methanol for the azoles. The concentrations were 64 mg/liter for ANF, 108 mg/liter for CSF, 40 mg/liter for CIS, 50 mg/liter for ISC, 30 mg/liter for AIS, 80 mg/liter for MCF, 124 mg/liter for PSC, and 96 mg/liter for VRC. The stock solutions of ANF, CSF, ISC, MCF, PSC, and VRC were spiked into plasma samples that were diluted 10 times with phosphate-buffered saline (PBS) to obtain working standard solutions containing the analytes. The calibration standards (CS) were prepared by further diluting the working standards with diluted plasma, since the preparation of CS in cell samples was not considered feasible. Diluted plasma samples were considered to be the most reliable matrix condition. The calibration curves consisted of eight standards containing the concentrations listed in Table S1 in the supplemental material. All CS solutions were stored at −20°C until analyzed.
Sample processing.
Whole blood was collected in two EDTA-treated tubes (16 ml). Blood was diluted with PBS to a volume of 24 ml and carefully layered onto a double-discontinuous Ficoll-Hypaque density gradient (Histopaque 1077 and 1119; Sigma-Aldrich, Munich, Germany) with equal volumes and spun at 850 × g for 30 min at 20°C. After centrifugation, the plasma was drawn from the upper layer, diluted 10 times with PBS, and stored at −20°C until used. PBMC and PMN were collected on the corresponding layers at densities ranging from 1.024 to 1.077 g/ml and 1.077 to 1.119 g/ml, respectively. A volume of 500 μl RBC was collected from the bottom of the tube. To ensure that the PBMC and PMN cells fractions were free of RBC, a hypotonic lysis with phosphate buffer (pH 7.4) was performed just before the washing steps. The cells were washed twice with PBS (pH 7.4, 150 mM). The absence of antifungals in the washing solution was checked by taking 500 μl of each solution. Counts were determined in a Neubauer chamber, and the cell pellets were stored at −20°C until analysis. The volume of each pellet was calculated based on the total cell count and the mean cell volume, i.e., 0.4 pl for PBMC, 0.334 pl for PMN, and 0.09 pl for RBC. The isolated PBMC and PMN cells (∼107 cells, i.e., 3 or 4 μl) were extracted with 100 μl acetonitrile containing internal standard by sonication for 10 min, vortexing for 1 min, and centrifugation for 10 min at 4°C and 15,000 × g. The calibration standards and diluted plasma samples, 30 μl for each preparation, were treated analogously. For the extraction of the RBC, 1 ml, instead of 100 μl, acetonitrile containing the internal standard was used.
Chromatography.
Aliquots containing 20 μl of the clear supernatants were injected onto the BetaBasic C4 column (100 by 3.0 mm, 5 μm) with its corresponding guard column (10 by 3.0 mm). The temperature of the column was maintained at 30°C, and the tray temperature in the autosampler was kept at 4°C. The system was equilibrated until the stability of total ion current (TIC) was reached for a minimum of 2 h. Subsequent to sample injection, the autosampler syringe and the injection needle were repeatedly rinsed with methanol-water (20:80 [vol/vol]). A gradient program was worked out for HPLC separation with a constant flow rate of 250 μl/min. The initial composition of eluents A and B was 50% each. Within 3.2 min, the fraction of eluent B was increased from 50% to 75% and increased further to 90% within 0.3 min. This composition of 10% eluent A and 90% eluent B remained constant for 3.3 min, before the composition was restored to 50% eluent A and 50% eluent B within 1.2 min. The retention times (RT) were 2.3 min for CSF and CIS, 4.0 min for MCF and VRC, 4.4 min for PSC, 4.6 min for ANF, 5.8 min for AIS, and 5.9 min for ISC (Table 1). The total duration of each run was 8 min.
TABLE 1.
Monitored transitions, retention times, and selected internal standardsa
| Compound | Mass transition (m/z) | Collision energy (eV) | Retention time (min) | Selected internal standard |
|---|---|---|---|---|
| ANF | 1,140.8 → 1,122.5 | 18 | 4.6 | CIS |
| CSF | 547.4 → 137.1 | 34 | 2.3 | CIS |
| CIS | 548.0 → 62.2 | 20 | 2.3 | NA |
| ISC | 438.2 → 224.0 | 28 | 5.9 | AIS |
| AIS | 705.2 → 392.1 | 40 | 5.8 | NA |
| MCF | 1,270.9 → 1,172.4 | 25 | 4.0 | CIS |
| PSC | 701.3 → 683.2 | 34 | 4.4 | AIS |
| VRC | 350.1 → 127.0 | 38 | 3.7 | AIS |
ANF, anidulafungin; CSF, caspofungin; CIS, caspofungin internal standard; ISC, isavuconazole; AIS, azole internal standard; ITC, itraconazole; MCF, micafungin; PSC, posaconazole; VRC, voriconazole.
Mass spectrometric conditions.
The detection was performed by a TSQ Quantum mass spectrometer fitted with an ESI source operating in positive ion and selected reaction monitoring mode. The following settings resulted in the best ion yield: capillary voltage, 3.4 kV; and desolvation temperature of heated capillary, 350°C. Nitrogen was used as sheath and auxiliary gas, set to 37 and 4 (arbitrary units), respectively. The mass spectrometer operated with a full width at half maximum (FWHM) of 0.7 Th for Q1 and Q3. The detection of the analytes was performed in SRM mode with a scan width of 0.5 Th for all channels. The argon collision gas pressure was set to 1.5 mtorr. The collision energy was set to 18 eV for ANF, 34 eV for CSF, 20 eV for CIS, 28 eV for ISC, 40 eV for AIS, 25 eV for MCF, 34 eV for PSC, and 38 eV for VRC. The following SRM transitions of [M+H]+ precursor ions to product ions were selected for each analyte (Table 1): m/z 1,140.8 → 1,122.5 for ANF; m/z 547.4 → 137.1 for CSF; m/z 548.0 → 62.2 for CIS; m/z 438.2 → 224.0 for ISC; m/z 705.2 → 392.1 for AIS; m/z 1270.9 → 1,172.4 for MCF; m/z 701.3 → 683.2 for PSC; and m/z 350.1 → 127.0 for VRC. The scan time (dwell time) for the SRM channels was set to 200 ms for all analytes.
Integration of peaks.
The Thermo Finnigan processing software LCquan (version 1.3) was used to integrate the peaks using the Interactive Chemical Information System (ICIS) peak detection and integration algorithm, which has advanced peak detection efficiency at low MS signal levels. To improve graphical appearance and hence the peak detection, the boxcar smoothing algorithm was applied to reduce the noise levels. All chromatograms were reviewed and, if necessary, reintegrated manually. However, if the change of the peak area was negligible (<1%) compared to the method setting, the integration was reset to the method setting.
Calibration and calculation.
At the end of each sample queue (maximum of 30 samples), the calibration curves were recorded using a minimum of three sets of eight different known concentrations of analytes (excluding blank values) (see Table S1 in the supplemental material). A weighted (1/concentration) linear least-squares regression of the peak area ratios of analyte to internal standard versus the concentration was used to obtain the calibration curves. These curves were then used to calculate the concentrations of the analytes.
Method validation.
The validation of the quantitation method was performed with respect to linearity, intra- and interday accuracy, and precision. The accuracy was calculated by the difference between observed and nominal concentrations expressed as percent bias. The precision was evaluated as the standard deviation of the observed concentration divided by the mean concentration expressed as percent relative standard deviation (RSD). The lower limit of detection (LOD) was calculated using the equation LOD = (3.3σ)/S′, where σ is the standard deviation for the calibration curve and S′ is its slope (20). For echinocandin antifungals, the lower limit of quantitation (LLOQ) was defined as the lowest concentration within the calibration range with an RSD of less than 20%. The LLOQ of the azoles was not covered by the calibration range; hence, it was determined using the equation LLOQ = (10σ)/S′, thus implying a signal-to-noise (S/N) ratio of 10 for the LLOQ. The stability of samples of all concentrations was investigated after four freeze-and-thaw cycles. During each cycle, the samples were thawed at room temperature for 1 h and again frozen at −20°C overnight. The recovery of the analytes from diluted blank plasma was assessed by comparing the peak intensities of the extracted samples in replicates of three with those of the unextracted standards, representing 100% extraction efficiency.
RESULTS
The calibration curves of all analytes were linear and fitted by using least squares with a weighting factor of the reciprocal concentration (1/x). The correlation coefficients of these curves were 0.99 or better. The precisions and accuracies were evaluated over the entire concentration range (see Table S2 in the supplemental material). The accuracies were within ±13.8% for ISC, ±10.9% for PSC, and ±12.2% for VRC. The intraday precisions (RSD) were within ±8.8%, ±6.3%, and ±6.9% for ISC, PSC, and VRC, respectively, while the interday assays of the azoles (i.e., ISC, PSC, and VRC) showed a variability of up to 12.8%.
The estimated LLOQs for the azoles were 4.5, 10, and 4.2 ng/ml for ISC, PSC, and VRC, respectively. For ANF, CSF, and MCF, the LLOQ was reached at 64, 108, and 160 ng/ml, respectively. The LODs and LLOQs are shown in Table 2. Above the LLOQ, the accuracies of the echinocandins were within ±11.7%, ±11.9%, and ±11.1% for ANF, CSF, and MCF, respectively. The intraday precisions (RSD) of the echinocandins above the LLOQ were ±15.4% for ANF, ±8.1% for CSF, and ±8.0% for MCF. The respective intraday and interday assays of all analytes are summarized in Table S2 in the supplemental material. In all stability tests (see Table S3 in the supplemental material), no significant decrease in drug concentrations was substantiated, i.e., mean changes in concentrations of the analytes were within the variability of the method.
TABLE 2.
LOD and LLOQa
| Compound | LOD (ng/ml) | LLOQ (ng/ml) |
|---|---|---|
| ANF | 8.3 | 64 |
| CSF | 31.5 | 108 |
| ISC | 1.5 | 4.5* |
| MCF | 97.7 | 160 |
| PSC | 3.3 | 10* |
| VRC | 1.4 | 4.2* |
Limit of detection (LOD = 3.3σ/S′; σ, standard deviation; S′, slope of the calibration curve) and lower limit of quantification (LLOQ) for ANF, CSF, ISC, MCF, PSC, and VRC. *, estimated: LLOQ = 10σ/S′.
The mean extraction efficiencies from diluted plasma were 94.0% for ANF, 68.0% for CSF, 78.0% for CIS, 89.5% for ISC, 93.7% for MCF, 98.1% for AIS, 93.3% for PSC, and 91.0% for VRC (see Table S4 in the supplemental material).
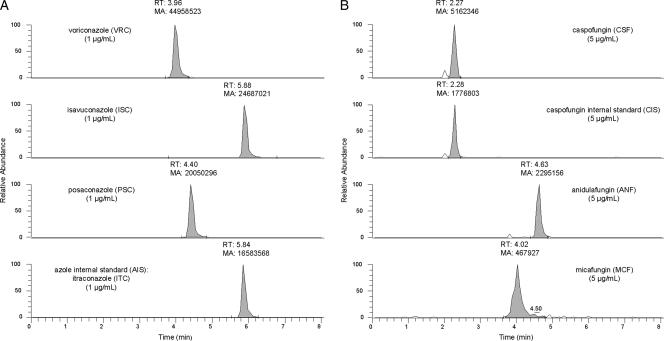
Figure 1 shows a chromatogram of diluted pooled blank plasma (Institute of Transfusion Medicine, Cologne, Germany) spiked with 5 μg/ml ANF, 5 μg/ml CSF, 5 μg/ml CIS, 1 μg/ml ISC, 1 μg/ml AIS, 5 μg/ml MCF, 1 μg/ml PSC, and 1 μg/ml VRC in one sample. The signals are clearly separated and well shaped. A chromatogram of pooled blank plasma is shown in Fig. S1 in the supplemental material.
FIG. 1.
Chromatogram of azole and echinocandin antifungals. (A) Azole antifungals voriconazole (VRC), isavuconazole (ISC), and posaconazole (PSC) and the azole internal standard (AIS) itraconazole (ITC), with 1 μg/ml each; (B) echinocardin antifungals caspofungin (CSF), anidulafungin (ANF), and micafungin (MCF) and the caspofungin internal standard (CIS), with 5 μg/ml each in spiked diluted plasma, demonstrating clearly separated and well-shaped peaks for all analytes.
Clinical application.
The quantitation method is currently in use to analyze samples collected as part of the Cologne biobank protocol (University of Cologne Ethics Committee; 08-160) on Improving Diagnosis of Severe Infections in Immunocompromised Patients (ISI) (9). Figures S2 to S4 in the supplemental material show representative chromatograms of PSC in PBMC, PMN, and RBC extracted from whole-blood samples from a patient receiving PSC at 200 mg three times a day (t.i.d.).
DISCUSSION
We describe a sensitive LC-MS/MS method to determine concentrations of ANF, CSF, ISC, MCF, PSC, and VRC in different compartments of the peripheral blood. This method can be used to measure this selection of antifungals by only one method, hence replacing various other methods currently used in parallel and thus optimizing the cost effectiveness of antifungal therapeutic drug monitoring, while ensuring a rapid turnaround (<1 day), regardless of the variety of antifungals currently in use. However, therapeutic drug monitoring is not a standard for the treatment with echinocandins, and it is unknown whether TDM in this setting has the potential to increase the efficacy of those regimens.
The lower limit of quantitation (LLOQ) was reached at 64, 108, and 160 ng/ml for ANF, CSF, and MCF, respectively. However, since the injection volume (20 μl) is relatively small compared to the extraction volume of 100 μl, a dry freezer could be facilitated to constrain the extract and therefore improve the sensitivity of the method.
Pascual et al. and Ballesta et al. both used radiometric assays to determine the intracellular uptake and elution of FLC and VRC, respectively (2, 26). Conte et al. as well as Nicasio et al. described methods (7, 23) which were modified for measuring PSC and MCF in bronchoalveolar lavage fluid and alveolar macrophages (7, 23). Although those authors presented a higher sensitivity, such low concentrations are actually not required for the purpose of our method. Our method was optimized with regard to the injection and sample volumes. In a previous report, an LC-MS/MS method developed for ISC in plasma and urine was also more sensitive than the method presented here (31, 32), but, compared to our method, a larger sample volume was required (50 μl versus 30 μl). Compared with published LC-MS/MS procedures for VRC in blood (13) and aqueous humor (40), the sensitivity of our method is similar to that of the published methods.
At present, no LC-MS/MS method for the determination of MCF has been published. A previous HPLC method for quantitation of MCF in plasma showed an LLOQ of 50 ng/ml using a 100-μl sample (24, 39). Despite efforts to enhance the signal intensity of MCF, we were not able to achieve the same signal intensities as that for the other molecules. There is also a tailing of MCF during chromatography (Fig. 1). Only a complete change of the gradient program would significantly improve the peak quality of MCF but would probably result in a significant loss in the peak quality of the other analytes. Two LC-MS/MS methods for ANF in human plasma, using either efavirenz (11) or loradine (12) as an internal standard, were described previously. However, of the internal standards used, efavirenz and loradine, neither is a member of the echinocandin class nor has a molecular mass (316 and 383 g/mol, respectively) similar to that of ANF (1,140 g/mol). Their chemical structures and physicochemical properties evidently differ largely from those of ANF. We used as internal standards structurally similar compounds, which mimic the properties of the analytes more closely.
The quantitation of CSF in human plasma samples by LC-MS/MS was previously described by Chavez-Eng et al. (6), Egle et al. (14), and Rochat et al. (29). In the method published by Chavez-Eng et al., similar product ions were acquired for both CSF and CIS. Rochat et al. and Egle et al. used an ion transition with different product ions (m/z 547.4 → 137.1 for CSF; m/z 548.0 → 62.2 for CIS). Chavez-Eng et al.'s LC-MS/MS method deployed pneumatically assisted electrospray (ion spray [ISP]) and turbo ISP (TISP) interfaces with injection volumes of 50 and 75 μl, respectively. Following a solid-phase extraction, with a sample volume of 1 ml plasma, the eluent was evaporated and reconstituted in a 100-μl mobile phase. Owing to the large sample volume, the LLOQs of this method were 10 ng/ml and 2.5 ng/ml using the ISP and TISP interface, respectively (6). Rochat et al. presented two different precipitation methods for the sample preparation, with sample volumes of 500-μl and 100-μl plasma samples, achieving LLOQs of 5 and 40 ng/ml, respectively. Like Rochat et al., we also deployed a precipitation method for the sample preparation; however, due to the fact that our sample volume was smaller (30 μl), our LLOQ was greater (108 ng/ml). The method described by Egle et al. consists of a column-switching technique, with inline extraction of CSF. The LLOQ of this method was 200 ng/ml using a 5-μl plasma sample. This column-switching method was too specific for our aim, and we had to make concessions with regard to sensitivity, while we developed a rapid method suitable for quantitating 6 antifungals in different compartments of the peripheral blood with a single method.
Supplementary Material
Acknowledgments
This work was supported by unrestricted grants from Astellas Pharma GmbH (Munich, Germany), Essex Pharma GmbH (Munich, Germany), and Pfizer GmbH (Karlsruhe, Germany).
Footnotes
Published ahead of print on 22 February 2010.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Astellas Pharma US I. 22 January 2008, posting date. Micafungin label information. Astellas Pharma US, Inc., Deerfield, IL.
- 2.Ballesta, S., I. Garcia, E. J. Perea, and A. Pascual. 2005. Uptake and intracellular activity of voriconazole in human polymorphonuclear leucocytes. J. Antimicrob. Chemother. 55:785-787. [DOI] [PubMed] [Google Scholar]
- 3.Bopp, L. H., A. L. Baltch, W. J. Ritz, P. B. Michelsen, and R. P. Smith. 2006. Antifungal effect of voriconazole on intracellular Candida glabrata, Candida krusei and Candida parapsilosis in human monocyte-derived macrophages. J. Med. Microbiol. 55:865-870. [DOI] [PubMed] [Google Scholar]
- 4.Boyd, A. E., S. Modi, S. J. Howard, C. B. Moore, B. G. Keevil, and D. W. Denning. 2004. Adverse reactions to voriconazole. Clin. Infect. Dis. 39:1241-1244. [DOI] [PubMed] [Google Scholar]
- 5.Brummer, E., P. R. Bhagavathula, L. H. Hanson, and D. A. Stevens. 1992. Synergy of itraconazole with macrophages in killing Blastomyces dermatitidis. Antimicrob. Agents Chemother. 36:2487-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chavez-Eng, C. M., M. Schwartz, M. L. Constanzer, and B. K. Matuszewski. 1999. Determination of a cyclic hexapeptide, a novel antifungal agent, in human plasma by high-performance liquid chromatography with ion spray and turbo ion spray tandem mass spectrometric detection. J. Chromatogr. B Biomed. Sci. Appl. 721:229-238. [DOI] [PubMed] [Google Scholar]
- 7.Conte, J. E., Jr., J. A. Golden, G. Krishna, M. McIver, E. Little, and E. Zurlinden. 2009. Intrapulmonary pharmacokinetics and pharmacodynamics of posaconazole at steady state in healthy subjects. Antimicrob. Agents Chemother. 53:703-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornely, O. A., A. Stollorz, C. Beisel, et al. 2007. Impact of posaconazole prophylaxis on the epidemiology of invasive aspergillosis, abstr. M-1175. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. Chicago, IL.
- 9.Cornely, O. A., J. J. Vehreschild, F. Farowski, and M. J. G. T. Rüping. 2008. Improving diagnosis of severe infections in immunocompromised patients (ISI). Klinik I für Innere Medizin, Koln, Germany. http://www.klinisches-studienzentrum.de/med1/en/trial/552.
- 10.Courtney, R., S. Pai, M. Laughlin, J. Lim, and V. Batra. 2003. Pharmacokinetics, safety, and tolerability of oral posaconazole administered in single and multiple doses in healthy adults. Antimicrob. Agents Chemother. 47:2788-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowell, J. A., J. Schranz, A. Baruch, and G. Foster. 2005. Safety and pharmacokinetics of coadministered voriconazole and anidulafungin. J. Clin. Pharmacol. 45:1373-1382. [DOI] [PubMed] [Google Scholar]
- 12.Dowell, J. A., M. Stogniew, D. Krause, T. Henkel, and B. Damle. 2007. Lack of pharmacokinetic interaction between anidulafungin and tacrolimus. J. Clin. Pharmacol. 47:305-314. [DOI] [PubMed] [Google Scholar]
- 13.Egle, H., R. Trittler, A. Konig, and K. Kummerer. 2005. Fast, fully automated analysis of voriconazole from serum by LC-LC-ESI-MS-MS with parallel column-switching technique. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 814:361-367. [DOI] [PubMed] [Google Scholar]
- 14.Egle, H., R. Trittler, and K. Kummerer. 2004. An advanced double column-switching technique (LC-LC) for liquid chromatography/electrospray ionisation tandem mass spectrometry for fully automated analysis of caspofungin. Rapid Commun. Mass Spectrom. 18:2871-2877. [DOI] [PubMed] [Google Scholar]
- 15.Glasmacher, A., C. Hahn, C. Leutner, E. Molitor, E. Wardelmann, C. Losem, T. Sauerbruch, G. Marklein, and I. G. Schmidt-Wolf. 1999. Breakthrough invasive fungal infections in neutropenic patients after prophylaxis with itraconazole. Mycoses 42:443-451. [DOI] [PubMed] [Google Scholar]
- 16.Gubbins, P. O., G. Krishna, A. Sansone-Parsons, S. R. Penzak, L. Dong, M. Martinho, and E. J. Anaissie. 2006. Pharmacokinetics and safety of oral posaconazole in neutropenic stem cell transplant recipients. Antimicrob. Agents Chemother. 50:1993-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reference deleted.
- 18.Hope, W. W., E. M. Billaud, J. Lestner, and D. W. Denning. 2008. Therapeutic drug monitoring for triazoles. Curr. Opin. Infect. Dis. 21:580-586. [DOI] [PubMed] [Google Scholar]
- 19.Hyland, R., B. C. Jones, and D. A. Smith. 2003. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab. Dispos. 31:540-547. [DOI] [PubMed] [Google Scholar]
- 20.ICH. November 2005, posting date. Validation of analytical procedures: text and methodology Q2(R1). International Conference on Harmonisation, Geneva, Switzerland.
- 21.Imhof, A., D. J. Schaer, U. Schanz, and U. Schwarz. 2006. Neurological adverse events to voriconazole: evidence for therapeutic drug monitoring. Swiss Med. Wkly. 136:739-742. [DOI] [PubMed] [Google Scholar]
- 22.Merck & Co., I. 29 July 2008, posting date. Caspofungin label information. Merck & Co., Whitehouse Station, NJ.
- 23.Nicasio, A. M., P. R. Tessier, D. P. Nicolau, R. F. Knauft, J. Russomanno, E. Shore, and J. L. Kuti. 2009. Bronchopulmonary disposition of micafungin in healthy adult volunteers. Antimicrob. Agents Chemother. 53:1218-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niwa, T., Y. Yokota, A. Tokunaga, Y. Yamato, A. Kagayama, T. Fujiwara, J. Hatakeyama, M. Anezaki, Y. Ohtsuka, and A. Takagi. 2004. Tissue distribution after intravenous dosing of micafungin, an antifungal drug, to rats. Biol. Pharm. Bull. 27:1154-1156. [DOI] [PubMed] [Google Scholar]
- 25.Pascual, A., T. Calandra, S. Bolay, T. Buclin, J. Bille, and O. Marchetti. 2008. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 46:201-211. [DOI] [PubMed] [Google Scholar]
- 26.Pascual, A., I. Garcia, C. Conejo, and E. J. Perea. 1993. Uptake and intracellular activity of fluconazole in human polymorphonuclear leukocytes. Antimicrob. Agents Chemother. 37:187-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfizer. 16 February 2006, posting date. Anidulafungin label information. Pfizer, New York, NY.
- 28.Poirier, J. M., and G. Cheymol. 1998. Optimisation of itraconazole therapy using target drug concentrations. Clin. Pharmacokinet. 35:461-473. [DOI] [PubMed] [Google Scholar]
- 29.Rochat, B., S. Bolay, A. Pascual, T. Calandra, and O. Marchetti. 2007. Liquid chromatography-mass spectrometry method for quantification of caspofungin in clinical plasma samples. J. Mass Spectrom. 42:440-449. [DOI] [PubMed] [Google Scholar]
- 30.Schering. 19 February 2009, posting date. Noxafil product information. Schering, Kenilworth, NJ.
- 31.Schmitt-Hoffmann, A., B. Roos, M. Heep, M. Schleimer, E. Weidekamm, T. Brown, M. Roehrle, and C. Beglinger. 2006. Single-ascending-dose pharmacokinetics and safety of the novel broad-spectrum antifungal triazole BAL4815 after intravenous infusions (50, 100, and 200 milligrams) and oral administrations (100, 200, and 400 milligrams) of its prodrug, BAL8557, in healthy volunteers. Antimicrob. Agents Chemother. 50:279-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitt-Hoffmann, A., B. Roos, J. Maares, M. Heep, J. Spickerman, E. Weidekamm, T. Brown, and M. Roehrle. 2006. Multiple-dose pharmacokinetics and safety of the new antifungal triazole BAL4815 after intravenous infusion and oral administration of its prodrug, BAL8557, in healthy volunteers. Antimicrob. Agents Chemother. 50:286-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reference deleted.
- 34.Smith, J., and D. Andes. 2008. Therapeutic drug monitoring of antifungals: pharmacokinetic and pharmacodynamic considerations. Ther. Drug Monit. 30:167-172. [DOI] [PubMed] [Google Scholar]
- 35.Tan, K., N. Brayshaw, K. Tomaszewski, P. Troke, and N. Wood. 2006. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J. Clin. Pharmacol. 46:235-243. [DOI] [PubMed] [Google Scholar]
- 36.Trifilio, S., G. Pennick, J. Pi, J. Zook, M. Golf, K. Kaniecki, S. Singhal, S. Williams, J. Winter, M. Tallman, L. Gordon, O. Frankfurt, A. Evens, and J. Mehta. 2007. Monitoring plasma voriconazole levels may be necessary to avoid subtherapeutic levels in hematopoietic stem cell transplant recipients. Cancer 109:1532-1535. [DOI] [PubMed] [Google Scholar]
- 37.Ullmann, A. J., O. A. Cornely, A. Burchardt, R. Hachem, D. P. Kontoyiannis, K. Topelt, R. Courtney, D. Wexler, G. Krishna, M. Martinho, G. Corcoran, and I. Raad. 2006. Pharmacokinetics, safety, and efficacy of posaconazole in patients with persistent febrile neutropenia or refractory invasive fungal infection. Antimicrob. Agents Chemother. 50:658-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walsh, T. J., I. Raad, T. F. Patterson, P. Chandrasekar, G. R. Donowitz, R. Graybill, R. E. Greene, R. Hachem, S. Hadley, R. Herbrecht, A. Langston, A. Louie, P. Ribaud, B. H. Segal, D. A. Stevens, J. A. van Burik, C. S. White, G. Corcoran, J. Gogate, G. Krishna, L. Pedicone, C. Hardalo, and J. R. Perfect. 2007. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin. Infect. Dis. 44:2-12. [DOI] [PubMed] [Google Scholar]
- 39.Yusuhiro, Y., K. Hayato, T. Kaoru, K. Masataka, I. Koji, K. Akio, T. Masato, and K. Akira. 2002. Simultaneous determination of antifungal drug, micafungin, and its two active metabolites in human plasma using high-performance liquid chromatography with fluorescence detection. Jpn. J. Chemother. 50:68-73. [Google Scholar]
- 40.Zhou, L., R. D. Glickman, N. Chen, W. E. Sponsel, J. R. Graybill, and K. W. Lam. 2002. Determination of voriconazole in aqueous humor by liquid chromatography-electrospray ionization-mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 776:213-220. [DOI] [PubMed] [Google Scholar]
- 41.Zonios, D. I., J. Gea-Banacloche, R. Childs, and J. E. Bennett. 2008. Hallucinations during voriconazole therapy. Clin. Infect. Dis. 47:e7-e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.